Abstract
Objectives
Muscle wasting is a recognised extra-pulmonary complication in chronic obstructive pulmonary disease and has been associated with increased risk of death. Acute respiratory exacerbations are associated with reduction of muscle function, but there is a paucity of data on their long-term effect. This study explores the relationship between acute respiratory exacerbations and long-term muscle loss using serial measurements of CT derived pectoralis muscle area (PMA).
Design and setting
Participants were included from two prospective, longitudinal, observational, multicentre cohorts of ever-smokers with at least 10 pack-year history.
Participants
The primary analysis included 1332 (of 2501) participants from Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) and 4384 (of 10 198) participants from Genetic Epidemiology of COPD (COPDGene) who had complete data from their baseline and follow-up visits.
Interventions
PMA was measured on chest CT scans at two timepoints. Self-reported exacerbation data were collected from participants in both studies through the use of periodic longitudinal surveys.
Main outcome measures
Age-related and excess muscle loss over time.
Results
Age, sex, race and body mass index were associated with baseline PMA. Participants experienced age-related decline at the upper end of reported normal ranges. In ECLIPSE, the exacerbation rate over time was associated with an excess muscle area loss of 1.3% (95% CI 0.6 to 1.9, p<0.001) over 3 years and in COPDGene with an excess muscle area loss of 2.1% (95% CI 1.2 to 2.8, p<0.001) over 5 years. Excess muscle area decline was absent in 273 individuals who participated in pulmonary rehabilitation.
Conclusions
Exacerbations are associated with accelerated skeletal muscle loss. Each annual exacerbation was associated with the equivalent of 6 months of age-expected decline in muscle mass. Ameliorating exacerbation-associated muscle loss represents an important therapeutic target.
INTRODUCTION
Sarcopenia is defined as an age-related loss of skeletal muscle mass and function and is closely linked to frailty.1 Over age 40, healthy adults lose muscle mass at a rate of 0.64%–0.7% per year for women and 0.8%–0.98% per year for men.2,3 Chronic disease can accelerate sarcopenia through inflammation, metabolic derangement, hospitalisation and activity reduction.4,5
There is an extensive body of literature demonstrating increased rates of sarcopenia in persons with COPD, but muscle wasting is not universal.5–8 van den Borst and colleagues found lower baseline fat free mass, but no difference in the rate of decline in fat free mass over 8 years of follow-up when comparing smokers with obstructive lung disease to smokers without obstruction.9 In the acute setting, respiratory events are associated with reductions in muscle function.10,11 Hopkinson and colleagues demonstrated subacute persistence of muscle volume loss at 1 year in those with two or more exacerbations in that period.12 These data suggest that respiratory events may contribute to chronic muscle wasting in smokers, but there is a paucity of longitudinal data to examine the durability of these effects.
Several methods for quantitatively measuring muscle mass have been endorsed in international guidelines including dual-energy X-ray absorptiometry, bioelectrical impedance analysis (BIA) and CT.1 Pectoralis muscle area (PMA), unlike lumbar, psoas or thigh muscle area, can be derived from routine chest CT. In patients with COPD, PMA correlates well with the whole-body fat free mass index derived from BIA and is more closely associated with mortality than body mass index (BMI).13–17
Using baseline and 3-year or 5-year follow-up data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) and Genetic Epidemiology of COPD (COPDGene) studies, we sought to identify the relationship between respiratory exacerbation rate over time and long-term muscle loss. We hypothesised that higher rates of respiratory exacerbation would be associated with an accelerated loss of skeletal muscle beyond what would be expected with age in ever smokers with and without COPD.
METHODS
Study population
The study populations used in our analysis were derived from two previously described multicentre, longitudinal, observational, cohort studies. The ECLIPSE (NCT00292552, SCO104960) study enrolled 2164 COPD patients and 337 controls, aged 45–75, with a smoking history of at least 10 pack years.18 Participants were enrolled from 26 centres across 12 countries and followed for a total of 3 years with assessments completed at baseline, 12 months and 36 months. For our primary analysis, we included those subjects who had complete data for both their baseline and 36-month visits.
The COPDGene study (NCT00608764) is ongoing and has enrolled 10 198 non-Hispanic White or African American ever smokers with and without COPD, aged 45–80, with a history of at least 10 pack years of smoking.19 Enrolment occurred at 21 centres in the USA with assessments every 5 years; the 10-year follow-up visit is ongoing. Our primary analysis used data from those participants with complete data for their baseline and year 5 visits.
Assessments
Both studies collected self-reported acute respiratory event data at each visit and between visits. Study visits were delayed if a patient was currently having an event. COPDGene participants were contacted every 6 months by telephone or web systems and ECLIPSE participants were contacted monthly by telephone.18,20 In both studies, an acute respiratory event (hereafter referred to as an exacerbation) was considered to be an increase in respiratory symptoms requiring antibiotics or systemic steroids; a severe event was one that required emergency department evaluation or hospitalisation.18,19 For each participant, the number of reported events was divided by the observed follow-up time to generate an annualised exacerbation rate.
Spirometry was obtained at baseline and each follow-up visit in both studies, before and after the administration of an inhaled short-acting beta agonist. Expiratory airflow obstruction was defined spirometrically by the presence of a post-bronchodilator FEV1/FVC ratio of less than 0.7. Additional categorisation of disease severity was performed based on decrements in the FEV1. COPD was defined as those with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 or higher disease (ie, FEV1 per cent predicted <0.8).21 Ever smokers without obstruction and those with an FEV1/FVC ratio less than 0.7, but an FEV1 >80% of predicted (GOLD 1), were categorised as ‘at risk’.
Six-minute walk testing was completed according to international guidelines at study visits for all participants in COPDGene and in participants diagnosed with COPD in ECLIPSE.22 The minimum clinically important change in distance on serial testing was taken as 30 m.23
Participants in both studies underwent CT scanning of the chest at full inflation at each in-person visit. 18,19 Quantitative assessments of the PMA were obtained as described previously from a single axial slice of the CT scan above the aortic arch.24,25 Each resulting segmentation was reviewed for quality control and segmentation failures (eg, malposition of the arm, distortion from an implanted device) were visually identified and excluded. The calculated PMA reported for each scan was expressed in cm2 and represented the aggregate cross-sectional area of the right and left pectoralis major and minor muscles.
Statistical analyses
Models were checked for normality of residuals, homoscedasticity and multicollinearity. When the 154 observations with residuals more than 2.5 SD away from the residuals mean were excluded, the effect estimates and CIs did not change. Variance inflation factors were between 1 and 2 for all covariates. Histogram and quantile-quantile plots revealed the distribution of the PMA values to be non-normal. Linear mixed effects models were used to fit the log-transform of PMA longitudinally. Primary predictors of interest in these models included phase of study (baseline or follow-up), annual exacerbation rate, and their interaction. The PMA loss associated with the interaction term is described as ‘excess loss’. Additional time-invariant covariates were race, sex and smoking history on enrolment (measured in pack years); time dependent covariates included age, BMI, chronic oral steroid use and current smoking status. Covariates were selected a priori as potential confounders of the relationship between exacerbations and PMA or their role as independent predictors of the outcome of interest. Repeated measures were accounted for by including random intercepts for subjects, which induced a compound symmetric covariance structure on the repeated measures. The Satterthwaite method for df was used to obtain p values for fixed effects.26 CIs were generated by the Wald method. Annual severe exacerbation rate and its effect on PMA was similarly and separately modelled. GOLD stage and participation in intercurrent pulmonary rehabilitation (PR) were explored as model covariates in separate, exploratory models.
Study participants were then divided into quintiles of per cent PMA decline, with quintile 0 representing those whom gained PMA and quintile 5 representing the largest decline over the study interval. The Kruskal-Wallis test was used to assess for differences in distribution of 6 min walk distance and BMI across quintiles. The Fisher exact test was used to assess for differences in the per cent of participants with a minimum clinically important gain or loss in 6 min walk distance across quintiles.
To account for bias due to missing data, we repeated the analysis in both cohorts including participants with complete data for only one of the time points, which we term the expanded cohort. For more details, see online supplemental file 1. All analyses were performed using R V.3.5.1, with longitudinal models using the lme4 and lmerTest packages.
RESULTS
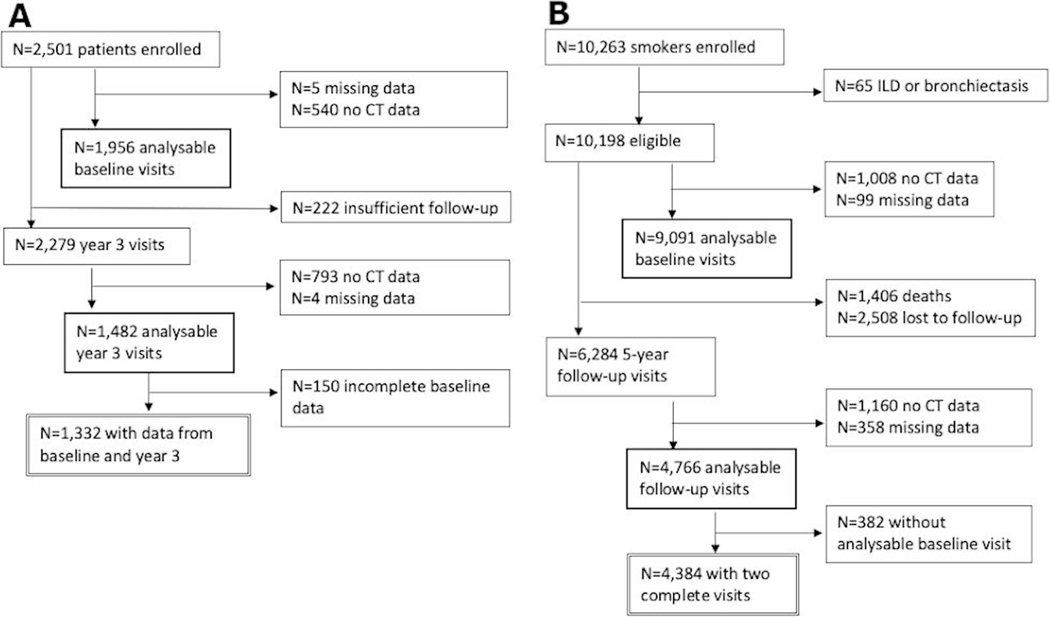
For the primary analysis, 1332 participants from ECLIPSE and 4384 participants from COPDGene had complete data for the baseline and follow-up visits (figure 1A,B, respectively). Baseline clinical characteristics of the cohorts are shown in table 1. The ECLIPSE cohort was 63.6% men, 97.7% non-Hispanic White, had a mean PMA of 36.6 cm2 (±10.9), and 0.5% of the participants reported using chronic oral steroids. The COPDGene cohort was 50.5% men, 70.6% non-Hispanic White, had a mean PMA of 41.3 cm2 (±15.3), and 1.5% of the participants reported using chronic oral steroids.
Figure 1.
Consort diagram. (A) Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints cohort; (B) Genetic Epidemiology of COPD cohort. ILD, interstitial lung disease.
Table 1.
Clinical characteristics of the cohorts
| ECLIPSE | COPDGene | |
|---|---|---|
| Baseline characteristics | ||
| Sample size | 1332 | 4384 |
| Age, years (mean, SD) | 61.8 (7.9) | 59.8 (8.6) |
| Male (n, %) | 847 (63.6) | 2214 (50.5) |
| Caucasian (n, %) | 1302 (97.7) | 3093 (70.6) |
| Current smoker (n, %) | 512 (38.4) | 2122 (48.4) |
| Pack years on study entry (mean, SD) | 45.8 (27.5) | 42.8 (23.8) |
| BMI (mean, SD) | 26.5 (5.1) | 29.1 (5.9) |
| Chronic oral steroid use (n, %) | 7 (0.5) | 64 (1.5) |
| Pectoral muscle area, cm2 (mean, SD) | 36.6 (10.9) | 41.3 (15.3) |
| 6-minute walk distance, m (mean, SD) | 386.4 (118.3) | 434.7 (113.1) |
| Baseline exacerbation history | ||
| An event in past year (%) | 45.9 | 20.5 |
| Mean rate, per year | 0.93 | 0.33 |
| A severe event in past year (%) | 15.3 | 4.6 |
| Mean severe rate, per year | 0.25 | 0.06 |
| Intercurrent exacerbation history | ||
| An event during follow-up (%) | 59.0 | 39.9 |
| ≥1 event per year (%) | 41.7 | 11.7 |
| Mean rate, per year | 0.94 | 0.33 |
| A severe event during follow-up (%) | 21.0 | 17.4 |
| ≥1 severe event per year (%) | 7.7 | 2.4 |
| Mean severe rate, per year | 0.19 | 0.09 |
Baseline exacerbation history represents participants’ self-reported 12-month exacerbation frequency at study enrolment. Intercurrent exacerbations are those that occurred during the study and were assessed using periodic surveys during the study follow-up period. These data are available by Global Initiative for Chronic Obstructive Lung Disease stage in online supplemental tables E1 and E2.
BMI, body mass index; COPDGene, Genetic Epidemiology of COPD; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints.
Exacerbations were common in both cohorts prior to enrolment as well as during the intercurrent follow-up interval (table 1). In the 12 months preceding study entry, 45.9% of participants in ECLIPSE and 20.5% of participants in COPDGene reported at least one exacerbation, with 15.3% and 4.6% reporting at least one severe exacerbation, respectively. During the follow-up interval, 59% of ECLIPSE participants and 39.9% of COPDGene participants reported at least one exacerbation, with 21.0% and 17.4% reporting at least one severe exacerbation, respectively.
In the mixed models for both cohorts, age(p<0.001), sex (p<0.001), BMI (p<0.001) and race (ECLIPSE p=0.004, COPDGene p<0.001) were significant correlates of PMA. Men and women experienced age-related decline at the upper end of reported normal ranges. Women lost 0.8% (95% CI 0.6 to 1.0, p<0.001) and 0.8% (95% CI 0.7 to 0.9, p<0.001) of their PMA annually in ECLIPSE and COPDGene, respectively while men lost 1.0% (95% CI 0.8 to 1.2, p<0.001) and 1.0% (95% CI 0.9 to 1.1, p<0.001) of their PMA annually in the ECLIPSE and COPDGene cohorts, respectively.
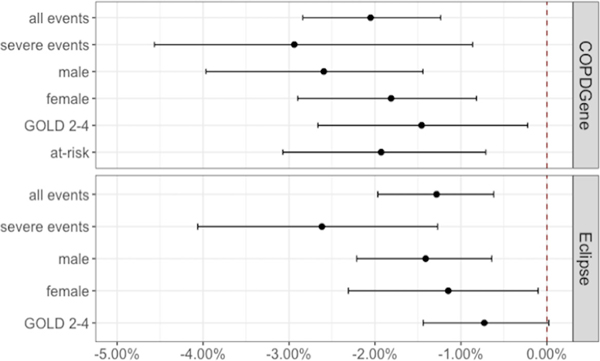
The exacerbation rate by time interaction was associated with a statistically significant excess PMA loss. In ECLIPSE, an annual exacerbation rate of one per year was associated with an excess loss of 1.3% (95% CI 0.6 to 1.9, p<0.001) over 3 years. The severe exacerbation rate by time interaction was associated with an excess PMA loss of 2.6% (95% CI 1.2 to 4.0, p<0.001) over that same time period. In COPDGene, an exacerbation rate of one per year was associated with an excess loss of 2.1% (95% CI 1.2 to 2.8, p<0.001) over 5 years. An equivalent severe exacerbation rate was associated with an excess PMA loss of 2.9% (95% CI 1.1 to 4.8, p=0.002) over that same time period (figure 2).
Figure 2.
Estimated excess per cent loss of pectoral muscle area per annual exacerbation. The 1332 participants from ECLIPSE were followed for 3 years and the 4384 participants in COPDGene were followed for 5 years. The dot represents the effect estimate for excess per cent muscle loss and the whiskers correspond to the 95% CI. COPDGene, Genetic Epidemiology of COPD; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The exacerbation rate by time interaction in women was associated with an excess PMA loss of 1.1% (95% CI 0.1 to 2.2, p=0.043) in ECLIPSE and 1.8% (95% CI 0.8 to 2.9, p=0.001) in COPDGene, compared with 1.4% (95% CI 0.6 to 2.2, p<0.001) in ECLIPSE and 2.6% (95% CI 1.4 to 4.0, p<0.001) in COPDGene for men (figure 2).
In both the at-risk and COPD groups, the exacerbation by time interaction was associated with excess loss of PMA (figure 2). In participants with COPD, an annual exacerbation rate of one per year was associated with a 0.7% (95% CI 0 to 1.4, p=0.050) excess loss of PMA in ECLIPSE and 1.5% (95% CI 0.3 to 2.6, p=0.017) in COPDGene. In participants at-risk, an effect estimate could not be calculated for those in ECLIPSE due to their low rates of exacerbation (online supplemental table 2), but for those in COPDGene, an annual exacerbation rate of one per year was associated with a 1.9% (95% CI 0.7 to 3.1, p=0.002) excess loss of PMA.
When included as a fixed effect in the model, GOLD stage was negatively associated with PMA (p=0.062 for GOLD 1 in COPDGene and p<0.001 for each GOLD 2–4 stage in both studies). When included as a fixed effect, intercurrent PR was also a significant predictor of PMA (p=0.001) in the COPDGene cohort. In the group whom attended PR (n=273), there was no significant excess decline in PMA (−0.2%, 95% CI −2.2 to 2.5, p=0.882) attributable to the exacerbation by time interaction despite a mean annual exacerbation rate of 1.0.
Participants whose PMA decreased over time had a corresponding decrease in BMI (p<0.001 for both cohorts, table 2). Greater decreases in PMA were not associated with changes in 6 min walk distance as measured by median per cent change (p=0.379 in ECLIPSE, p=0.363 in COPDGene), per cent gaining 30 m or more metres (p=0.965 in ECLIPSE, p=0.738 in COPDGene) or per cent losing 30 m or more metres (p=0.332 in ECLIPSE, p=0.520 in COPDGene, table 3).
Table 2.
Percent change in pectoral muscle area and BMI by quintile of PMA loss
| ECLIPSE |
COPDGene |
|||||
|---|---|---|---|---|---|---|
| Quintile of PMA decline | n | Median % change PMA | Median % change BMI | n | Median % change PMA | Median % change BMI |
| 0 | 503 | 8.2 | 0.8 | 1516 | 9.4 | 1.8 |
| 1 | 166 | −2.1 | −0.3 | 574 | −2.5 | 0.1 |
| 2 | 166 | −6.3 | −1.3 | 574 | −7.6 | 0.1 |
| 3 | 166 | −9.7 | −2.5 | 573 | −12.5 | −1.4 |
| 4 | 166 | −14.7 | −2.2 | 574 | −18.6 | −1.2 |
| 5 | 165 | −22.4 | −2.6 | 573 | −27.9 | −3.9 |
The 0th quintile represents those subjects who had an increase in their PMA during the study.
BMI, body mass index; COPDGene, Genetic Epidemiology of COPD; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; PMA, pectoral muscle area.
Table 3.
Changes in 6 min walk testing by quintile of PMA loss
| ECLIPSE* |
COPDGene† |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quintile of PMA decline | n | Median % change 6MW distance | % with ≥30 m gain | % with ≥30 m loss | n | Median % change 6MW distance | % with ≥30 m gain | % with ≥30 m loss |
| 0 | 357 | −3.1 | 25.5 | 42.3 | 1482 | −8.2 | 22.3 | 53.8 |
| 1 | 113 | −2.8 | 26.5 | 39.8 | 560 | −9.2 | 20.2 | 54.1 |
| 2 | 121 | −2.2 | 24.0 | 37.2 | 561 | −5.8 | 22.3 | 49.5 |
| 3 | 136 | −3.4 | 23.5 | 39.0 | 559 | −7.3 | 23.1 | 53.1 |
| 4 | 121 | −3.6 | 26.4 | 38.0 | 556 | −8.1 | 24.1 | 54.1 |
| 5 | 131 | −5.9 | 22.1 | 49.6 | 560 | −9.4 | 22.7 | 54.5 |
The 0th quintile represents those subjects who had an increase in their PMA during the study.
N=353 subjects from ECLIPSE did not have 6 min walk data available.
N=106 subjects from COPDGene did not have 6 min walk data available.
COPDGene, Genetic Epidemiology of COPD; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; 6MW, 6 min walk; PMA, pectoral muscle area.
DISCUSSION
The results of this longitudinal study in two observational cohorts demonstrate that acute respiratory exacerbations are associated with accelerated loss of skeletal muscle cross-sectional area over 3-year and 5-year periods of observation. The association was present in both men and women, as well as both those diagnosed with COPD, and current and former smokers at risk for COPD. In annualised terms, our data suggest that for each exacerbation, a person loses the equivalent of 6 months of age-related pectoral muscle area. That is, a person who has one exacerbation per year would be expected to lose 1.5 times their age-expected muscle area and a person who has two exacerbations per year would lose two times their age expected muscle area in that year.
Our findings extend and reinforce the conclusions of Hopkinson and colleagues who found that persons with two or more exacerbations in 1 year of follow-up had a larger decrease in fat free mass.12 Two or more exacerbations in 12 months allows little recovery time, making their described change in body composition potentially attributable to the acute effect of those exacerbations. By observing participants for multiple years, our study demonstrates a persistent decrement despite periods of intercurrent stability and provides a potential explanation as to why people with COPD may have accelerated muscle loss.
Similar to van den Borst and colleagues, we found that persons with COPD had lower baseline muscle mass than those without obstruction. However, van den Borst found no difference in the body composition trajectories over 8 years comparing participants with COPD to smoking controls.9 Their study did not report data on exacerbations and had only 260 obstructive lung disease participants, of whom one third were GOLD stage 1. It is possible that their cohort had a low exacerbation frequency, which limited their power to detect differences attributable to exacerbations. Taken in concert, our two studies suggest that exacerbations are a potential mechanism for accelerated decline in muscle mass and that absence of exacerbations could result in a body composition trajectory similar to that of non-obstructed individuals.
Exercise programmes, such as PR, are the most widely available, recommended intervention in persons with skeletal muscle dysfunction.1,27,28 Exercise programmes improve skeletal muscle function and increase muscle mass in the short-term.8,29–31 For the small number of people in this study whom underwent intercurrent PR, there was no association between exacerbations over time and PMA. This raises the possibility that PR attenuates the effect of exacerbations on the skeletal musculature; however, replication in a larger cohort is necessary to confirm this hypothesis. PR immediately following exacerbations may be of particular benefit; multiple studies have demonstrated improvement in 1-year mortality when PR was initiated within 90 days of hospital discharge.32,33 Unfortunately, long-term mortality has not shown a similar improvement, which may be related to the inconsistency with which PR results in sustained increases in physical activity.29,32,34
Surprisingly, those individuals with the greatest percentage of PMA loss did not have reduced performance on 6 min walk testing compared with those with little or no PMA loss (table 3). This may reflect the fact that the 6 min walk is a submaximal test and is more akin to a test of functional performance rather than a test of endurance exercise capacity or force generation. Muscle weakness does manifest predominantly in the lower limbs; however, a disconnect between skeletal muscle mass and quadriceps weakness has also been previously described.5,8,35 This underscores the point that low muscle mass does not necessarily equate to muscle weakness and that a test of functional performance is not necessarily a strong correlate of muscle mass. Similarly, following BMI clinically would underestimate the impact on muscle mass, as we found the change in skeletal muscle area to be 10-fold the change in BMI (table 2). Identifying and potentially intervening in those patients with muscle mass loss, regardless of BMI or physical performance, is important, however, as muscle wasting is demonstrated to carry a poor prognosis.13,36–38 Indeed, weight loss, even in the absence of low BMI, is associated with increased mortality in COPD, likely because it often represents loss of muscle, rather than fat, mass.39
Strengths of our study include the replicability in two large, multicentre cohorts, our longitudinal model design, and our examination of both muscle mass and function. The smaller effect estimates reported for the ECLIPSE cohort are likely a reflection of the shorter duration of follow-up: 3 years in ECLIPSE compared with 5 years in COPDGene, given the annualised effects were consistent between cohorts. While loss to follow-up presents a concern for bias in our results, our expanded analysis using partial data and application of inverse probability weighting methods suggest that missing data did not alter estimates notably (see online supplemental file 1).
Other limitations to our investigation include lack of data on physical activity, having only two time points, and lack of causal inference. Due to the long time intervals used, we could not observe acute muscle decrement associated with exacerbations and the potential mechanism of incomplete recovery remains a hypothesis. The low exacerbation rate in the at-risk subjects in the ECLIPSE cohort prevented us from replicating the effect noted in the at-risk subjects in the COPDGene cohort. Nonetheless, fat free mass index has been previously associated with higher risk of exacerbations in ECLIPSE, corroborating our findings.40 The true effect on those at-risk or with mild disease is of interest not only for the possibility of early intervention, but because lung function decline was most prominent in GOLD stage 1 participants in a recent longitudinal analysis of the COPDGene cohort.41 Further, all of the exacerbation data was self-reported, which introduces recollection bias, especially in the baseline data in which subjects were asked to recall the prior 12 months. Subjects in COPDGene were contacted every 6 months, compared with monthly in ECLIPSE, which may have resulted in relative under-reporting of exacerbations in the COPDGene cohort.
In summary, we used clinical and radiological data from two cohorts of patients at risk for, or diagnosed with, COPD to demonstrate an association between exacerbation rate over time and an accelerated loss of skeletal muscle. Neither BMI nor 6 min walk performance reflected the degree of skeletal muscle loss. CT phenotyping could prove to be a useful methodology for assessing body composition as many patients already undergo imaging scans for lung cancer screening or clinical concerns. Further work is needed to determine whether PR attenuates muscle loss and whether intervention to preserve muscle mass can alter prognosis.
Supplementary Material
Key messages.
What is the key question?
► Are respiratory exacerbations associated with chronic muscle loss in ever-smokers?
What is the bottom line?
► Each annual exacerbation is associated with excess muscle loss equivalent to 6 months of age-related decline.
Why read on?
► Muscle wasting is noted across the spectrum of obstructive lung disease severity and is associated with increased risk of death; finding and addressing potential drivers represents an important therapeutic avenue.
Acknowledgments
Funding This work was supported by NIH HL089856 and HL089897 and T32 HL007633 (SEM). The COPDGene (Genetic Epidemiology of COPD) project is also supported by the COPD Foundation through contributions made to an industry advisory board comprised AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
Competing interests MTD reports grant funding from the National Institutes of Health relevant to the submitted work. Outside the submitted work, he reports grants from the American Lung Association, Department of Veterans Affairs, and the Department of Defense. He reports consulting fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, PneumRx, Quark Pharmaceuticals and Mereo. He is involved in contracted clinical trials with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Yungjin, PneumRx, Pulmonx, Boston Scientific, Gala and Nuvaira. RC reports grant funding from the National Institutes of Health relevant to the submitted work. Outside the submitted work, he reports consulting fees or advisory board membership from Boehringer Ingelheim, GlaxoSmithKline, Astra Zeneca, Abbott, Genentech and Regeneron. He is involved in contracted clinical trials with Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Genentech and Regeneron. HR reports grant funding from the National Institutes of Health and the Tobacco Related Disease Research Program relevant to the submitted work. Outside the submitted work, he is involved in contracted clinical trials with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, Genentech and Regeneron. WWL reports funding from the National Institutes of Health, non-financial support from Pulmonx, and personal fees from Konica Minolta, outside of the submitted work. MKH reports grant funding from the National Institutes of Health relevant to the submitted work. Outside the submitted work she reports grant funding from Verona, Mylan and Merck. She reports personal fees from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim. She reports other support from Novartis and Suovion. KAY and MS report grant funding from the National Institutes of Health relevant to the submitted work. RaulSJE reports funding from the National Institutes of Health relevant to the submitted work. Outside the submitted work, he reports grants from Boehringer Ingelheim and personal fees from Leuko labs and Chiesi. He is also a founder and co-owner of Quantitative Imaging Solutions which is a company that provides image based consulting and develops software to enable data sharing. BJM reports grants from AstraZeneca, GlaxoSmithKline, Sunovion, Pearl Research and the National Heart Lung Blood Institute. He reports trial related activity from AzatraZeneca, Spiration, GlaxoSmithKline, Sunovision, Third Pole and Takeda. He reports CME related support from Mt Sinai, WebMD, National Jewish Health, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, Medscape, Ultimate Medical Academy, Eastern Pulmonary Society, Catamount Medical, Eastern Va Medical Center, Academy Continued Health Learning and Wolters Kluwer Health, from outside the submitted work. He is involved with the medical advisory board for Verona, Boehringer Ingelheim, Theravance, Circassia, Phillips, Science 24/7, AstraZeneca and GlaxoSmithKline. All activities are outside of the submitted work. GRW reports grants from the National Institutes of Health, Boehringer Ingelheim, BTG Interventional Medicine, and Janssen Pharmaceuticals. He reports consulting work for Boehringer Ingelheim, PulmonX, Janssen Pharmaceuticals, GlaxoSmithKline, Novartis, Vertex and CSL Behring. He reports advisory board involvement with Boehringer Ingelheim, Vertex and CSL Behring. He is also a founder and co-owner of Quantitative Imaging Solutions which is a company that provides image based consulting and develops software to enable data sharing. All activities are outside the submitted work.
Footnotes
Twitter Stefanie Elizabeth Mason @stef_mason
Disclaimer The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the report or the decision to publish.
Patient consent for publication Not required.
Ethics approval Each study was approved by the relevant Institutional Review Board(s). For a complete list of the sites and corresponding IRB approval numbers, please see online supplemental tables E4 and E5. Written informed consent was obtained from all participants.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available. Investigators can contact the data coordinating centers for each study with data requests. ECLIPSE: https://eclipse-copd.com/COPDGene: http://www.copdgene.org/
REFERENCES
- 1.Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging 2018;22:1148–61. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 2018;47:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell WK, Williams J, Atherton P, et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res 2017;29:11–17. [DOI] [PubMed] [Google Scholar]
- 5.Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. what we know and can do for our patients. Am J Respir Crit Care Med 2018;198:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev 2019;28. doi: 10.1183/16000617.0049-2019. [Epub ahead of print: 31 Dec 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddocks M, Kon SSC, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016;71:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SE, Maddocks M, Kon SSC, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015;70:213–8. [DOI] [PubMed] [Google Scholar]
- 9.van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax 2011;66:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003;58:752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129:536–44. [DOI] [PubMed] [Google Scholar]
- 12.Hopkinson NS, Tennant RC, Dayer MJ, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res 2007;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald M- LN, Diaz AA, Rutten E, et al. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur Respir J 2017;50. doi: 10.1183/13993003.01134-2017. [Epub ahead of print: 14 Dec 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809–13. [DOI] [PubMed] [Google Scholar]
- 15.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City heart study. Am J Respir Crit Care Med 2006;173:79–83. [DOI] [PubMed] [Google Scholar]
- 16.Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. novel chest computed Tomography-derived index for prognosis. Ann Am Thorac Soc 2016;13:334–41. [DOI] [PubMed] [Google Scholar]
- 17.McDonald M-LN, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014;11:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (eclipse). Eur Respir J 2008;31:869–73. [DOI] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart JI, Moyle S, Criner GJ, et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD 2012;9:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary. Am J Respir Crit Care Med 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- 22.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. Ats statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 23.Celli B, Tetzlaff K, Criner G, et al. The 6-Minute-Walk distance test as a chronic obstructive pulmonary disease stratification tool. insights from the COPD biomarker qualification Consortium. Am J Respir Crit Care Med 2016;194:1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreta-Martinez RO, Onieva J, Pascau J. Pectoralis muscle and subcutaneous adipose tissue segmentation on CT images based on convolutional networks. Int J Comput Assist Radiol Surg 2017;12:31–3. [Google Scholar]
- 25.Cano-Espinosa C, Gonzalez G, Washko GR, et al. Biomarker localization from deep learning regression networks. IEEE Trans Med Imaging 2020;39:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017;82:26. [Google Scholar]
- 27.Maltais F, Decramer M, Casaburi R, et al. An official American thoracic Society/European respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;189:e15–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic Society/European respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. [DOI] [PubMed] [Google Scholar]
- 29.Spruit MA, Pitta F, McAuley E, et al. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:924–33. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura Y, Wakabayashi H, Yamada M, et al. Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc 2017;18:553.e1–553.e16. [DOI] [PubMed] [Google Scholar]
- 31.De Brandt J, Spruit MA, Hansen D, et al. Changes in lower limb muscle function and muscle mass following exercise-based interventions in patients with chronic obstructive pulmonary disease: a review of the English-language literature. Chron Respir Dis 2018;15:182–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryrsø CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med 2018;18:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA 2020;323:1813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;12:CD005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2000;20:353–60. [DOI] [PubMed] [Google Scholar]
- 36.Diaz AA, Martinez CH, Harmouche R, et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res 2018;19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celis-Morales CA, Welsh P, Lyall DM, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 2018;361:k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massierer D, Alsowayan W, Lima VP, et al. Prognostic value of simple measures of physical function and muscle strength in COPD: a systematic review. Respir Med 2020;161:105856. [DOI] [PubMed] [Google Scholar]
- 39.McDonald M- LN, Wouters EFM, Rutten E, et al. It’s more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res 2019;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutten EPA, Spruit MA, McDonald M- LN, et al. Continuous fat-free mass decline in COPD: fact or fiction? Eur Respir J 2015;46:1496–8. [DOI] [PubMed] [Google Scholar]
- 41.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.