Abstract
Objective:
Alcohol use disorder (AUD) remains an urgent public health problem. Longitudinal data are needed to clarify the role of acute subjective responses to alcohol in the development and maintenance of excessive drinking and AUD. We now report on 10-years of repeated examination of acute alcohol responses in the Chicago Social Drinking Project.
Methods:
Young adult drinkers (N=190) participated in an initial alcohol challenge testing (0.8 g/kg alcohol vs. placebo) that was repeated 5 and 10 years later. They were also assessed on drinking behavior and AUD symptoms at numerous intervals over the decade. Retention was high as 184 of the 186 (99%) non-deceased active participants completed the 10-year follow-up, and 91% (163/179) of those eligible for alcohol consumption engaged in repeated laboratory testing at this interval.
Results:
At the end of the decade, 21% of participants met criteria for past year AUD. Individuals who reported the greatest alcohol stimulation, liking, and wanting at the initial alcohol challenge were most likely to have developed AUD 10 years later. Further, alcohol-induced stimulation and wanting increased in re-examination testing among those with the highest AUD symptoms as the decade progressed.
Conclusions:
Initial stimulant and rewarding effects of alcohol predicted heavy alcohol use and the magnitude of these positive subjective effects increased over a 10-year period in those developing AUD versus those who did not develop the disorder. The findings demonstrate systematic changes in subjective responses to alcohol over time, providing an empirical basis for prevention, early intervention and treatment strategies.
Keywords: alcohol response, stimulation, reward, incentive-sensitization, allostasis, alcohol use disorder (AUD)
PLAIN LANGUAGE SUMMARY
Knowledge of factors that lead to and sustain excessive drinking are crucial in developing effective prevention and intervention programs. The authors provided the first repeated assessment of acute alcohol responses in the same people over a substantial period of time. They found that persons developing alcohol use disorder had higher initial pleasurable responses to alcohol and these stimulating and rewarding responses were magnified over time. This suggests that long-held views of tolerance to alcohol’s pleasurable effects in those becoming addicted were not substantiated.
INTRODUCTION
Heavy drinking is increasing in US adults (1–3) and remains a major preventable contributor to disability and mortality worldwide (4). It is also a strong predictor of subsequent alcohol use disorder (AUD) (5,6) which carries additional serious consequences for health and functioning (7). Given the global burden of alcohol misuse, identifying factors that increase the susceptibility to the development and maintenance of AUD is a critical public health need (8).
One way to examine vulnerability to AUD is to characterize acute subjective responses to alcohol at different stages of development of the disorder (9). Two large prior studies found that greater initial stimulation and reward responses to alcohol (10,11), as well as lesser intoxicating and sedating effects (12) predict future drinking problems through young adulthood. These acute responses may change with chronic heavy drinking, due to neuroadaptations that result in either tolerance or sensitization. Tolerance, or a diminished response to the same dose of a drug after repeated use, is a diagnostic criterion for AUD. Conversely, sensitization, or an increase drug effect with repeated exposure, has also been linked to addiction (13,14). Although both tolerance and sensitization are integral components of neurobiological theories of the development and progression of addiction (14–16), most of the empirical evidence is based on studies using animal models.
Examination of adaptive responses to alcohol in humans requires rigorous longitudinal investigations in persons developing AUD or progressing in severity of the disorder. Such prospective, repeated-measurement studies of acute alcohol responses are challenging, time- and labor-intensive, and require high retention rates. Yet the knowledge from such an investigation is necessary to adequately test the translational significance of animal models of addiction to humans (17) and thus inform empirically-based strategies for prevention, early intervention and treatment. Whether the excitatory, euphoric, or sedating effects of alcohol increase, decrease, or remain constant over time in problem drinkers an unresolved issue in clinical psychiatry.
In the Chicago Social Drinking Project (CSDP), we have undertaken such an analysis in our first cohort and documented that, compared with lighter drinkers, young adult heavy drinkers were more sensitive to the stimulating, motivating (“wanting”), and rewarding effects (“liking”) of alcohol, and were less sensitive to its sedative effects (10). Although stimulation and sedation were inversely correlated (18), we found that higher alcohol stimulation, wanting, and liking, and not lower sedation, predicted progression of AUD symptoms 5–6 years after the initial alcohol challenge in heavy drinkers (11). Further, when participants were re-tested with alcohol 5–6 years later, heavy drinkers who developed more AUD symptoms reported persistently greater stimulation, wanting and liking after consuming alcohol (at both initial test and 5 years later), whereas light drinkers did not report experiencing these euphoric and motivating responses (19). However, the testing was limited to a five year re-examination period when participants were just entering their fourth decade of life which may not have been sufficient to fully examine AUD development. In addition, the previous report focused mainly on heavy drinkers and did not allow for an integrated examination of the trajectory of both light and heavy drinkers.
We now report on a more comprehensive and extended 10-year follow-up and re-examination of acute responses to alcohol in the CSDP. We shift the analytical focus to examine differences in the acute responses to alcohol, based on who, among the entire sample, did or did not manifest AUD after a decade of natural drinking. The approach comprises a three-phase assessment, i.e., initial, 5- and 10-yr examinations of alcohol responses in young adulthood through middle age. We evaluated the existence of adaptive changes in subjective responses to alcohol consumption in the development and progression of addiction. Our goal was to determine whether AUD is marked by increases, decreases, or no change in the sensitivity to alcohol’s stimulating, motivating, rewarding, or sedating effects.
METHODS
The CSDP is a multi-cohort, within-subject, double-blinded study with repeated, within-subject laboratory assessments of acute responses to alcohol vs. placebo, combined with long-term follow-up of drinking behaviors and AUD symptoms. The study was approved by the University of Chicago Institutional Review Board and participants provided written information consent. The project conducted testing sessions at the Clinical Addictions Research Laboratory at the University of Chicago. Participants undertook initial laboratory sessions from March 2004 to July 2006. They underwent regular follow-up interviews and were invited to participate in identical, double-blinded alcohol and placebo re-examinations five and ten years later (see CONSORT diagram, Suppl. Figure 1).
Initial screening and enrollment
Recruitment employed local media and internet advertisements and word-of-mouth referrals. Initial inclusion criteria were: age 21–35 years, weight 110–210 pounds, good general health, not pregnant or lactating, no current or past major medical or psychiatric disorders including DSM-IV alcohol and substance dependence (other than nicotine), and no current use of any psychotropic medications. Participants were included if they met criteria for either a high or low-risk drinker. This distinction was defined by a predominant adult pattern of drinking (and minimally for the past two years). High-risk, heavy drinkers reported weekly binge drinking of ≥5 drinks for men, ≥4 for women, and regular consumption of 10–40 drinks per week. Low-risk, light drinkers reported rare/no binges and regular consumption of 1–5 drinks per week. These criteria were based upon established high- and low-risk drinking guidelines (20,21) and were consistent with prior studies (22–25). Candidates underwent medical and psychiatric screening to determine eligibility for an alcohol challenge (for details, see Suppl. Methods).
Laboratory Procedures
At each of the three testing phases, participants attended two individual 4–5 hour laboratory sessions; one with alcohol, the other placebo, in randomized order and under double blind conditions. Sessions were conducted in a comfortable living-room like environment, separated by at least 24 hours. The sessions were identical except for the alcohol content of the beverage, 0.8 g/kg alcohol (adjusted for sex), or placebo with a 1% alcohol taste mask. Upon arrival, objective breath tests confirmed compliance with recent alcohol abstinence, and a urine sample was collected for women to verify non-pregnancy. Subjective, objective and breathalyzer tests were obtained throughout the session (for more details, see Suppl. Methods).
Starting at experimental time 0, the participant received his/her beverages in lidded, clear plastic cups in two equal portions and, in the presence of the research assistant, consumed each portion over two 5 minute periods, separated by a 5 minute rest (22,23,26). The Alternative Substance Paradigm (27) was employed to reduce expectancy effects by informing each participant the beverage may contain a stimulant, sedative, alcohol, a placebo, or a combination of these substances. At the end of each approximate 4-hour session, when breath alcohol concentration (BrAC) was <0.04 g/dl (28), the participant was transported home by a car service.
Identical procedures and instructions were followed for the 5- and 10-year laboratory testing re-examination phases. For ethical reasons, participants with major medical or psychiatric contraindications (n=3), pregnancy or nursing (n=3), or alcohol abstinence (n=3) were not eligible for the final 10-year re-examination sessions. As nearly half (49%) of the sample no longer resided in the area, transportation to Chicago, lodging accommodations, and other per diem expenses were provided, as warranted. Re-examination sessions were scheduled for the same month as initial testing; the mean (±SD) interval from participants’ initial to year 5 sessions was 61±3.0 months and to year 10 sessions was 122±3.7 months.
Outcome and Measures:
In the testing sessions, measures were obtained before beverage consumption and repeated 30, 60, 120, and 180 minutes after consumption. To maintain blinding, the breathalyzer tests (Alco-Sensor IV, Intoximeter; St. Louis, MO) displayed 0.000 g/dl during real-time assessments with actual values downloaded later. The primary dependent measures were stimulation and sedation subscale scores from the 14-item Biphasic Alcohol Effects Scale (BAES) (29) and two items from the Drug Effects Questionnaire (DEQ; 100 mm visual analogue scale) for hedonic reward (“do you LIKE the effect you are feeling now?”) and motivational salience (“would you like MORE of what you consumed, right now?”) (30,31). The surveys instructed participants to focus on their current mood state and did not reveal the beverage content (32). Stimulation, sedation, liking, and wanting were examined across the BrAC curve by subtracting the placebo from alcohol response at each time point. Secondary measures were general drug (DEQ “feel-drug” item) and physiological responses, including heart rate and salivary cortisol.
Follow-up Assessments
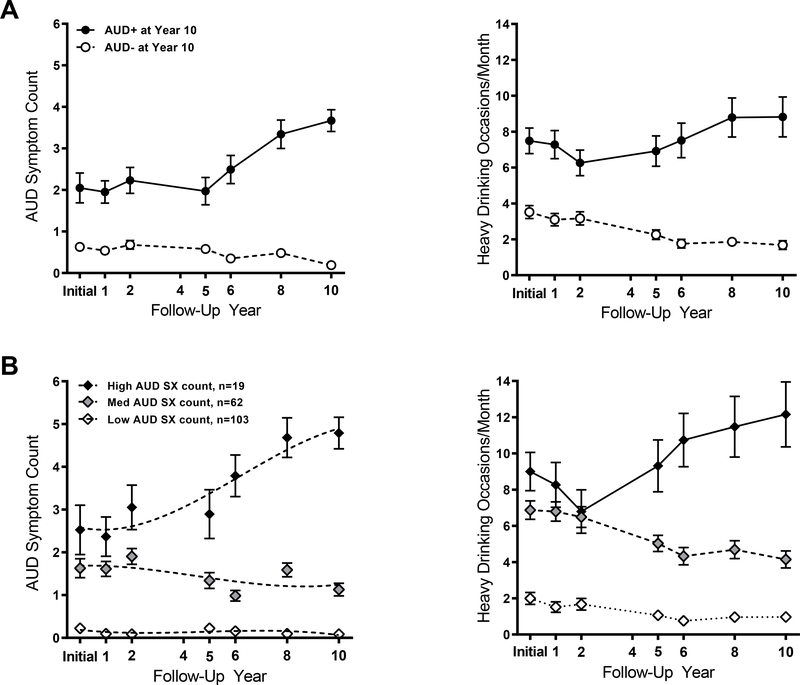
After each participant’s initial testing, follow-up assessments were conducted at 1, 2, 4, 5, 6, 8, and 10 years. No subject was lost to follow-up, i.e., unreachable throughout this decade of participation, reflecting our protocol to attain strong retention (33). There was 98% (186/190) retention of the sample, with one death and three study dropouts. The follow-up assessments included internet or mailed surveys and a telephone or in-person SCID interview to determine AUD symptoms. The main outcome from these assessments was the presence (AUD+) or absence (AUD−) of the disorder in the last year of follow-up. Twenty-one percent of participants (n=39) met DSM-5 criteria for AUD at year 10, forming the AUD+ group. Of these, 20 met criteria for mild AUD, 10 moderate, and 9 severe AUD. The remaining 79% did not meet AUD criteria and comprised the AUD− group. Of these, 124 reported no AUD symptoms and 21 reported one symptom. Reviewing the prior 10 years, the AUD+ group showed more AUD symptoms than the AUD− group, and reported heavier drinking (Figure 1A).
Figure 1: Mean AUD symptom count and heavy drinking occasions per month over 10 years of follow-up for A) AUD+/− (as determined at Year 10) groups and B) AUD SX subgroups.
Data are shown as mean (SEM), with the left panel depicting AUD symptoms at each follow-up interval for each group and the right panel depicting binge drinking frequency over follow-up. A) AUD+ and AUD− groups were identified based on AUD symptom counts per the DSM-5 at Year 10 follow-up. B) Three trajectory subgroups were identified based on symptom count from DSM-IV alcohol abuse and alcohol dependence; 0–11 possible) over 10 years of follow-up occurring at initial testing and years 1, 2, 4, 5, 6, 8, and 10 (dotted lines represent model fits). As the study began before the DSM-5 was developed, symptom counts for years 1–5 were based on the 11 criteria comprising DSM-IV alcohol abuse and dependence (First et al., 2002). For unity, these DSM-IV criteria were also used for follow-ups in years 6–10 for trajectory analysis. Notably, only 1 symptom is different between DSM-IV alcohol abuse and dependence criteria versus those for DSM-5 AUD.
In addition to AUD group classifications based on the 10-year follow-up, we employed a complementary approach to determine trajectory subgroups based on the growth and progression (or regression) in AUD symptom severity throughout all follow-ups (10,11,19). These were based on DSM-IV symptom count as DSM-5 was not yet developed when the follow-ups initiated in 2005. This trajectory analysis (34) included a zero-inflated Poisson mixture model with a cubic trajectory for each subgroup. The number of trajectory groups was determined by model Bayesian Information Criterion (BIC) using 2ln(B10) ≥ 10 as strong evidence for rejecting the null model. This analysis showed that a three-trajectory group model best fit the data, including a low AUD symptom subgroup (low AUD SX, n=103), an intermediate AUD symptom subgroup (intermediate AUD SX, n=62), and a high AUD symptom group (high AUD SX, n=19). The AUD symptoms across these subgroups corresponded to heavy drinking frequency over follow-up (Figure 1B). Of note, the classifications of individuals in the AUD symptom trajectory subgroup were fairly stable, relative to the classifications of initial heavy drinkers in the 5-year analysis (11). Eighty-one percent of the participants remained in their respective low, intermediate, and high AUD SX subgroup, while 15% moved by one category to the next higher SX group, and 4% changed to the next lower group.
Statistical Analysis
Demographic and drinking characteristics were compared between AUD+ and AUD− groups by t-tests and Chi-Square, as appropriate. Acute alcohol responses were analyzed by Generalized Estimating Equations (GEE) (35) models including group, time, and phase with the latter two variables treated as continuous variables. To assess potential nonlinearity over time, models for each alcohol response included linear, square and cubic terms of time. Results revealed that only BAES stimulation (and not sedation, liking or wanting) required the second and third order polynomial terms. This is consistent with prior work showing rapid increases in stimulation during the ascending BrAC limb, followed by an inflection point and sharp declines during the descending BrAC limb (10). GEE models were also used for secondary measures including general subjective response (DEQ feel drug) and physiological effects (heart rate and cortisol secretion) sensitive to alcohol (36–38). The AUD SX subgroups were examined on alcohol responses in GEE models similar to those conducted in the main analysis to examine subgroup, time, and phase effects and their interactions. All GEE models adjusted for age, race, education, FH (39), BrAC (as a time-varying covariate), and the number of baseline AUD symptoms.
RESULTS
CSPD participant retention was high with 99% (184/186) of the non-deceased active participants completing follow-up at 10 years (i.e., 184/190 of original sample, 97%). The 5- and 10-year laboratory re-examination sessions were conducted in 156 and 163 participants, respectively. These rates represent 88% and 91% of the 178 and 179 participants eligible for re-examination at each phase, respectively.
The AUD+ and AUD− groups at 10 years did not differ on most demographic characteristics except, as expected, the AUD+ group reported higher alcohol consumption and drinking problems than the AUD− group at the 10-year re-examination as well as initial testing phase (Table 1). Across the test phases, the groups did not differ significantly on average BrAC (Figure 2) with both showing a rapid rising BrAC limb to peak level 60 minutes after the initiation of the beverage consumption, followed by a slow BrAC declining limb [group x time x phase: χ2 (8)=5.78, p=0.672]. Thus, while reflecting individual variability in alcohol pharmacokinetics, particularly in absorption for oral administration (40), the AUD group means did not substantially differ on BrAC levels across testing phases.
Table 1.
Characteristics of AUD+ and AUD− groups at Year 10 at Initial and Year 10 testing
| AUD+ at Year 10 (n=39) | AUD− at Year 10 (n=145) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Testing | Year 10 | Initial Testing | Year 10 | ||||||
| N | % | N | % | N | % | N | % | Significance Testing | |
| Male | 25 | 64 | -- | -- | 75 | 52 | -- | -- | ns |
| White a | 32 | 82 | -- | -- | 108 | 74 | -- | -- | ns |
| Family history positive (AUD) b | 7 | 18 | -- | -- | 35 | 24 | -- | -- | ns |
| Married/living with partner | 3 | 8 | 17 | 44 | 20 | 14 | 98 | 68 | ns |
| Nicotine dependence c | 6 | 15 | 10 | 26 | 3 | 2 | 6 | 4 | group, p<.01 |
| Substance dependence c | 3 | 8 | 1 | 3 | 1 | 1 | 0 | 0 | ns |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 24.6 | 3.0 | 34.6 | 3.0 | 25.9 | 3.2 | 35.9 | 3.2 | ns |
| Education (years) | 15.5 | 1.7 | 16.4 | 2.1 | 16.2 | 1.8 | 17.7 | 3.0 | ns |
| Beck Depression Inventory | 4.1 | 3.3 | 6.5 | 5.7 | 2.2 | 2.6 | 3.2 | 3.8 | ns |
| Spielberger State Anxiety (t-score) | 49.2 | 8.9 | 51.8 | 7.8 | 43.6 | 6.8 | 46.6 | 5.9 | ns |
| Aspartate aminotransferase (AST; units/L) | 21.7 | 6.7 | 21.0 | 5.3 | 22.6 | 9.5 | 20.5 | 10.4 | ns |
| Alanine aminotransferase (ALT; units/L) | 20.1 | 10.6 | 22.1 | 11.3 | 22.1 | 17.2 | 21.5 | 16.8 | ns |
| Drinking days in past month d | 14.3 | 6.9 | 20.6 | 6.3 | 9.7 | 5.1 | 15.0 | 6.9 | group, p<.05 |
| Heavy drinking days in past month d | 7.5 | 4.5 | 13.7 | 6.6 | 3.5 | 4.3 | 4.9 | 4.9 | group*phase, p<.01 |
| Alcohol problems (AUDIT)e | 11.3 | 5.0 | 18.7 | 6.2 | 6.8 | 4.6 | 8.6 | 5.4 | group*phase, p<.01 |
| AUD symptoms during follow-upe | -- | -- | 4.5 | 1.8 | -- | -- | 1.2 | 1.5 | group, p<.001 |
Note.
Race was provided by participants among a list of options consistent with NIH classifications
Family history positive defined as having one biological primary or two or more biological secondary relatives with AUD
From Structural Clinical Interview for DSM-IV interview indicating number of participants meeting past year criteria at Year 1 and during any follow-up across ten years
From Timeline Follow-Back calendar for the month preceding initial testing and maxima across follow-ups for days that any alcohol was consumed and days with heavy drinking defined as ≥5 drinks per occasion for males and ≥4 drinks for females and based on standard definition of one drink = 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor
From Alcohol Use Disorders Identification Test (AUDIT) and Structural Clinical Interview for DSM interview indicating past year at initial testing and maxima score or number of 11 DSM-IV symptoms met across follow-ups for ten years.
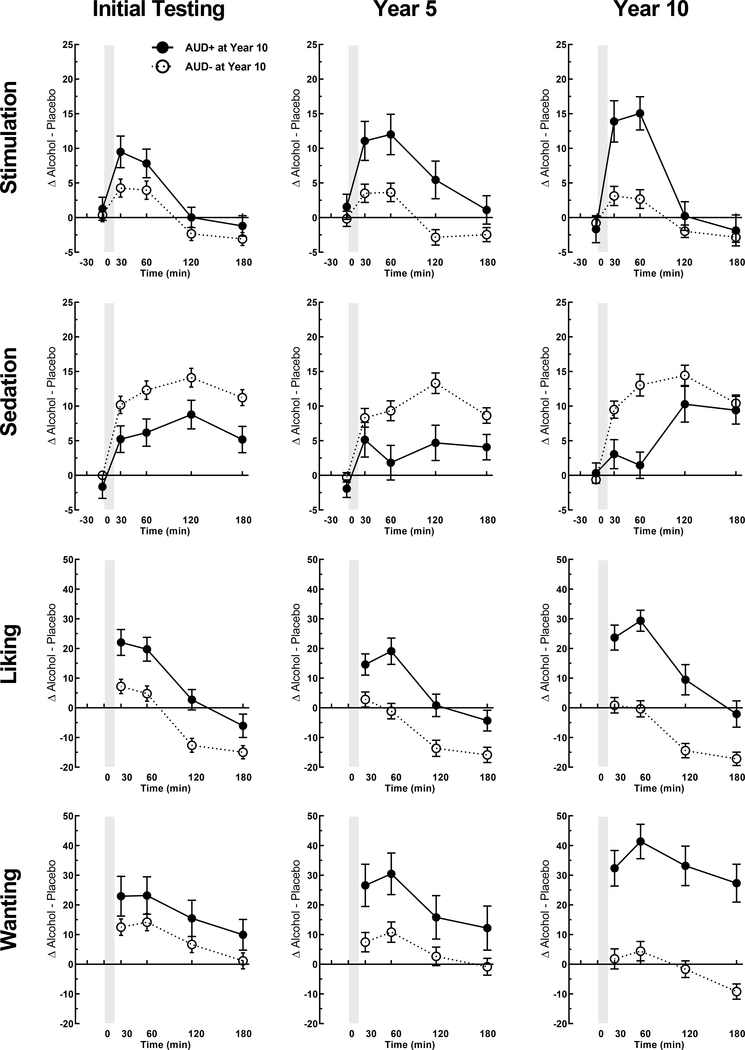
Figure 2: Subjective alcohol responses at initial, 5- and 10-year re-examinations in AUD+ and AUD− groups (as determined at Year 10).
Data are shown as means (±SEM) of change scores (alcohol session minus placebo session) for the four primary subjective response measures each time point and each testing phase. for AUD+ at Year 10 (n=39) and AUD− at Year 10 (n=145). AUD+ and AUD− groups were identified based on AUD symptom counts per the DSM-5 at Year 10 follow-up. Y-axis range for liking and wanting differ slightly given the range of values and that liking is based on a scale with mid-point as neutral. Details on the statistical testing are in Table 3.
In terms of subjective response, alcohol produced markedly different effects in the 10-year AUD+ and AUD− groups. These differences were evident starting at initial testing and increased further over the course of the re-examination phases (Figure 3). Overall, the AUD+ exhibited increasing sensitivity for pleasurable alcohol responses over the decade of participation, while AUD− showed low initial levels on these measures with little to no change through testing phases. Specifically, alcohol produced initially higher stimulation in AUD+ (vs. AUD−) during the early portion of the BrAC curve that increased in intensity through the re-examination phases [group x time x phase, p=0.016; Table 2]. Alcohol also initially increased ratings of motivational salience (wanting) in 10-year AUD+ relative to AUD− across the entire BrAC curve, and wanting also increased in intensity through the re-examination phases [group x phase, p=0.001]. Initial hedonic reward (liking) from alcohol was also higher in the AUD+ [group, p=0.017] and this effect increased through re-examination, but the escalation was not statistically significant [group x phase, p=0.104]. Finally, both groups showed increases in alcohol-induced sedation, but this effect did not differ by group or test phase [group x phase, p>0.385]. Re-analyzing the data based on DSM-IV alcohol dependence yielded similar results.
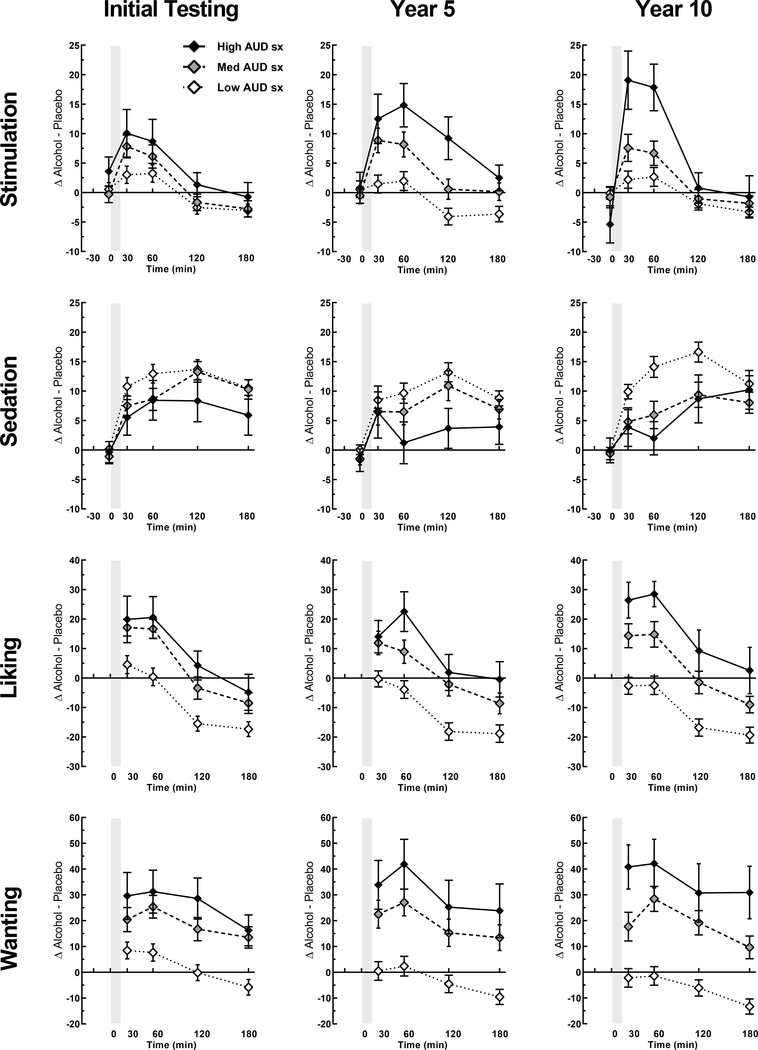
Figure 3: Subjective alcohol responses at initial, 5- and 10-year re-examinations for AUD SX trajectory subgroups.
Data are shown as means (SEM) for low AUD sx count (n=103), intermediate AUD sx count (n=62), and high AUD sx count (n=19) trajectory groups at initial and re-examination phases. Subjective response data are change scores (alcohol session minus placebo session) at each time point. Y-axis range for liking and wanting differ slightly given the range of values and that liking is based on a scale with mid-point as neutral. Details on the statistical testing are in Table 3.
Table 2:
Characteristics of AUD SX Trajectory Subgroups at Initial Testing and Year 10 Testing
| Low AUD SX (n=103) | Intermediate AUD SX (n=62) | High AUD SX (n=19) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Testing | Year 10 | Initial Testing | Year 10 | Initial Testing | Year 10 | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | Significance Testing | |
| Male | 48 | 47 | -- | -- | 40 | 65 | -- | -- | 12 | 63 | -- | -- | ns |
| White a | 75 | 73 | -- | -- | 50 | 81 | -- | -- | 15 | 79 | -- | -- | ns |
| Family history positive (AUD) b | 28 | 27 | -- | -- | 10 | 16 | -- | -- | 4 | 21 | -- | -- | ns |
| Married/living with partner | 15 | 15 | 71 | 69 | 6 | 10 | 37 | 60 | 2 | 10 | 7 | 37 | ns |
| Nicotine dependence c | 2 | 2 | 3 | 3 | 4 | 6 | 6 | 10 | 3 | 16 | 7 | 37 | group, p<.05 |
| Substance dependence c | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 16 | 1 | 5 | ns |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 26.2 | 3.3 | 36.2 | 3.3 | 24.8 | 3.0 | 34.7 | 3.0 | 25.3 | 2.9 | 35.3 | 2.9 | ns |
| Education (years) | 16.4 | 1.8 | 18.0 | 3.2 | 15.9 | 1.5 | 17.0 | 1.8 | 15.1 | 2.2 | 15.6 | 2.2 | group*phase, p<.05 |
| Beck Depression Inventory | 1.8 | 2.3 | 2.9 | 3.2 | 3.2 | 3.3 | 4.0 | 3.6 | 5.1 | 3.8 | 9.1 | 6.2 | group x phase, p<.001 |
| Spielberger State Anxiety (t-score) | 50.1 | 12.7 | 46.5 | 4.6 | 49.6 | 10.1 | 48.2 | 6.9 | 49.2 | 11.4 | 53.4 | 8.2 | group x phase, p<.001 |
| Aspartate aminotransferase (AST; units/L) | 22.2 | 4.8 | 19.3 | 7.4 | 22.5 | 6.8 | 22.8 | 12.9 | 23.0 | 7.7 | 20.8 | 6.5 | ns |
| Alanine aminotransferase (ALT; units/L) | 21.9 | 11.9 | 19.6 | 14.6 | 21.2 | 9.5 | 24.6 | 17.8 | 22.1 | 14.0 | 22.9 | 14.6 | ns |
| Drinking days in past month d | 8.0 | 4.3 | 13.3 | 6.8 | 13.6 | 5.5 | 18.8 | 5.7 | 15.4 | 7.1 | 22.8 | 5.5 | group x phase, p<.01 |
| Heavy drinking days in past month d | 2.0 | 3.4 | 3.0 | 3.8 | 6.9 | 4.0 | 10.0 | 4.5 | 9.0 | 4.6 | 16.7 | 6.9 | group x phase, p<.01 |
| Alcohol problems (AUDIT)e | 5.0 | 3.3 | 6.4 | 3.8 | 11.0 | 4.3 | 14.4 | 4.8 | 12.4 | 5.4 | 21.9 | 6.2 | group x phase, p<.01 |
| AUD symptoms during follow-up e | -- | -- | 0.5 | 0.7 | -- | -- | 2.9 | 1.4 | -- | -- | 6.1 | 1.3 | group, p<.001 |
Note.
Race was provided by participants among a list of options consistent with NIH classifications
Family history positive defined as having one biological primary or two or more biological secondary relatives with AUD
From Structural Clinical Interview for DSM-IV interview indicating number of participants meeting past year criteria at Year 1 and during any follow-up across ten years
From Timeline Follow-Back calendar for the month preceding initial testing and maxima across follow-ups for days that any alcohol was consumed and days with heavy drinking defined as ≥5 drinks per occasion for males and ≥4 drinks for females and based on standard definition of one drink = 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor
From Alcohol Use Disorders Identification Test (AUDIT) and Structural Clinical Interview for DSM interview indicating past year at initial testing and maxima score or number of 11 DSM-IV symptoms met across follow-ups for ten years.
A similar pattern of results emerged for alcohol responses with analyses based on the AUD SX subgroups (Table 2). Relative to the low AUD SX subgroup, the intermediate and high AUD SX subgroups showed initially heightened alcohol stimulation, wanting and liking. For alcohol stimulation and wanting, these responses were potentiated through the re-examination phases (i.e., augmented over the decade of participation) with liking remaining elevated but not increasing further as the decade progressed (Figure 3). Initial subjective responses to alcohol also predicted the frequency of heavy drinking, another outcome used in our prior work (10), and evident for liking and wanting (rs=0.19, ps<.01), stimulation (r=0.14, p=.06), and sedation (r=−0.17, p<.05).
On secondary measures, alcohol increased feel-drug ratings during the rising BrAC limb [time, p=0.026; Suppl Table 1]; this response became more intense through the re-examination for AUD+ [group x phase, p=0.004]. Alcohol-induced heart rate and cortisol increases did not differ by group or across test phases (Table 2; Suppl. Figure 2). The same results were observed when based on analysis of AUD SX subgroups. For all analyses, there were no sex differences.
DISCUSSION
Alcohol use disorder is phenotypically complex (41) and a better understanding of factors that increase vulnerability to sustained excessive drinking are crucial for effective prevention, early intervention, and treatment development (42). The current study provided the first repeated assessment of acute alcohol responses in the same people over a substantial 10-year period of adulthood. Results comprise two main findings: a) heightened sensitivity to the pleasurable effects of alcohol (stimulation, liking, and wanting) in young adulthood preceded the development of AUD through middle age; b) sensitivity to alcohol stimulation and wanting increased in repeat testing over a decade in persons developing AUD. Trajectory analyses of AUD symptom progression over follow-up confirmed these findings by demonstrating amplification of alcohol stimulation and wanting as a function of AUD severity over a decade. Notably, physiological responses such as heart rate and cortisol did not predict AUD development. Taken together, these findings support the idea that the positive stimulating and motivating effects of alcohol increase during the development and maintenance of AUD. The findings do not support the idea that low sensitivity to alcohol increases the risk for AUD (12) or that a reward deficit stage emerges in the progression to the “dark side of addiction” (43).
The study results are consistent with the central tenet of the incentive-sensitization theory – that motivational (“wanting”), but not hedonic (“liking”) processes become sensitized in the progression to addiction (14). In our study, the individuals who developed AUD over the 10-year period reported increases in wanting alcohol over time, while liking of alcohol remained high but stable over time. The incentive-sensitization theory is based on behavioral and pharmacological studies in rodents demonstrating wanting and liking as distinct components of reward and mediated by separate neural systems. In the animal studies, repeated exposure to the drug produces incremental neuroadaptations in the circuits mediating motivational salience, rendering them hypersensitive to the drug and its associated stimuli (14,44). Until now, however, this idea has not been examined in a longitudinal manner in humans. Although cross-sectional studies of alcohol use severity continuum support this idea (45–49), to our knowledge, our study, which includes repeated alcohol challenge testing in the same participants over an extensive period, provides the first longitudinal support for the central tenet of this theory.
There has been substantial disagreement among clinicians and researchers as to whether AUD is progressive, what behavioral or subjective changes occur in response to alcohol over extended periods of use, and what processes underlie neuroadaptive changes that result from chronic drinking. It is known that chronic exposure to alcohol leads to pharmacodynamic tolerance such that users can withstand higher BrAC than inexperienced drinkers before experiencing stupor, coma and eventual death (50). Although tolerance to the subjective effects of alcohol is a hallmark symptom of AUD, it has been challenging to track the development of such tolerance in humans. In a similar vein, it has been difficult to document the progressive stages of allostasis, another construct believed to contribute to the disorder.
Several issues are relevant in reconciling the present study results with existing theory. First, retrospective patient reports suggesting the need for more alcohol to get the same effects as compared to when one first started drinking regularly is not sufficient support for chronic tolerance. The well-controlled present study findings are not consistent with robust chronic tolerance to alcohol’s subjective effects suggesting that some of the users’ reports of lessened effects may be influenced by other factors. Second, studies of chronic exposure to alcohol derived from animal models of addiction, while providing critical information about the behavioral and physiological correlates of consumption (51), are not able to shed light on changes in the euphoric effects of drinking in humans, and how these change over time. Third, some theories of addiction, such as allostasis, indicate development of a deficit in reward, or a change in aversive states and stress responses. Although our data do not provide support for this idea, it is possible this putative stage requires more than a decade to development or that it exists only in those prone to negative affect or extremely aversive withdrawal. Alternatively, while certain tenets of the allostasis theory may relate to animal models, they may not translate to manifestations of AUD as observed within the demographics of the current study sample. Indeed, cross-sectional neuroimaging studies suggest that individuals with more severe heavy drinking histories exhibit a reduced activation of the nucleus accumbens after a single dose of alcohol (52–54). In contrast, our within-subject findings demonstrated that the perceived pleasurable alcohol effects are not diminished as AUD severity progressed over a 10-year period. Although it is difficult to reconcile the contrasting findings, given the differences in samples and methodologies, we may speculate that other neurobiological substrates (i.e. basal ganglia) or circuits underlie the persistent positive subjective response to alcohol. Nevertheless, this possibility highlights the necessity for studies employing both neurobiological techniques and validated subjective measures.
There are several strengths of the CSDP including a prospective design with alcohol and placebo testing at three phases, outstanding retention, and determination of drinking and AUD symptoms from early to middle adulthood. Most notably, we were able to attain near-perfect retention of participants across the decade. The inclusion of dual follow-up outcomes, i.e., categorical AUD diagnosis and trajectory analyses of symptom growth over time, provide a powerful picture of the development of alcohol problems (55). Related, as this longitudinal study began in 2004, many years prior to the 2013 release of the DSM-5, trajectory analysis was based on DSM-IV symptom counts for alcohol abuse and dependence. Thus, symptom counts may have been “undercounts” with actual symptom count severity likely higher if DSM-5 was available because the DSM-IV included the item for legal problems, which was determined to have infrequent endorsement (56), and did not include the item for craving, which is more often endorsed (57).
The study also had limitations. First, the standardized, body-weight and sex-adjusted fixed dose of alcohol was chosen to produce a sharp rise in BrAC to minimize variability. But this procedure did not allow for other clinically-relevant aspects of drinking, including both self-paced drinking and choice to drink in the presence of both alcohol and other reinforcers or consequences. Second, we were not able to test participants under 21 years of age, and it is possible that alcohol-related changes had already occurred before the participants enrolled in the CSDP. Recent work shows that older adolescent heavy drinkers exhibit sensitivity to alcohol stimulation with heightened tonic wanting (Chavarria et al., under review), so prospective studies with younger participants, perhaps in locations where adolescent drinking is permitted, would be valuable in determining the earliest precipitants of adaptive responses to alcohol. Third, although our sample developed AUD at a higher rate than the 12.7% general population rate (1), the final AUD+ sample size was modest. However, using two similar but not overlapping outcome approaches may ameliorate concerns about sample size. Using AUD+ vs AUD− at year 10 provided a practical clinical endpoint used by clinicians, and notably, 64% of AUD+ subjects met DSM-IV alcohol dependence at some point through follow-up, versus 7% in the AUD− group. The AUD SX trajectories construct provided a data-driven approach providing consistency with our prior work (11,19). All of the participants in the high AUD SX subgroup met alcohol dependence through follow-up, compared with 26% and 0% in the intermediate and low AUD SX groups. While details on consequences and distress related to excessive drinking was beyond the scope of this paper, persons in the AUD+ group had a higher likelihood of seeking advice or help on drinking or seriously considering getting help relative to the AUD− group (49% vs 8%).
In sum, this study is the most extensive repeated human testing study of its kind, examining the adaptive processes underlying the development and maintenance of AUD. Alcohol-induced subjective stimulation increased over time, and euphoric processes did not wane as AUD symptoms emerged. Instead, these effects either stayed the same or increased, challenging the notion that conventional chronic tolerance to alcohol’s subjective effects plays a key role in escalation of excessive drinking. The findings are consistent with the incentive-sensitization theory as alcohol wanting increased over a decade in persons developing AUD. But study results were not consistent with the allostasis model of a progressive deficit in reward. In terms of prevention of alcohol problems, rather than a sole focus on tolerance, young adults might be informed that marked stimulant-like, pleasurable and appetitive effects after consuming alcohol are risk factors for the development and maintenance of addiction. Finally, pharmacological and behavioral interventions focused on reduce positive reinforcing effects and motivational salience may be crucial in medications development and the future treatment of AUD.
Supplementary Material
Table 3:
GEE Analysis summary of primary alcohol response outcomes in AUD +/− groups at Year 10 and AUD SX trajectory subgroups
| Primary Outcomes | AUD Group | Time | Phase | AUD Group x Phase | AUD Group x Time | AUD Group x Time x Phase | ||||||||||||
| Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | |
| Stimulation | 1.126 | 2.192 | 0.607 | −4.089 | 4.383 | 0.351 | −0.037 | 0.137 | 0.788 | −0.305 | 0.289 | 0.292 | 6.027 | 6.300 | 0.339 | 2.541 | 0.987 | 0.010 |
| Sedation | −1.980 | 1.958 | 0.312 | 1.885 | 0.373 | <0.001 | −0.030 | 0.099 | 0.760 | −0.182 | 0.210 | 0.385 | −1.250 | 0.789 | 0.113 | 0.159 | 0.124 | 0.198 |
| Wanting | 5.534 | 6.093 | 0.364 | −2.819 | 1.172 | 0.016 | −0.830 | 0.332 | 0.012 | 2.203 | 0.702 | 0.002 | −1.367 | 2.368 | 0.564 | 0.268 | 0.371 | 0.470 |
| Liking | 11.454 | 4.691 | 0.015 | −8.699 | 0.996 | <0.001 | −0.574 | 0.282 | 0.042 | 1.091 | 0.598 | 0.068 | −1.463 | 2.016 | 0.468 | −0.148 | 0.316 | 0.639 |
| Primary Outcomes | AUD SX Group | Time | Phase | AUD SX Group x Phase | AUD SX Group x Time | AUD SX Group x Time x Phase | ||||||||||||
| Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | Coef | SE | p | |
| Stimulation | 0.759 | 1.380 | 0.582 | −9.354 | 7.061 | 0.185 | 0.326 | 0.297 | 0.273 | −0.281 | 0.176 | 0.111 | 5.104 | 3.820 | 0.181 | 1.818 | 0.600 | 0.002 |
| Sedation | −0.927 | 1.270 | 0.465 | 1.822 | 0.812 | 0.025 | 0.123 | 0.216 | 0.569 | −0.125 | 0.128 | 0.328 | −0.133 | 0.479 | 0.781 | −0.001 | 0.075 | 0.991 |
| Wanting | 10.741 | 3.792 | 0.005 | −4.727 | 2.475 | 0.056 | −1.959 | 0.733 | 0.008 | 1.054 | 0.433 | 0.015 | 1.033 | 1.456 | 0.478 | −0.090 | 0.229 | 0.693 |
| Liking | 8.712 | 2.972 | 0.003 | −8.215 | 2.079 | <0.001 | −1.194 | 0.616 | 0.052 | 0.567 | 0.364 | 0.119 | −0.553 | 1.223 | 0.651 | −0.075 | 0.192 | 0.696 |
Note. Data are results from GEE analysis and include the cubic term for stimulation and linear terms for sedation, wanting and liking as the best fit for these four variables. For stimulation, the three-way interaction of AUD Group x Time x Phase and AUD Group x Time2 x Phase were also significant. All GEE analysis controlled for baseline AUD count, BrAC, sex, race, and family history of alcoholism. Bold represents significant finding(s).
ACKNOWLEDGEMENTS
This research was supported by grant R01-AA013746 (AK) from the National Institute on Alcohol Abuse and Alcoholism for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review and approval of the manuscript; and decision to submit the manuscript for publication, T32-DA043469 (HD, AV) and R01-AA023839 (DC) for the management, analysis, and interpretation of the data, the Indiana Alcohol Research Center P60-AA07611 (SO) and R01-AA025309 and R01-DA018652 and the New York State Psychiatric Institute (DH) for the interpretation of the data; and preparation, review and approval of the manuscript. Appreciation is extended to Patrick McNamara for project coordination, data collection and database management and to Royce Lee, MD and Jon Grant, MD for medical supervision and oversight.
Footnotes
Clinical Trials Name: Chicago Social Drinking Project
URL: http://clinicaltrials.gov/ct2/show/NCT00961792
Registration #: NCT00961792
References
- 1.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74(9):911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKetta S, Keyes KM. Heavy and binge alcohol drinking and parenting status in the United States from 2006 to 2018: An analysis of nationally representative cross-sectional surveys. PLoS Med 2019;16(11):e1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasin DS, Shmulewitz D, Keyes K. Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug Alcohol Depend 2019; 205:107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chassin L, Pitts SC, Prost J. Binge Drinking Trajectories From Adolescence to Emerging Adulthood in a High-Risk Sample. J Consult Clin Psychol 2002;70(1):67–78. [PubMed] [Google Scholar]
- 6.Zucker RA, Wong MM, Clark DB, Leonard KE, Schulenberg JE, Cornelius JR, et al. Predicting risky drinking outcomes longitudinally: What kind of advance notice can we get? In: Alcoholism: Clinical and Experimental Research 2006, p. 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry CD, Patra J, Rehm J. Alcohol consumption and non-communicable diseases: Epidemiology and policy implications. Addiction 2011;106(10):1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehm J The risks associated with alcohol use and alcoholism. Alcohol Res Heal. 2011;34(2):135–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Ray LA, Bujarski S, Roche DJO. Subjective Response to Alcohol as a Research Domain Criterion. Alcohol Clin Exp Res. 2016;40(1):6–17. [DOI] [PubMed] [Google Scholar]
- 10.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, Stimulant, and Sedative Alcohol Responses and Relationship to Future Binge Drinking. Arch Gen Psychiatry 2011;68(4):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol Psychiatry 2014;75(10):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151(2):184–9. [DOI] [PubMed] [Google Scholar]
- 13.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 1987;94(4):469–92. [PubMed] [Google Scholar]
- 14.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–91. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997;278(5335):52–8. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AJ, Heyser CJ, Maunj Cole BS, Peter Griffin BS, Koob GF. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology 2000;22(6):581–94. [DOI] [PubMed] [Google Scholar]
- 17.Spanagel R Animal models of addiction. Dialogues Clin Neurosci. 2017;19(3):247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King AC, Cao D, deWit H, O’Connor SJ, Hasin DS. The role of alcohol response phenotypes in the risk for alcohol use disorder. BJPsych Open. 2019;5(3):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry 2016;79(6):489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcohol Clin Exp Res 2000;24(12):1820–9. [PubMed] [Google Scholar]
- 21.Rethinking Drinking Homepage - NIAAA [Internet]. Available from: https://www.rethinkingdrinking.niaaa.nih.gov/
- 22.King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 2002;26(6):827–35. [PubMed] [Google Scholar]
- 23.King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29(4):547–52. [DOI] [PubMed] [Google Scholar]
- 24.McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 2000;22(5):480–92. [DOI] [PubMed] [Google Scholar]
- 25.Wiers RW, Van De Luitgaarden J, Van Den Wildenberg E, Smulders FTY. Challenging implicit and explicit alcohol-related cognitions in young heavy drinkers. Addiction 2005;100(6):806–19. [DOI] [PubMed] [Google Scholar]
- 26.Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend 2007;91(1):10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad M, McNamara P, King A. Alternative substance paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharmacol. 2012. October;20(5):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Advisory Council on Alcohol Abuse and Alcoholism. Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. 2005. Available from: https://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm#levels [DOI] [PMC free article] [PubMed]
- 29.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and Validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 1993;17(1):140–6. [DOI] [PubMed] [Google Scholar]
- 30.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: Diazepam. Psychopharmacology (Berl). 1980. December;71(3):269–73. [DOI] [PubMed] [Google Scholar]
- 31.Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 2013;227(1):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rueger SY, McNamara PJ, King AC. Expanding the utility of the biphasic alcohol effects scale (BAES) and initial psychometric support for the brief-BAES (B-BAES). Alcohol Clin Exp Res. 2009;33(5):916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LJ, McNamara PJ, King AC. Optimizing follow-up and study retention in the 21st century: Advances from the front line in alcohol and tobacco research. Drug Alcohol Depend 2017;175:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones BL, Nagin DS . A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociol Methods Res 2013;42(4):608–13. [Google Scholar]
- 35.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73(1):13–22. [Google Scholar]
- 36.Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl). 2001;157(1):20–30. [DOI] [PubMed] [Google Scholar]
- 37.Newlin DB, Byrne EA, Porges SW. Vagal Mediation of the Effect of Alcohol on Heart Rate. Alcohol Clin Exp Res. 1990;14(3):421–4. [DOI] [PubMed] [Google Scholar]
- 38.King A, Munisamy G, De Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006. March;59(3):203–9. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol Problems in Adoptees Raised Apart From Alcoholic Biological Parents. Arch Gen Psychiatry 1973;28(2):238–43. [DOI] [PubMed] [Google Scholar]
- 40.Ramchandani VA, Plawecki M, Li TK, O’Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009. May;33(5):938–44. [DOI] [PubMed] [Google Scholar]
- 41.Reilly MT, Noronha A, Goldman D, Koob GF. Genetic studies of alcohol dependence in the context of the addiction cycle. Vol. 122, Neuropharmacology. Elsevier Ltd; 2017. p. 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder a review. JAMA - J Am Med Assoc. 2018;320(8):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008;59:29–53. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. [DOI] [PubMed] [Google Scholar]
- 45.Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: A test of the incentive-sensitisation theory. Psychopharmacology (Berl). 2005. April;178(4):493–9. [DOI] [PubMed] [Google Scholar]
- 46.Ostafin BD, Marlatt GA, Troop-Gordon W. Testing the incentive-sensitization theory with at-risk drinkers: Wanting, liking, and alcohol consumption. Psychol Addict Behav. 2010;24(1):157–62. [DOI] [PubMed] [Google Scholar]
- 47.Cofresí RU, Bartholow BD, Piasecki TM. Evidence for incentive salience sensitization as a pathway to alcohol use disorder. Vol. 107, Neuroscience and Biobehavioral Reviews. Elsevier Ltd; 2019. p. 897–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: An examination of Koob’s allostatic model in humans. Drug Alcohol Depend 2014;140:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bujarski S, David Jentsch J, Roche DJO, Ramchandani VA, Miotto K, Ray LA. Differences in the subjective and motivational properties of alcohol across alcohol use severity: Application of a novel translational human laboratory paradigm. Neuropsychopharmacology 2018;43(9):1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le AD, Mayer JM. Aspects of Alcohol Tolerance in Humans and Experimental Animals. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; 1995, p. 251–68. [Google Scholar]
- 51.Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- 52.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37(2):467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, et al. Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38(12):3024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuckit MA. The answer you get depends on the question you ask. Biol Psychiatry 2014;75(10):754–5. [DOI] [PubMed] [Google Scholar]
- 56.Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36(7):931–41. [DOI] [PubMed] [Google Scholar]
- 57.Murphy CM, Stojek MK, Few LR, Rothbaum AO, MacKillop J. Craving as an alcohol use disorder symptom in DSM-5: An empirical examination in a treatment-seeking sample. Exp Clin Psychopharmacol. 2014;22(1):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.