Abstract
Purpose:
To examine associations between physiologic stress and delirium in the setting of a direct neurologic injury.
Materials and Methods:
We obtained initial neutrophil-to-lymphocyte ratio (NLR), glucose, and troponin in consecutive non-comatose patients with non-traumatic intracerebral hemorrhage (ICH) over 1 year, then used multivariable regression models to determine associations between each biomarker and incident delirium. Delirium diagnoses were established using DSM-5-based methods, with exploratory analyses further categorizing delirium as first occurring <24 hours (“early-onset”) or >24 hours after presentation (“later-onset”).
Results:
Of 284 patients, delirium occurred in 55% (early-onset: 39% [n=111]; later-onset: 16% [n=46]). Patients with delirium had higher NLR (mean 9.0±10.4 vs. 6.4±5.5; p=0.01), glucose (mean 146.5±59.6 vs. 129.9±41.4 mg/dL; p=0.008), and a higher frequency of elevated troponin (>0.05 ng/mL; 21% vs. 10%, p=0.02). In adjusted models, elevated NLR (highest quartile: OR 3.4 [95% CI 1.5–7.8]), glucose (>180 mg/dL: OR 3.1 [95% CI 1.1–8.2]), and troponin (OR 3.0 [95% CI 1.2–7.2]) were each associated with delirium, but only initial NLR was specifically associated with later-onset delirium and with delirium in non-mechanically ventilated patients.
Conclusions:
Stress-related biomarkers corresponding to multiple organ systems are associated with ICH-related delirium. Early NLR elevation may also predict delayed-onset delirium, potentially implicating systemic inflammation as a contributory delirium mechanism.
Keywords: Delirium, intracerebral hemorrhage, biomarkers
Introduction
Delirium occurs frequently in patients with stroke [1], especially in those with intracerebral hemorrhage (ICH) where prevalence may be higher than 50% [2, 3]. However, its occurrence is often unpredictable from one patient to the next even though certain stroke characteristics have been identified as potential delirium risk factors [3]. This suggests that delirium pathogenesis may be multifactorial even in patients with comparable underlying structural lesions and clinical characteristics.
Inflammatory and stress-related mechanisms have been implicated in delirium pathogenesis in non-neurologic populations [4] and may also play a role in post-stroke delirium. However, it is unclear whether such processes are responsible for producing a deliriogenic substrate and precede the development of delirium, arise concurrently with delirium due to shared physiologic pathways, or if they represent a physiologic stress response to delirium onset. For example, elevated levels of cortisol from stress may lead to neutrophil-predominant leukocytosis and hyperglycemia, while agitated delirium may lead to cardiac sequelae such as tachycardia, hypertension, and coronary ischemia, which can cause elevated troponin levels. Further, such phenomena are also common after stroke, which is known to be accompanied by elevated markers of physiologic stress such as neutrophil-to-lymphocyte ratio (NLR) [5], glucose [6], and troponin [7].
Though prior studies in non-neurologic populations have shown that a variety of inflammatory and other biomarkers may be elevated in patients who develop delirium [8, 9], it is unclear whether such markers can similarly predict the development of post-stroke delirium. We therefore aimed to determine associations between early levels of commonly measured biomarkers of physiologic stress and incident delirium in a cohort of patients with ICH, with the hypothesis that early NLR, glucose, and troponin elevations would each be associated with delirium. We chose these specific biomarkers due to their wide use and availability, their known association with physiologic stress [10–12], and their representation of three distinct physiologic systems (immunologic, endocrine, and cardiac, respectively).
Materials and Methods
Study population
We performed a retrospective cohort study using prospectively collected data from the ICH registry at our academic medical center’s Comprehensive Stroke Center. We included consecutive patients who were determined to have a non-traumatic ICH by two attending neurocritical care and/or vascular neurologists over a 12-month period from February 16th, 2018 (the registry’s start date) to February 15th, 2019. Patients diagnosed with ICH due to hemorrhagic conversion of a known ischemic stroke or a known intracranial malignancy at the time of admission were excluded from the registry. Additionally, patients who had no evidence of purposeful response to any stimulus (corresponding to a Richmond Agitation Sedation Scale [RASS] score of −5) for the duration of their hospitalization were considered persistently comatose and therefore excluded from this study. The use of data for this study was approved by our hospital’s Institutional Review Board, and the requirement for informed consent was waived.
Data collection
All data related to standard clinical stroke care were prospectively collected in a REDCap database (Vanderbilt University, Nashville, TN) [13, 14] as part of an ongoing institutional quality improvement project. These data included patient demographics, comorbidities, neuroimaging, and other diagnostic testing. ICH-related clinical predictors, including hematoma location and size, ICH score, and etiology were adjudicated by two attending neurologists with board certification in neurocritical care and/or vascular neurology until consensus was achieved.
Clinical management
Intensive care unit (ICU) management for all patients conformed to institutional and American Heart Association guidelines [15]. Clinical surveillance via neurologic assessment was performed by dedicated neuroscience nurses every 1–2 hours for the duration of each patient’s ICU admission. Intracranial hypertension and cerebral edema were treated with hypertonic saline and/or mannitol, with escalation to neurosurgical interventions as required. Fevers were treated with antipyretic medications, with escalation to temperature regulation devices for persistent fevers. Antiepileptic drugs were used only for seizure treatment and were not used prophylactically. Sedation for mechanically ventilated patients was limited to the lowest possible doses necessary to maintain wakefulness with minimal stimulation (RASS score 0 to −1) except in cases of refractory intracranial hypertension, status epilepticus, shivering, or similar contraindication.
Delirium diagnosis
Delirium was diagnosed in all cases by an attending neurointensivist with additional training in delirium (MR) according to reference-standard DSM-5 criteria [16]: disturbances in attention and awareness (often accompanied by disturbances in other cognitive domains, such as psychomotor slowing or agitation, disorientation, disorganized thinking, impaired executive function, or perceptual disturbance) that develop over a short period of time and tend to fluctuate, represent a change in function, and are due to an underlying medical condition or toxic/withdrawal syndrome. As described previously [2, 17], we considered a broad scope of manifestations of attention and awareness, especially in the context of severe stroke-related deficits such as aphasia or decreased arousal. These included patients’ distractibility, attention and awareness of visual and auditory stimuli, and ability to shift focus between multiple stimuli. Information from multiple time points was typically necessary to determine whether symptoms were fluctuating and out of proportion to patients’ expected ICH-related deficits, with specific consideration given toward distinguishing acute delirium symptoms from those attributable to a focal neuroanatomic lesion.
In approximately one-third of cases, diagnoses were made prospectively, either as part of a nested research study [2] or in the course of standard clinical care. In all other cases, delirium diagnoses were determined retrospectively via detailed chart review (again by MR) using the same DSM-5 criteria, with established chart-based methods that have been previously described [17, 18]. These methods were informed by Confusion Assessment Method for the ICU (CAM-ICU) scores [19] documented as part of clinical care for all patients, though the CAM-ICU was not used as a primary source of delirium diagnosis given previously identified concerns about its accuracy in this patient population [2].
Outcomes
Our primary outcome was any incident delirium during a patient’s hospitalization. We classified patients as “never delirious” if they did not meet criteria for delirium at any time during their hospitalization using the above assessment methods. For the purposes of exploratory analyses, we also defined an additional secondary outcome in which we classified delirium as “early-onset” if it was present on arrival or first occurred within 24 hours of hospital presentation, and “later-onset” if it first occurred at any subsequent point during hospitalization.
Laboratory measurements
We retrospectively abstracted initial laboratory values from peripheral blood obtained at the time of hospital presentation for each patient. These included white blood cell (WBC) counts (with percent differentials), glucose, and troponin. We calculated NLR from WBC differentials, which we treated as a categorical variable divided into quartiles, then categorized serum troponin as not elevated (≤0.05 ng/mL) or elevated (>0.05 ng/mL), and serum glucose as not elevated (<130 mg/dL), moderately elevated (130–180 mg/dL), or high (>180 mg/dL). We treated these markers as categorical variables due to convention as well as the non-linear associations with outcome that have been described for NLR [20], glucose [21, 22], and troponin [23] in critically ill and stroke patients.
Statistical analysis
We used standard descriptive statistics to report patient characteristics, with data that had a normal distribution described with means and standard deviations (SD), and non-normal data described with medians and interquartile ranges (IQR). Differences between continuous variables were analyzed using t-tests or the Mann-Whitney U-test, as appropriate. Differences between categorical variables were analyzed using the Chi-square test.
We then used separate multivariable logistic regression models calculating odds ratios (OR) and exact confidence intervals (CI) to determine associations between incident delirium and each of the following predictor variables: NLR, treated as a quartile-based categorical variable; glucose, treated as a categorical variable; and troponin, treated as a binary variable. Our multivariable models were adjusted for demographics (age, sex, and race classified as white vs. nonwhite), ICH volume, location (supratentorial vs. infratentorial), presence of intraventricular hemorrhage (IVH), and relevant comorbidities (recent corticosteroid use for analyses of NLR, diabetes mellitus for analyses of glucose, and coronary artery disease and chronic kidney disease for analyses of troponin).
In our primary analyses, we used binary logistic regression models with any incident delirium as the outcome of interest. We performed further subgroup analyses in patients who did not require early mechanical ventilation (i.e., within the first day of hospitalization), given the likelihood that mechanical ventilation would have contributed additional physiologic stress at the time of laboratory measurement. In additional exploratory analyses, we included interaction terms between each biomarker and ICH volume (as a representation of ICH severity), and also further categorized our outcome as either early-onset or later-onset delirium using multinomial logistic regression models. In a post-hoc analysis, we plotted the interaction effect between NLR and ICH volume in patients with supratentorial ICH, while keeping other variables in the model at mean values for the sample. This prompted an additional post-hoc subgroup analysis of patients with supratentorial hematomas smaller than 10 cc in order to further explore the association between NLR and delirium in patients with less severe ICH, while also determining the potential utility of NLR in predicting delirium in such cases using area under the receiver operating characteristic curve (AUROC) analysis. Finally, we performed a post-hoc confirmatory analysis in which we considered the association between WBC and delirium in place of NLR.
All hypothesis-testing was two-sided, and the threshold of significance was set at alpha = .05. Given the hypothesis-generating nature of our study, we did not utilize correction methods for multiple testing. Statistical analysis was performed using Stata/MP 11 (College Station, TX).
Results
Baseline characteristics & delirium incidence
There were 284 patients with ICH during the study period who were not persistently comatose, of whom 51% were male and 83% were white, with 34% of patients (n=97) assessed for delirium prospectively. Mean age was 70.1 years (SD 15.8), median ICH score was 1 (IQR 1–2), and 55% of patients (n=157) developed delirium during their hospitalization (including 65% of prospectively assessed patients and 50% of those who were retrospectively rated). Early-onset delirium occurred in 39% of patients (n=111), with later-onset delirium occurring in 16% (n=46). Compared to patients without delirium, delirious patients had larger ICH volumes, were more likely to have IVH, and less likely to have infratentorial hematomas, while the difference in age between groups did not reach statistical significance (Table 1). There were 40 patients who required early mechanical ventilation, of whom 95% (n=38) had delirium during their hospitalization.
Table 1.
Baseline characteristics for patients with intracerebral hemorrhage (ICH) with and without delirium during their hospitalization.
| Any delirium (n = 157) | No delirium (n = 127) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, years, mean (SD) | 71.7 (16.2) | 68.3 (15.1) | 0.07 |
| Male, n (%) | 80 (51%) | 65 (51%) | 0.97 |
| White, n (%) | 128 (82%) | 109 (86%) | 0.33 |
| Comorbidities, n (%) | |||
| Hypertension | 114 (73%) | 78 (61%) | 0.045 |
| Atrial fibrillation | 35 (22%) | 24 (19%) | 0.48 |
| Coronary artery disease | 30 (19%) | 15 (12%) | 0.09 |
| Chronic obstructive pulmonary disease | 13 (8%) | 13 (10%) | 0.56 |
| Diabetes mellitus | 40 (25%) | 28 (22%) | 0.50 |
| Chronic kidney disease | 10 (6%) | 8 (6%) | 0.98 |
| Dementia | 22 (14%) | 6 (5%) | 0.01 |
| Prior stroke | 18 (11%) | 9 (7%) | 0.21 |
| Recent corticosteroid use | 7 (4%) | 3 (2%) | 0.34 |
| ICH characteristics | |||
| ICH score, median (IQR) | 2 (1–3) | 1 (0–1) | < 0.001 |
| ICH volume, cc, mean (SD) | 23.3 (24.6) | 7.0 (10.6) | < 0.001 |
| Intraventricular hemorrhage, n (%) | 87 (55%) | 28 (22%) | < 0.001 |
| Infratentorial location, n (%) | 16 (10%) | 30 (24%) | 0.002 |
| Admission laboratory values | |||
| WBC, per μL, mean (SD) | 12.3 (4.9) | 9.8 (3.5) | < 0.001 |
| NLR, mean (SD) | 9.0 (10.4) | 6.4 (5.5) | 0.01 |
| Serum glucose, mg/dL, mean (SD) | 146.5 (59.6) | 129.9 (41.4) | 0.008 |
| Serum troponin, ng/mL, median (IQR)* | 0.015 (0–0.039) | 0.011 (0–0.024) | 0.13 |
| Elevated troponin, n (%)* | 29 (21%) | 10 (10%) | 0.02 |
Abbreviations: SD, standard deviation; IQR, interquartile range; WBC, white blood cell count; NLR, neutrophil-to-lymphocyte ratio
Data not available for 45 patients
Primary analyses
Data for initial NLR were available for 99% of patients (n=282). Compared to never delirious patients, those with delirium at any point during their hospitalization had higher initial NLR (Table 1), even after adjusting for ICH severity (adjusted mean difference 2.4 [95% CI 0.1–4.7], p=0.04). In our primary multivariable logistic regression analyses, initial elevated NLR was significantly associated with incident delirium after adjusting for relevant confounders (highest NLR quartile: OR 3.4 [95% CI 1.5–7.8], p=0.003). In the subgroup of patients who did not require early mechanical ventilation, elevated NLR remained significantly associated with incident delirium (highest NLR quartile: OR 3.0 [95% CI 1.1–8.1], p=0.03).
Data for initial serum glucose levels were available for all patients. Although patients with delirium during their hospitalization had higher initial glucose levels compared to non-delirious patients (Table 1), this difference was not significant after adjusting for relevant confounders (adjusted mean difference 10.1 [95% CI −2.1–22.2], p=0.10). In our primary multivariable logistic regression analyses, a high initial glucose was significantly associated with incident delirium after adjusting for relevant confounders (glucose >180 mg/dL: OR 3.0 [95% CI 1.1–8.2], p=0.03; glucose 130–180 mg/dL: OR 2.0 [95% CI 1.0–3.8], p=0.051). In the subgroup of patients who did not require early mechanical ventilation, the association between elevated glucose and delirium was no longer significant (glucose >180 mg/dL: OR 1.9 [95% CI 0.6–6.5], p=0.29; glucose 130–180 mg/dL: OR 1.7 [95% CI 0.8–3.6], p=0.18).
Data for initial troponin levels were available for 84% of patients (n=239). Although initial troponin levels were not significantly higher in patients with delirium compared to never delirious patients, patients with delirium were significantly more likely to have elevated initial troponin levels (Table 1). In our primary multivariable logistic regression analyses, an elevated initial troponin level was significantly associated with incident delirium after adjusting for relevant confounders (OR 3.0 [95% CI 1.2–7.2], p=0.01). In the subgroup of patients who did not require early mechanical ventilation, the association between elevated troponin and delirium did not reach statistical significance (OR 2.3 [95% CI 0.9–6.0], p=0.10).
Exploratory analyses
In additional exploratory analyses, we included interaction terms between each biomarker and ICH volume (as a representation of ICH severity), and also further categorized patients by delirium timing (early-onset vs. later-onset). We found that the interaction between ICH volume and NLR was significant (p=0.02), while corresponding interactions with glucose and troponin were not significant (p=0.71 and p=0.47, respectively). A plot of the interaction effect between NLR and ICH volume showed that NLR may be especially predictive of delirium in patients with smaller hemorrhages (Figure 1). In a post-hoc subgroup analysis in patients with supratentorial hematomas smaller than 10 cc, we found that elevated NLR was strongly associated with delirium in our multivariable model (highest NLR quartile: OR 5.6 [95% CI 1.9–16.4], p=0.002), while it was also a reasonably accurate predictor of delirium onset on its own (AUROC 0.693 [95% CI 0.601–0.786]).
Figure 1.
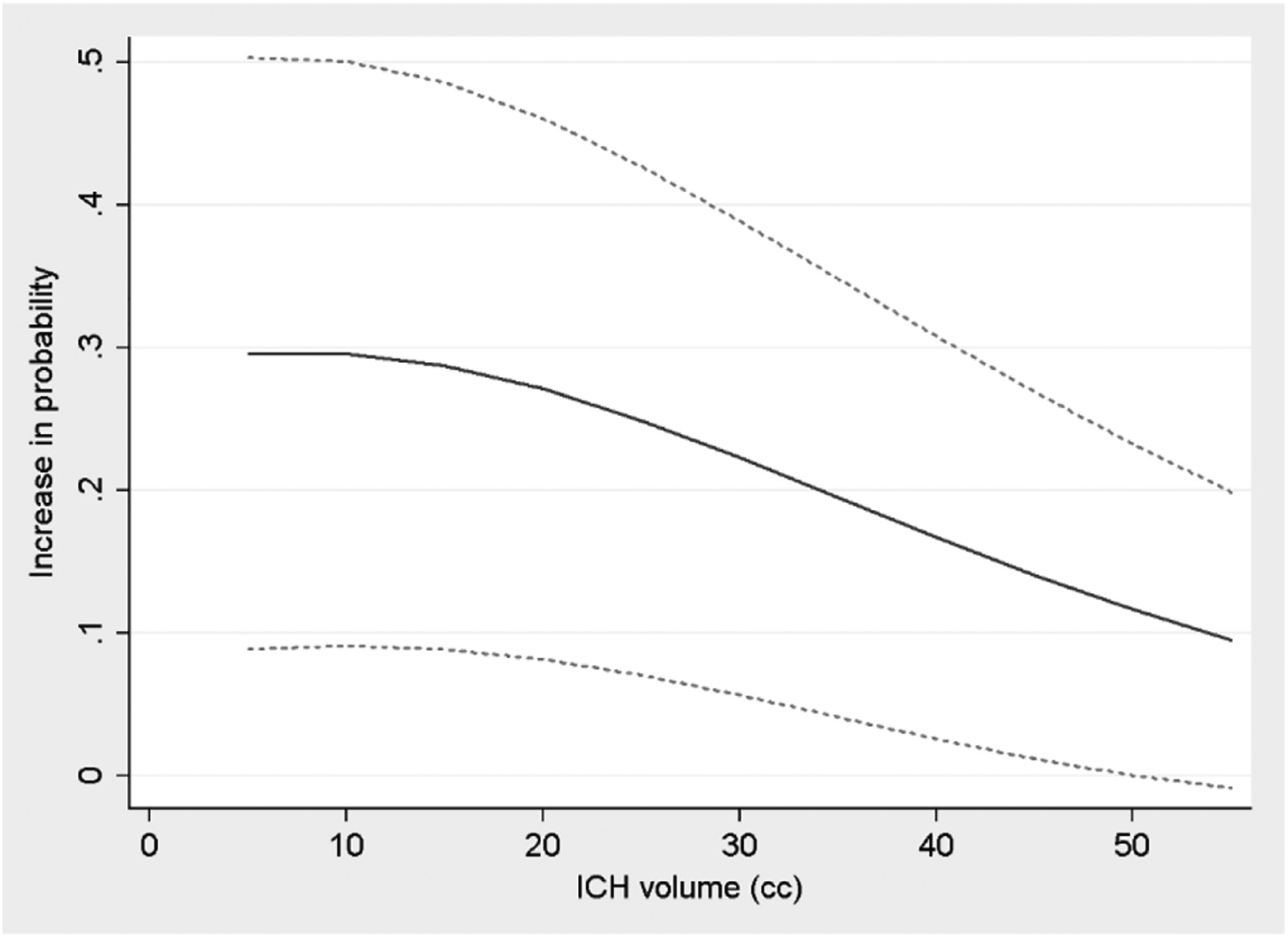
Change in predicted probability of delirium associated with highest quartile of neutrophil-to-lymphocyte ratio (NLR), according to intracerebral hemorrhage (ICH) volume. Analysis includes only patients with supratentorial ICH, while age and other ICH-related factors in the model were held at mean values for the sample. Dotted lines represent upper and lower bounds of 95% confidence intervals
Further, initial NLR was higher in patients with later-onset delirium compared to non-delirious patients (adjusted mean difference 3.4 [95% CI 0.2–6.5], p=0.04), while the difference for patients with early-onset delirium was not statistically significant (adjusted mean difference 2.0 [95% CI −0.5–4.4], p=0.11) (Figure 2a). Multinomial logistic regression analysis showed that initial elevated NLR was significantly associated with both early-onset delirium (highest NLR quartile: OR 4.8 [95% CI 1.7–13.3], p=0.003) and later-onset delirium (highest NLR quartile: OR 4.1 [95% CI 1.1–15.8], p=0.04). Results were similar when considering WBC instead of NLR (highest WBC quartile, early-onset delirium: OR 7.1 [95% CI 2.7–18.0], p<0.001; later-onset delirium: OR 3.7 [95% CI 1.1–12.3], p=0.03).
Figure 2.
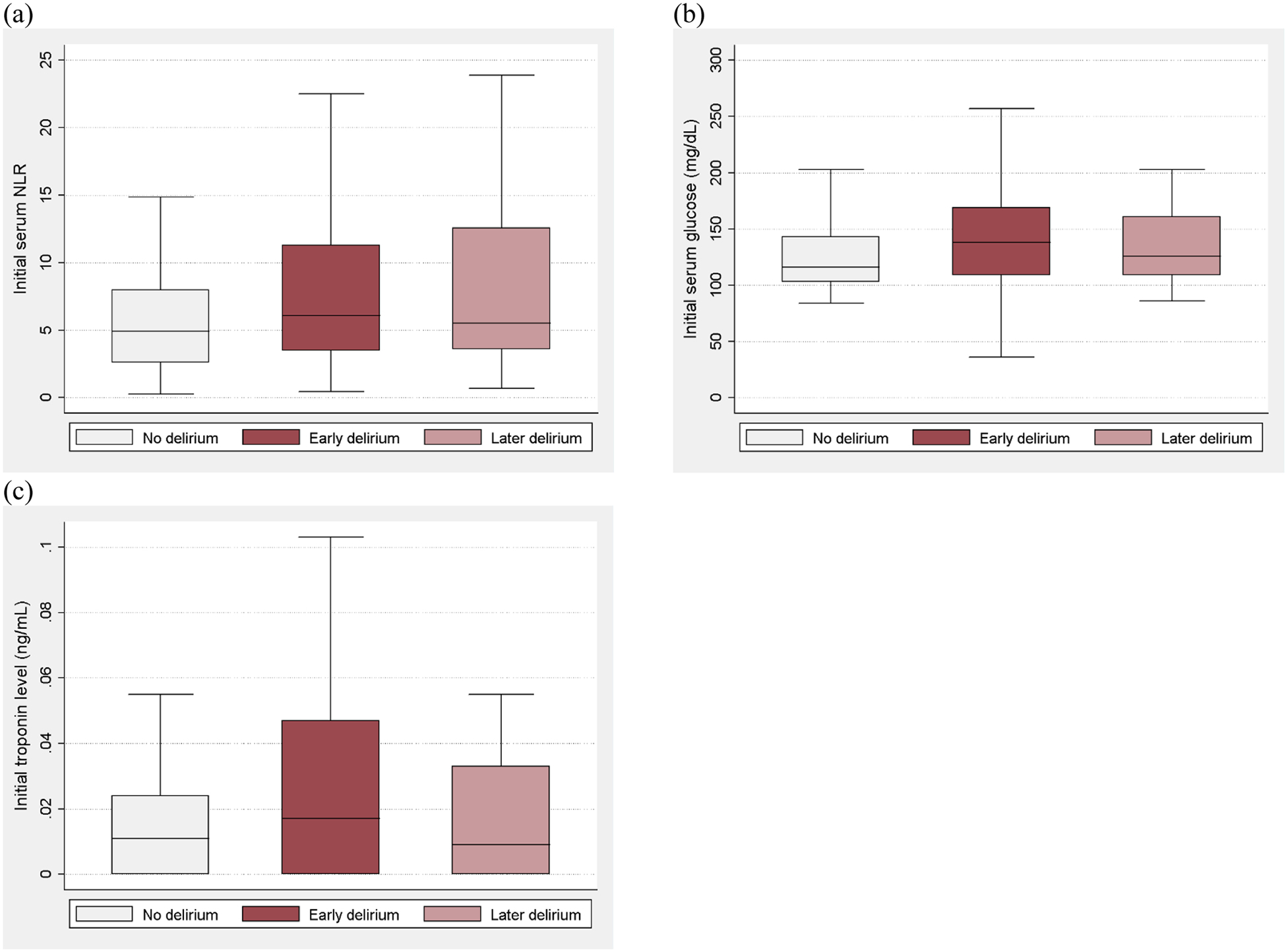
Boxplots comparing (a) initial neutrophil-to-lymphocyte ratio (NLR), (b) glucose, and (c) troponin measured on the day of hospital presentation between groups of patients who did not have delirium, those with early delirium, and those with later delirium
Categorizing patients by delirium onset also suggested a higher initial glucose level in those with early-onset delirium compared to non-delirious patients, but there was not a statistically significant difference in either early-onset or later-onset delirium groups (early-onset: adjusted mean difference 12.7 [95% CI −0.3–25.7], p=0.055; later-onset: adjusted mean difference 3.4 [95% CI −13.3–20.1], p=0.69) (Figure 1b). Multinomial logistic regression analysis showed that a high initial glucose was significantly associated with early-onset delirium (glucose >180 mg/dL: OR 3.7 [95% CI 1.1–11.7], p=0.03; glucose 130–180 mg/dL: OR 2.1 [95% CI 1.0–4.6], p=0.051), but not with later-onset delirium (glucose >180 mg/dL: OR 2.4 [95% CI 0.5–12.7], p=0.29; glucose 130–180 mg/dL: OR 1.6 [95% CI 0.6–4.0], p=0.36).
Finally, among those who did develop delirium, the frequency of elevated troponin was not significantly higher in those with early-onset delirium compared to those with later-onset delirium (24% [24/99] vs. 13% [5/39], p=0.14). Multinomial logistic regression analysis showed that an elevated initial troponin level was significantly associated with early-onset delirium (OR 3.2 [95% CI 1.0–10.1], p=0.045), but not with later-onset delirium (OR 2.0 [95% CI 0.5–8.9], p=0.35) (Figure 1c).
Discussion
We found that early levels of several commonly measured biomarkers of physiologic stress were associated with delirium incidence in patients with ICH. Because these markers represent the involvement of multiple organ systems (cardiac, endocrine, and immunologic), delirium at ICH onset may correspond to a more robust overall physiologic stress response. However, only NLR remained significantly associated with delirium in patients who did not have the additional physiologic stress of mechanical ventilation, and only initial NLR and WBC were also associated with delirium that had a delayed onset. This suggests that evidence of systemic inflammation may exist prior to the development of delirium, and that inflammatory markers may potentially predict post-stroke delirium independent of intrinsic stroke characteristics or external factors. Further, the association between NLR and delirium appeared to be most prominent in patients with smaller hematomas, suggesting that the inflammatory response in patients with delirium is not driven exclusively by ICH severity. These findings in a population of patients with a direct neurologic insult are hypothesis-generating, and may also reinforce the prevailing hypothesis that systemic inflammation is a major factor in the development of delirium in general.
The neuroinflammatory hypothesis has been a popular mechanism implicated in delirium pathogenesis for some time, and proposes that central nervous system (CNS) inflammatory cascades may be induced by peripheral inflammation from an extrinsic insult coupled with subsequent blood-brain barrier (BBB) breakdown [4]. This in turn may result in downstream neuronal network dysfunction that leads to overt cognitive manifestations. However, BBB breakdown is an expected consequence of stroke and other forms of direct neurologic injury, and such patients may therefore be especially susceptible to the effects of systemic inflammation [24]. There may also be shared pathways between neuroinflammation, stroke, and delirium. As a result, differences in the systemic inflammatory response may partially explain why some patients with otherwise similar stroke-related clinical factors may develop delirium while others do not. On a broader scale, systemic and neuroinflammatory processes have also been implicated in overall post-stroke outcomes [25].
Various inflammatory biomarkers have been studied as potential delirium predictors, including IL-6, TNF-α, and C-reactive protein (CRP), although results have thus far been mixed. Among these, CRP may have the most evidence supporting its role in predicting delirium in non-neurologic patients, as in post-operative [26, 27] and medical ICU populations [28]. Inflammatory markers such as CRP may potentially have utility in predicting delirium in neurologic patients as well; in one recent study, several such markers, including NLR and CRP, were elevated in patients with early-onset delirium after ischemic stroke [29]. Given possible shared pathways between delirium and stroke, such patients may represent an ideal model population to continue investigations into the neuroinflammatory hypothesis of delirium, with future studies also ideally investigating correlations between peripheral and CNS inflammatory markers. Additional studies considering the utility of serial inflammatory marker measurements in the prediction of delirium incidence and resolution may also be warranted, as such biomarkers may represent surrogates of persistent or resolving inflammation.
Our study has several limitations. First, as a single-center study, it may have reduced generalizability based on patient demographics and other clinical factors, though our rates of delirium are similar to those described in other studies. Second, delirium diagnoses were made retrospectively in most cases, though a sizeable subset of patients (greater than one-third) were prospectively assessed by an expert clinician, and similar diagnostic criteria were used in both cases. Third, because of the retrospective nature of our study, our ability to study biomarkers suggestive of physiologic stress was limited to common laboratory tests measured as part of standard clinical stroke care, which are relatively non-specific. Fourth, we did not have details on other factors that may have affected biomarker levels, such as concurrent infections that may have caused an elevated NLR, nor did we have detailed data on oxygenation, cerebral perfusion, or other physiologic factors that may have contributed to acute cognitive dysfunction. Finally, we did not have details on the underlying etiology for each case of delirium, which may have further elucidated associations between our biomarkers of interest and delirium that had a later onset. These limitations make a future prospective study using a comprehensive panel of inflammatory biomarkers measured in a serial fashion a logical next step in establishing a temporal link between systemic inflammation and post-ICH delirium.
Conclusions
While stress-related biomarkers corresponding to multiple organ systems are associated with delirium after ICH, early NLR may also predict delayed-onset delirium, potentially implicating systemic inflammation as a major contributory delirium mechanism.
Highlights.
Delirium pathophysiology is likely multifactorial, even in neurologic patients
Biomarkers related to physiologic stress are associated with delirium after ICH
Elevated neutrophil-to-lymphocyte ratio may also predict delayed-onset delirium
Systemic inflammation may contribute to delirium, even in neurologic patients
Financial support:
LAD is supported by NIH grant R01AG058648.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared.
References
- [1].Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in Acute Stroke: A Systematic Review and Meta-Analysis. Stroke 2012;43(3):645–9. [DOI] [PubMed] [Google Scholar]
- [2].Reznik ME, Drake J, Margolis SA, Moody S, Murray K, Costa S, et al. Deconstructing Poststroke Delirium in a Prospective Cohort of Patients With Intracerebral Hemorrhage*. Read Online: Critical Care Medicine | Society of Critical Care Medicine 2020;48(1):111–8. [DOI] [PubMed] [Google Scholar]
- [3].Carin-Levy G, Mead GE, Nicol K, Rush R, van Wijck F. Delirium in acute stroke: screening tools, incidence rates and predictors: a systematic review. Journal of neurology 2012;259(8):1590–9. [DOI] [PubMed] [Google Scholar]
- [4].Maldonado JR. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. The American Journal of Geriatric Psychiatry 2013;21(12):1190–222. [DOI] [PubMed] [Google Scholar]
- [5].Song S-Y, Zhao X-X, Rajah G, Hua C, Kang R-J, Han Y-P, et al. Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients With Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front Neurol 2019;10:1032-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients. Stroke 2001;32(10):2426–32. [DOI] [PubMed] [Google Scholar]
- [7].Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated Troponin after Stroke: A Systematic Review. Cerebrovascular Diseases 2009;28(3):220–6. [DOI] [PubMed] [Google Scholar]
- [8].Khan BA, Zawahiri M, Campbell NL, Boustani MA. Biomarkers for Delirium—A Review. Journal of the American Geriatrics Society 2011;59(s2):S256–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Michels M, Michelon C, Damásio D, Vitali AM, Ritter C, Dal-Pizzol F. Biomarker Predictors of Delirium in Acutely Ill Patients: A Systematic Review. Journal of Geriatric Psychiatry and Neurology 2019;32(3):119–36. [DOI] [PubMed] [Google Scholar]
- [10].Boland TA, Lee VH, Bleck TP. Stress-Induced Cardiomyopathy. 2015;43(3):686–93. [DOI] [PubMed] [Google Scholar]
- [11].Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet (London, England) 2009;373(9677):1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-Induced Leukocytosis: Early Observations, Current Research, and Future Directions. Brain, Behavior, and Immunity 1996;10(2):77–91. [DOI] [PubMed] [Google Scholar]
- [13].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2015;46(7):2032–60. [DOI] [PubMed] [Google Scholar]
- [16].Diagnostic and statistical manual of mental disorders : DSM-5. Fifth edition. Arlington, VA: : American Psychiatric Association, [2013]; 2013. [Google Scholar]
- [17].Reznik ME, Moody S, Murray K, Costa S, Grory BM, Madsen TE, et al. The impact of delirium on withdrawal of life-sustaining treatment after intracerebral hemorrhage. Neurology 2020: 10.1212/WNL.0000000000010738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pisani MA, Araujo KLB, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Critical Care 2006;10(4):R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mitasova A, Kostalova M, Bednarik J, Michalcakova R, Kasparek T, Balabanova P, et al. Poststroke delirium incidence and outcomes: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)*. Critical care medicine 2012;40(2):484–90. [DOI] [PubMed] [Google Scholar]
- [20].Hwang SY, Shin TG, Jo IJ, Jeon K, Suh GY, Lee TR, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. The American Journal of Emergency Medicine 2017;35(2):234–9. [DOI] [PubMed] [Google Scholar]
- [21].Investigators TN-SS. Intensive versus Conventional Glucose Control in Critically Ill Patients. New England Journal of Medicine 2009;360(13):1283–97. [DOI] [PubMed] [Google Scholar]
- [22].Ntaios G, Egli M, Faouzi M, Michel P. J-Shaped Association Between Serum Glucose and Functional Outcome in Acute Ischemic Stroke. Stroke 2010;41(10):2366–70. [DOI] [PubMed] [Google Scholar]
- [23].Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, Falcou A, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. Journal of Neurology, Neurosurgery & Psychiatry 2005;76(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. American Journal of Physiology-Cell Physiology 2019;316(2):C135–C53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/Brain Biomarkers of Inflammation After Stroke and Their Association With Outcome: From C-Reactive Protein to Damage-Associated Molecular Patterns. Neurotherapeutics 2016;13(4):671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ayob F, Lam E, Ho G, Chung F, El-Beheiry H, Wong J. Pre-operative biomarkers and imaging tests as predictors of post-operative delirium in non-cardiac surgical patients: a systematic review. BMC Anesthesiology 2019;19(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu X, Yu Y, Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLOS ONE 2018;13(4):e0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khan BA, Perkins AJ, Prasad NK, Shekhar A, Campbell NL, Gao S, et al. Biomarkers of Delirium Duration and Delirium Severity in the ICU. Critical care medicine 2019;Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kotfis K, Bott-Olejnik M, Szylińska A, Listewnik M, Rotter I. Characteristics, Risk Factors And Outcome Of Early-Onset Delirium In Elderly Patients With First Ever Acute Ischemic Stroke - A Prospective Observational Cohort Study. Clin Interv Aging 2019;14:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


