Abstract
Microglia are long-lived resident macrophages of the brain with diverse roles that span development, adulthood, and aging. Once thought to be a relatively homogeneous population, there is a growing recognition that microglia are highly specialized to suit their specific brain region. Cerebellar microglia represent an example of such specialization, exhibiting a dynamic, transcriptional, and immunological profile that differs from other microglial populations. Here we review the evidence that cerebellar microglia shape the cerebellar environment and are in turn shaped by it. We examine the roles microglia play in cerebellar function, development, and aging. The emerging findings on cerebellar microglia may also provide insights into disease processes involving cerebellar dysfunction.
Keywords: Immune surveillance, Purkinje cell, phagocytosis, immune activation, synapse elimination
Cerebellar microglia in the light of microglial heterogeneity
Microglia, the innate immune cells of the central nervous system (CNS), directly shape and influence neural development and function. Classically studied for their roles in pathology, it is becoming increasingly clear that even under homeostatic conditions microglia are not quiescent sentinels but actively survey and patrol the CNS and are able to respond rapidly to chemotactic cues [1,2]. As they do so, they make frequent transient contacts with neural elements [3], eliminate synapses [4,5], and influence the formation of dendritic spines [6]. Microglia-mediated plasticity depends upon a number of signaling pathways including complement signaling [4,5], purinergic signaling [7], β-adrenergic signaling [8], fractalkine signaling [9], neurotrophic factors [10], inflammatory cytokines [11,12], and superoxide levels [13]. Disturbances to these pathways have been shown to result in aberrant neural development and plasticity [14,15] (Box 1).
Box 1: Technical approaches to deciphering microglial roles in altering synapses and circuits.
A common approach to investigating how microglia interact with neurons is to alter microglial function and observe the effects on neuronal properties, circuit function or animal behavior. Early studies relied on pharmacological methods to interfere with microglial immune function, but were limited in their specificity to microglia (e.g. drugs like minocycline affect astrocytes and other cell types in addition to microglia) and did not address non-immune microglial pathways [102,103]. Pharmacological and genetic microglial depletion experiments have illuminated the diverse roles microglia play in brain function, but lessons learned from the removal of such a large number of cells may not accurately mirror normal function [60,104,105]. The CX3CR1-creERT mouse revolutionized the study of microglia by allowing the conditional expression and removal of target genes in microglia, facilitating the modification of specific pathways at distinct developmental time points [10,106]. Unbiased genomic profiling approaches have generated candidate pathways for manipulation, also highlighting microglial changes throughout the lifespan or in disease, and exposing differences between microglial populations [41,43,56]. Bulk RNAseq [20], single cell RNAseq [79,107], and translating ribosome affinity purification (TRAP) sequencing [19], have revealed differential gene expression patterns between cerebellar microglia and other microglial cell populations. These powerful genomic approaches, however, must be carefully vetted as changes at the mRNA level do not always reflect changes in protein expression or activity, and do not provide detailed spatiotemporal information, a limitation which may be partly alleviated by new spatial transcriptomic methods [108,109]. In parallel, in vivo imaging techniques allowed the assessment of these reactive cells in their native milieu and revealed the unprecedented dynamics of microglial structures, as well as their ability to dynamically interact with other brain cells [1,2]. While technical challenges remain in imaging undisturbed microglia, and depth limitations prohibit access to some microglial populations [110,111], the different dynamics of cerebellar and cortical microglia underscore this cell type’s functional heterogeneity [29]. Technical advances now allow further characterization of cerebellar microglia and their functions. Transcriptomic approaches could further elucidate cerebellar microglial subtypes while in vivo imaging paired with electron microscopy could illuminate the precise nature of microglia-neural contacts, not just with Purkinje cells but with climbing fibers, parallel fibers, interneurons, and granule cells [3,112]. Electrophysiology combined with genetic knockout studies and microglia depletion paradigms could be used to demonstrate the functions of microglia within the cerebellar circuit [11,66,85]. Lastly, the same tools could be paired with cerebellar specific behavioral paradigms, such as the optokinetic and vestibule-ocular reflexes [113] to better understand the effect of microglia and microglia signaling pathways on behaviors mediated by the cerebellum.
Much of our knowledge of microglia-neuron interactions derives from the study of cortical or hippocampal microglia, which have largely been assumed to be representative of all microglia. The notion that microglia are relatively homogeneous in their phenotypes and behaviors may stem in part from the fact that all microglia are thought to originate from the same early yolk sac progenitors [16-18]. These early progenitors infiltrate the brain before the blood brain barrier fully matures, and proliferate to populate all brain regions, becoming the CNS’s first line “immune system” (Fig 1), a function that could be envisioned to be largely uniform throughout the brain. Given our current understanding of the expanded roles of microglia and the different functions, structures, cellular profiles of different brain areas, it seems likely that the microglia embedded in these different milieus exhibit some degree of regional specification to fit the demands of surrounding neurons. Indeed, evidence is emerging that microglia are not homogeneous and vary greatly between brain regions in terms of morphology, signaling pathways and gene expression patterns [19-22]. For instance, adjacent basal ganglia nuclei, such as the ventral tegmental area and the substantia nigra pars reticulata, contain microglia characterized by distinct morphologies, electrophysiological signatures, and transcriptional profiles [23]. These microglial characteristics appear to be dependent upon highly specific, local, developmentally regulated, signaling cues embedded within each brain structure [18,23,24]. Thus, while cortical and hippocampal microglia have been intensely studied, they may not be representative of other microglial populations.
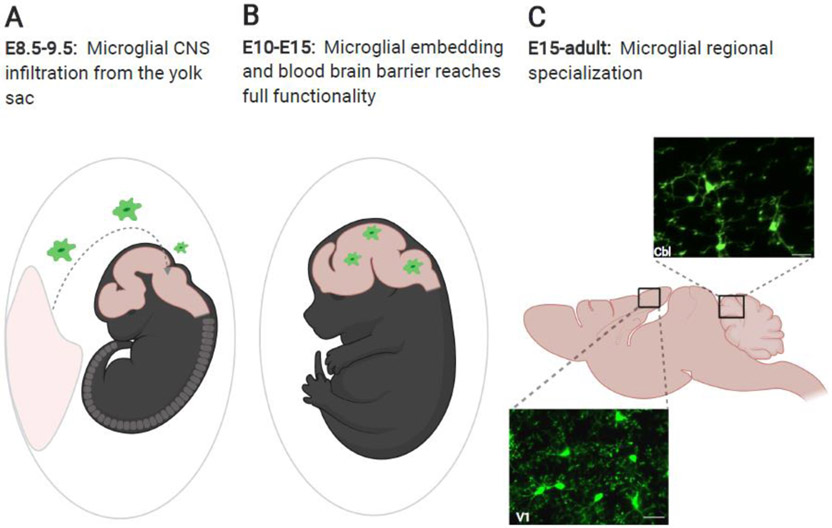
Figure 1: Microglial infiltration of the CNS and transformation by local cues.
(A) Microglia are born in the yolk sac and in mice, infiltrate the embryonic CNS between embryonic day (E)8.5 and E9.5 before the closure of the blood brain barrier. (B) Between E10 and E15 the blood brain barrier reaches full functionality, and microglia distribute within the CNS. (C) From E15 onward, influenced by local cues, microglia specialize to suit their local microenvironment. Pictured here are in vivo images of microglia from primary visual cortex (V1) and cerebellum (Cbl) in CX3CR1-GFP mice (unpublished images by Mark Stoessel and Ania Majewska). Scale bars = 25 μm. Figure created with Biorender.
The cerebellum is an evolutionarily ancient structure, responsible for motor coordination and other functions, displaying a unique architecture of neurons organized into stereotyped input and output circuits (Box 2; see also refs [25-27]). In addition, the cerebellum possesses a unique astrocyte population, which constitutes by itself an area of intense study (Box 3). Given the radically different function, cytoarchitecture, macroglial populations, and levels of activity between the cerebellum and other brain regions [27], it is perhaps not surprising that microglia in the cerebellum display unique characteristics. In this review, we will examine the lines of evidence suggesting that cerebellar microglia are a distinct microglial population with unique profiles driven by the cerebellar environment. Intriguingly, many of the differences observed in cerebellar microglia may have their origins in development, and we will explore how the microglial functions during this early period may shape their long-term characteristics. We will then conclude with a discussion of future directions and potential applications of the new understanding of cerebellar microglia to neurodevelopmental disorders and aging processes.
Box 2: Cerebellar anatomy and structure.
The cerebellum is at once a deceptively simple, and wonderfully complex structure. Canonically thought to be primarily involved in motor coordination and balance, a growing body of research is revealing vast arrays of novel emotional and cognitive functions [11,66,101,114-117]. Cerebellar cytoarchitecture was famously described by Santiago Ramón y Cajal and Camilio Golgi in early 20th century [118]; however, the assemblage of this unique structure is still the topic of intense research and necessitates the accurate and functional formation of billions of synapses. Incorrect assembly can lead to many motor and cognitive deficits, often through impairment of long term depression which has been shown to be critical to cerebellar learning [119]. The cerebellar cortex is organized into three layers, the molecular layer (ML), the Purkinje cell layer (PCL), and the granule cell layer (GCL) with the cerebellar white matter located furthest from the ML (Fig I). On the circuit level, the cerebellum contains millions of repeated cellular units, with each unit centering around the Purkinje cell. In the adult mammalian cerebellum these complex ramified cells are the only output of cerebellum, sending inhibitory projections to one of the deep cerebellar nuclei, which in turn send projections to a wide range of regions of the CNS. Purkinje cells receive two distinct excitatory inputs; one from climbing fibers (CFs) and the other from parallel fibers (PFs). CFs send vestibular information and project from the inferior olivary nucleus. Each CF innervates only one Purkinje cell, but in doing so makes hundreds of thousands of synapses onto the proximal dendritic arbor. PFs are the axons of granule cells, which receive inputs from mossy fibers. Mossy fiber inputs carry information from many sources in the CNS all via the pontocerebellar tract. In contrast to CFs, each Purkinje cell receives functionally weak but numerous inputs from PFs to its distal dendrites [119,120]. It remains unclear how the cerebellum’s unique structure alters the microglia residing in the cerebellum and how these microglia, in turn, alter cerebellar structure and function.
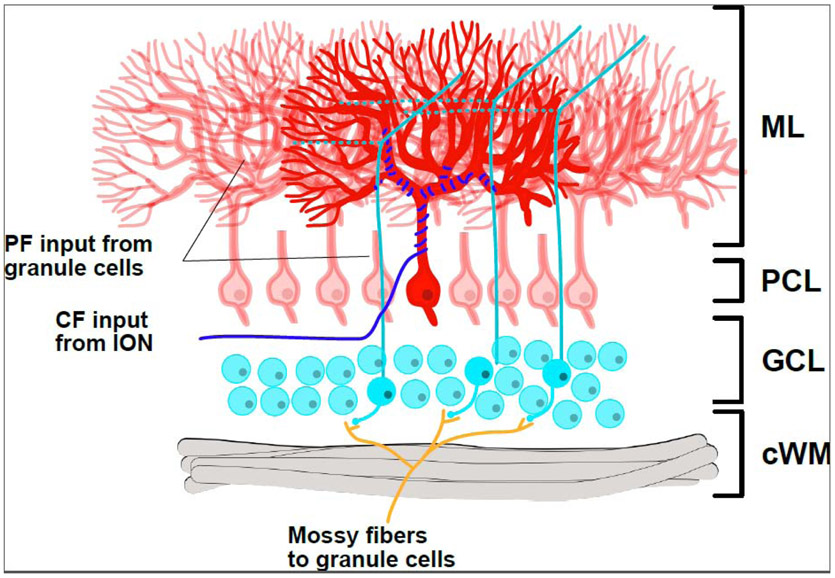
Figure I: Cerebellar lamination and circuitry.
The mature cerebellum is composed of three principal layers: the molecular layer (ML), the Purkinje cell layer (PCL), and the granular cell layer (GCL), as well as a white matter tract (cWM). The ML is a layer of dense neuropil, containing the Purkinje cell arbor. The PCL contains large Purkinje cell bodies (red) in a monolayer. The GCL contains the much smaller cerebellar granule cells (cyan), which receive inputs via mossy fibers (orange) and then send their axon arbors into the ML to become parallel fibers (PFs; cyan/green; running into (solid) and out of (dashed) the plane of the page in 3D). Each PF makes synapses with hundreds of thousands of Purkinje cells. In contrast to this, each Purkinje cell is innervated by one CF (blue), which makes thousands of synapses onto that cell and receives input from the inferior olivary nucleus (ION). In addition to the cell types shown here, there are several classes of inhibitory interneurons which provide GABAergic inhibition to both the GCL and ML.
Box 3: Cerebellar astrocytic heterogeneity.
Glia specialization in the cerebellum is not limited to microglia but extends to astrocytes as well. Cerebellar astrocytes are often grouped into three main forms: white matter astrocytes, granular layer astrocytes, and Bergmann glia (BG). Cerebellar astrocytes are thought to share a common lineage, all arising from cerebellar radial glial during development [121]. These same radial glia give rise to cerebellar oligodendrocytes and molecular layer neurons as well, though it is unclear if a cerebellar radial glia may become all cell types or if there are further lineage restriction mechanisms at play [121]. Cerebellar white matter astrocytes are typically compared to fibrous astrocytes seen in other white matter tracts in the CNS, and possess similar morphologies, fulfilling functions similar to those of other white matter astrocytes. Granular layer astrocytes are typified by their “bushy” morphology with many fine diffuse processes and are thought to carry out many of the homeostatic and support functions carried out elsewhere in the CNS by protoplasmic astrocytes. BG are perhaps the most unique cerebellar astrocyte population, with distinct morphologies and specific interactions with other cell types. BG are often compared to radial glia in the neocortex. In mice, BG originate from cerebellar radial glia at approximately E14.5-E18 [122]. They serve a similar function, though later in development, guiding Purkinje cell precursor cells to their eventual location. Importantly BG remain in the cerebellum throughout life, unlike their cortical radial glia counterparts.
As in other regions of the brain, cerebellar astrocytes perform vital neuronal support functions. Astrocytic synaptic contacts in the cerebellum facilitate synaptogenesis, and maintain synaptic health, and synaptic persistence [88,123,124], similar to other regions of the brain [125]. The synaptic roles of cerebellar astrocytes may be particularly important as nearly all synapses within the cerebellar cortex are ensheathed in astrocytic processes, as opposed to 50% or less in the hippocampus and neocortex [87-89,126]. It has been shown that association of BG, in particular, with cerebellar Purkinje cells is critical in maintaining key electrophysiological properties—specifically bistability—of these neurons [124]. Such mechanisms may be similar to astrocyte regulation of neuronal networks in the hippocampus and neocortex [127].
Adult cerebellar microglia represent a distinct microglial population
Cerebellar microglia show unique functional dynamics
As mentioned, microglial dynamics have thus far been studied largely in the context of cortical microglia. Cortical microglia are highly ramified and regularly tile the neocortex, maintaining non-overlapping territories. In vivo and brain slice time lapse imaging experiments have shown that the cell bodies of these microglia are fairly stationary, while a very motile process arbor samples the defined territory of each cell. These microglia also rapidly respond to injury. For instance, following a high-intensity, focal laser applied to the tissue, microglia rapidly extend their processes to surround the injury core [7]. These dynamic changes in microglial morphology are thought to be critical to their function, both in physiological processes, where they facilitate interactions with other cell types such as neurons, and during injury and disease, where they allow microglia to rapidly restore homeostasis [2,7].
Studies have shown that the description of “canonical” microglial features based on cortical microglia do not hold true for the cerebellum. Cerebellar microglia are less ramified and are sparsely distributed within the tissue [28-31]. As a consequence, they survey less of the cerebellar parenchyma in the same amount of time compared to their cortical counterparts, despite their motile processes [29] (Box 1). Perhaps in order to compensate for decreased density and surveillance, cerebellar microglia exhibit somatic motility, by which their somas rapidly translocate, moving through the cerebellum on a timescale of tens of microns an hour. While adult cortical microglial somas are largely stationary, cortical microglial soma motility has been reported in aged mice [32,33], although not nearly to the degree observed in young adult cerebellar microglia [29]. It remains unclear what triggers the motility of cerebellar microglial somata. This motility may be directed, as in the chemotactic response to chemoattractants, or may be a random sampling of the environment, as is thought to be the case for the surveillant motility of microglial processes. While the mechanisms of soma motility in the cerebellum are not known, it is possible that the process is similar to the migration observed in other peripheral macrophage populations, whose morphology resembles that of the less ramified cerebellar microglia. In the periphery, macrophages migrate through tissues under steady state conditions, sampling the microenvironment for antigens and replacing tissue specific monocytes as needed [34]. Peripheral macrophage motility appears to be partly under control of MHC-II (see Glossary) and it is perhaps worth noting that MHC-II related genes are upregulated in cerebellar microglia [20]. No studies have, as of yet, determined the function of MHC-II in homeostatic microglia, though in neurodegenerative conditions, knocking out MHC-II prevents microglial activation [35]. Establishing the mechanisms that control soma motility in cerebellar microglia may provide insights into the function of this phenomenon.
Possibly as a result of differential surveillance dynamics, cerebellar microglia exhibit different focal injury responses than their cortical counterparts. In the cerebellum, microglial processes are recruited from a greater distance but arrive at the injury core in approximately half the time of cortical microglia [29]. This may be indicative of distinct signaling pathways that control chemoattraction and directed motility, and may be expressed differently in cerebellar microglia than those in the neocortex. P2Y12 is a key mediator of directed motility during the injury response in the neocortex [7,36], and it is possible that it mediates differential microglial responses in these two parts of the brain. However, P2Y12 is expressed to a roughly similar extent in cerebellar and cortical microglia, when assayed at both the RNA and protein level [20,29]. This suggests that changes in downstream intracellular effector pathways may be at play, or that different receptors sense damage in the two microglial populations. To further elucidate the role of P2Y12 in the cerebellum, comparative immunohistochemical or in situ hybridization studies may be necessary and could be combined with electron microscopy to compare subcellular localization of P2Y12 in microglia at rest and during injury response.
Are cerebellar microglia a uniform cell population?
Most studies have not attempted to classify different sub-populations of microglia within the cerebellum. However, there is some evidence to suggest that cerebellar microglia are heterogeneous and that layer specific differences exist. Histological studies have reported differences in microglial morphology based on cerebellar layer, with microglia in the molecular layer being less arborized than those in the granule cell layer [31], and microglia in the granule cell layer being more densely packed, although still much less so than in the neocortex [29]. Process motility is also slightly higher in the Purkinje cell layer than the molecular layer [29]. Further studies have suggested lobule specific differences in the developmental phagocytic activity of cerebellar microglia [37,38] Additionally, some cerebellar microglia are more susceptible to acute ethanol intoxication, with microglia in the Purkinje cell layer but not the molecular layer responding to ethanol with hyperramification of the arbor and decreased arbor dynamics [39]. More experiments will be needed to better elucidate layer-specific differences within the cerebellar microglia population. Techniques such as layer-specific RNA sequencing or cerebellar slice two photon microscopy can better define markers for different populations that may exist in separate layers, identify differential signaling pathways, and describe functional differences in microglial behavior.
Cerebellar microglia have a distinct immune status
The distinct morphological and dynamic phenotypes of cerebellar microglia may be driven by the fact that microglia in the cerebellum have altered immune signaling compared to cortical and hippocampal microglia. RNA profiling of microglia isolated from different brain regions of adult mice has demonstrated that cerebellar microglia show enrichment of pathways involved in pathogen recognition such as lectins (Clec4e and Clec7a), interferon pathway genes (Stat1, Stat4, Irf7), and genes involved in antigen presentation such as MHC-I (H2-D1 and H2-K1) and MHC-II (H2-Aa, H2-Ab1), leading to the idea that these cells are more “immunovigilant” than their cortical and hippocampal counterparts and more responsive to various immune signals [20]. While cerebellar microglia express signature microglial genes, including those that comprise the microglial “sensome”, they express lower levels of canonical sensome genes that are thought to be involved in reducing inflammatory signaling, such as CX3CR1 [40,41]. Likely as a result of these expression patterns, cerebellar microglia show an increased capacity to clear apoptotic cells and exhibit greater numbers of pyknotic cups than cortical microglia at baseline [19,38], although the ability to clear pathogens has not been compared across these microglial populations. This increased phagocytic capacity may be driven by intrinsic signaling within cerebellar microglia or by on-going reciprocal microglia-astrocyte or microglia-neuron interactions which may be distinct in the cerebellum as compared to other brain regions.
It is likely that cerebellar microglia morphology and the motility of their somata arise from this elevated immune activity. Microglia morphology and function are closely linked (Fig 2) [42], and these highly plastic cells are known to alternate between highly ramified morphologies—such as those seen in the neocortex during periods of synaptic reorganization—and much less ramified or even ameboid morphologies—such as those typically associated with disease processes [7,43]. In general, highly ramified microglia are thought to carry out surveillance and homeostatic functions, while less ramified cells are thought to be phagocytically active and inflammatory [1,42]. In the adult CNS, microglia exist on a reversible continuum between these states, and are able to rapidly alter their phenotype and dynamics based on local cues. Thus, the less ramified morphology is likely the result of the increased immune signaling of cerebellar microglia. It has been observed that activated microglia adopt such a morphology upon stimulation with lipopolysaccharide (LPS) or a similar danger signal, with microglia retracting their processes and becoming ameboid in shape [44,45]. The gene expression patterns of cerebellar microglia, however, are distinct from those observed after immune challenges that elicit classical or alternative microglial activation. For example, Nos2 and Arg1, which typify classical and alternative activation respectively, are absent from the transcriptional profile of cerebellar microglia, suggesting a distinct immune profile, at least from a transcriptional standpoint [20]. As discussed above, soma motility may also arise from their particular immune state, though this link is not well established [29]. However, increased expression of immunoregulatory genes in cerebellar microglia is accompanied by increased expression of genes involved in cellular metabolism and energy production [20]. Increased metabolic capacity may be required in these cells to meet the demands of surveying a larger area by physically migrating the cell body. The augmented response to focal injury may also be a consequence of enhanced immune signaling. This may suggest that cerebellar microglia have common phenotypes with “primed” microglia which are also more responsive to injury and pathogens. In fact, microglia that are challenged during development with infection, toxicants or even alcohol exposure, are more responsive to a “second hit”, which can include an enhanced response to focal injury [46-49]. It is interesting to note that cerebellar microglia may acquire their immune phenotype early due to the unique environment of the developing cerebellum similar to “primed” microglia which are generated by insults that also occur during development (see section “Microglia are essential to cerebellar development” below).
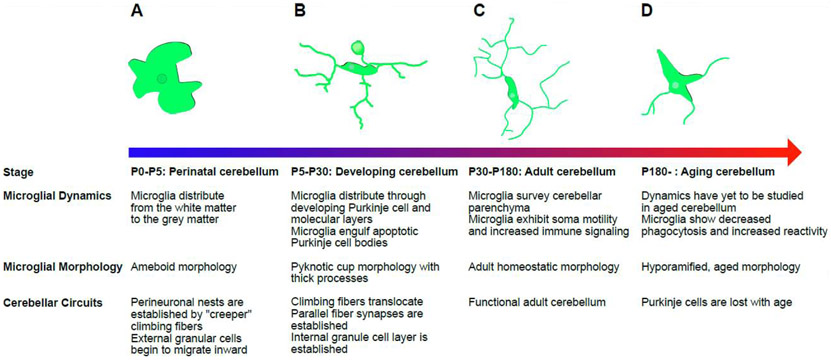
Figure 2: Cerebellar microglial dynamics and morphologies in development and in the adult.
While many different morphologies may be present in the cerebellum at any given time, this figure seeks to give an overview and provide examples of the dominant morphology at developmentally relevant points. Developmental stages refer to postnatal day (P) in mice. (A) In the perinatal cerebellum, microglia are primarily ameboid in morphology and confined to what will become the cerebellar white matter. At P5 they begin to travel outward, towards the developing Purkinje cell layer and external granule cell layer. (B) Microglia distribute themselves within the developing cerebellum and participate in cerebellar development. Microglia engulf apoptotic Purkinje cells, and as such are often observed with more pyknotic cups and thicker processes than adult cerebellar microglia. It is during this period that the cerebellar circuit is established. (C) In the adult cerebellum, microglia have distributed themselves throughout the layers of the cerebellum and actively survey the cerebellar parenchyma. Microglia exhibit soma motility. Microglial morphology at this stage is characterized by long thin processes and some ramification, though not to the degree of their cortical counterparts. (D) As the cerebellum ages, some degree of this ramification is lost, as is the phagocytic capacity. Immune reactivity is hypothesized to increase. It is unclear whether aging affects soma and process motility.
Immune challenges during early development can modify microglia, affecting their function and reactivity throughout the lifespan, and set the stage for microglial behavior in the context of age-related and neurodegenerative disease. During aging, microglia throughout the brain decrease their phagocytic capacity, while increasing the severity of the reactive state [50,51], with such shifts being accompanied by bioenergetic and transcriptomic changes [40,52]. RNA profiling suggests that microglia upregulate pathogen sensing genes, while downregulating receptors to recognize endogenous ligands [40]. Cerebellar microglia appear to be particularly sensitive to the aging process, with changes in their expression profiles occurring steadily from mid-life into old age. As they age, cerebellar microglia become distinct from other microglial populations such as those in the neocortex and hippocampus, upregulating expression of immunoregulatory genes (such as Stat1), maintaining levels of antigen presentation genes (such as H2-Aa), and decreasing their expression of microglial signature genes (such as P2Y12, Tmem119) [20,53]. Such patterns suggest a cerebellar microglia specific aging process which exacerbates the reactive state in these cells, rendering them more susceptible to disease processes and environmental insults.
Cerebellar microglia phenotypes arise from differential signaling
Though still an emerging field of study, it is likely that many of the regulatory cues for microglial specialization arise from epigenetic modifications, similar to what is observed in other myeloid cell populations. Epigenetic modifications have been shown to influence expression of key myeloid genes, often converging around the transcription factor PU.1 and its effectors [54,55]. It has been further shown that microglia use epigenetic modifications to modulate their response to environmental stimuli. Microglia lacking histone deacetylase complexes (HDAC) 1 and 2 were unable to respond appropriately to treatment with LPS [55]. The epigenetic modification H3Kme2 has also been implicated in the maintenance of the disease associated microglia (DAM) phenotype [56].
In a similar manner, expression of many of the inflammatory genes and the phagocytic capacity of cerebellar microglia discussed above have been shown to depend on the removal of the repressive epigenetic histone modification, H3K27me3. H3K27me3 is an important modulator of regional identity of other tissue specific macrophages, and it would follow that H3K27me3 plays a role in determining regional differences between microglial populations as well [57,58]. H3K27me3 is deposited on histones by polycomb repressive complex 2 (PRC2) and removed by two demethylases KMD6a and KMD6b. PRC2 and KDM6a/KDM6b act in opposition, with PRC2 being active in homeostatic microglia (those of neocortex and striatum) and KDM6a and KDM6b being active in immunologically primed microglia (those of cerebellum). Inactivation of PRC2 in striatal microglia which results in loss of H3K27me, leads these microglia to adopt a “cerebellar-like” phenotype [19]. Such mechanisms may explain the process by which microglia receive region specific cues and specialize to their environments.
One such possible environmental cue is transduced by the colony stimulating factor-1 receptor (CSF-1R), which is expressed by all microglia and whose signaling is required for microglial maintenance. Deletion or blockade of CSF-1R causes widespread microglial depletion [16,59,60]. CSF-1R binds two structurally similar ligands, colony stimulating factor-1 (CSF-1) and interleukin 34 (IL-34) [59,61] whose spatial expression is tightly regulated. In the CNS, IL-34 is produced by neurons of the neocortex and hippocampus, but not the cerebellum, while CSF-1 is expressed by astrocytes, oligodendrocytes, and microglia throughout the brain [62-64]. IL-34 has been shown to be crucial for the development and maintenance of cortical microglia, and the same was assumed of cerebellar microglia [65]. However, it has been recently demonstrated that deletion of CSF-1 causes a significant reduction of cerebellar microglia, leaving cortical microglia largely unaffected [66]. Furthermore, application of IL-34 to cerebellar microglia drives a cortical profile, whereas application of CSF-1 to cortical microglia drives a cerebellar profile [66]. This provides an example as to how differential signaling through common microglial receptors can influence regional specificity in microglial populations as well as a potential tool to dissect cortical and cerebellar microglia.
Intriguingly, cerebellar microglia share this dependence on CSF-1 signaling with white matter microglia. When CSF-1 is removed using an antibody sequestration method, white matter microglia are also selectively depleted [63]. Furthermore, similar to cerebellar microglia, white matter microglia have been shown to exhibit lower densities, hyporamification, higher expression of genes related to phagocytosis, especially early in development (P0-P10 in mice), and an exaggerated response to insults such as LPS (Fig. 2 see section “Microglia are essential to cerebellar development”) [30,51,67,68]. Though many questions remain, the similarities between cerebellar and white matter microglial populations may be due to the fact that cerebellar microglia emerge from the cerebellar white matter postnatally, from ~P4-P8 in the mouse, which is later than microglia in the neocortex and hippocampus [69]. As a consequence of their prolonged residence in the white matter in development, cerebellar microglia may maintain some of the characteristics and transcriptional patterns of their white matter counterparts well into adulthood, especially as some of those characteristics are essential to the proper development of cerebellar circuits.
Microglia are essential to cerebellar development
Microglia are integral to the developing nervous system and have been shown to influence synaptic remodeling in diverse regions such as the developing lateral geniculate nucleus (LGN) [4,5], primary visual [3,7,29] and somatosensory [6,70] cortices, and the hippocampus [14,15,71]. In these areas, microglia interact with synapses to restructure synaptic inputs, often in an activity-/experience-dependent manner that has been linked to their ability to phagocytose or trogocytose synaptic material [5,7,14,72]. The clearance activities of microglia are augmented in the cerebellum due to epigenetic changes which increase phagocytic potential [19]. These in turn may be set in place due to the demands placed on microglia by developing cerebellar neurons and nascent circuits. Here, we will review cerebellar specific developmental roles of microglia and potential consequences for adult cerebellar transcriptional and dynamic profiles. Though we focus on certain types of microglial interactions with the developing cerebellar circuit such as phagocytosis, much of this landscape remains unexplored, and it remains unclear what role, if any, microglia play in many cerebellar processes such as granule cell maturation, molecular layer interneuron development, axonal guidance, and vascularization [25,73]. Microglial involvement in these cerebellar specific processes is an important future direction of study.
Microglia clearance activity ensures Purkinje cell function
Throughout cerebellar development, microglia clear apoptotic Purkinje cell bodies [74]. Shortly after birth in mice, Purkinje cells undergo a period of apoptosis, which peaks at approximately P10 [75]. Cerebellar microglia trigger apoptosis in immature Purkinje cells by producing superoxide ions and then proceed to engulf the debris [74]. This clearance activity was shown to depend upon caspase-3 activity within the apoptotic Purkinje cells [74]. As expected, this period coincides with increases in cerebellar microglia phagocytosis and pyknotic cup-like morphology [37,38]. Microglia-mediated clearance activity is vital to proper establishment of cerebellar circuits: preventing microglial activity or depleting cerebellar microglia has been shown to result in aberrations in Purkinje cell development—such as overlapping, multiplanar arbors and ectopic cell body insertion—and disruption of cerebellum-dependent behaviors, as seen for instance in movement disorders and social deficits [66]. Microglial clearance of neuronal cell bodies also occurs in other brain regions, principally those known to undergo adult neurogenesis, such as the olfactory bulb and the dentate gyrus of the hippocampus [76-78]. Neuron elimination in these areas is also caspase-3 dependent, but it does not occur on the same scale, nor does it have the same functional importance as in the cerebellum. This developmental phagocytic activity likely shapes future microglia transcriptional profiles, as cerebellar and hippocampal microglia share some transcriptional characteristics [19,20,79]. Exposing forebrain microglia to apoptotic cells in vitro also induces the expression of “cerebellar” microglia genes, suggesting that triggering phagocytosis may alter microglial phenotypes and immune signaling [19]. Intriguingly, single cell RNA sequencing data shows that cerebellar microglia express similar suites of genes throughout different stages of life – from development, when they first embed in the cerebellum and undergo a sustained period of phagocytosis, into adulthood [79]. This suggest that cerebellar microglia retain some of their developmental qualities throughout the lifespan of the animal.
Microglia shape cerebellar circuits
In addition to their contributions to Purkinje cell development, microglia also play a crucial role in shaping the connectivity and function of these cells. Microglia are critical to the formation of climbing fiber (CF) synapses and in CF translocation on Purkinje cell arbors (Box 4). In mice, at P0, Purkinje neurons are innervated by multiple immature CFs of relatively equal input strength. However, from P7-P12, the excess fibers undergo elimination and a single fiber is translocated to the proximal Purkinje cell dendritic arbor [80,81]. This elimination of excess synapses is akin to developmental synaptic refinement in the thalamus, neocortex, and hippocampus, where microglia have been shown to be critical players in the removal of synapses after an early period of exuberant synaptogenesis [25,69,80]. CF elimination requires both GABAergic transmission and the presence of microglia, as disruptions of either of these from P10-P16 impairs excess CF elimination [82]. Interestingly, the phenotype observed in both impaired GABA transmission and microglial ablation conditions could be rescued with local application of diazepam, a GABAAR agonist, suggesting a link between GABA transmission and microglia’s role in excess CF elimination [69,82]. In fact, recently, microglia have been shown to be a part of a negative feedback loop that dampens neuronal activity, supporting the idea that inhibitory neuronal signaling and microglia may work together to shape circuits [83]. Direct microglial phagocytosis of CFs was rarely observed [69], which is surprising given previously described roles of microglia in the developing cerebellum and other areas of the brain [74]. Microglial release of neurotrophic factors (e.g. BDNF) has been shown to be crucial in controlling synapse remodeling and plasticity [10]. Therefore, it is possible that non-phagocytic mechanisms mediate microglial roles in CF elimination, as has been shown in other brain regions [84]. It is also possible, however, that cerebellar microglia use different mechanisms to interact with synapses than microglia in other brain regions. In fact, fractalkine signaling, which mediates plasticity in the somatosensory cortex and hippocampus, is not involved in CF elimination [85]. This finding is in keeping with reports that the importance of fractalkine signaling to neuronal development varies regionally [6,9,70,86]. It is also possible that microglia affect CF elimination through actions (including phagocytosis) at a different synapse type that is critical to GABAergic signaling within the circuit, such as at the synapse between molecular layer interneurons and Purkinje cells. In fact, astrocytes show a much greater level of ensheathment of cerebellar synapses than those in neocortex and hippocampus (Box 3), suggesting that glia may have extended roles in the remodeling of most synapses in the cerebellum [87-89]. While the role of microglia at the much more diffuse and weak PF synapses is currently unknown, the fact that developmental loss of cerebellar microglia leads to an increase in excitatory synaptic events in Purkinje cells [66] may suggest that microglia also affect non-CF inputs to Purkinje cells as well. Microglia may also shape cerebellar circuits by altering intrinsic excitability of cerebellar neurons [90]. Though not unique to cerebellum, intrinsic excitability is pivotal in shaping cerebellar firing properties, and thereby affecting behavior by modulating membrane properties through calcium induced potassium (SK) channels to change conductance and signal propagation within the dendrite [91]. Microglial inflammatory signaling in the cerebellum was recently shown to directly impact intrinsic excitability and SK channel activation through the cytokine TNFα [11].
Box 4: Climbing fiber selection during development.
Immature Purkinje cells typically send dendrites into many planes within the molecular layer. Through a series of complex molecular cues, an orientation of the Purkinje cell is established and extraplanar arbors are eliminated from approximately postnatal day (P)7 to P30 (in mice) [25,81,128]. These immature Purkinje cell bodies are innervated by multiple CFs in what has been dubbed the “creeper” stage (Fig I). They are more ramified than mature CFs and from P3-P7 establish synapses onto perisomatic protrusions which emerge from the Purkinje cell bodies. By P7, the creeper CFs have essentially enveloped the Purkinje cell body in a structure called the pericellular nest [129]. Translocation, or the process of CF selection and excess synapse elimination, begins around this time. Translocation is thought to occur in two distinct phases, an early phase (P7-P12) and a late phase (P12-P20). During the early phase, the pericellular nest is composed of both large and small CFs, with larger CFs innervating the soma and the developing proximal dendrites, while smaller CFs being generally restricted to the soma. The eventual strength of this synapse is determined both by the presynaptic CF and the postsynaptic Purkinje cell. Identical stimulation patterns that pair pre- and postsynaptic activity in large and small CFs cause LTP and LTD, respectively, strengthening the large winning CF and weakening the smaller CF [130-132]. This is mediated by the magnitude of Ca2+ elevations postsynaptically, which are greater in the dendrites due to differential Cav2.1 expression and the larger surface area to volume ratio in those dendrites. The later phases of translocation are characterized by elimination of excess CF synapses and the smaller fibers. This requires Purkinje cell expression of glutamate receptor δ2 subunit (GluRδ2) [133], the metabotropic glutamate receptor subtype-1 (mGluR1) [133], the complement family protein C1ql1 [134], current through NMDA channels [135] and the immediate early gene Arc/Arg3.1 [136]. These pathways allow for retrograde signaling from the Purkinje cell to the CF, primarily using semaphorin class signaling molecules Sema7A and Sema3A [137]. Disruption of any these genes or their corresponding proteins has profound consequences for circuit development. As previously mentioned, this period is also marked by growth of the Purkinje cell dendritic arbor and the pruning of extraplanar arbors. As the cell matures, one primary plane of growth is established. It has been suggested that extraplanar dendritic outgrowths are innervated by weaker CFs [81], although how exactly these extraplanar dendrites are eliminated is still unclear. Other factors, such as the presence of microglia and GABAergic transmission have been shown to be required for proper CF elimination as well [69,82].
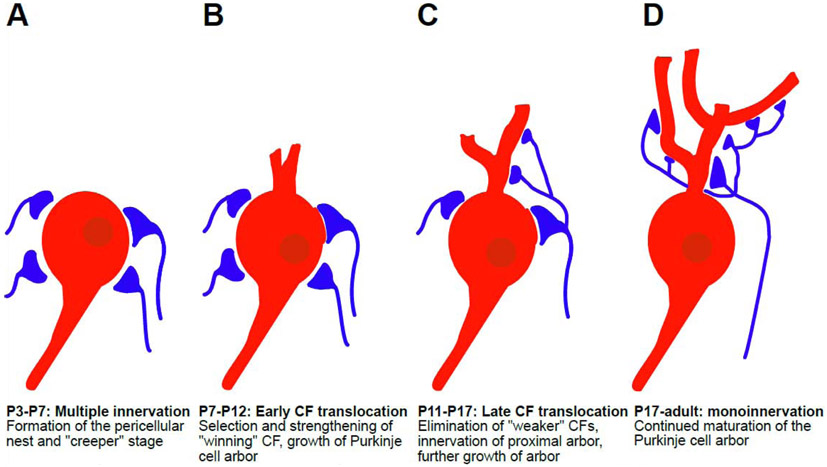
Figure I: Climbing fiber (CF) translocation is a multistep process that occurs postnatally in the mouse.
(A) From P3-P7, multiple (~4-5) CF afferents innervate a single immature Purkinje cell. All of these make several large synapses with the Purkinje cell soma, all of equal strength during the “creeper” stage. This creates the pericellular nest. (B) From P7-P12, during early CF translocation one of these fibers, the “winning” fiber starts to strengthen, in an activity-dependent manner. The Purkinje cell arbor begins to grow into the molecular layer. (C) From P11-P17, during late CF translocation, the “winning” CF begins to move to the developing proximal dendritic arbor of the Purkinje cell, while “weaker” CFs are eliminated. The arbor grows further and begins to segregate into a single plane. (D) By ~P17 the “winning” CF has fully translocated to the dendritic arbor and mono-innervation of each Purkinje cell has been achieved.
Microglial modulation of cerebellar neurons and synapses has functional effects. Microglial depletion has been shown to alter both cerebellar neuronal firing properties and increase cerebellar linked anxiety behaviors [66]. Further, cerebellar microglial inflammatory activation changed Purkinje cell excitability, communication from the cerebellum to prefrontal cortex and promoted repetitive and anxiety like behaviors [11]. While it is clear that microglia are active participants in shaping cerebellar function, more work will be needed to elucidate the cellular and synaptic partners of microglia, and how these interactions alter different behaviors. Microglial depletion, possibly using a conditional CNS specific CSF-1 knockout to ablate cerebellar microglia during development vs. adulthood [66], could be used to systematically assay which cerebellar learning paradigms or tasks require microglia. The circuits underlying these tasks could then be probed to determine the locus and mechanisms through which microglia contribute.
Concluding Remarks and Future Directions
Mounting evidence suggests that cerebellar microglia are distinct from other microglial cell populations, as demonstrated by their differential morphology, distribution and dynamics [29]. These characteristics arise from altered transcriptomics, epigenetics, and signaling pathways within cerebellar microglia and cerebellar-specific environmental cues [11,19,20,66]. Many of these differences may have their origins in cerebellar development, where microglia clear entire apoptotic neuronal cell bodies and participate in excess CF elimination [69,74,79]. In particular, the clearance of entire cell bodies in the cerebellum is notable, and this early developmental phagocytic role may contribute to the adult phenotype and a differential aging process [20,21,79].
Given that cerebellar microglia in the adult share many similarities with microglia in early developmental stages, and that cerebellar microglia have a distinct aging process compared to other brain regions, it is possible that this microglial subpopulation is more susceptible to disease than other microglia populations. Both developing and aging microglia possess a greater sensitivity to environmental insult [46,48,92]. As such, cerebellar microglia may be a unique nexus between the environment and the brain and be key to many aspects of cerebellar disease. For example, in fetal alcohol spectrum disorder and chronic alcoholism, cerebellar microglia and Purkinje cells are some of the first cell types to be affected [39,46,93-95]. Furthermore, there is a growing appreciation of the cerebellum as a critical contributor to dysfunction in disorders that involve altered brain-wide connectivity patterns, including autism spectrum disorder, schizophrenia, and to a lesser extent Alzheimer’s disease [93,96-99]. While historically appreciated as a self-contained circuit that comprises “the little brain” and controls motor activities, the cerebellum actually makes wide-ranging connections, and growing evidence suggests that most motor and cognitive functions are smoothed and corrected by the cerebellum [93,100,101]. The computations underlying these corrections are distributed throughout the cerebellum, feeding back to cortical and motor areas through cerebellocortical connections that are vital to many cognitive processes and everyday life. Given the sensitivity of cerebellar microglia to developmental insults and their impact on the maturation of the cerebellum and its functions, it is imperative to clarify how and through which molecular mechanisms microglia contribute to the normal workings of the cerebellum (see Outstanding Questions). This knowledge may facilitate the design of better interventions for disorders involving cerebellar dysfunction and expand our understanding of how neural and glial cells function within the CNS as a cohesive unit.
Outstanding Questions:
Astrocytes and microglia both interact with synapses and contribute to the maintenance and remodeling of synaptic function. Both of these cell types have distinct phenotypes in the cerebellum that set them apart from their counterparts in other areas of the CNS. Do these differences call for adjustments in the conception of the “quad-partite synapse” in the cerebellum, and if so in what ways?
Microglia are required for the elimination of “weaker” CFs, although direct phagocytosis has yet to be observed. What are the mechanisms by which microglia contribute to CF elimination? Do microglia play a role in the elimination of excess PF synapses?
How are the unique dynamic properties of cerebellar microglia regulated by canonical microglial signaling pathways known to be important in other parts of the CNS, such as complement, purinergic, adrenergic, and fractalkine signaling?
It is known that microglia associate and make contact with cerebellar neurons. What pathways control associations with different classes of cerebellar neurons? What functional roles do these contacts play in cerebellar neuronal properties? How does this affect cerebellar-mediated behaviors?
Developing microglia are vulnerable to environmental insult and cerebellar microglia may be particularly sensitive due to their enhanced immune profile. What role might this susceptibility have in diseases of the cerebellum? Does the dysfunction of cerebellar microglia contribute to other neurological and psychiatric disorders?
Highlights:
Microglia, the innate immune cells of the central nervous system (CNS), directly shape and influence neural development and function.
Much of our knowledge of microglia-neuron interactions derives from the study of cortical or hippocampal microglia, which may not fully represent other microglial populations. Microglia in the cerebellum represent an extreme case of regional specification and differ from microglia residing in the cortex and hippocampus phenotypically and functionally.
Cerebellar microglia show distinctive functional dynamics compared to other microglial populations studied to date and their properties may stem from their gene expression profiles which are enriched for immune pathways.
Microglia play cerebellar-specific roles in development, phagocytosing apoptotic Purkinje cells and facilitating the development of the cerebellar circuit.
The developmental roles of cerebellar microglia, such as pronounced phagocytic activity, likely influence their distinctive adult phenotype, however the cerebellar environment in adulthood may also play a role in maintaining microglial characteristics.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants R01s NS114480, AA02711 (AKM), and F31NS120609 (MBS), as well as grants from the Schmitt and Mangurian Foundations.
Glossary:
- Climbing fiber (CF)
A projection fiber from the inferior olivary nucleus to cerebellar Purkinje cells, thought to encode sensory information. Each Purkinje cell is innervated by a single climbing fiber, although this single fiber makes thousands of synapses onto the Purkinje cell dendritic arbor. The CF is one of the two excitatory inputs to Purkinje cells.
- Disease associated microglia (DAM)
a transcriptomic signature defined as suites of differentially expressed genes in microglia during disease processes when compared with microglia in a healthy environment.
- Histone deacetylase complex (HDAC)
a complex of enzymes involved in epigenetic regulation which remove acetyl groups from histones.
- Lateral geniculate nucleus (LGN)
A thalamic nucleus and part of the visual system, which receives input from retinal ganglion cells. Synaptic pruning and eye specific segregation occurs in the LGN early in the development of the visual system. It was shown in several landmark studies that microglia are required for this segregation through the process of synaptic pruning, mediated by the complement cascade.
- Lipopolysaccharide (LPS)
large compounds found on the cell walls of gram-negative bacteria such as E. coli. Commonly used as a systemic immune challenge to assess microglial responses.
- Major histocompatibility complex II (MHC-II)
a key complex of antigen presenting molecules present on the surfaces of many immune cells, notably dendritic cells and macrophages.
- Parallel fiber (PF)
each parallel fiber makes contact with ~150,000 Purkinje cells, with ~1-2 synapses per Purkinje cell. Granule cells receive inputs through the mossy fibers, which originate in the pontocerebellar tract.
Footnotes
Declaration of Interests
The authors declare no competing interests in relation to this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Nimmerjahn A et al. Resting Microglial Cells Are Hightly Dynamic Surveillants of Brain Parenchyma in Vivo. . (2005), Science, 1314–1317 [DOI] [PubMed] [Google Scholar]
- 2.Davalos D et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci 8, 752–758 [DOI] [PubMed] [Google Scholar]
- 3.Tremblay MĚ et al. (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens B et al. (2007) The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 131, 1164–1178 [DOI] [PubMed] [Google Scholar]
- 5.Schafer DP et al. (2012) Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto A et al. (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sipe GO et al. (2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stowell RD et al. (2019) Noradrenergic signaling in wakeful states inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci 22, 1782–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshiko M et al. (2012) Deficiency of the Microglial Receptor CX3CR1 Impairs Postnatal Functional Development of Thalamocortical Synapses in the Barrel Cortex. J. Neurosci 32, 15106–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkhurst CN et al. (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M et al. (2019) Microglia-Triggered Plasticity of Intrinsic Excitability Modulates Psychomotor Behaviors in Acute Cerebellar Inflammation. Cell Rep. 28, 2923–2938.e8 [DOI] [PubMed] [Google Scholar]
- 12.Kaneko M et al. (2008) Tumor Necrosis Factor-α Mediates One Component of Competitive, Experience-Dependent Plasticity in Developing Visual Cortex. Neuron 58, 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J et al. (2014) Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 82, 195–207 [DOI] [PubMed] [Google Scholar]
- 14.Paolicelli RC et al. (2011) Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science (80-. ). 33, 1456–1458 [DOI] [PubMed] [Google Scholar]
- 15.Zhan Y et al. (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci 17, 400–406 [DOI] [PubMed] [Google Scholar]
- 16.Ginhoux F et al. (2010) Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science (80-. ). 701,841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kierdorf K et al. (2013) Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nat. Neurosci 16, 273–280 [DOI] [PubMed] [Google Scholar]
- 18.Matcovitch-Natan O et al. (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science (80-. ). 353, [DOI] [PubMed] [Google Scholar]
- 19.Ayata P et al. (2018) Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci 21, 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabert K et al. (2016) Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci 19, 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvin A and Ginhoux F (2018) Microglia heterogeneity along a spatio–temporal axis: More questions than answers. Glia DOI: 10.1002/glia.23458 [DOI] [PubMed] [Google Scholar]
- 22.Süß P et al. (2020) Chronic Peripheral Inflammation Causes a Region-Specific Myeloid Response in the Central Nervous System. Cell Rep. 30, 4082–4095.e6 [DOI] [PubMed] [Google Scholar]
- 23.De Biase LM et al. (2017) Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 95, 341–356.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q et al. (2019) Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 101, 207–223.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leto K et al. (2016) Consensus Paper: Cerebellar Development. The Cerebellum 15, 789–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eccles JC et al. (1967) The Cerebellum as a Neuronal Machinele, Springer-Verlag. [Google Scholar]
- 27.Ito M (2006) Cerebellar circuitry as a neuronal machine. Prog. Neurobiol 78, 272–303 [DOI] [PubMed] [Google Scholar]
- 28.Ashwell K (1990) Microglia and cell death in the developing mouse cerebellum. Dev. Brain Res 55, 219–230 [DOI] [PubMed] [Google Scholar]
- 29.Stowell RD et al. (2018) Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev. Neurobiol 78, 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson LJ et al. (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 [DOI] [PubMed] [Google Scholar]
- 31.Vela JM et al. (1995) Morphology and distribution of microglial cells in the young and adult mouse cerebellum. J. Comp. Neurol 361, 602–616 [DOI] [PubMed] [Google Scholar]
- 32.Hefendehl JK et al. (2014) Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell 13, 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyo UB et al. (2018) P2Y12R-Dependent Translocation Mechanisms Gate the Changing Microglial Landscape. Cell Rep. 23, 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakubzick C et al. (2013) Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harm AS et al. (2013) MHCII is required for α-Synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci 33, 9592–9600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes SE et al. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci 9, 1512–1519 [DOI] [PubMed] [Google Scholar]
- 37.Perez-Pouchoulen M et al. (2019) Regulatory Control of Microglial Phagocytosis by Estradiol and Prostaglandin E2 in the Developing Rat Cerebellum. Cerebellum 18, 882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Pouchoulen M et al. (2015) Morphological and Phagocytic Profile of Microglia in the Developing Rat Cerebellum. eNeuro 2, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stowell RD and Majewska AK (2020) Acute ethanol exposure rapidly alters cerebellar and cortical microglial physiology. Eur. J. Neurosci DOI: 10.1111/ejn.14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickman SE et al. (2013) The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci 16, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butovsky O et al. (2014) Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci 17, 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karperien A et al. (2013) Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci 7, 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasemann S et al. (2017) The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del M. Fernández-Arjona M et al. (2017) Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front. Cell. Neurosci 11, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis EJ et al. (1994) Cellular forms and functions of brain microglia. Brain Res. Bull 34, 73–78 [DOI] [PubMed] [Google Scholar]
- 46.Wong EL et al. (2018) Developmental alcohol exposure impairs synaptic plasticity without overtly altering microglial function in mouse visual cortex. Brain. Behav. Immun 67, 257–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong EL et al. (2017) What the spectrum of microglial functions can teach us about fetal alcohol spectrum disorder. Front. Synaptic Neurosci 9, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolton JL et al. (2017) Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front. Synaptic Neurosci 9, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williamson LL et al. (2011) Microglia and memory: Modulation by early-life infection. J. Neurosci 31, 15511–15521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritzel RM et al. (2015) Age- and location-related changes in microglial function. Neurobiol. Aging 36, 2153–2163 [DOI] [PubMed] [Google Scholar]
- 51.Hart AD et al. (2012) Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain. Behav. Immun 26, 754–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flowers A et al. (2017) Proteomic anaysis of aged microglia: Shifts in transcription, bioenergetics, and nutrient response. J. Neuroinflammation 14, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett ML et al. (2016) New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U. S. A 113, E1738–E1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gosselin D et al. (2014) Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datta M et al. (2018) Histone Deacetylases 1 and 2 Regulate Microglia Function during Development, Homeostasis, and Neurodegeneration in a Context-Dependent Manner. Immunity 48, 514–529.e6 [DOI] [PubMed] [Google Scholar]
- 56.Keren-Shaul H et al. (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e17 [DOI] [PubMed] [Google Scholar]
- 57.Kruidenier L et al. (2012) A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Santa F et al. (2007) The Histone H3 Lysine-27 Demethylase Jmjd3 Links Inflammation to Inhibition of Polycomb-Mediated Gene Silencing. Cell 130, 1083–1094 [DOI] [PubMed] [Google Scholar]
- 59.Nandi S et al. (2012) The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol 367, 100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmore MRP et al. (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin H et al. (2008) Discovery of a Cytokine and Its Receptor by Functional Screening of the. Science (80-. ). 707, 807–811 [DOI] [PubMed] [Google Scholar]
- 62.Wang Y et al. (2012) IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol 13, 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Easley-Neal C et al. (2019) CSF1R Ligands IL-34 and CSF1 Are Differentially Required for Microglia Development and Maintenance in White and Gray Matter Brain Regions. Front. Immunol 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greter M et al. (2012) Stroma-Derived Interleukin-34 Controls the Development and Maintenance of Langerhans Cells and the Maintenance of Microglia. Immunity 37, 1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kana V et al. (2019) CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J. Exp. Med DOI: 10.1084/jem.20182037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staszewski O and Hagemeyer N (2019) Unique microglia expression profile in developing white matter. BMC Res. Notes 12, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammond TR et al. (2019) Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 50, 253–271.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakayama H et al. (2018) Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum. Nat. Commun 9, 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunner G et al. (2019) Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci 22, 1075–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stellwagen D et al. (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci 25, 3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinhard L et al. (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biswas S et al. (2020) Neuronal and glial regulation of CNS angiogenesis and barriergenesis. Dev. 147, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marín-Teva JL et al. (2004) Microglia Promote the Death of Developing Purkinje Cells. Neuron 41, 535–547 [DOI] [PubMed] [Google Scholar]
- 75.Cheng XS et al. (2011) Neuronal Apoptosis in the Developing Cerebellum. J. Vet. Med. Ser. C Anat. Histol. Embryol 40, 21–27 [DOI] [PubMed] [Google Scholar]
- 76.Sierra A et al. (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diaz-Aparicio I et al. (2020) Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci 40, 1453–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wallace JL et al. (2017) Development and Refinement of Functional Properties of Adult-Born Neurons. Neuron 96, 883–896.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda T et al. (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 [DOI] [PubMed] [Google Scholar]
- 80.Hashimoto K et al. (2009) Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 63, 106–118 [DOI] [PubMed] [Google Scholar]
- 81.Kaneko M et al. (2011) Remodeling of monoplanar purkinje cell dendrites during cerebellar circuit formation. PLoS One 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakayama H et al. (2012) GABAergic Inhibition Regulates Developmental Synapse Elimination in the Cerebellum. Neuron 74, 384–396 [DOI] [PubMed] [Google Scholar]
- 83.Badimon A et al. (2020) Negative feedback control of neuronal activity by microglia. Nature 10, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheadle L et al. (2020) Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron DOI: 10.1016/j.neuron.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaiser N et al. (2020) Undisturbed climbing fiber pruning in the cerebellar cortex of CX3CR1-deficient mice. Glia DOI: 10.1002/glia.23842 [DOI] [PubMed] [Google Scholar]
- 86.Lowery RL et al. (2017) The microglial fractalkine receptor is not required for activity-dependent plasticity in the mouse visual system. Glia 65, 1744–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buosi AS et al. (2017) Heterogeneity in Synaptogenic Profile of Astrocytes from Different Brain Regions. Mol. Neurobiol DOI: 10.1007/s12035-016-0343-z [DOI] [PubMed] [Google Scholar]
- 88.Lippman JJ et al. (2008) Morphogenesis and regulation of Bergmann glial processes during Purkinje cell dendritic spine ensheathment and synaptogenesis. Glia 56, 1463–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witcher MR et al. (2010) Three-dimensional relationships between perisynaptic astroglia and human hippocampal synapses. Glia 58, 572–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belmeguenai A et al. (2010) Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J. Neurosci 30, 13630–13643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohtsuki G et al. (2012) SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 75, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kracht L et al. (2020) Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science (80-. ). 369, 530–537 [DOI] [PubMed] [Google Scholar]
- 93.Stoodley CJ et al. (2018) Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci 21, 1016. [DOI] [PubMed] [Google Scholar]
- 94.Goodlett CR et al. (1990) a Single Day of Alcohol Exposure During the Brain Growth Spurt Induces Brain-Weight Restriction and Cerebellar Purkinje-Cell Loss. Alcohol 7, 107–114 [DOI] [PubMed] [Google Scholar]
- 95.Belmeguenai A et al. (2008) Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J. Neurophysiol 100, 3167–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Mello AM et al. (2016) Cerebellar gray matter differentiates children with early language delay in autism. Autism Res. 9, 1191–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoxha E et al. (2018) The emerging role of altered cerebellar synaptic processing in Alzheimer’s disease. Front. Aging Neurosci 10, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andreasen NC and Pierson R (2008) The Role of the Cerebellum in Schizophrenia Recent Evidence for the Role of the Cerebellum in Cognition. Biol. Psychiatry 64, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moberget T et al. (2018) Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Mol. Psychiatry 23, 1512–1520 [DOI] [PubMed] [Google Scholar]
- 100.Schmahmann JD et al. (2019) The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci 42, 337–364 [DOI] [PubMed] [Google Scholar]
- 101.Guell X et al. (2018) Functional gradients of the cerebellum. Elife 7, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yrjänheikki J et al. (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. U. S. A 95, 15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Möller T et al. (2016) Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64, 1788–1794 [DOI] [PubMed] [Google Scholar]
- 104.Han J et al. (2017) An updated assessment of microglia depletion: Current concepts and future directions. Mol. Brain 10, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spangenberg E et al. (2019) Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun 10, 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yona S et al. (2013) Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 38, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Q et al. (2019) Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 101, 207–223.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sankowski R et al. (2019) Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci 22, 1–13 [DOI] [PubMed] [Google Scholar]
- 109.Ståhl PL et al. (2016) Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science (80-. ). 353, 78–82 [DOI] [PubMed] [Google Scholar]
- 110.Xu HT et al. (2007) Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci 10, 549–551 [DOI] [PubMed] [Google Scholar]
- 111.Tischbirek CH et al. (2017) In vivo deep two-photon imaging of neural circuits with the fluorescent Ca2+ indicator Cal-590. J. Physiol 595, 3097–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bisht K et al. (2016) Dark microglia: A new phenotype predominantly associated with pathological states. Glia 64, 826–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawato M and Gomi H (1992) The cerebellum and VOR / OKR learning models. Trends Neurosci. 15, 445–452 [DOI] [PubMed] [Google Scholar]
- 114.Strata P (2015) The Emotional Cerebellum. Cerebellum 14, 570–577 [DOI] [PubMed] [Google Scholar]
- 115.Miquel M et al. (2019) A Working Hypothesis for the Role of the Cerebellum in Impulsivity and Compulsivity. Front. Behav. Neurosci 13, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piochon C et al. (2014) Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai PT et al. (2018) Sensitive Periods for Cerebellar-Mediated Autistic-like Behaviors. Cell Rep. 25, 357–367.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sotelo C (2011) Camillo Golgi and Santiago Ramon y Cajal: The anatomical organization of the cortex of the cerebellum. Can the neuron doctrine still support our actual knowledge on the cerebellar structural arrangement? Brain Res. Rev 66, 16–34 [DOI] [PubMed] [Google Scholar]
- 119.Eccles JC et al. (1967) The cerebellum as a neuronal machine, Springer-Verlag. [Google Scholar]
- 120.Ito M (1972) Neural Design of the Cerebellar Motor Control System. Brain Res. 40, 81–84 [DOI] [PubMed] [Google Scholar]
- 121.Buffo A and Rossi F (2013) Origin, lineage and function of cerebellar glia. Prog. Neurobiol 109, 42–63 [DOI] [PubMed] [Google Scholar]
- 122.He L et al. (2018) Transcriptional regulator ZEB2 is essential for bergmann glia development. J. Neurosci 38, 1575–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Zeeuw CI and Hoogland TM (2015) Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Front. Cell. Neurosci 9, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang F et al. (2012) Bergmann glia modulate cerebellar Purkinje cell bistability via Ca 2+-dependent K+ uptake. Proc. Natl. Acad. Sci. U. S. A 109, 7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sofroniew MV and Vinters HV (2010) Astrocytes: Biology and pathology. Acta Neuropathol. 119, 7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matias I et al. (2019) Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci 11, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Letellier M et al. (2016) Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc. Natl. Acad. Sci. U. S. A 113, E2685–E2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lefebvre JL et al. (2012) Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 488, 517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chedotal A and Sotelo C (1993) The “creeper stage” in cerebellar climbing fiber synaptogenesis precedes the “pericellular nest” - ultrastructural evidence with parvalbumin immunocytochemistry. Dev. Brain Res 76, 207–220 [DOI] [PubMed] [Google Scholar]
- 130.Hashimoto K et al. (2011) Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc. Natl. Acad. Sci. U. S. A 108, 9987–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohtsuki G and Hirano T (2008) Bidirectional plasticity at developing climbing fiber-Purkinje neuron synapses. Eur. J. Neurosci 28, 2393–2400 [DOI] [PubMed] [Google Scholar]
- 132.Bosman LWJ and Konnerth A (2009) Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience 162, 612–623 [DOI] [PubMed] [Google Scholar]
- 133.Hashimoto K et al. (2001) Roles of glutamate receptor δ2 subunit (GluRδ2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J. Neurosci 21, 9701–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kakegawa W et al. (2015) Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85, 316–329 [DOI] [PubMed] [Google Scholar]
- 135.Kakizawa S et al. (2000) Critical period for activity-dependent synapse elimination in developing cerebellum. J. Neurosci 20, 4954–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mikuni T et al. (2013) Arc/Arg3.1 Is a Postsynaptic Mediator of Activity-Dependent Synapse Elimination in the Developing Cerebellum. Neuron 78, 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Uesaka N et al. (2014) Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science (80-. ). 344, 1020–1023 [DOI] [PubMed] [Google Scholar]