Abstract
Basic and clinical research have shown that the expression of molecules involved in the hepatocellular carcinoma (HCC) cell signaling pathway is related to the sensitivity to molecular-targeted agents. We herein report a case of HCC that was effectively treated with lenvatinib after a poor response to sorafenib. The tumor showed a high expression of fibroblast growth factor receptor 4, which is reportedly related to the sensitivity to lenvatinib in vitro. The information obtained from this case and from our literature review highlights the importance of assessing the expression of the molecules involved in tumors for effective precision medicine.
Keywords: hepatocellular carcinoma, FGFR4, FGF19, lenvatinib, molecular targeting agents
Introduction
With the development of various molecular-targeting agents (MTAs) and immune checkpoint inhibitors, the therapeutic options for hepatocellular carcinoma (HCC) have become varied in the past decade (1-4). Although there are guidelines for the management of HCCs (5), with the treatment approaches depending on hepatic reserve, predictive biomarkers assessing the therapeutic efficacy of these treatment options need to be studied further in order to optimize the use of the appropriate agents. For example, the REFLECT trial showed that lenvatinib (LEN), an MTA classified as a tyrosine kinase inhibitor (TKI), was non-inferior to sorafenib (SOR), the first approved TKI for advanced HCC (6). However, biomarkers capable of predicting a patient's response to these TKIs for HCC remain largely unknown.
With increases in the genetic information on HCCs (7), substantial knowledge concerning the gene expression and molecular mechanisms has been aggregated (8). Based on the available information, it has been determined that LEN inhibits vascular endothelial growth factor receptors (VEGFRs) 1-3, fibroblast growth factor receptors (FGFRs) 1-4, c-Kit, and ret proto-oncogene (RET) more strongly than SOR (9-12). Autocrine activation of this FGF signaling involving FGFR4 and its cognate ligand FGF19 is reported in about 30-50% of HCC patients (13-16). In addition, aberrant expression of FGF19-FGFR4 have been reported to be correlated with tumor progression and a poorer prognosis of HCC, making them a novel therapeutic target for HCC therapy (17-19). LEN also reportedly inhibits this FGF19-FGFR4 signaling pathway more efficiently than SOR, as evidenced by the half maximal inhibitory concentration (IC50) for FGFR4 (IC50=43) with LEN being significantly lower than that of SOR (IC50=3,400) (9).
We herein report a case of HCC expressing a high level of FGFR4 that showed a favorable response to LEN compared to SOR. The results support the importance of assessing the genetic expression in order to choose the most appropriate MTA.
Case Report
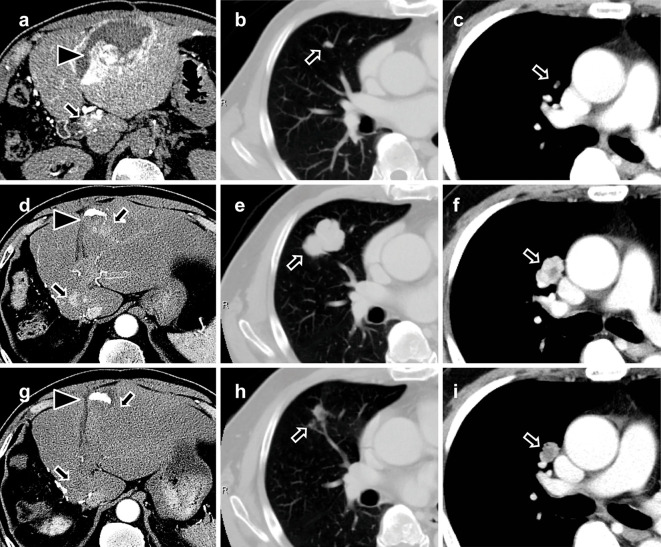
A 69-year-old man with nonalcoholic steatohepatitis was referred to our hospital with a 12-cm hepatocellular carcinoma in the posterior segment of his liver, which was treated with curative extended right hepatectomy in December 2015 (Fig. 1). The tumor was nodular with an extranodular growth pattern and histologically moderately differentiated without microvascular invasion. Seven months later, contrast-enhanced computed tomography (CT) showed multiple intrahepatic, lung, and mediastinal metastases (Fig. 2a-c). In addition, one of the hepatic lesions showed intra-tumoral bleeding (Fig. 2a). The courses of the images of the liver, lung, and mediastinal lesions are shown in Fig. 2d-i.
Figure 1.
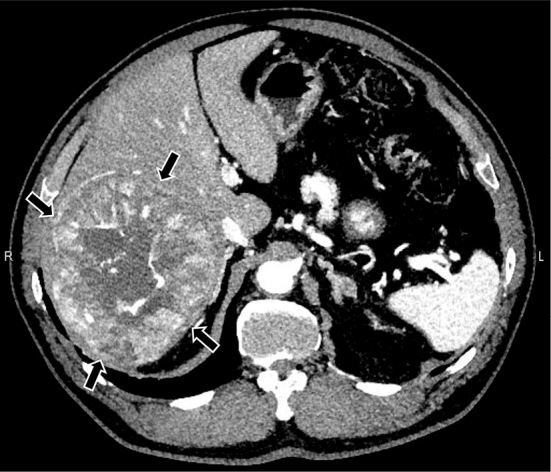
Computed tomography findings of HCC. Arterial phase of contrast-enhanced dynamic computed tomography (CT) of the primary tumor. A 12-cm, well-enhanced tumor in the posterior segment of the liver (black arrows). HCC: hepatocellular carcinoma
Figure 2.
Time-dependent change in CT images. (a-c) CT before the SOR administration. (a) Abdominal CT angiography demonstrated multiple liver tumors (black arrow) and intra-tumoral bleeding of one of the tumors in the liver (black arrowheads). Chest CT demonstrated multiple lung (b, black arrow) and mediastinal metastases (c, black arrow). (d-f) CT after two years of SOR and before LEN administration. (d) The intrahepatic metastases of HCC (black arrows). The main tumor in the liver showed therapeutic effect of TACE (black arrowhead). The progression of the lung (e, black arrow) and mediastinal metastases (f, black arrow). (g-i) CT after two months of LEN administration. The intrahepatic (g, black arrowhead and black arrows), lung (h, black arrow), and mediastinal metastases (i, black arrow) showed a response to LEN in two months. SOR: sorafenib, LEN: lenvatinib, HCC: hepatocellular carcinoma, TACE: transcatheter arterial chemoembolization
As there was no response after 2 courses of continuous systemic infusion of low-dose cisplatin (CDDP 7 mg/day) and 5-fluorouracil (5-FU 300 mg/day), SOR was initiated at 400 mg orally, with 2 doses a day. Since then, the lesions have been controlled with multimodal therapy, including SOR, transcatheter arterial infusion of cisplatin, and transcatheter arterial chemoembolization (TACE). The dose of 400 mg was continued, as the tumor showed stable disease response with the dose and the adverse events of increase of blood pressure and grade 1 hand-foot skin reaction were managed with the calcium channel blocker and urea-based cream.
The metastatic tumor in the lung and mediastinum showed progressive disease after two years of stability with SOR (Fig. 2e, f). There was no response to an increased dose of SOR, and due to the profile of the adverse events with SOR and based on the expression of FGFR4 in the tumor, the patient was administered LEN at a dose of 12 mg/day as the secondary treatment, instead of regorafenib, which carried a risk of similar and more severe adverse events than SOR (1).
Upon the initiation of this therapy, the laboratory data showed a mild increase in hepatic enzymes: alpha-fetoprotein (AFP) of 113 ng/mL and Des-gamma-carboxy prothrombin (DCP) of 843.6 ng/mL (Table 1). Two months after the successful continuation of LEN, CT showed a prominent decrease in hepatic (Fig. 2g), lung (Fig. 2h), and mediastinal masses (Fig. 2i), showing central necrotic changes without any enhancement. Tumor markers showed a significant decrease: AFP and DCP to 20 ng/mL and 205.6 ng/mL, respectively. Fortunately, no significant changes in the hepatic reserve function were seen after LEN administration (Table 2).
Table 1.
Results of Laboratory Investigation before LEN Administration.
| Hematology | Biochemistry | Tumor marker | |||||||||||
| WBC | 5,460 | /μL | TP | 7.6 | g/dL | AFP | 113 | ng/mL | |||||
| RBC | 456×104 | /μL | Alb | 4.2 | g/dL | AFP-L3 | 61.6 | % | |||||
| Hb | 15.3 | g/dL | BUN | 17.0 | mg/dL | DCP | 843.6 | ng/mL | |||||
| PLT | 17.3×104 | /μL | Cre | 0.9 | mg/dL | ||||||||
| T-Bil | 1.0 | mg/dL | |||||||||||
| AST | 49 | IU/L | |||||||||||
| ALT | 60 | IU/L | |||||||||||
| Coagulation | LDH | 265 | IU/L | ||||||||||
| PT | 113 | % | ALP | 310 | IU/L | ||||||||
| APTT | 31.5 | s | γGTP | 185 | IU/L | ||||||||
| CRP | 1.3 | mg/dL | |||||||||||
LEN: lenvatinib, WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, PLT: platelet, PT: prothrombin time, APTT: activated partial thromboplastin time, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, Cre: creatinine, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γGTP: gamma-glutamyl transpeptidase, CRP: C-reactive protein, AFP: alpha-fetoprotein, AFP-L3: lectin-reactive alpha-fetoprotein, DCP: Des-gamma-carboxy prothrombin
Table 2.
Results of Laboratory Investigation after LEN Administration.
| Hematology | Biochemistry | |||||||
| WBC | 4,490 | /μL | TP | 7.9 | g/dL | |||
| RBC | 467×104 | /μL | Alb | 4.0 | g/dL | |||
| Hb | 15.5 | g/dL | BUN | 15.0 | mg/dL | |||
| PLT | 16.8×104 | /μL | Cre | 0.9 | mg/dL | |||
| T-Bil | 1.1 | mg/dL | ||||||
| AST | 62 | IU/L | ||||||
| ALT | 76 | IU/L | ||||||
| Coagulation | LDH | 231 | IU/L | |||||
| PT | 105 | % | ALP | 361 | IU/L | |||
| APTT | 34.4 | s | γGTP | 211 | IU/L | |||
| CRP | 1.7 | mg/dL | ||||||
LEN: lenvatinib, WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, PLT: platelet, PT: prothrombin time, APTT: activated partial thromboplastin time, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, Cre: creatinine, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γGTP: gamma-glutamyl transpeptidase, CRP: C-reactive protein, AFP: alpha-fetoprotein, AFP-L3: lectin-reactive alpha-fetoprotein, DCP: Des-gamma-carboxy prothrombin
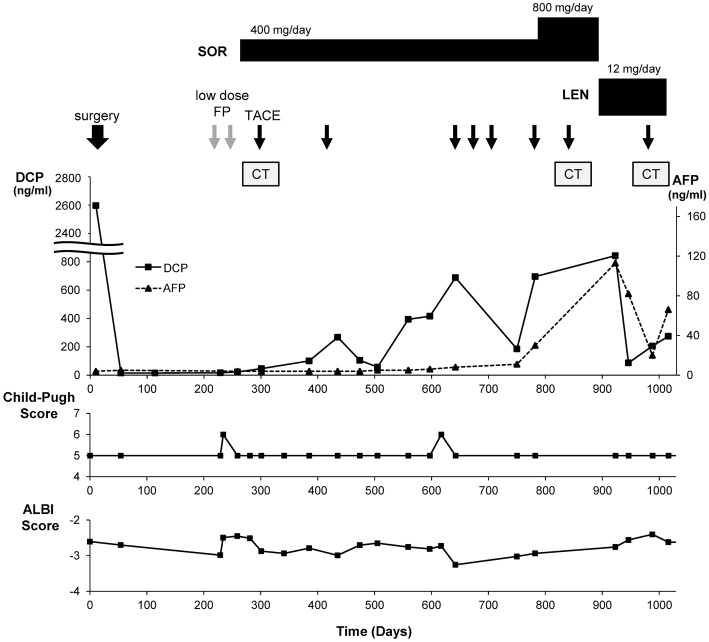
The clinical course is summarized in Fig. 3. Thus far, the patient has shown stable disease with LEN therapy and is alive with a good general condition while continuing the calcium channel blocker and urea-based cream, with no significant adverse events confirmed.
Figure 3.
Clinical course of the case. SOR: sorafenib, LEN: lenvatinib, FP: fluorouracil and cisplatin, TACE: transarterial chemoembolization, CT: computed tomography, AFP: alpha fetoprotein, DCP: Des-gamma-carboxy prothrombin, ALBI: albumin-bilirubin
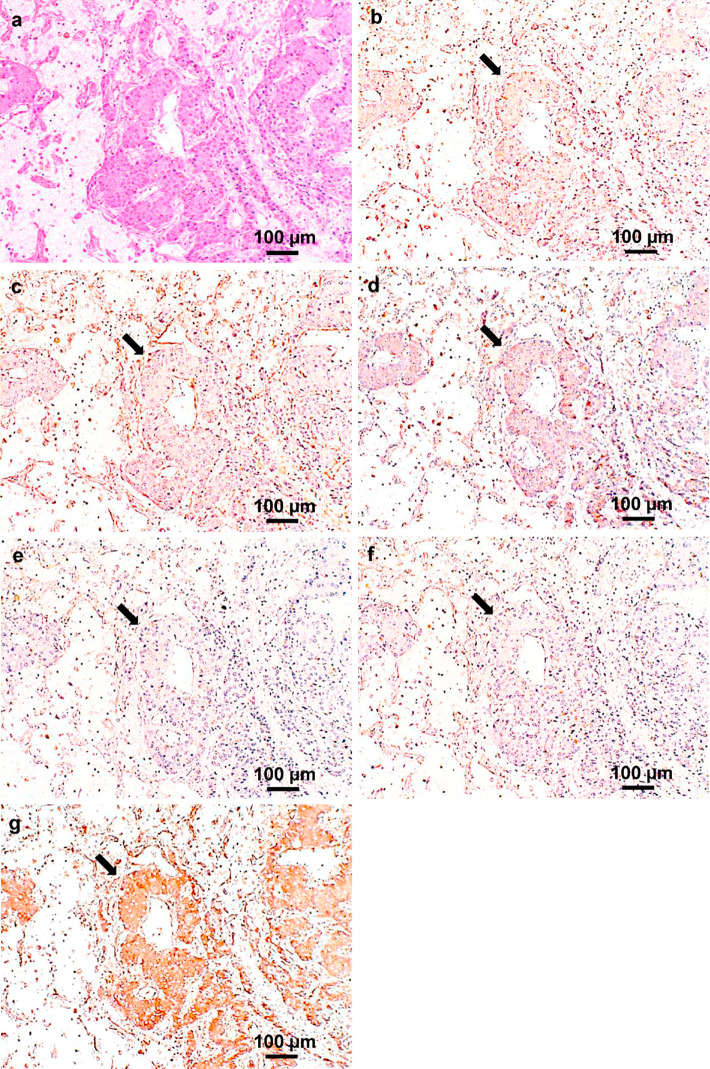
To verify the differences in the therapeutic effect of SOR and LEN, an immunohistochemical examination of the resected tumor specimen was performed (Fig. 4). While the tumor was negative for FGFR2 (Fig. 4e) and FGFR3 (Fig. 4f), FGFR4 staining was strongly positive (Fig. 4g) and platelet-derived growth factor receptor (PDGFR) α (Fig. 4b), PDGFRβ (Fig. 4c), and FGFR1 (Fig. 4d) showed mildly positive staining.
Figure 4.
Results of a histological analysis of the tumor. (a) Hematoxylin and Eosin staining and (b-g) representative immunohistochemical staining of (b) PDGFRα, (c) PDGFRβ, (d) FGFR1, (e) FGFR2, (f) FGFR3, and (g) FGFR4. Immunohistochemical staining showed mild positivity for PDGFRα, PDGFRβ, and FGFR1; negativity for FGFR2 and FGFR3; and strong positivity for FGFR4. Black arrows indicate the tumor cells. PDGFR: platelet-derived growth factor receptor, FGFR: fibroblast growth factor receptor
Discussion
HCC is the most common type of liver cancer, accounting for 70-90% of cases, and is one of the leading causes of cancer-related death worldwide (2,20,21). Despite advancements in therapeutic strategies, the response rate and overall survival rate are still low (21,22). To improve the outcomes of unresectable HCC, various MTAs have been developed and their efficacy was tested. Among the TKIs used for HCC, LEN has been reported to more strongly inhibit VEGFR1-3, FGFR1-4, c-Kit, and RET in HCC than SOR (10,11). Potent activity against FGFR4 is a distinctive feature of LEN compared with SOR, and the IC50 values of FGFR4 for LEN and SOR are 43 and 3,400, respectively (9).
Although these promising TKIs have been approved and shown therapeutic effects (1-4), only a few reports have described the predictors of a treatment response and survival in patients treated with TKIs (Table 3). Kim et al. reported that serum levels of basic fibroblast growth factor (bFGF) were significantly higher in the progressive disease (PD) group than in the non-PD group treated with SOR (mean bFGF 3.07 vs. 1.65 pg/mL; p<0.001) (23). And Finn et al. reported that patients with higher serum FGF21 levels (LEN, n=70; SOR, n=27) had a longer overall survival (OS) in the LEN arm than in the SOR arm (median OS 10.9 vs. 6.8 months; Pinteraction = 0.04) (24). Furthermore, the analyses of HCC tissue samples (LEN, n=34; SOR, n=24) revealed that patients with higher VEGF- and FGF-family gene expression had a longer OS when treated with LEN than when treated with SOR (25). In addition, the expression of FGF19-FGFR4 complex was recently shown to enhance the progression of HCC and to be associated with a poor prognosis (13-19,26,27). Matsuki et al. also reported that LEN inhibited tumor growth by suppressing the FGF signaling pathway in FGF19-FGFR4-overexpressing HCC cell lines in vitro and in vivo, and this effect was not seen with SOR administration (28). A Phase I clinical trial of specific FGFR4 inhibitor for HCC patients showed an overall response rate of 17% in FGF19-positive HCC patients and 0% in FGF19-negative HCC patients. This finding suggests that the expression of FGF19-FGFR4 can be a therapeutic target, and since LEN induces a better therapeutic effect for cases of HCC with FGF-FGFR4 expression than for those without it (28,29), FGFR4 expression can be a predictor of therapeutic efficacy when using MTAs.
Table 3.
Summary of Literatures.
| Ref | Medication | Number of case | Proteins assessed | Samples | Results |
|---|---|---|---|---|---|
| 23 | SOR 400 mg, BID | 124 | bFGF | Serum | Serum bFGF was significantly higher in the PD group (n=80, mean bFGF of 3.07 pg/mL) than non-PD group (n=44, mean bFGF of 1.65 pg/mL) in HCC patients treated with SOR. |
| 24 | LEN (>60 kg: 12 mg/day; <60 kg: 8 mg/day) or SOR 400 mg BID |
97 (LEN, n=70; SOR, n=27) | FGF21 | Serum | Patients with higher baseline serum FGF21 levels had a longer OS with LEN than that of SOR. (median OS 10.9 vs. 6.8 months; Pinteraction=0.04) |
| 25 | LEN (>60 kg: 12 mg/day; <60 kg: 8 mg/day) or SOR 400 mg, BID |
58 (LEN, n=34; SOR, n=24) | VEGF- and FGF-family | Tissue | Median OS was longer with LEN with high levels of VEGF- and FGF-family expression but SOR showed no effect on the group. |
| 30 | SOR 400 mg, BID | 54 | pERK, VEGFR2 | Tissue | High levels of pERK and VEGFR2 expression were associated with longer TTP. |
| 31 | SOR 400 mg, BID | 33 | pERK | Tissue | High level of pERK expression had a longer TTP compared to those with lower expression. |
SOR: sorafenib, LEN: lenvatinib, BID: twice daily, FGF: fibroblast growth factor, bFGF: basic fibroblast growth factor, VEGF: vascular endothelial growth factor, pERK: phospho extracellular signal-regulated kinase, VEGFR: vascular endothelial growth factor receptor, OS: overall survival, TTP: time to progression
Supporting these results, Yamauchi et al. reported that the FGFR4 expression in the tumor tissue was significantly related to the efficacy of LEN on the response and survival benefit (30). They also showed that the positive immunohistochemistry for FGFR4 in the tumor was a potential predictor of the efficacy of LEN treatment (30). These previous findings support the outcome in the present case showing a favorable response to LEN.
However, as SOR inhibits the mitogen-activated protein kinase pathway through Raf and the extracellular signal-regulated kinase (ERK) pathway, patients with HCC expressing high level of phosphorylated ERK showed a longer time to progression (TTP) when treated with SOR than patients with low level of phosphorylated ERK (31,32) (Table 3). Therefore, determining these molecules' expression in HCC lesions is important for selecting the most appropriate MTA.
In a recent report, combination therapy with the programmed death ligand 1 inhibitor atezolizumab and the VEGF inhibitor bevacizumab improved the OS and progression-free survival in patients with advanced-stage HCC compared to SOR (33). With progress in our understanding of molecular signaling in HCC (34), the urgent clinical need for predictive biomarkers to guide the effective use of these therapy options can finally be met.
Our study was limited by the fact that the immunohistochemical analyses were performed using the HCC tumor in the liver and not the tumors collected from the lung or mediastinum, which might have had different characteristics. In addition, various treatments, including low-dose cisplatin and 5-fluorouracil, SOR, and TACE, might have modified the FGFR4 expression in the remaining tumor cells, either with suppression or further activation. With the increase in the number of HCC cases treated with LEN (1,6,35), randomized trials based on the FGFR expressions in the target tumors and its inhibitor will guide our management of HCC cases in a more precise manner (36). In addition, the combination of these MTAs will bring about a better response in cases of HCC with molecular heterogeneity.
In summary, we experienced a case of HCC with a high FGFR4 expression that was effectively treated with LEN after a poor response to SOR. The results suggest that LEN should be considered as a first-line treatment in patients with a high or aberrant expression of FGFR4 while cautiously monitoring for side effects. The accumulation of information from more cases is necessary; however, the report indicates the importance of analyzing the gene expression in tumors to ensure the effective application of precision medicine.
Materials and methods
Standard immunohistochemistry was performed using rabbit anti-FGFR1 antibody (#9740, 1:250 dilution; Cell Signaling Technology, Danvers, USA), rabbit anti-FGFR2 antibody (#23328, 1:100 dilution; Cell Signaling Technology), rabbit anti-FGFR3 antibody (#4574, 1:25 dilution; Cell Signaling Technology), mouse anti-FGFR4 antibody (sc-136988, 1:50 dilution; Santa Cruz Biotechnology, Dallas, USA), rabbit anti-PDGFRα antibody (#5241, 1:100 dilution; Cell Signaling Technology), rabbit anti-PDGFRβ antibody (#4564, 1:100 dilution; Cell Signaling Technology), and a DAB chromogen tablet (Muto Pure Chemicals, Tokyo, Japan).
Informed consent was obtained from all individual participants included in the study.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kudo M. Systemic therapy for hepatocellular carcinoma: latest advances. Cancers (Basel) 10: 412, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16: 589-604, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150: 835-853, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2: 16018, 2016. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69: 182-236, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol 71: 616-630, 2019. [DOI] [PubMed] [Google Scholar]
- 8. Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci 20: 1358, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014: 638747, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 6: 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol 2: 3005, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122: 664-671, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Desnoyers LR, Pai R, Ferrando RE, et al. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 27: 85-97, 2008. [DOI] [PubMed] [Google Scholar]
- 14. French DM, Lin BC, Wang M, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 7: e36713, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hyeon J, Ahn S, Lee JJ, Song DH, Park CK. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci 58: 1916-1922, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Repana D, Ross P. Targeting FGF19/FGFR4 pathway: a novel therapeutic strategy for hepatocellular carcinoma. Diseases 3: 294-305, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149: 121-130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao H, Lv F, Liang G, et al. FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3β/β-catenin signaling cascade via FGFR4 activation. Oncotarget 7: 13575-13586, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miura S, Mitsuhashi N, Shimizu H, et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 12: 56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Huang S, He X. The role of microRNAs in liver cancer progression. Br J Cancer 104: 235-240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HY, Lee DH, Lee JH, et al. Novel biomarker-based model for the prediction of sorafenib response and overall survival in advanced hepatocellular carcinoma: a prospective cohort study. BMC Cancer 18: 307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finn RS, Kudo M, Cheng AL, et al. Final analysis of serum biomarkers in patients from the Phase 3 study of lenvatinib in unresectable hepatocellular carcinoma (REFLECT). Ann Oncol 29(Suppl): viii17-viii18, 2018. [Google Scholar]
- 25. Finn RS, Kudo M, Cheng AL, et al. Analysis of serum biomarkers (BM) in patients (PTS) from a Phase 3 study of lenvatinib (LEN) vs. sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma (uHCC). Ann Oncol 28(Suppl): v605-v649, 2017. [Google Scholar]
- 26. Matakidou A, El Galta R, Rudd MF, et al. Further observations on the relationship between the FGFR4 Gly388Arg polymorphism and lung cancer prognosis. Br J Cancer 96: 1904-1907, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X, Ge H, Lemon B, et al. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem 285: 5165-5170, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuki M, Hoshi T, Yamamoto Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med 7: 2641-2653, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim RD, Sarker D, Meyer T, et al. First-in-human phase I study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov 9: 1696-1707, 2019. [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi M, Ono A, Ishikawa A, et al. Tumor fibroblast growth factor receptor 4 level predicts the efficacy of Lenvatinib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol 11: e00179, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen D, Zhao P, Li SQ, et al. Prognostic impact of pERK in advanced hepatocellular carcinoma patients treated with sorafenib. Eur J Surg Oncol 39: 974-980, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 4293-4300, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Cheng AL, Qin S, Ikeda M, et al. Combination of immunotherapy and VEGF inhibitor improves survival in HCC. ESMO Asia Congress 2019. [Google Scholar]
- 34. Kamimura K, Yokoo T, Abe H, Terai S. Gene therapy for liver cancers: current status from basic to clinics. Cancers 11: 1865, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh A liver function: a proof-of-concept study. Cancers 11: 1084, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sawey ET, Chanrion M, Cai C, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell 19: 347-358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]