Abstract
Increasing the T-cell immune response to Mycobacterium tuberculosis with an anti-programmed cell death 1 (anti-PD-1) antibody may ultimately have detrimental effects. We present the case of a patient with advanced non-small cell lung cancer who developed active tuberculosis (TB) after initial treatment with pembrolizumab, an anti-PD-1 antibody. Pembrolizumab was resumed after completing anti-TB treatment, and no relapse of TB was observed clinically or radiologically. Checkpoint inhibitor-related pneumonitis (CIP) is first suspected when a pulmonary shadow presents during treatment with an anti-PD-1 antibody. It is sometimes difficult to diagnose CIP using computed tomographic images alone. Careful testing, including bacterial examinations and bronchoscopic biopsy, should be performed.
Keywords: Mycobacterium tuberculosis, immune checkpoint inhibitor, non-small cell lung cancer, pembrolizumab
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) and programmed death ligand 1 (PD-L1) have revolutionized therapy for several advanced types of cancer, including non-small cell lung cancer (NSCLC). The inhibition of the interaction between PD-1 and PD-L1 by an anti-PD-1 antibody causes the reactivation of cytotoxic T cells and stimulates antitumor immune responses (1). However, an overactivated immune response can have unique side effects, known as ‘immune-related adverse events’ (irAEs) (2). Among the reported irAEs is checkpoint inhibitor-related pneumonitis (CIP), which develops in 5-10% of patients (3-5). CIP presents as a spectrum of radiographic patterns (3-6); thus, it can be difficult to distinguish from infectious pneumonia based on radiological findings alone. It remains unclear how ICIs affect the infectious immune response; however, cases involving the reactivation of pulmonary tuberculosis (TB) during anti-PD-1 antibody immunotherapy have been reported (7). The readministration of ICIs after the improvement of irAE is associated with a risk of irAE recurrence (8) and should only be considered after careful scrutiny. The safety of resuming ICIs after TB infection has not been well evaluated because few cases have been reported (7). We herein report a case in which treatment with the anti-PD1 antibody pembrolizumab was recommenced after the completion of TB treatment in a patient with advanced NSCLC who developed TB during initial pembrolizumab therapy.
Case Report
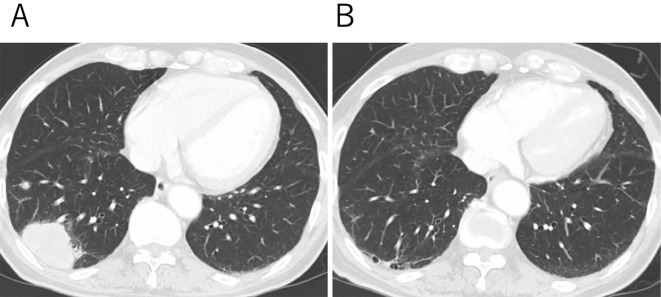
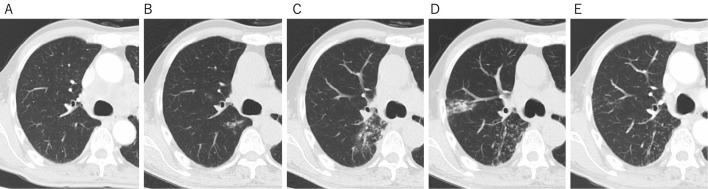
The patient was a 73-year-old man who was diagnosed with T3N3M1a stage IV lung adenocarcinoma in the right lower lobe of the lung (Fig. 1A), without driver mutations-including epidermal growth factor receptor mutations and anaplastic lymphoma kinase translocations-but with the elevated expression of PD-L1 on 90% of tumor cells. His performance status was 0. He had no history of active TB and computed tomography (CT) scans showed no abnormalities suggestive of latent TB, such as calcification of the hilar or mediastinal lymph nodes (Fig. 2A). The patient had been treated with pembrolizumab, an anti-PD-1 monoclonal antibody (200 mg, every 3 weeks), as a first-line therapy since July 2017. After five cycles of pembrolizumab, the patient developed muscle pain in his shoulder and thigh, suggesting myositis as an irAE of pembrolizumab. Pembrolizumab was discontinued and treatment with oral prednisolone (15 mg/day for 2 weeks) was commenced, resulting in the resolution of his muscle pain after 2 weeks. No additional steroid therapy was administered and his symptoms were well controlled with nonsteroidal anti-inflammatory drugs (NSAIDs). In January 2018, a CT scan showed tumor shrinkage; however, a new faint shadow was detected in the right lower lobe (Fig. 1B, 2B). Three months later, the lesion had expanded, with ground-glass opacity and centrilobular nodules (Fig. 2C), but the patient did not present cough, sputum, fever, or other symptoms. Because CIP was suspected, steroid therapy was initiated (prednisolone, 0.5 mg/kg/day) and gradually tapered off over a period of approximately 3 months. However, the ground-glass opacity with nodular shadowing did not improve with steroidal therapy; thus, radiological follow-up was continued without drugs. In January 2019, the patient developed cough and sputum without fever. Chest CT revealed expanded consolidation and a nodular lesion in the right upper lobe (Fig. 2D). Ziehl-Neelsen staining of a sputum smear was negative; however, a mycological culture of a sputum sample was positive for acid-fast bacilli, which were confirmed by polymerase chain reaction (PCR) to be Mycobacterium tuberculosis (Mtb). Thus, the patient received standard anti-TB chemotherapy, involving a 6-month period of combination treatment with isoniazid, rifampicin, pyrazinamide, and ethambutol, followed by a regimen of isoniazid and rifampicin from February 2019. In August 2019 (Fig. 2E), the pulmonary shadows in the right upper and lower lobes had disappeared but swelling of the abdominal paraaortic lymph nodes was detected on CT, with simultaneously elevation of his carcinoembryonic antigen (a tumor marker) level, indicating the recurrence of lung cancer. Pembrolizumab was therefore resumed; after 15 cycles, no relapse of TB was observed, either clinically or radiologically, and a partial tumor response was detected on CT in April 2020.
Figure 1.
Computed tomography (CT) images of the primary lesion during the patient’s clinical course. (A) A CT scan at the time of the diagnosis showed a mass lesion in the right lower lobe. (B) The tumor regressed after five cycles of pembrolizumab.
Figure 2.
Computed tomography (CT) images of pulmonary opacities during the patient’s clinical course. (A) A CT scan of the right S6 segment at the time of the diagnosis. (B) A CT scan showing the development of a new faint pulmonary shadow in the right S6 segment. (C) A CT scan obtained when checkpoint inhibitor-related pneumonitis was initially suspected. (D) A CT scan at the time of the diagnosis of TB. (E) A CT scan after the completion of anti-TB treatment.
Discussion
Several reports have described the reactivation of TB during anti-PD-1 antibody immunotherapy (7). In many cases, ICI was stopped after the development of TB and anti-TB treatment was commenced. There is concern that the renewed administration of ICI could exacerbate TB. TB did not worsen in some cases in which ICIs were readministered; however, the number of these cases was small and was not sufficient to establish the safety of recommencing ICI treatment. In our patient, we recommenced pembrolizumab after the completion of TB treatment. At present, 10 months since the recommencement of pembrolizumab, this treatment is ongoing, with no recurrence of TB. The long-term safety data provided by this case support the readministration of ICI after TB treatment.
The reactivation of TB is usually attributed to an immune system weakened by disease, including by malignant tumors, or by cytotoxic chemotherapy or immunosuppressive medications, such as corticosteroids or anti-tumor necrosis factor α (anti-TNF-α) antibody (9,10). A weakened immune system can no longer contain the mycobacteria in the granuloma and the bacteria are released into the extracellular space. Our patient received prednisolone (PSL) (15 mg/day) for 2 weeks to treat his muscle pain, which was considered an irAE of pembrolizumab. However, the period of oral steroid administration was not long. We therefore assume that it had little effect on the relapse of TB. The PD-1/PD-L1 pathway has been implicated in the pathophysiology of chronic infections, including TB and fungal infections. PD-1 is expressed on T cells and the exhaustion of T-cell immunity, which is similar to cancer immunity, has been demonstrated (11,12). Based on this fact, ICIs-including anti-PD-1 antibodies-are usually expected to promote the immune response to microorganisms. Some in vitro studies have shown that the anti-PD-1 blockade imposed by human peripheral blood mononuclear cells from TB patients restores responsiveness to TB antigens and cytokine secretion (13,14). In a PD-1-deficient mouse model, increased cytokine production against Mtb-specific antigens was observed; however, survival was significantly worse than in the wild-type mice (15,16). Moreover, pathological overinflammation and excessive necrosis were also detected in PD-1-deficient mice (15). These findings indicate that enhancing T-cell immunity directed against Mtb could ultimately have detrimental effects. TNF-α is the dominant cytokine affecting Mtb growth and an excess of TNF-α accelerates Mtb growth (17). PD-1 may regulate the immune reaction to Mtb, and anti-PD-1 antibodies could exacerbate TB infections via the excessive secretion of TNF-α (17). The expression of PD-1 on Mtb-specific CD4+ T cells is associated with the bacterial load during TB infection (18). Thus, PD-1 levels decrease after the completion of anti-TB treatment relative to the pretreatment levels (18,19). It may therefore be reasonable to restart anti-PD-1 antibody therapy after the completion of anti-TB treatment in cancer patients. In a systematic review of the development of active TB after ICI therapy in 16 patients, two patients died from complications of TB infection (7). ICI therapy was continued in only one case; however, no details were given (20). In seven cases, ICI therapy was recommenced before or after the anti-TB treatment was completed (7); however, there were no cases of worsening TB. In the present case, pembrolizumab was resumed after completing anti-TB treatment because the tumor had continued to shrink until the end of anti-TB treatment. Although resuming ICI treatment during TB treatment is an alternative option, it would depend upon the condition of the cancer.
CIP shows a broad spectrum of radiographic patterns on chest CT images, which can be classified as the cryptogenic organizing pneumonia pattern, the hypersensitivity pneumonia pattern, and the diffuse alveolar damage pattern (3-5). However, some cases have shown a CT pattern with bronchitis-like centrilobular nodules (6). When such nodular patterns are present, it can be difficult to distinguish between CIP and infection. In the present case, centrilobular nodules were detected in the right lower lobe on CT scans (Fig. 2C). Based on the CT findings, infection was indisputable; however, the patient did not present cough, sputum, fever, or other symptoms. Thus, we initially suspected CIP, and the period from the appearance of nodular lesions to the diagnosis of pulmonary TB was approximately 1 year. Based on a systematic review, the median time to the diagnosis of TB after the initiation of ICI therapy was 6.3 months (range: 1-24 months) (7). In 15 of 16 cases, the diagnosis of TB was confirmed with either Mtb culture or the detection of tubercular DNA by PCR. Pathological assessments were also made in 10 patients and confirmed the presence of granulomatous inflammation. The median time for the development of CIP after the initiation of ICI therapy was approximately 2.3 months (range: 0.2-27.4) (5-21). Thus, the times at which CIP and TB develop after the initiation of ICI therapy are similar. Therefore, when bronchitis-like centrilobular nodules or pulmonary consolidation mimicking infectious disease are present in the lung field on CT, careful testing is required, such as a bacterial examination, PCR analysis of sputum samples, or a bronchoscopic biopsy.
The reactivation of TB is one of the most important complications of anti-TNF-α antibody therapy (10). An interferon-γ-release assay (IGRA), which detects the immune response to specific Mtb antigens, shows good sensitivity and specificity for the diagnosis of TB infection; thus, screening for latent TB with an IGRA is recommended before anti-TNF-α antibody therapy is initiated in patients with rheumatoid arthritis (22). Isoniazid prophylaxis is offered to patients with evidence of latent TB infection (22). However, there are no data on the risk of the reactivation of latent TB in patients treated with ICIs. The utility of screening for latent TB before commencing ICI therapy in cancer patients and whether prophylaxis can prevent the reactivation of TB are unclear. In a previous case report, a patient with a history of TB treatment showed a negative IGRA result at their first visit, but developed TB reactivation during treatment with durvalumab, an anti-PD-L1 antibody, after chemoradiotherapy for stage III NSCLC. False-negative results are a major obstacle to screening for latent TB, and approximately 8-19% of patients show a negative IGRA result even when presenting with active TB (23). Indeterminate IGRA results are frequently reported in patients with malignancies and patients undergoing immunosuppressive therapy (24).
In conclusion, we presented a case of active TB that developed after the initiation of pembrolizumab treatment in a patient with advanced NSCLC. The readministration of pembrolizumab after the completion of anti-TB treatment had no adverse effects on the course of TB. However, the safety of the readministration of ICIs after anti-TB treatment is still controversial and the exacerbation of TB after ICI treatment has been reported in patients with previously treated TB. Thus, further cases must be evaluated to establish a suitable management strategy for active TB during ICI therapy.
Author's disclosure of potential Conflicts of Interest (COI).
Terufumi Kato: Employment, MSD; Honoraria, MSD. Haruhiro Saito: Research funding, MSD.
References
- 1. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375: 1767-1778, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54: 139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 22: 6051-6060, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35: 709-717, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 69: 15-22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 125: 150-156, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Zaemes J, Kim C. Immune checkpoint inhibitor use and tuberculosis: a systematic review of the literature. Eur J Cancer 132: 168-175, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6: 1093-1099, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai CC, Lee MT, Lee SH, Lee SH, Chang SS, Lee CC. Risk of incident active tuberculosis and use of corticosteroids. Int J Tuberc Lung Dis 19: 936-942, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345: 1098-1104, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Wherry EJ. T cell exhaustion. Nat Immunol 12: 492-499, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486-499, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jurado JO, Alvarez IB, Pasquinelli V, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 181: 116-125, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Singh A, Dey AB, Mohan A, Mitra DK. Programmed death-1 receptor suppresses gamma-IFN producing NKT cells in human tuberculosis. Tuberculosis (Edinb) 94: 197-206, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Lazar-Molnar E, Chen B, Sweeney KA, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A 107: 13402-13407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tousif S, Singh Y, Prasad DV, Sharma P, Van Kaer L, Das G. T cells from programmed death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PloS one 6: e19864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tezera LB, Bielecka MK, Ogongo P, et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-alpha. Elife 9: e52668, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Day CL, Abrahams DA, Bunjun R, et al. PD-1 expression on Mycobacterium tuberculosis-specific CD4 T cells is associated with bacterial load in human tuberculosis. Front Immunol 9: 1995, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hassan SS, Akram M, King EC, Dockrell HM, Cliff JM. PD-1, PD-L1 and PD-L2 gene expression on t-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PloS one 10: e0137646, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Picchi H, Mateus C, Chouaid C, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect 24: 216-218, 2018. [DOI] [PubMed] [Google Scholar]
- 21. Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 50: 1700050, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 625-639, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamasue M, Komiya K, Usagawa Y, et al. Factors associated with false negative interferon-gamma release assay results in patients with tuberculosis: a systematic review with meta-analysis. Sci Rep 10: 1607, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komiya K, Ariga H, Nagai H, et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-gamma release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern Med 49: 1849-1855, 2010. [DOI] [PubMed] [Google Scholar]