Abstract
The prefrontal cortex (PFC) orchestrates higher brain function and becomes disrupted in many mental health disorders. The rodent medial PFC (mPFC) possesses an enormous variety of projection neurons and interneurons. These cells are engaged by long-range inputs from other brain regions involved in cognition, motivation and emotion. They also communicate in the local network via specific connections between excitatory and inhibitory cells. In this review, we describe the cellular diversity of the rodent mPFC, the impact of long-range afferents, and the specificity of local microcircuits. We highlight similarities and differences with other cortical areas, illustrating how the circuit organization of the mPFC may give rise to its unique functional roles.
Keywords: Prefrontal cortex, projection neuron, interneuron, circuits, cell types, synapses
Cell- and Synapse-Specific Wiring Rules in the Medial PFC
The PFC plays an essential role in higher brain function, including cognition, motivation, reward and emotion [1,2]. Dysfunction of the PFC is also implicated in diverse neuropsychiatric disorders, including schizophrenia, ADHD, addiction, and depression [3,4]. The behavioral and computational roles of the rodent mPFC have been extensively studied for several decades. However, our understanding of cortical circuitry has predominantly emerged from studies of primary sensory cortices [5,6]. Importantly, the mPFC displays a distinct architecture [7], potentially enabling different forms of processing. In particular, the mPFC receives a variety of long-range excitatory inputs and sends diverse outputs to many other brain regions (Fig. 1A). These input and output pathways are connected via the local circuit, in which different excitatory and inhibitory neurons communicate with each other through specific wiring rules.
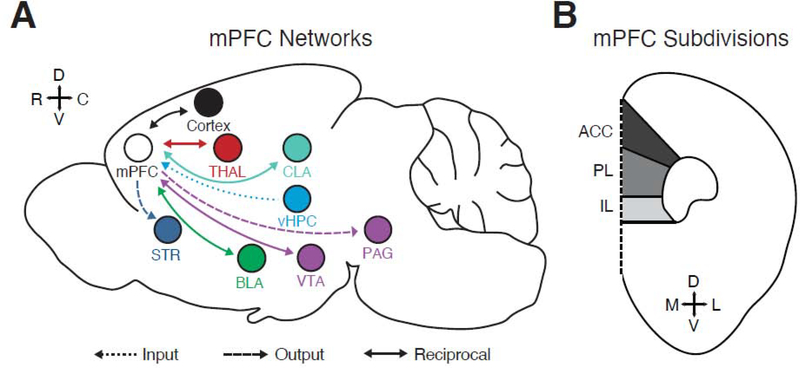
Figure 1. General organization of the rodent mPFC.
A) Key brain regions that communicate with the mPFC by providing input to it, receiving output projections from the mPFC, or making reciprocal connections with the mPFC. BLA = basolateral amygdala, CLA = claustrum, PAG = periaqueductal gray, STR = striatum, THAL = thalamus, vHPC = ventral hippocampus, VTA = ventral tegmental area. Note that many connections are reciprocal.
B) Distribution of the subdivisions of the mPFC along the dorso-ventral axis. ACC = anterior cingulate cortex, PL = prelimbic, IL = infralimbic.
Recent work using a variety of approaches has made significant strides towards revealing the specificity of mPFC circuits. Anatomical tracing of mPFC afferents and efferents has characterized highly organized brain networks [8–13]. While some connections are unidirectional, most are organized as reciprocal loops, allowing the mPFC to orchestrate diverse behaviors across time by continually updating neural activity [14,15]. Slice electrophysiology and optogenetics have enabled mechanistic dissection of local and long-range circuits [16]. Many connections are highly specific, with the identity of presynaptic and postsynaptic cells determining signal flow within the network. Lastly, in vivo electrophysiology, imaging, optogenetics and chemogenetics have shown how individual cell types and connections contribute to behavior and disorders [17–21]. Here we review recent progress in understanding the circuit organization of the mPFC in rodents, focusing on emerging rules of cell- and synapse-specific connectivity. Some variations exist in the literature with regards to the precise definition of the rodent mPFC, and for the purpose of this article, the mPFC refers to the collection of anterior cingulate (ACC), prelimbic (PL) and infralimbic (IL) cortices. Much of the work on cell type-specific circuit mapping of the mPFC has been conducted so far in mice, due to the availability of genetic tools in this model. Accordingly, the discussions in this review will focus primary on the mouse mPFC, and particularly the PL. Examining whether and how these findings generalize to other species remains an important goal for future research.
General organization of the mPFC
The cerebral cortex is organized into layers, in which neurons sample long-range inputs and interact via local connections [5,6]. Sensory cortex is divided into 6 main layers, whose structure and function are broadly conserved across modalities. Layer (L)1 contains the apical dendrites of pyramidal neurons and several populations of GABAergic interneurons [22–24]. L2 and L3 are typically grouped as L2/3, containing intratelencephalic (IT) cells that project to other cortical regions [25,26]. L4 is a major input layer, with either pyramidal cells or stellate cells that make local connections within the cortex. L5 is a major output layer, with a separate group of IT cells, and pyramidal tract-like (PT) cells that target subcortical regions [5,27]. L6 is another output layer, home to the corticothalamic (CT) cells that project to thalamic relay nuclei and the thalamic reticular nucleus [27,28]. A variety of interneurons are distributed across L1 to L6, including parvalbumin (PV+), somatostatin (SOM+), and vasoactive intestinal peptide (VIP+) cells [5,29]. There are well-defined rules for connections between pyramidal cells and interneurons within and across layers [5]. Long-range inputs also target specific layers, with primary thalamic inputs to L4 and L5b, higher-order thalamic inputs to L1 and L5a [5,30], and cortical inputs to superficial and deep layers, depending on hierarchical relationships between connected cortical regions [5].
There are several major differences between this traditional laminar view of the sensory cortex and the rodent mPFC. First, the mPFC is agranular, lacking a canonical thalamo-recipient L4, instead possessing inputs across L1, L2/3, L5 and L6. Consistent with a lack of L4, there is minimal ROR-beta labeling in the mPFC, which defines this layer in sensory and motor cortices [5,31]. Second, projection neurons are located across L2 to L6, which often engage in reciprocal circuits with other brain regions. This includes multiple types of IT, PT and CT cells, which also communicate with each other via local excitation [5,16,26,32,33]. Third, there are different allocations of GABAergic interneuron subtypes, which mediate local inhibition [34]. There appear to be fewer PV+ cells but more SOM+ cells, which may give rise to differences in local computations. Fourth, long-range inputs arrive across L1 to L6, which convey signals related to action, cognition, reward, and emotion. These inputs contact and drive projection neurons and interneurons, with wiring rules depending on presynaptic and postsynaptic cells. Understanding mPFC computations therefore requires a detailed knowledge of cell types, activation by long-range inputs, and processing via local circuits. In many cases, determining the intrinsic physiology of individual cells and synaptic properties of specific connections is critical in order to assess organization of these networks.
It is important to note that rodent mPFC consists of multiple regions, including anterior cingulate (ACC), prelimbic (PL) and infralimbic (IL) (Fig. 1B), whose roles in cognition gradually shift along the dorso-ventral axis, from decision making and action to motivation and emotion. They also have distinct inputs and outputs, with differences in connectivity likely underlying behavioral roles. Comparison of the PFC across species is an active area of debate [35–37], and beyond the scope of the current review. However, the rodent mPFC shares many features with the medial agranular cingulate cortex of humans and primates, and is considered to be distinct from the dorsolateral PFC. In this review, we will focus primarily on the mouse PL, where most studies on cell type-specific circuitry have been performed, drawing comparisons to ACC, IL and other cortical areas whenever possible.
Diversity of projection neurons in the mPFC
The mPFC possesses a variety of excitatory projection neurons that span multiple layers and target diverse brain regions. These projection neurons allow the mPFC to influence activity in other parts of the brain and exert top-down control of behaviors. Recent work highlights the properties of these cells and the rules by which they respond to excitatory and inhibitory inputs. Across the cortex, the three main subclasses of projection neurons are IT, PT and CT cells, which can be readily identified by retrograde labeling [5] (Fig. 2A). These cells have stereotyped morphology and intrinsic physiology [5] (Fig. 2B & C), and have distinct gene expression profiles that allow for genetic access [38]. However, expression profiles in the mPFC often differ from elsewhere in cortex, with major markers often failing to assume characteristic lamination patterns. Moreover, the traditional IT / PT / CT categorization is clearly an oversimplification of projection neuron diversity. Instead, individual neurons send axon collaterals to multiple targets, but not all neurons in a given class project to all known targets of that class [25,39]. Thus, each population can be further subdivided based on their projection targets, laminar location, and connectivity.
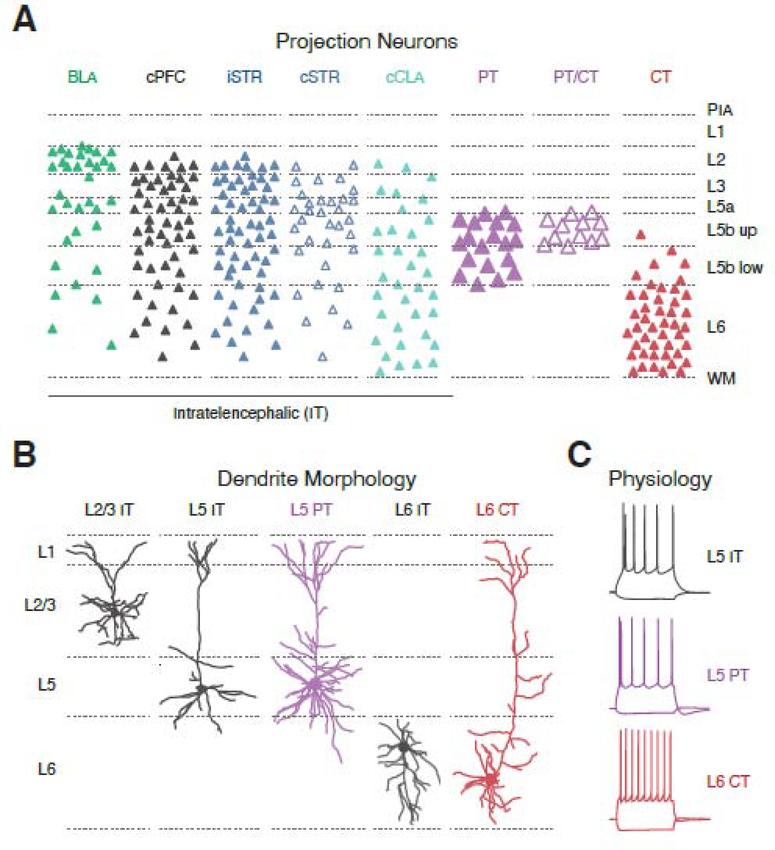
Figure 2. mPFC projection neuron diversity.
A) Laminar distributions of projection neurons in the mPFC, identified by injection of retrograde tracers into target brain regions. BLA = basolateral amygdala, cPFC = contralateral PFC, iSTR = ipsilateral striatum, cSTR = contralateral striatum, cCLA = contralateral claustrum, PT = pyramidal tract, PT / CT = PT cells that send collaterals to the thalamus, CT = corticothalamic, non-PT cells. PIA = pial surface, WM = white matter, L5b up = upper L5b, L5b low = lower L5b. Note that projection neurons are found from L2 to L6.
B) Dendritic morphologies of mPFC projection neurons. From left to right: L2/3 intratelencephalic (IT) cell, L5 IT cell, L5 pyramidal tract (PT) cell, L6 IT cell, and L6 corticothalamic (CT) cell. Note that L5 PT cells have more elaborate dendrites than adjacent IT cells, and that L6 CT cells have apical dendrites that often extend to L1, unlike L6 IT cells that can display inverted pyramidal morphologies.
C) Intrinsic firing properties of mPFC projection neurons. From top to bottom: L5 IT cells, L5 PT cells and L6 CT cells. Note that L5 IT cells lack Ih-mediated voltage sag, L5 PT cells display strong Ih-mediated voltage sag, and L6 CT cells display highly non-adapting firing patterns.
Panel A adapted from references 16 & 32. Data in panels B & C used with permission from references 16, 32 & 42,
IT cells in the mPFC are present across L2-L6, and project to other parts of the cortex, basolateral amygdala (BLA), striatum, and claustrum (Fig. 2A). As seen in other cortices [25,26], L2/3 IT cells segregate into distinct output pathways, with cortico-amygdala neurons distinct from cortico-cortical or cortico-striatal neurons [40,41]. L5 and L6 IT cells have markedly distinct morphologies and physiology from nearby L5 PT and L6 CT cells [42–44] (Fig. 2B & C). The axons of L5 IT cells branch to many regions, comprising cortico-cortical, cortico-striatal, cortico-amygdala and cortico-claustral projections [32,45]. While also present in other cortices, cortico-striatal and cortico-claustral neurons appear more numerous and broadly distributed across layers in the mPFC [32,46,47]. Lastly, the mPFC sends strong, bilateral projections to both the ipsi- and contra-lateral claustrum, which appears unique to frontal cortex [48].
Across the cortex, PT cells are confined to L5b (Fig. 2A), with elaborate dendrites and elevated HCN channel expression [42–44] (Fig. 2B & C). PT cells in the mPFC target diverse subcortical brain regions, including ipsilateral striatum, thalamus, pons, periaqueductal grey, and multiple neuromodulatory centers [16,49]. These diverse outputs are an important feature of the mPFC, which allows PT cells to influence multiple aspects of higher-order behavior. In motor cortex, PT cells fall into two subpopulations, with upper L5b innervating the pons and thalamus and lower L5b bypassing the thalamus to target the medulla and spinal cord [39]. While PT cells in upper L5b of the mPFC also target thalamus [16], the targets of lower L5b have not been fully explored, but are expected to include different sets of neuromodulatory, midbrain and hindbrain areas [49].
Lastly, L6 CT cells are distinguished by their distinct morphology and highly non-adapting firing properties [16,27] (Fig. 2A–C). The apical dendrites of CT cells in the mPFC extend to the pial surface, suggesting they sample inputs across all layers. Projection neurons in L5 and L6 target multiple thalamic nuclei, including both mediodorsal (MD) and ventromedial (VM) thalamus. The release properties of L5 PT and L6 CT inputs to thalamus follow the “driver” and “modulator” classification seen in sensory thalamus, respectively [16]. Interestingly, a subset of both PT and CT cells send bifurcating projections to both MD and VM [16], an arrangement not typically found in sensory cortex. CT cells also target thalamic reticular nucleus (TRN) to drive inhibition of thalamus, which may regulate attention [28,50]. Lastly, L6 CT cells are interspersed with L6 IT cells, which again lack HCN channels and often have unusual, multipolar or even inverted dendritic morphologies, suggesting they receive distinct afferents (Fig. 2A–C).
Long-range inputs to the mPFC
The mPFC processes long-range inputs from many other brain regions, including other cortical areas, thalamus, BLA, ventral hippocampus (vHPC), and claustrum (Fig. 3A). In vivo studies highlight distinct functional roles for these afferents in cognitive and emotional behavior [51–54]. Slice recordings also show how each input preferentially activates specific types of projection neurons in the mPFC.
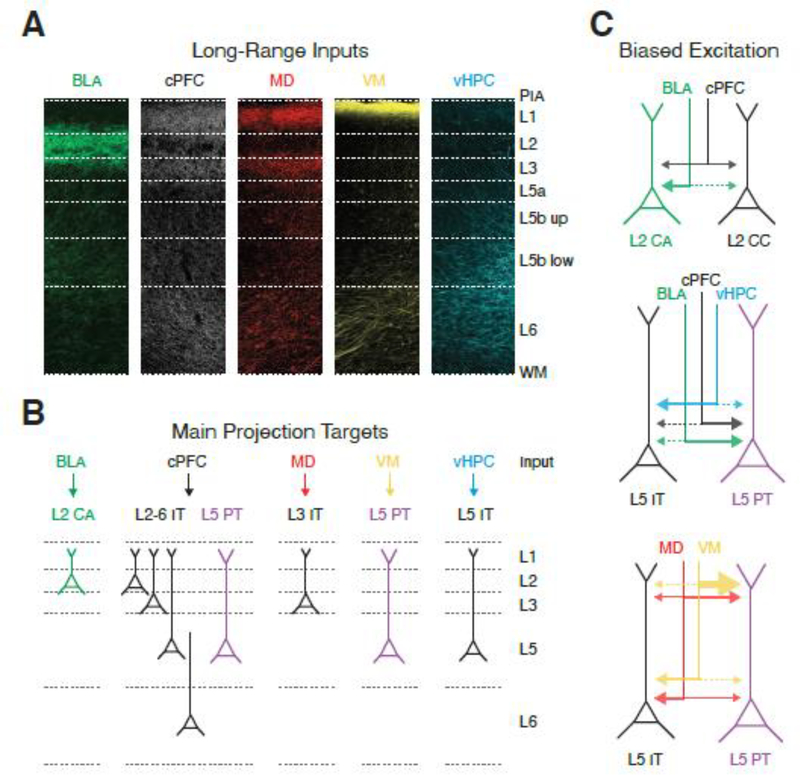
Figure 3. Long-range excitatory inputs to the mPFC.
A) Laminar distribution of axons to the prelimbic mPFC from different brain regions. From left to right: BLA = basolateral amygdala, cPFC = contralateral PFC, MD = mediodorsal thalamus, VM = ventromedial thalamus, vHPC = ventral hippocampus. Note that inputs arrive in characteristic patterns across L1 to L6.
B) Summary of the main projection neuron(s) contacted by long-range excitatory inputs. BLA activates reciprocally-projecting L2 cortico-amygdala (CA) neurons. cPFC innervates more broadly, including L5 PT cells and a range of IT cells. MD strongly activates L3 IT cells, while VM engages the dendrites of L5 PT cells. vHPC primarily targets L5 IT cells. Note that the most strongly driven targets are shown for simplicity, but most inputs provide some excitation to all cells in the network.
C) Biased long-range excitatory inputs onto neighboring projection neurons. Top, BLA preferentially contacts L2 CA neurons over L2 cortico-cortical (CC) neurons, whereas cPFC shows no bias. Middle, BLA and cPFC preferentially contact L5 PT cells over L5 IT cells, with vHPC showing opposite bias. Bottom, MD preferentially contacts L5 IT cells over L5 PT cells, but make stronger contacts onto dendrites of L5 PT cells. VM inputs make highly enriched inputs to L5 PT apical dendrites, where they can evoke dendritic Ca2+ spikes.
Images in panel A adapted with permission from reference 23 and unpublished data from the Carter lab,
Inputs from the contralateral PFC (cPFC) support prefrontal activity and share information between hemispheres [55]. cPFC inputs are distributed across all layers (Fig. 3A), and contact IT cells in superficial and deep layers [40,56,57], cortico-amygdala neurons in L2 [40], and PT cells in L5 [42,58] (Fig. 3B). Overall, cPFC connectivity appears relatively egalitarian, making similar strength connections onto L2 cortico-amygdala and cortico-cortical neurons (Fig. 3C). Differences in targeting of L5 IT and PT cells are compensated by differences in input resistances, yielding responses of similar amplitude [42,58]. The subcellular targeting of cPFC synapses is also largely uniform across the dendrites of L2 and L5 pyramidal neurons [42,56,58]. However, an important caveat is that previous work has considered the entirety of callosal input, and it may be that IT cells residing in different layers contact distinct targets in the contralateral hemisphere.
Thalamic inputs to the mPFC support a wide array of cognitive functions, including working memory, learning, attention and arousal [51,52,59,60]. While most work has focused on MD thalamus, inputs arise from several nuclei, including VM thalamus [10,11,16,23]. MD inputs terminate in two prominent bands of axons found in L1 and L3 (Fig. 3A). MD input strongly engages L3 IT cells, which are readily driven to fire action potentials [16] (Fig. 3B). This input also contacts L5 pyramidal cells, preferentially targeting the soma of IT cells over nearby PT cells (Fig. 3C). In principle, this may ensure that thalamic inputs are processed locally by the mPFC before being relayed back to the thalamus. However, MD input to deep L1 (L1b) also contacts the apical dendrites of L5 PT cells (Fig. 3C). Ultimately, MD has a variety of influences on both the superficial and deep layers of mPFC, but appears to primarily activate L3 IT cells, which may help support local processing via recurrent interactions in the local circuit [14].
In contrast, VM inputs to the mPFC terminate in a dense band in superficial L1 (L1a), similar to other “matrix” thalamic inputs (Fig. 3A). VM inputs are relatively weak at IT cells in L2 to L5 [16,61] (Fig. 3B). Instead, VM inputs are highly enriched at the distal apical dendrites of PT cells [16,23], which is more pronounced than for MD inputs, and not seen for other long-range inputs [42,58,62] (Fig. 3C). This dendrite targeting could provide a powerful way for thalamic input to activate PT cells and thus enhance mPFC output [23]. Similar connectivity is seen for higher-order thalamic inputs to other frontal cortices [28], but equivalent inputs to somatosensory cortex are not enriched onto PT apical dendrites [63], suggesting this thalamic connectivity motif may be a distinguishing feature of higher-order frontal circuits. Lastly, the mPFC may have weaker thalamic input to L6 than sensory cortex, with no prominent band of thalamic axons in deeper layers [16,30].
The mPFC also markedly differs from sensory cortices with major input from regions that convey emotional signals. Connections with the BLA are important for the encoding, expression and reversal of emotional states, including aversive memories [21,54]. BLA inputs are particularly enriched in L2 of PL (Fig. 3A), where reciprocally-connected cortico-amygdala neurons are also prominent [40,49]. Indeed, BLA inputs to mPFC selectively targets cortico-amygdala neurons over intermingled cortico-cortical or cortico-striatal neurons, providing the synaptic basis for a strong reciprocal loop [40,41] (Fig. 3B & C). However, BLA axons display pronounced differences along the dorso-ventral axis, with more input to L2 of PL, but L5 of IL [64]. Interestingly, BLA inputs are stronger at PT over IT cells in L5 of IL, as seen for callosal inputs, but opposite to thalamic inputs [16,64] (Fig. 3C). Interestingly, biased connectivity is also seen in the reverse direction, with mPFC making stronger connections onto amygdala-cortical cells in the BLA, providing a synaptic basis for strong, reciprocal connectivity between these regions [65].
Lastly, hippocampal input to the mPFC provides contextual information and plays a role in mnemonic encoding and emotional control [53,66] (Fig. 3A). Hippocampal inputs arise from pyramidal cells in vHPC, including both CA1 and the subiculum [62]. Recent studies indicate a variety of projection cells in the vHPC, which have distinct physiological properties and connectivity [66]. vHPC inputs to the mPFC are region-specific, targeting multiple layers in IL, largely restricted to L5 in PL, but mostly absent from ACC [62]. vHPC inputs are also layer-specific, with strongest input to pyramidal neurons in IL L5, followed by IL L2/3 and PL L5, with weak input to PL L2/3 and L6 [62] (Fig. 3B). Finally, vHPC inputs are cell-type specific, targeting IT cells over PT cells in L5 of IL [62], which is similar to thalamic inputs, but opposite to BLA and cPFC inputs (Fig. 3C). Lastly, dorsal hippocampus also has a functional impact on the mPFC, but there are few direct connections, suggesting this influence is via polysynaptic circuits.
In addition to these main afferents, there are several other long-range inputs to the mPFC, including from the claustrum and inhibitory regions, about which relatively little is known. It will be important to assess which projection neurons are most strongly engaged by these inputs.
Local excitatory circuits in the mPFC
Once long-range inputs drive projection neurons in the mPFC, their influence also reflects local excitation. In sensory cortex, there is a canonical flow of excitation between layers, with L4 signaling to L2/3, which then signals to L5 (Fig. 4A). While the equivalent organization has not been extensively studied in the mPFC, it is likely similar to other frontal and motor cortices, which also show a clear hierarchy of connectivity from the superficial to deep layers (Fig. 4A). For example, thalamo-recipient pyramidal cells in L3 send ascending connections to L2 pyramidal cells [31]. L2/3 pyramidal cells then make descending connections to L5 pyramidal cells, preferentially contacting PT cells [16,67]. In a further refinement, descending connections may also depend on the sublayer of presynaptic and postsynaptic cells [68,69]. For example, PT cells in lower L5b receive little local input, and instead mainly process long-range afferents [70]. Moreover, L5 IT cells send ascending projections to L2/3, whereas L5 PT cells provide minimal feed-back [71]. Little is currently known about connections onto IT and CT cells in L6, including how these neurons are engaged locally and influenced by long-range inputs [72].
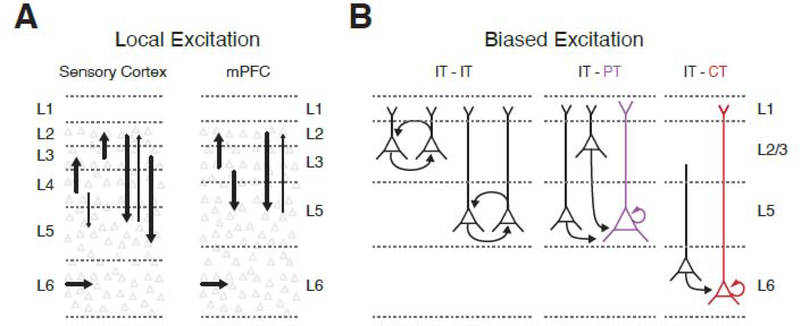
Figure 4. Local excitatory connections in the mPFC.
A) Simplified wiring diagram of the local excitatory circuit in sensory cortex (left) and mPFC (right). Individual arrows represent major excitatory connections, with the weight of the arrow indicating connection strength.
B) Biased local excitatory connectivity within and across layers of the mPFC. Note that IT cells, PT cells and CT cells make within-class connections, and that IT cells also make between-class connections onto PT and CT cells, which are the major outputs to subcortical brain regions.
Pyramidal cells also robustly communicate within individual layers, with wiring rules often determined by presynaptic and postsynaptic cell type [5,26,33] (Fig. 4B). Connections within the same cell type may be particularly strong in the mPFC, displaying short-term dynamics predicted to sustain reverberant activity [73]. For example, robust connections between L2/3 pyramidal cells may amplify inputs arriving from MD [14]. Similarly, facilitating connections between PT cells may ensure reliable output to subcortical targets [73]. Connections between cell types can also be highly selective, providing directionality of signaling within and between cortical layers. For example, IT cells strongly innervate PT cells, which may ensure local computations are relayed via output pathways [5,16,33]. However, the reverse connection is weak, perhaps ensuring output signals do not immediately re-enter the cortical network. Overall, both local and long-range inputs make precise connections onto specific projection neurons in the mPFC.
Interneurons and local inhibitory circuits in the mPFC
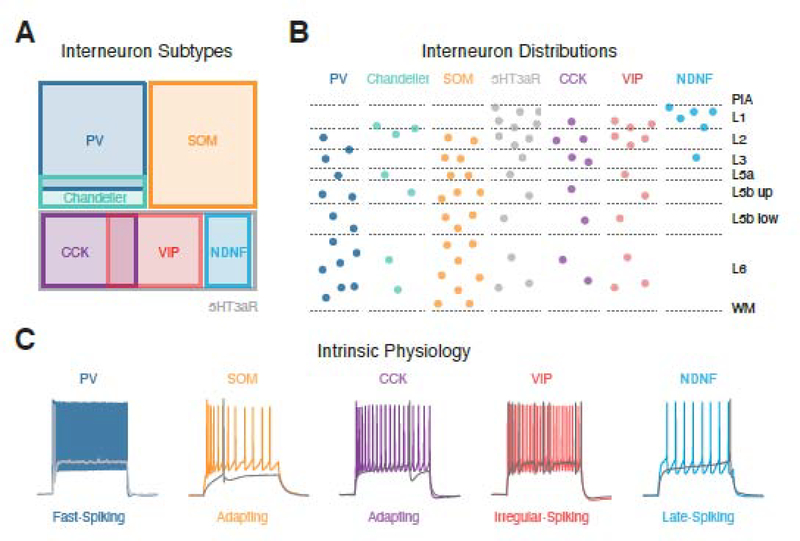
The ability of projection neurons to respond to long-range and local excitation is also strongly shaped by inhibitory interneurons, which are the subject of several reviews [29,74,75]. Across the cortex, GABAergic interneurons are divided into three largely non-overlapping populations expressing PV, SOM or serotonin receptor 3a (5HT3aR) [29] (Fig. 5A). The latter are further subdivided by expression of cholecystokinin (CCK), VIP, reelin or neuron derived neurotrophic factor (NDNF) [22,29]. All of these interneurons are found in the mPFC and display broadly similar lamination patterns to other cortical areas (Fig. 5B). They also have characteristic physiological and morphological properties that determine how they respond to inputs and inhibit pyramidal cells (Fig. 5C). However, recent work suggests there may be important differences in the properties of interneurons in the mPFC [76]. For example, there appear to be fewer PV+ cells and more SOM+ and CCK+ cells compared to other cortical areas [34,77,78]. The dearth of PV+ cells in superficial layers, particularly in PL and IL, may instead be compensated by other interneurons. It remains unclear if these differences reflect shifts in the relative ratios of cardinal interneuron subtypes, additional subtypes of interneurons in the mPFC, or some combination of factors.
Figure 5. GABAergic interneuron subtypes in the mPFC.
A) Segregation of different subpopulations of interneurons based on gene expression and morphological properties. PV = parvalbumin, SOM = somatostatin, 5HT3aR = serotonin receptor 3a, CCK = cholecystokinin, VIP = vasoactive intestinal peptide, NDNF = neuron derived neurotrophic factor.
B) Laminar distributions of interneurons based on labelling with transgenic mouse lines. PIA = pial surface, WM = white matter, L5b up = upper L5b, L5b low = lower L5b.
C) Characteristic firing properties of different interneurons. Grey traces show peri-threshold spikes and colored traces show sustained firing in response to larger current steps. Data in panel B adapted from references 23, 32, 78 & 94. Traces in panel C adapted with permission from reference 23 and unpublished data from the Carter lab,
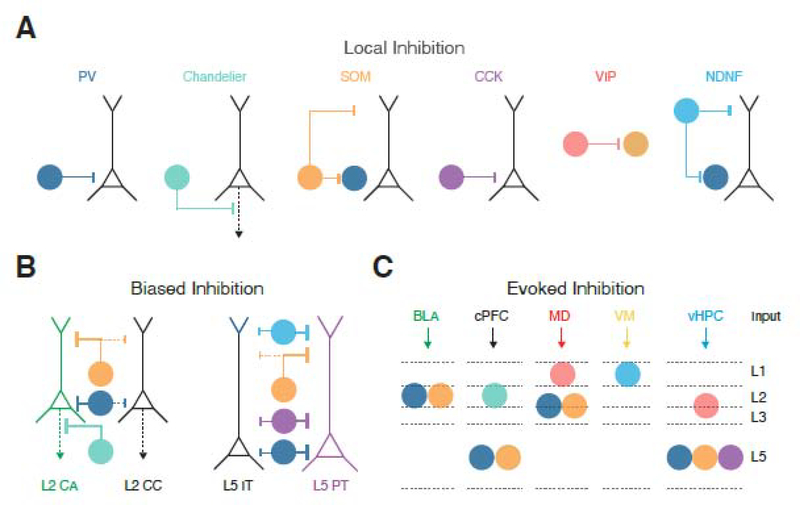
Inhibitory connections onto pyramidal cells also display class-specific wiring rules at the cellular and subcellular levels [29] (Fig. 6A). For example, PV+ cells inhibit near the soma and axons of nearby pyramidal cells, SOM+ cells primarily inhibit the dendrites, whereas VIP+ cells primarily target other classes of interneurons. Although not as well studied, CCK+ cells also target the soma of pyramidal cells, and NDNF+ cells the distal apical tufts [23,24,78]. Recently, interneurons have been proposed to provide a “blanket” of inhibition onto neighboring pyramidal cells [79]. However, in the mPFC, inhibitory connections neurons often show pronounced selectivity. In superficial layers, PV+ and SOM+ cells preferentially target L2 cortico-amygdala over adjacent cortico-cortical and cortico-striatal neurons [41,80] (Fig. 6B). This biased connectivity is also seen in deeper layers, where PV+, SOM+, CCK+ and NDNF+ interneurons all preferentially target L5 PT cells over neighboring IT cells [23,42,57,78] (Fig. 6B). Nevertheless, it remains uncertain if this biased connectivity reflects distinct cohorts of interneurons that each target specific types of pyramidal cells [81], or if each class of interneuron provides widespread inhibition but makes stronger or more numerous connections onto certain types of projection neurons.
Figure 6: Feed-forward inhibitory circuits in the mPFC.
A) Targeting of pyramidal cells by PV+ basket cells, PV+ chandelier cells, SOM+ cells, CCK+ cells and NDNF+ cells, along with targeting of SOM+ cells by VIP+ cells and PV+ cells by SOM+ cells and NDNF+ cells.
B) Biased inhibitory inputs onto neighboring projection neurons. PV+ (basket and chandelier cells) and SOM+ cells preferentially contact L2 CA neurons over L2 CC neurons. PV+, SOM+, CCK+ and NDNF+ preferentially contact L5 PT cells over L5 IT cells.
C) Summary of the currently known interneurons contacted by long-range excitatory inputs. BLA activates L2 PV+ and SOM+ cells. cPFC innervates L2 Chandelier cells, in addition to L5 PV+ and SOM+ cells. MD engages both L3 PV+ and SOM+ cells, as well as L1 VIP+ cells. VM drives L1 NDNF+ cells. vHPC activates L2/3 VIP+ cells and L5 PV+, SOM+ and CCK+ cells. Note that the distinct presynaptic release properties of inputs onto these cells mean they also fire during different phases of activity.
Activation of inhibitory circuits in the mPFC
In addition to activating projection neurons, long-range inputs engage a variety of interneurons in the mPFC. In many cases, interneurons are activated before projection neurons, thereby contributing to feed-forward inhibition [41,42,74]. Once the local network is activated, feed-back inhibition is also engaged, contributing to complex network dynamics [29,74,75].
PV+ interneurons across the cortex comprise two main subtypes: PV+ basket cells and PV+ chandelier cells. PV+ basket cells are found in L2 to L6 and make fast, depressing connections near the cell body of pyramidal cells (Fig. 6A). In contrast, PV+ chandelier cells are prevalent at the L1/2 border and target the axon initial segment of pyramidal cells (Fig. 6A). Feed-forward inhibition across the cortex is primarily mediated by PV+ basket cells, which gate responses to long-range inputs, and thus control signal transmission [41,42,74]. In the mPFC, cortical, thalamic, BLA, vHPC and claustral inputs all strongly activate PV+ basket cells, albeit in different layers [41,42,78,82–85] (Fig. 6C). While less studied, cortical inputs also engage PV+ chandelier cells, which preferentially target L2 cortico-amygdala neurons to regulate their output [80].
The sparsity of PV+ interneurons in the mPFC suggests other interneurons may also contribute to feed-forward inhibition. Indeed, whole-brain rabies tracing studies show that several other interneuron subtypes receive long-range inputs [10,11,23]. For example, our group recently showed that CCK+ interneurons are excited by vHPC inputs to mediate feed-forward inhibition in IL [78] (Fig. 6C). These CCK+ interneurons target the soma of L5 pyramidal cells (Fig. 6A), and show biased connections onto PT cells, similar to PV+ interneurons (Fig. 6B). CCK+ synapses also undergo endocannabinoid-mediated presynaptic modulation via CB1 receptors. Surprisingly, this modulation is only observed at L5 IT cells [78], which represent the main target of vHPC inputs to IL [62], suggesting endocannabinoids may play a role in regulating specific networks within the mPFC, including vHPC-evoked inhibition.
Across cortex, SOM+ interneurons are usually thought to mediate feed-back inhibition at the dendrites of pyramidal cells [29,81]. However, cortical, thalamic, BLA and vHPC inputs also innervate and drive SOM+ interneurons in the mPFC [10,11,41,42,78] (Fig. 6C). In each case, these connections undergo pronounced facilitation, similar to local excitatory inputs from nearby pyramidal cells [41]. Equivalent targeting is also found in sensory cortex, with gradual transition from PV- to SOM-mediated inhibition during ongoing activity [29]. Together, these findings suggest that a variety of long-range afferents can drive robust feed-forward inhibition in the mPFC, which shifts from the soma to dendrites of pyramidal cells during repeated activation.
5HT3aR+ interneurons are primarily found in superficial layers and can also contribute to feed-forward inhibition [23,29]. In sensory cortex, L1 interneurons are subdivided into NDNF+, VIP+, and alpha-7 nicotinic receptor expressing cells, with the NDNF+ cells further sub-divided by expression of neuropeptide Y (NPY+) (NDNF+/NPY+ and NDNF+/NPY-) [22]. In the mPFC, NDNF+ cells are prominent in L1a, whereas VIP+ cells are absent from L1a, instead found in L1b and L2/3 [23]. These interneurons are targeted by particular long-range inputs, with recent work from our lab showing VM drives NDNF+ cells in L1a, whereas MD engages VIP+ cells in L1b [23] (Fig. 6C). vHPC inputs may also target VIP cells in L2/3 [86], whereas the claustrum activates a separate NPY+ population in deeper layers [85]. Importantly, L1 interneurons are also major recipients of many major neuromodulatory inputs, which are more abundant in mPFC than sensory cortex, influencing many receptors and channels to regulate interneuron function [32,87].
Lastly, subsets of interneurons mediate multiple forms of “disinhibition” by selectively targeting other interneurons. As in sensory cortex, VIP+ cells target SOM+ cells, which in turn also target PV+ cells [23,88–90] (Fig. 6A). Recent work shows NDNF+ cells in L1 target PV+ cells in L2/3 in a separate disinhibitory circuit [23]. By contacting VIP+ and NDNF+ cells, long-range afferents can thus drive different disinhibitory networks involving SOM+ or PV+ cells (Fig. 6C). Ultimately, the impact of a given input on the mPFC depends on the complex interplay of excitation, inhibition and disinhibition. A goal for future work is to establish which of these responses dominates in vivo activity, and how these different circuit motifs contribute to behaviors involving the PFC.
Functional implications of mPFC connectivity
We are beginning to understand how specific circuits in the mPFC communicate with the rest of the brain to shape high level behaviors. One important feature of the mPFC is that long-range inputs directly target and activate specific types of projection neurons, with several reciprocally organized networks, including with cortex, thalamus and BLA. These circuits are well placed to support sustained, delay-period activity observed in the PFC during short-term memory tasks [14,51,52]. This organization is very different from sensory cortex, where thalamic inputs primarily engage local circuit neurons and are processed in a hierarchical manner. Additional studies are now needed to explore other long-range inputs, including excitatory connections from the ipsilateral cortex and claustrum, as well as inhibitory inputs from the basal ganglia.
While long-range inputs to mPFC directly engage projection neurons, they also trigger local communication. For example, IT cells receive input from BLA (in L2), thalamus (in L3), and vHPC (in L5), before relaying information to other projection neurons [16,33]. Thus, L5 PT cells are directly activated by long-range inputs, while also integrating the activity of IT cells distributed over multiple layers. Interestingly, the activity of PT cells may be coordinated via enriched thalamic input to their apical dendrites, a notable difference between mPFC and sensory cortex. This connectivity is instead reminiscent of circuits in the hippocampus, where perforant path inputs contact the apical dendrites of pyramidal cells, allowing them to gate responses to other inputs that target proximal dendrites [91]. In the future, it will be interesting to assess the functional implications of VM inputs to PT cells, including regulation of synaptic plasticity.
The targeting of long-range inputs to different classes of interneurons also sculpts functional responses in the mPFC. By engaging distinct local inhibitory circuits, long-range inputs can have unexpected and interesting influences. For example, VM strongly engages L1 NDNF+ cells to inhibit the dendrites of PT cells, effectively silencing dendritic excitation [23]. In contrast cPFC inputs target PV+ interneurons, controlling activity at the soma [42,57]. The activation of PV+ interneurons is reduced during ongoing activity, whereas SOM+ cells becoming increasingly engaged by repetitive stimulation [41]. Finally, vHPC inputs target CCK+ interneurons, whose presynaptic terminals are uniquely sensitive to endocannabinoid signaling [78]. Moving forward, it is important to assess the functional implications of these findings and how different forms of inhibition are regulated, including by neuromodulators that shape in vivo dynamics and behavior.
Lastly, it is important to remember that wiring rules can differ across subregions of the mPFC that play distinct roles in behavior [92,93]. For example, afferents from vHPC and BLA show graded innervation along the dorso-ventral axis of mPFC. They also display distinct laminar targeting in each subregion, with BLA and vHPC input segregating in L2 and L5 of PL but overlapping in L5 of IL. Moreover, they show varying cell-type specificity across subregions, with BLA targeting L2 cortico-amygdala neurons in PL but L5 PT cells in IL [40,64], and vHPC targeting L5 IT cells in IL [62]. By contacting different subregions, layers and projection neurons, each long-range input has distinct effects on the local circuit, and thus has unique impact on downstream brain regions.
CONCLUDING REMARKS
Different cortical regions share many common features, including a laminar structure, excitatory and inhibitory cells, and specific wiring rules [5,34,38]. However, major regional variation also exists, with the fundamental building blocks of the cortex assembled in unique ways to enable specialized functions. Among the keys to understanding the cortex is to appreciate that these differences between regions are at least as important as the similarities. The mPFC is distinguished from primary sensory cortex in several important ways, including being agranular and displaying distinct connectivity characterized by enormous variety of both projection neurons and long-range inputs. Understanding the specific cell types, local connections and long-range networks of the mPFC provides an important framework for assessing how the properties of mPFC circuits contribute to specific functions, including how these networks integrate multiple types of task-relevant information, support delay-period activity, and ultimately how specific mPFC circuit elements control behavior (see Outstanding Questions) [18,51–54].
OUTSTANDING QUESTIONS.
How many cell types are present in the rodent mPFC? Given its distinct cytoarchitecture, diverse projection neurons, and different interneuron allocations, there is need to establish how the neuronal complement within mPFC differs from other cortical regions.
How do subregions of the mPFC communicate with each other? While much has been learned about thalamic, BLA and vHPC inputs, current understanding of cortical and claustral inputs is still limited. It will be important to establish how ipsilateral cortical inputs link different regions of the mPFC, including ACC, PL and IL, along the dorso-ventral axis.
How do mPFC network elements contribute to behavior? Better understanding of mPFC circuits will help establish the distinct functional roles of cells and connections.
How does neuromodulation influence different neurons and connections? It is likely that dopamine, noradrenaline, acetylcholine and endocannabinoids control specific circuit elements to shape in vivo dynamics and behavior. New advances in genetically encoded sensors for neuromodulators may help answer this question.
How are mPFC circuits disrupted in mental health disorders? Having learned about circuits in the healthy, mature brain, we now need to understand the extent to which molecular cues and activity patterns influence specific cell types and connections, and the functional consequences when these processes are perturbed in both development and disease.
HIGHLIGHTS.
The rodent medial prefrontal cortex (mPFC) shows key differences from sensory cortex in the composition and organization of local and long-range circuits.
Long-range inputs from other brain regions display distinct lamination patterns, which contribute to these projections’ cellular and subcellular targeting.
Long-range inputs to the mPFC contact distinct populations of both projection neurons and interneurons, potentially explaining these inputs’ unique behavioral roles.
In many cases, long-range inputs preferentially target neurons that project back to the input region, providing a synaptic substrate for strong reciprocal loops.
Projection neurons and interneurons also make specific local connections that shape how functional signals are routed through the mPFC, linking inputs with outputs.
Acknowledgements:
We would like to thank members of the Carter lab, Christine Constantinople, and David Schneider for helpful discussions and comments on the manuscript. This work was supported by NIH R01 MH085974 (AGC). PGA is supported by Marie Skłodowska-Curie Actions (European Commission), a NARSAD YI Grant (Brain & Behaviour Research Foundation) and a Springboard Award (The Academy of Medical Sciences).
Footnotes
Declaration of interests: The authors declare no competing interests in relation to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miller EK and Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202 [DOI] [PubMed] [Google Scholar]
- 2.Robbins TW and Arnsten AFT (2009) The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu Rev Neurosci 32, 267–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare BD and Duman RS (2020) Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Molecular Psychiatry DOI: 10.1038/s41380-020-0685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten AFT and Rubia K (2012) Neurobiological Circuits Regulating Attention, Cognitive Control, Motivation, and Emotion: Disruptions in Neurodevelopmental Psychiatric Disorders. Journal of the American Academy of Child & Adolescent Psychiatry 51, 356–367 [DOI] [PubMed] [Google Scholar]
- 5.Harris KD and Shepherd GMG (2015) The neocortical circuit: themes and variations. Nature Neuroscience 18, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adesnik H and Naka A (2018) Cracking the Function of Layers in the Sensory Cortex. Neuron 100, 1028–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van De Werd HJ et al. (2010) Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain structure & function 214, 339–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S et al. (2016) Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat Neurosci 19, 1733–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingg B et al. (2014) Neural Networks of the Mouse Neocortex. Cell 156, 1096–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ährlund-Richter S et al. (2019) A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nature Neuroscience 22, 657. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q et al. (2019) A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nat Neurosci 22, 1357–1370 [DOI] [PubMed] [Google Scholar]
- 12.Oh SW et al. (2014) A mesoscale connectome of the mouse brain. Nature 508, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooks BM et al. (2018) Topographic precision in sensory and motor corticostriatal projections varies across cell type and cortical area. Nat Commun 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X-J (2001) Synaptic reverberation underlying mnemonic persistent activity. Trends in Neurosciences 24, 455–463 [DOI] [PubMed] [Google Scholar]
- 15.Fuster JM (2001) The Prefrontal Cortex—An Update: Time Is of the Essence. Neuron 30, 319–333 [DOI] [PubMed] [Google Scholar]
- 16.Collins DP et al. (2018) Reciprocal Circuits Linking the Prefrontal Cortex with Dorsal and Ventral Thalamic Nuclei. Neuron 98, 366–379.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warden MR et al. (2012) A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weele CMV et al. (2018) Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature DOI: 10.1038/s41586-018-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan M et al. (2017) Combined Social and Spatial Coding in a Descending Projection from the Prefrontal Cortex. Cell 171, 1663–1677 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama H et al. (2018) Cell-Type-Specific Contributions of Medial Prefrontal Neurons to Flexible Behaviors. J Neurosci 38, 4490–4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloodgood DW et al. (2018) Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translational Psychiatry 8, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuman B et al. (2019) Four Unique Interneuron Populations Reside in Neocortical Layer 1. J. Neurosci. 39, 125–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastasiades PG et al. (2021) Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 109:314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abs E et al. (2018) Learning-Related Plasticity in Dendrite-Targeting Layer 1 Interneurons. Neuron DOI: 10.1016/j.neuron.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Y et al. (2018) The logic of single-cell projections from visual cortex. Nature 556, 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueta Y et al. (2019) Ipsi- and contralateral corticocortical projection-dependent subcircuits in layer 2 of the rat frontal cortex. Journal of Neurophysiology 122, 1461–1472 [DOI] [PubMed] [Google Scholar]
- 27.Baker A et al. (2018) Specialized Subpopulations of Deep-Layer Pyramidal Neurons in the Neocortex: Bridging Cellular Properties to Functional Consequences. J Neurosci 38, 5441–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo K et al. (2018) Anterolateral Motor Cortex Connects with a Medial Subdivision of Ventromedial Thalamus through Cell Type-Specific Circuits, Forming an Excitatory Thalamo-Cortico-Thalamic Loop via Layer 1 Apical Tuft Dendrites of Layer 5B Pyramidal Tract Type Neurons. The Journal of Neuroscience 38, 8787–8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay R et al. (2016) GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crandall SR et al. (2017) Infrabarrels Are Layer 6 Circuit Modules in the Barrel Cortex that Link Long-Range Inputs and Outputs. Cell Reports 21, 3065–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamawaki N et al. (2014) A genuine layer 4 in motor cortex with prototypical synaptic circuit connectivity. Elife 3, e05422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anastasiades PG et al. (2019) Cell-Type-Specific D1 Dopamine Receptor Modulation of Projection Neurons and Interneurons in the Prefrontal Cortex. Cereb Cortex 29, 3224–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown SP and Hestrin S (2009) Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y et al. (2017) Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 171, 456–469 e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise SP (2008) Forward Frontal Fields: Phylogeny and Fundamental Function. Trends Neurosci 31, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laubach M et al. (2018) What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlén M (2017) What constitutes the prefrontal cortex? Science 358, 478–482 [DOI] [PubMed] [Google Scholar]
- 38.Tasic B et al. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Economo MN et al. (2018) Distinct descending motor cortex pathways and their roles in movement. Nature 563, 79. [DOI] [PubMed] [Google Scholar]
- 40.Little JP and Carter AG (2013) Synaptic Mechanisms Underlying Strong Reciprocal Connectivity between the Medial Prefrontal Cortex and Basolateral Amygdala. J. Neurosci. 33, 15333–15342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGarry LM and Carter AG (2016) Inhibitory Gating of Basolateral Amygdala Inputs to the Prefrontal Cortex. J. Neurosci. 36, 9391–9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anastasiades PG et al. (2018) Cell-Type Specificity of Callosally Evoked Excitation and Feedforward Inhibition in the Prefrontal Cortex. Cell Reports 22, 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dembrow NC et al. (2010) Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci 30, 16922–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gee S et al. (2012) Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. J Neurosci 32, 4959–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama H et al. (2018) Cell-Type-Specific Contributions of Medial Prefrontal Neurons to Flexible Behaviors. J Neurosci 38, 4490–4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohur US et al. (2014) Anatomic and molecular development of corticostriatal projection neurons in mice. Cereb Cortex 24, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wall NR et al. (2013) Differential Innervation of Direct- and Indirect-Pathway Striatal Projection Neurons. Neuron 79, 347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q et al. (2017) Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol 525, 1317–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabbott PLA et al. (2005) Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology 492, 145–177 [DOI] [PubMed] [Google Scholar]
- 50.Wimmer RD et al. (2015) Thalamic control of sensory selection in divided attention. Nature 526, 705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolkan SS et al. (2017) Thalamic projections sustain prefrontal activity during working memory maintenance. Nature Neuroscience 20, 987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt LI et al. (2017) Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spellman T et al. (2015) Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgos-Robles A et al. (2017) Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci 20, 824–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N et al. (2016) Robust neuronal dynamics in premotor cortex during motor planning. Nature 532, 459–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little JP and Carter AG (2012) Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J Neurosci 32, 12808–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee AT et al. (2014) Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron 81, 61–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dembrow NC et al. (2015) Temporal dynamics of L5 dendrites in medial prefrontal cortex regulate integration versus coincidence detection of afferent inputs. J Neurosci 35, 4501–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parnaudeau S et al. (2013) Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77, 1151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honjoh S et al. (2018) Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nature Communications 9, 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cruikshank SJ et al. (2012) Thalamic Control of Layer 1 Circuits in Prefrontal Cortex. J. Neurosci. 32, 17813–17823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X and Carter AG (2018) Ventral Hippocampal Inputs Preferentially Drive Corticocortical Neurons in the Infralimbic Prefrontal Cortex. J Neurosci 38, 7351–7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petreanu L et al. (2009) The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheriyan J et al. (2016) Specific Targeting of the Basolateral Amygdala to Projectionally Defined Pyramidal Neurons in Prelimbic and Infralimbic Cortex. eNeuro 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGarry LM and Carter AG (2017) Prefrontal cortex drives distinct projection neurons in the basolateral amygdala. Cell Rep 21, 1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sánchez-Bellot C and MacAskill AF (2019) Push-pull regulation of exploratory behavior by two opposing hippocampal to prefrontal cortex pathways. bioRxiv DOI: 10.1101/2019.12.18.880831 [DOI] [Google Scholar]
- 67.Cheriyan J and Sheets PL (2018) Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J Neurosci 38, 4829–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson CT et al. (2010) Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci 13, 739–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Otsuka T and Kawaguchi Y (2011) Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci 31, 3862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hooks BM et al. (2013) Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci 33, 748–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirai Y et al. (2012) Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J Neurosci 32, 1898–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zolnik TA et al. (2020) Layer 6b Is Driven by Intracortical Long-Range Projection Neurons. Cell Reports 30, 3492–3505.e5 [DOI] [PubMed] [Google Scholar]
- 73.Wang Y et al. (2006) Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci 9, 534–542 [DOI] [PubMed] [Google Scholar]
- 74.Isaacson JS and Scanziani M (2011) How inhibition shapes cortical activity. Neuron 72, 231–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kepecs A and Fishell G (2014) Interneuron cell types are fit to function. Nature 505, 318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Granger AJ et al. (2020) Cortical ChAT+ neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. eLife 9, e57749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whissell PD et al. (2015) Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Frontiers in neuroanatomy 9, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X et al. (2020) Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex. eLife 9, e55267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fino E and Yuste R (2011) Dense inhibitory connectivity in neocortex. Neuron 69, 1188–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu J et al. (2017) Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat Neurosci 20, 1377–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hilscher MM et al. (2017) Chrna2-Martinotti Cells Synchronize Layer 5 Type A Pyramidal Cells via Rebound Excitation. PLOS Biology 15, e2001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee SH et al. (2014) Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delevich K et al. (2015) The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci 35, 5743–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marek R et al. (2018) Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci 21, 384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson J et al. (2018) Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron 99, 1029–1039 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee AT et al. (2019) VIP Interneurons Contribute to Avoidance Behavior by Regulating Information Flow across Hippocampal-Prefrontal Networks. Neuron 102, 1223–1234.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Letzkus JJ et al. (2015) Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron 88, 264–276 [DOI] [PubMed] [Google Scholar]
- 88.Pi H-J et al. (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H et al. (2013) Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77, 155–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cummings KA and Clem RL (2020) Prefrontal somatostatin interneurons encode fear memory. Nature neuroscience 23, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bittner KC et al. (2015) Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nature Neuroscience 18, 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sierra-Mercado D et al. (2011) Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dalley JW et al. (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews 28, 771–784 [DOI] [PubMed] [Google Scholar]
- 94.Taniguchi H et al. (2013) The spatial and temporal origin of chandelier cells in mouse neocortex. Science 339, 70–4 [DOI] [PMC free article] [PubMed] [Google Scholar]