Abstract
Background:
Signet ring cell breast carcinoma (SRCBC) is a rare variant of invasive lobular carcinoma and there are no large series characterizing its long-term prognosis.
Materials and Methods:
The NCDB was queried from 2004-2016 to identify SRCBC patients. Patients were excluded if they had non-invasive tumors, multiple malignancies, or incomplete surgical data. Univariate analysis was performed utilizing chi-squared and Fischer’s Exact tests. Kaplan-Meier and Cox proportional hazard models were used for survival analysis.
Results:
324 patients met inclusion criteria. Patients were mostly White (75.3%), ≥50 years of age (88.2%), female (98.5%), and had a low Charlson-Deyo score (82.7%). 34.5% had Stage IV disease and 78.1% had ER+ tumors. In patients with non-Stage IV disease, 91.5% received surgery: 49.5% had lumpectomy and 50.5% underwent mastectomy. Radiation therapy was used in 40.7% (71.4% with lumpectomy and 35.8% with mastectomy) and 50% received chemotherapy. Significant differences in unadjusted overall survival (OS) were seen at 5 and 10 years based on stage (p<0.001). On multivariate analysis, ER+ patients showed an improved survival (HR 0.5, p<0.01) but there was no difference in survival if ER+ patients received endocrine therapy (ET) (HR 0.9, p=0.57). Non-metastatic patients who underwent surgery had improved OS compared to those that did not (HR 0.5, p=0.02), but there was no survival difference based upon type of breast operation (p=0.8).
Conclusion:
SRCBC frequently presents at an advanced stage. While ER+ patients appear to have improved survival, there was no clear survival benefit to receiving ET in ER+ patients.
Keywords: Breast cancer, Signet ring cell, Endocrine therapy, Mastectomy, Lumpectomy
Introduction:
Breast cancer is the most frequently diagnosed cancer in women in the United States, and is the second most common cause of cancer death 1. Signet ring cell breast carcinoma (SRCBC) is classified as a rare variant of invasive lobular carcinoma and was first described in 1976 by Dr. Jerry S. Steinbrecher and Dr. Steven G. Silverberg. Its prevalence is postulated as high as 2- 4.5% of all breast carcinomas 2,3. While initial investigations of SRCBC characterized this entity as a derivative of lobular, ductal, and colloid carcinoma, in 2003 it was classified as a unique type of mucin-producing carcinoma by the World Health Organization (WHO) 4. Histologically, SRCBC is defined by the presence of > 20% of tumor cells containing intracytoplasmic, mucin-rich vacuoles that displace the nucleus toward one pole of the cell, forming a crescent or signet ring shape 3,5.
Signet ring cell carcinomas can be found in a variety of anatomical locations, but little has been published on the clinical and prognostic outcomes of SRCBC. However, most studies do report that this histological variant has a poor prognosis with high rates of metastatic disease 2,3,5,6. This study aims to better clinically characterize breast SRCBC and describe its short- and long-term prognosis utilizing the National Cancer Database (NCDB).
Methods:
A retrospective analysis of the NCDB was performed between the years 2004 - 2016 utilizing the 2016 Participant User File (PUF) to identify all cases of breast SRCBC. The NCDB is a national, hospital-based cancer database administered by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The database records approximately 70% of all cancer cases in the United States and provides information on patient demographics, cancer staging, and treatment(s)7. NCDB data is compliant with the privacy requirements of the Health Insurance Portability and Accountability Act (HIPAA). This study was exempt from formal committee review and had approved waiver of consent by the Medical College of Wisconsin Institutional Review Board.
Cases of breast SRCBC were identified through International Classification of Diseases for Oncology codes (ICD-O) 8490 and 8520/3. Those with non-invasive disease were excluded. Patients who received a portion or all of their first treatment at any location other than their designated Commission on Cancer (CoC) site reporting facility were excluded. Patients with more than one malignancy and those with non-Stage IV disease who had missing surgical information were also excluded given that treatment data, often surgical data, was incomplete. Patient demographics, tumor characteristics, and treatment(s) were included in the analysis. Staging was performed using the American Joint Commission on Cancer (AJCC) 7th edition utilizing pathological stage when available; however, if pathological stage was unavailable, notably in Stage 4 patients who did not receive surgery, then clinical stage was used. Univariate analysis was performed utilizing chi-squared tests and Fischer’s Exact tests. The Kaplan-Meier method was used for overall survival analysis. Cox proportional-hazard analysis was performed to assess the effect of multiple variables on survival. All statistical analyses were performed using Rv3.6.3.
Results:
Out of the 2,696,734 patients with breast cancer in the NCDB PUF, a total of 528 (0.02%) patients were identified through the ICD-O codes, of which 324 (0.01%) met inclusion criteria (Figure 1). The majority of patients were White (75.3%), female (98.5%), and healthy (82.7%) as defined by a Charleson-Deyo score (CDS) of 0 8,9 (Table 1). Mean patient age was 63.7 years and median age was 64.0 (range 28-90). Most patients had Medicare (46.3%) and private insurance (38.3%). Treatment centers were primarily comprehensive community cancer programs (36.1%) and academic programs (35.8%). Median follow-up time was 43.2 months.
Figure 1:
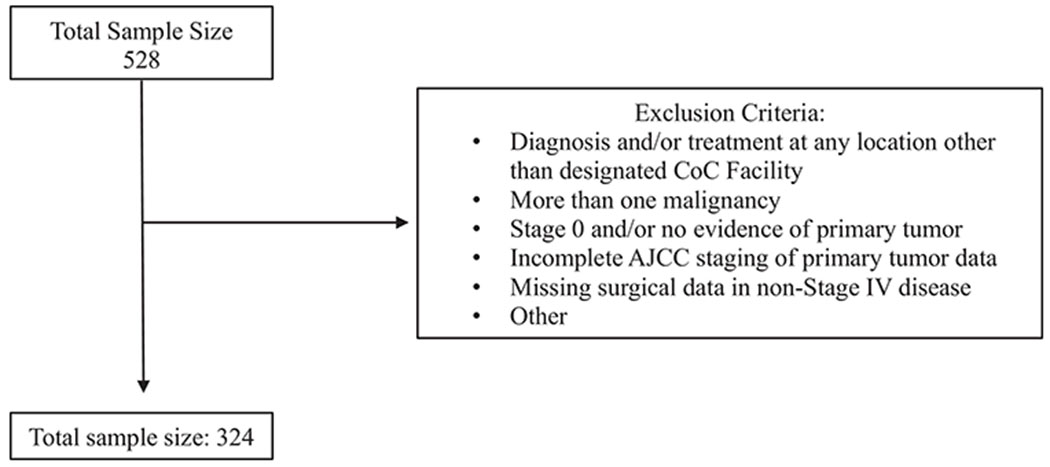
Flow Diagram with exclusion criteria for the cohort from the National Cancer Data Base Stage I-IV SRCBC Patients from 2004 to 2016.
Table 1.
Cohort demographics and clinicopathological characteristics of SRCBC Stage I-IV patients, National Cancer Data Base (NCDB), 2004-2016 (n=324)
| VARIABLE | Total Cohort = 324 n (%) |
|---|---|
| Age (years) | |
| <50 | 38 (11.7) |
| 50-59 | 68 (21.0) |
| 60-69 | 109 (33.6) |
| ≥ 70 | 109 (33.6) |
| Sex | |
| Male | 5 (1.5) |
| Female | 319 (98.5) |
| Race/Ethnicity | |
| White | 244 (75.3) |
| Black | 25 (7.7) |
| Hispanic | 26 (8.0) |
| Other | 29 (9.0) |
| Charleson-Deyo Score | |
| 0 | 268 (82.7) |
| 1 | 42 (13.0) |
| 2 | 11 (3.4) |
| ≥3 | 3 (0.9) |
| Insurance | |
| Not insured | 7 (2.2) |
| Private Insurance | 124 (38.3) |
| Medicaid | 17 (5.2) |
| Medicare | 150 (46.3) |
| Other Government | 3 (0.9) |
| Unknown | 23 (7.1) |
| Treating Facility Geographic Region | |
| New England | 24 (7.4) |
| Middle Atlantic | 67(20.7) |
| South Atlantic | 48 (14.8) |
| East North Central | 65 (20.1) |
| East South Central | 16 (4.9) |
| West North Central | 15 (4.6) |
| West South Central | 40 (12.3) |
| Mountain | 14 (4.3) |
| Pacific | 33 (10.2) |
| Unknown | 2 (0.6) |
| Facility Type | |
| Community | 34 (10.5) |
| CCC | 117 (36.1) |
| Academic | 116 (35.8) |
| INCC | 55 (17.0) |
| Unknown | 2 (0.6) |
| Survival at last follow up | |
| Alive | 150 (46.3) |
| Deceased | 149 (46.0) |
| Unknown | 25 (7.7) |
| Primary Tumor size (cm) | |
| ≤2 | 100 (30.9) |
| 2 - 5 | 107 (33.0) |
| ≥5 | 54 (16.6) |
| Unknown | 63 (19.4) |
| Stage* | |
| I | 63 (19.4) |
| II | 85 (26.2) |
| III | 64 (19.8) |
| IV | 112 (34.5) |
| Tumor Grade | |
| I | 16 (4.9) |
| II | 120 (37.0) |
| III | 101 (31.2) |
| Unknown | 87 (26.8) |
| ER | |
| Positive | 253 (78.1) |
| Negative | 46 (14.2) |
| Unknown | 25 (7.7) |
| PR | |
| Positive | 190 (58.6) |
| Negative | 101 (31.2) |
| Unknown | 33 (10.2) |
| Her2 Neu | |
| Positive | 18 (5.6) |
| Negative | 97 (29.9) |
| Borderline | 5 (1.5) |
| Unknown | 184 (56.8) |
| Nodal Involvement | |
| Positive | 115 (35.5) |
| Negative | 95 (29.3) |
| Unknown | 114 (35.2) |
| Number of Nodes Examined | |
| 0 | 96 (29.6) |
| 1-5 | 92 (28.4) |
| >5 | 112 (34.6) |
| Unknown | 24 (7.4) |
| Breast Surgery Type | |
| Lumpectomy | 99 (30.6) |
| Mastectomy | 108 (33.3) |
| No surgery | 116 (35.8) |
| Unknown | 1 (0.3) |
| Systemic Chemotherapy | |
| Yes | 163 (50.3) |
| No | 154 (47.5) |
| Unknown | 7 (2.2) |
| Endocrine therapy for ER+ (n=253) | |
| Yes | 165 (65.2) |
| No | 74 (29.2) |
| Unknown | 14 (5.5) |
| Radiation therapy | |
| Yes | 132 (40.7) |
| No | 188 (58.0) |
| Unknown | 4 (1.2) |
| Immunotherapy | |
| Yes | 13 (4.0) |
| No | 303 (93.5) |
| Unknown | 8 (2.5) |
Key: ER = estrogen receptor, PR=progesterone receptor, CCC= community cancer center, INCC=integrated network cancer center
=Stage based on AJCC 7th Edition
Over a third of patients had stage IV disease (34.5%) while 26.2% had stage II disease, 19.8% had stage III disease, and only 19.4% had stage I disease. The majority (78.1%) of patients had estrogen-receptor (ER) positive tumors, while 58.6% had both ER and progesterone-receptor (PR) positive tumors, and <1% of patients had ER−/PR+ disease. Data on Her2 status was only available for 120 patients (37%), of which 18 patients (5.6%) were positive , 97 (29%) were negative, and 5 (1.5%) were borderline. Only 10 patients could be formally classified as triple-negative; however, given the large amount of the number of patients with unknown Her2 status, this value is likely underestimated.
Over half of the cohort had primary breast tumors smaller than 5cm in size (63.9%). Of the 210 patients who had surgical nodal staging performed, 54.8% were node positive and 45.2% were node negative. Within the entire cohort, 207 (63.9%) received surgery: 47.8% received breast conserving surgery and 52.2% underwent mastectomy. In patients with non-Stage IV disease, 91.5% received surgery: 49.5% had lumpectomy and 50.5% underwent mastectomy. In those patients who underwent mastectomy, 11.1% had post-mastectomy breast reconstruction. Radiation therapy was used in 40.7% of patients (71.4% with lumpectomy and 35.8% with mastectomy), 50% received systemic chemotherapy, and only 4% received immunotherapy. There were 13 patients with ER− tumors who did not receive chemotherapy; 77% of which had Stage II disease or greater. The majority (64.2%) of patients with ER+ tumors received endocrine therapy.
The cohort was also analyzed by disease stage and no differences were seen in age, gender, race, CDS, type of insurance, geographic region, facility type, or receipt of immunotherapy (p>0.05, Table 2). As expected, disease stage showed differences in tumor size and nodal status (p<0.001). Lower stage patients had higher rates of locoregional therapy with surgery and radiation (p<0.001) while higher stage patients were more likely to receive systemic chemotherapy (p<0.001). Although there was no difference in ER status by stage, in patients with ER+ disease, there was a trend towards lower receipt of endocrine therapy with increasing stage: 78.2% for Stage I, 72.9% for Stage II, 63.8% for Stage III, and 50.6% for Stage IV (p=0.001) (Table 2).
Table 2:
Demographics and clinicopathological characteristics of the cohort by Stage, analysis based on chi-squared tests for SRCBC Stage I-IV patients, National Cancer Data Base (NCDB), 2004-2016 (n=324)
| Variable | Stage 1 | Stage 2 | Stage 3 | Stage 4 | p-value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| 63 (19.4) | 85 (26.2) | 64 (19.8) | 112 (34.6) | ||
| Age (years) | |||||
| <50 | 5 (7.9) | 11 (12.9) | 8 (12.5) | 14 (12.5) | 0.46 |
| 50-59 | 12 (19.0) | 12 (14.1) | 14 (21.9) | 30 (26.8) | |
| 60-69 | 24 (38.1) | 30 (35.3) | 25 (39.1) | 30 (26.8) | |
| ≥70 | 22 (34.9) | 32 (37.6) | 17 (26.5) | 38 (33.9) | |
| Gender | |||||
| Male | 1 (1.6) | 1 (1.2) | 0 | 3 (2.7) | 0.87a |
| Female | 62 (98.4) | 84 (98.8) | 64 (100) | 109 (97.3) | |
| Race | |||||
| White | 52 (82.5) | 65 (76.5) | 49 (76.5) | 78 (69.6) | |
| Black | 3 (4.8) | 7 (8.2) | 2 (3.1) | 13 (11.6) | 0.31 |
| Hispanic | 6 (9.5) | 6 (7.1) | 7 (10.9) | 7 (6.3) | |
| Other | 2 (3.2) | 7 (8.2) | 6 (9.4) | 14 (12.5) | |
| CDS | |||||
| 0 | 52 (82.5) | 64 (75.3) | 56 (87.5) | 96 (85.7) | |
| 1 | 8 (12.7) | 17 (20.0) | 7 (10.9) | 10 (8.9) | 0.65a |
| 2 | 3 (4.8) | 3 (3.5) | 1 (1.6) | 4 (3.6) | |
| ≥3 | 0 (0) | 1 (1.2) | 0 (0) | 2 (1.8) | |
| Insurance | |||||
| None | 0 | 1 (1.2) | 1 (1.6) | 5 (4.5) | |
| Private | 24 (38.1) | 27 (31.8) | 31 (48.4) | 42 (37.5) | |
| Medicaid | 2 (3.2) | 4 (4.7) | 3 (4.7) | 8 (7.1) | 0.49a |
| Medicare | 31 (49.2) | 46 (54.1) | 22 (34.4) | 51 (45.5) | |
| Other | 6 (9.5) | 7 (8.2) | 7 (10.9) | 6 (5.4) | |
| Treating Facility Geographic Region | |||||
| New England | 3 (4.8) | 7 (8.2) | 7 (10.9) | 7 (6.3) | |
| Mid-Atlantic | 13 (20.6) | 21 (24.7) | 12 (18.8) | 21 (18.8) | |
| South Atlantic | 7 (11.1) | 15 (17.6) | 11 (17.2) | 15 (13.4) | |
| East North Central | 12 (19.0) | 16 (18.8) | 10 (15.6) | 27 (24.1) | |
| East South Central | 2 (3.2) | 1 (1.2) | 3 (4.7) | 10 (8.9) | 0.49a |
| West North Central | 6 (9.5) | 1 (1.2) | 4 (6.3) | 4 (3.6) | |
| West South Central | 10 (15.9) | 11 (12.9) | 8 (12.5) | 11 (9.8) | |
| Mountain | 2 (3.2) | 3 (3.5) | 5 (7.8) | 4 (3.6) | |
| Pacific | 8 (12.7) | 10 (11.8) | 4 (6.3) | 11 (9.8) | |
| Facility Type | |||||
| Community | 5 (7.9) | 10 (11.8) | 5 (7.8) | 14 (12.5) | |
| CCC | 25 (39.7) | 28 (32.9) | 28 (43.8) | 36 (32.1) | |
| Academic | 23 (36.5) | 32 (37.6) | 22 (34.4) | 39 (34.8) | 0.90a |
| INCC | 10 (15.9) | 15 (17.6) | 9 (14.0) | 21 (18.8) | |
| Unknown | 0 | 0 | 0 | 2 (1.8) | |
| Tumor Size (cm) | |||||
| ≤ 2 | 60 (95.2) | 19 (22.4) | 7 (10.9) | 14 (12.5) | |
| >2 - 5 | 1 (1.6) | 56 (65.9) | 24 (37.5) | 26 (23.2) | <0.001a |
| ≥ 5 | 1 (1.6) | 7 (8.2) | 26 (40.6) | 20 (17.9) | |
| Unknown | 1 (1.6) | 3 (3.5) | 6 (9.4) | 52 (46.4) | |
| Node Status | |||||
| Positive | 1 (1.6) | 40 (47.1) | 52 (81.3) | 22 (19.6) | <0.001a |
| Negative | 59 (93.6) | 31 (36.5) | 5 (7.8) | 0 | |
| Unknown | 3 (4.8) | 14 (16.5) | 7 (10.9) | 90 (80.4) | |
| Nodes Examined | <0.001a | ||||
| 0 | 2 (3.2) | 10 (11.8) | 2 (3.1) | 82 (73.2) | |
| 1-5 | 47 (74.6) | 29 (34.1) | 6 (9.4) | 10 (8.9) | |
| >5 | 10 (15.8) | 41 (48.2) | 50 (78.1) | 12 (10.7) | |
| Unknown | 4 (6.3) | 5 (5.9) | 6 (9.4) | 8 (7.1) | |
| ER status | |||||
| ER + | 55 (87.3) | 70 (82.4) | 47 (73.4) | 81 (72.3) | 0.13a |
| ER− | 6 (9.5) | 13 (15.3) | 15 (23.4) | 12 (10.7) | |
| Unknown | 2 (3.2) | 2 (2.3) | 2 (3.1) | 19 (17.0) | |
| Endocrine Therapy for ER+ Patients (n=253) | |||||
| Yes | 43 (78.2) | 51 (72.9) | 30 (63.8) | 41 (50.6) | 0.001 |
| No/Unknown | 12 (21.8) | 19 (27.1) | 17 (36.2) | 40 (49.4) | |
| Operation | |||||
| Lumpectomy | 43 (68.3) | 39 (45.9) | 14 (21.9) | 3 (2.7) | |
| Mastectomy | 19 (30.2) | 36 (42.4) | 43 (67.2) | 10 (8.9) | <0.001a |
| None | 1 (1.6) | 10 (11.8) | 7 (10.9) | 98 (87.5) | |
| Unknown | 0 | 0 | 0 | 1 (0.9) | |
| Radiation Therapy | |||||
| Yes | 36 (57.1) | 33 (38.8) | 37 (57.8) | 26 (23.2) | <0.001a |
| No | 27 (42.9) | 51 (60.0) | 25 (3.9) | 85 (75.9) | |
| Unknown | 0 | 1 (1.2) | 2 (3.1) | 1 (0.9) | |
| Chemotherapy | |||||
| Yes | 13 (20.6) | 36 (42.3) | 56 (87.5) | 58 (51.8) | <0.001a |
| No | 48 (76.2) | 47 (55.3) | 6 (9.4) | 53 (47.3) | |
| Unknown | 2 (3.2) | 2 (2.4) | 2 (3.1) | 1 (0.9) | |
| Immunotherapy | |||||
| Yes | 3 (4.8) | 1 (1.2) | 4 (6.3) | 5 (4.5) | 0.43a |
| No | 59 (93.6) | 81 (95.3) | 57 (89.1) | 106 (94.6) | |
| Unknown | 1 (1.6) | 3 (3.5) | 3 (4.7) | 1 (0.9) | |
| Survival at last follow-up | |||||
| Alive | 48 (76.2) | 47 (55.3) | 38 (59.4) | 17 (15.2) | |
| Deceased | 13 (20.6) | 27 (31.8) | 22 (34.4) | 87 (77.7) | <0.001 |
Key: p-value based upon excluding unknowns, ER=estrogen receptor, PR=progesterone receptor, CCC=community cancer center, INCC=integrated network cancer center
In patients who underwent surgery, lower stage patients had higher rates of breast conserving surgery while more advanced stages had higher rates of mastectomy (p<0.01). Increasing stage demonstrated an increase in utilization of post-mastectomy radiation (p<0.001) (Table 3). However, in patients who underwent breast conserving surgery, increasing stage was actually associated with lower rates of receiving post-lumpectomy radiation (p=0.024).
Table 3:
Local therapy treatment details by disease stage, based on Fischer’s Exact tests for SRCBC Stage I-IV patients, National Cancer Data Base (NCDB), 2004-2016 (n=193). Only patients with both surgical and radiation therapy data included.
| Surgery | Radiation | Stage 1 n=60 n (%) |
Stage 2 n=70 n (%) |
Stage 3 n=51 n (%) |
Stage 4 n=12 n (%) |
p-value |
|---|---|---|---|---|---|---|
|
Mastectomy n=95 |
<0.001 | |||||
| Radiation | 1 (1.67) | 7 (10.0) | 24 (47.1) | 2 (16.7) | ||
| No Radiation | 16 (26.7) | 24 (34.3) | 14 (28.0) | 7 (58.3) | ||
|
Lumpectomy n=98 |
0.024 | |||||
| Radiation | 35 (58.3) | 26 (37.1) | 9 (17.6) | 0 (0) | ||
| No Radiation | 8 (13.3) | 13 (18.6) | 4 (7.8) | 3 (25.0) |
Significant differences in unadjusted overall survival (OS) were seen at 5 and 10 years based on disease stage. Lower stage patients exhibited greater OS than later stage patients (p<0.001) (Figure 2). Five- and ten-year survival analysis, respectively, based on stage was 89.7% and 72.8% for stage I, 80.1% and 59.9% for stage II, 68.2% and 48.2% for stage III, and 26.9% and 8.8% for stage IV.
Figure 2:
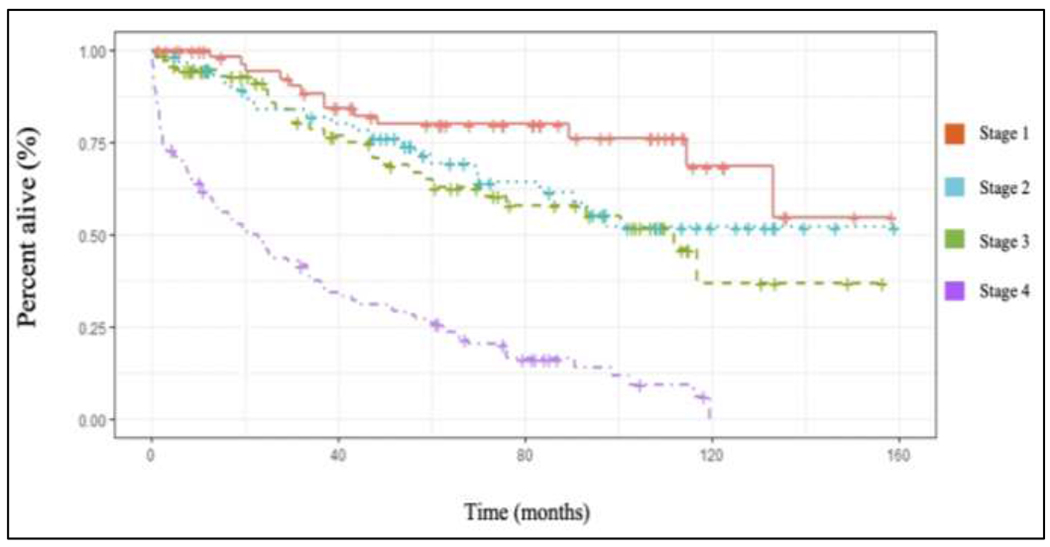
Kaplan-Meier unadjusted overall survival curves for SRCBC based upon disease stage (p<0.001) using the NCDB 2004-2016 PUF. Image should be in color.
Unadjusted OS based on ER status (positive versus negative) revealed no significant survival difference at 5 or 10 years (p=0.079) (Figure 3); however, a survival benefit was seen on multivariate analysis (HR 0.5, 95% CI 0.3-0.9, p<0.01, Table 4). ER+ patients who received endocrine therapy had a statistically higher unadjusted OS compared to patients who did not receive endocrine therapy (p=0.006); however, this did not remain significant when stratifying by disease stage (p=0.35), or on multivariate analysis (HR 0.8, 95% CI 0.6 – 1.3, p=0.57) (Figure 4 and Table 4). Patients with non-metastatic disease who underwent surgery had improved overall long-term survival compared to those that did not receive surgery (HR 0.5, 95% CI 0.3-0.9, p=0.02, Figure 5 and Table 4); however, the majority of patients who did not undergo surgery had more advanced disease (Table 2). There was no difference in survival based upon type of breast operation (p=0.8) (Figure 5).
Figure 3:
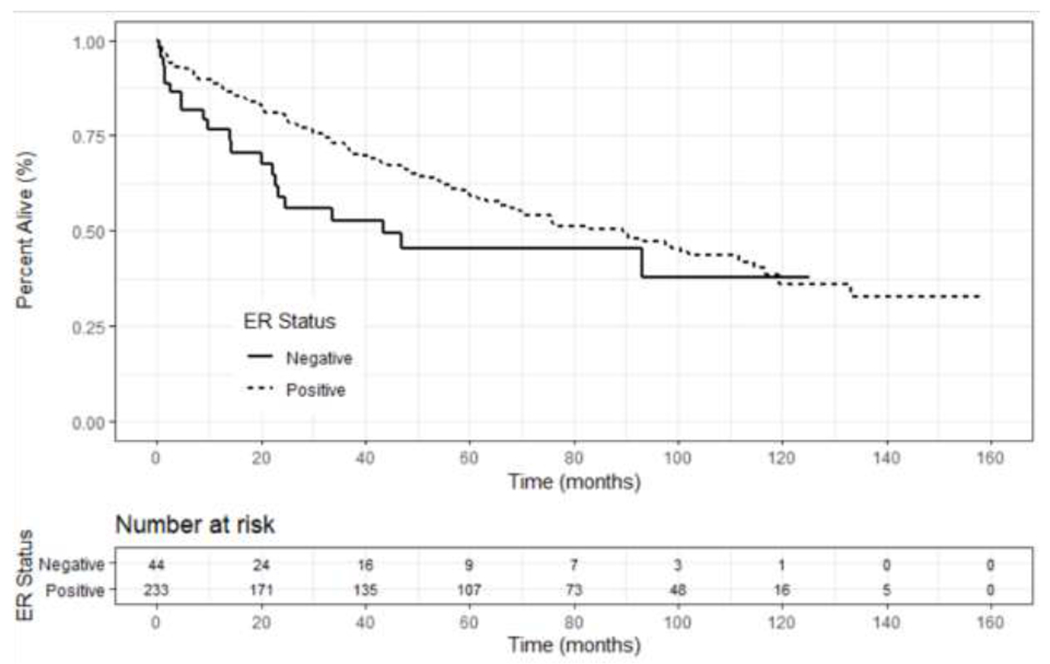
Kaplan-Meier unadjusted overall survival curves based upon estrogen receptor status for patients with Stage I-IV SRCBC utilizing the NCDB 2004-2016. (p=0.079).
Table 4.
Cox proportional-hazard analysis for SRCBC Stage I-IV patients, National Cancer Data Base (NCDB), 2004-2016
| Variable | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Underwent Surgerya | |||
| No | Reference | ||
| Yes | 0.5 | 0.3- 0.9 | 0.02 |
| Stage | |||
| Stage I | Reference | ||
| Stage II | 2.2 | 1.1 – 4.4 | 0.03 |
| Stage III | 1.6 | 0.8 – 3.4 | 0.22 |
| Stage IV | 4.2 | 1.9 – 9.4 | <0.001 |
| Estrogen Receptor Status | |||
| Negative | Reference | ||
| Positive | 0.5 | 0.3 – 0.8 | <0.01 |
| Received Endocrine Therapyb | |||
| No | Reference | ||
| Yes | 0.9 | 0.6 – 1.3 | 0.57 |
=patients with Stage IV disease excluded.
=only for those with ER+ disease
Figure 4:
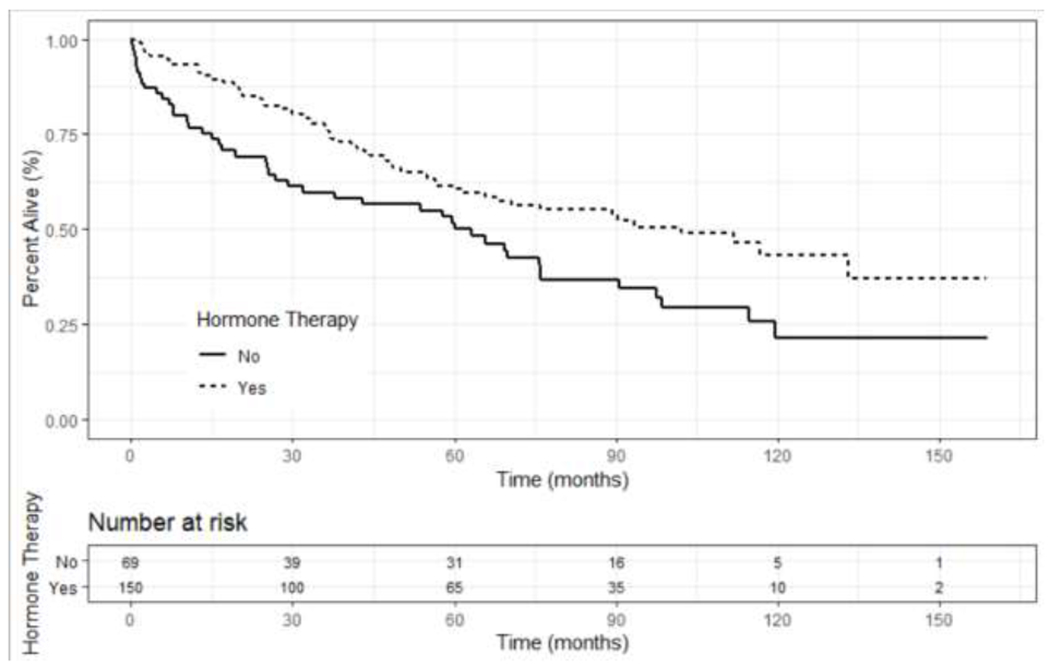
Kaplan Meier unadjusted overall survival curves for ER+ patients based on treatment with endocrine therapy for patients with Stage I-IV SRCBC utilizing the NCDB 2004-2016 (p=0.35).
Figure 5:
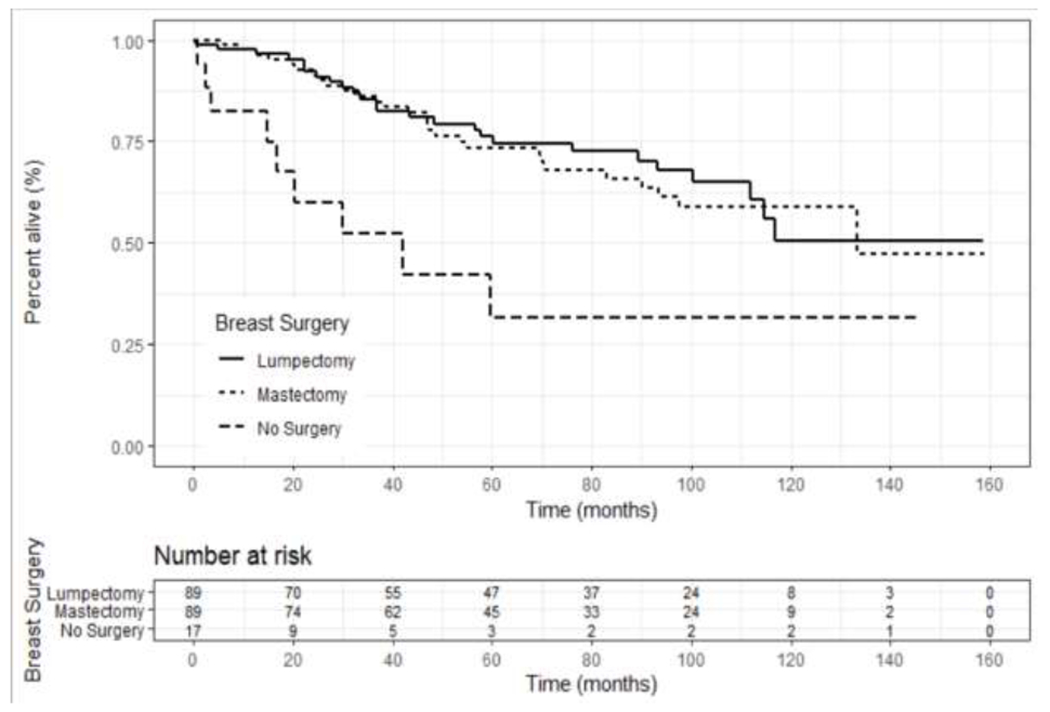
Kaplan-Meier unadjusted overall survival curves for patients with Stage I-III SRCBC utilizing the NCDB 2004-2016 who underwent surgery, and which type of operation, versus those that did not. Patients who underwent surgery showed a survival benefit (p<0.001) but there was no difference in survival based on type of operation (p=0.8).
Discussion:
In this analysis, we found that SRCBC was predominantly found in White women over the age of 50, was usually ER+ and grade 2 or 3, and over a third of patients had metastatic disease at the time of initial presentation. Overall disease stage was largely predictive of OS. While ER+ patients had an improved survival, there was no difference in OS in ER+ patients based on receipt of endocrine therapy on multivariate analysis.
Signet ring cell carcinoma (SRCC) is a rare subtype of mucin-producing adenocarcinoma of which more than 90% of cases arise from stomach, breast, and colon10. SRCC is frequently associated with diagnosis at advanced disease stage, elevated risk of local occurrence, and a limited benefit has been seen from systemic chemotherapy in primary tumors arising from the stomach, gallbladder, prostate, bladder, lung, and colon11–17. Other locations of SRCC, such as lung, gastrointestinal tract, and reproductive organs, are usually a result of metastatic spread from a primary source of signet ring cell breast carcinoma18,19. The growing infiltrative and destructive nature of SRCC, whether primary or metastasis, makes treatments of great clinical research value.
ER and PR expression are important prognostic factors for response to treatment and OS in breast cancer, with hormone-receptor positive tumors demonstrating a more favorable outcome 20–23. Administration of endocrine therapy for ER+ SRCBC patients did not statistically or clinically demonstrate a survival benefit. This finding could be attributed to the smaller cohort size or lack of patient compliance adhering to endocrine therapy which has been well-documented 24,25. In recent years, there have been growing concerns regarding the development of endocrine therapy resistance with reports of approximately 30-40% of patients acquiring resistance to endocrine therapy26. Endocrine resistance is defined as progression or relapse of hormone-receptor positive disease during treatment with adjuvant endocrine therapy and may possibly explain the lack of survival benefit in ER+ SRCBC with the use of endocrine therapy 26.
Patients with ER− tumors often receive systemic chemotherapy 23. In this cohort, there were 13 patients with ER− tumors who did not receive chemotherapy; 77% of which had Stage II disease or greater. Previous publications have demonstrated that administration of chemotherapy significantly reduces the risk of recurrence in patients diagnosed with ER negative breast tumors 23. Unfortunately, data on decision-making regarding systemic therapy is not available through the NCDB and thus it is unclear why these patients did not receive chemotherapy. Additionally, data on Her2 status did not begin to enter the NCDB until 2010, and thus most patients did have not have data on Her2 status available.
Like that of other breast cancer subtypes, significant differences in survival outcomes based upon tumor stage were found. The 5-year unadjusted OS rates of SRCBC are overall lower when compared to that of non-triple negative breast cancer patients in the NCDB from 2010-2014: 89.7% vs 91% for Stage I disease, 80.1% vs 87% for Stage II disease, 68.2% vs 75% for Stage III disease, and 26% vs 43% for Stage IV disease 27. Earlier stage patients were also more likely to receive locoregional therapy with surgery and/or radiation and was an expected finding. This analysis found that in patients who underwent breast conserving surgery, increasing stage was associated with lower rates of receiving post-lumpectomy radiation, but may be attributable to the fact that lumpectomy may have only been performed for palliative reasons in Stage IV patients. While patients with stage III disease had lower rates of post-lumpectomy radiation, they did demonstrate the highest rates of post-mastectomy radiation, which would be expected given a larger local tumor burden.
Over a third of the patients within this cohort had Stage IV disease, similar to previous publications which have demonstrated that SRCBC patients have more advanced disease and higher mortality rates compared to other histological breast cancer subtypes 2,3,5,6. A previous retrospective series found disease-specific mortality to be as high as 60% at 7 years for SRCBC 3. When examining Stage I disease only, tumors consisting of 10% or more signet ring cells have been found to exhibit a greater metastatic potential compared to those under 10%6.
The limitations of this analysis include its retrospective nature and possible inconsistencies in data entry into the NCDB. Intrinsic to the NCDB, a lack of disease-specific mortality and disease recurrence data restrict long-term findings. Additionally, patient specific factors such as smoking status and BMI are not available through the dataset and may be associated with outcomes. However, notable strengths of this analysis include that it is the first study demonstrating long-term outcomes for breast SRCBC, utilizes a large national dataset, and includes demographic, tumor, and treatment characteristics.
Future studies on SRCBC should aim to identify if there are other factors that may be better prognostic indicators than hormone-receptor status, such as tumor genomic markers. There are emerging sequencing techniques identifying oncogene driver mutations and chromosomal abnormalities, especially in more aggressive forms of breast cancer which may provide better treatment strategies 28,29. Additionally, the lack of benefit of endocrine therapy seen in this series can provide the basis for future studies to ascertain if this finding persists in other large data sets or in a multi-institutional prospective clinical setting. In patients with ER+ SRCBC who are being treated with endocrine therapy and experiencing moderate side effects, it may be reasonable for providers to discuss the potential lack of benefit of endocrine therapy.
Conclusion:
This study is the largest analysis of patients with SRCBC and provides data on patient characteristics and outcomes. A large portion of patients present with metastatic disease, and while those with ER+ disease have better survival outcomes, treatment with endocrine therapy did not demonstrate a survival benefit in ER+ patients. Future studies should aim to assess these findings amongst other large databases of SRCBC patients and to identify other genomic or clinical characteristics that may offer more accurate prognostic information.
Highlights:
Signet ring cell carcinoma breast carcinoma often presents with advanced disease.
There is a survival benefit for patients who undergo surgery for non-metastatic disease.
Treatment with endocrine therapy for ER+ patients did not demonstrate a survival benefit.
Funding:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interests: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: A Cancer Journal for Clinicians 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Hull MT, Seo IS, Battersby JS, Csicsko JF. Signet-ring Cell Carcinoma of the Breast: A Clinicopathologic Study of 24 Cases. American Journal of Clinical Pathology 1980;73:31–5. [DOI] [PubMed] [Google Scholar]
- 3.Merino MJ, Livolsi VA. Signet ring carcinoma of the female breast: a clinicopathologic analysis of 24 cases. Cancer 1981;48:1830–7. [DOI] [PubMed] [Google Scholar]
- 4.Ellis I, Schnit S, Sastre-Garau X, et al. Tumors of the Breast and Female Genital System. 2003. [Google Scholar]
- 5.Frost AR, Terahata S, Yeh IT, Siegel RS, Overmoyer B, Silverberg SG. The significance of signet ring cells in infiltrating lobular carcinoma of the breast. Arch Pathol Lab Med 1995;119:64–8. [PubMed] [Google Scholar]
- 6.Wu X, Zhang Z, Li X, et al. Poorer Prognosis of Primary Signet-Ring Cell Carcinoma of the Breast Compared with Mucinous Carcinoma. PloS one 2016;11:e0162088–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.About the National Cancer Database. American College of Surgeons website 1996-2020. [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 10.Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004;121:884–92. [DOI] [PubMed] [Google Scholar]
- 11.Min BS, Kim NK, Ko YT, et al. Clinicopathological features of signet-ring cell carcinoma of the colon and rectum: a case-matched study. Hepatogastroenterology 2009;56:984–8. [PubMed] [Google Scholar]
- 12.el-Zimaity HM, Itani K, Graham DY. Early diagnosis of signet ring cell carcinoma of the stomach: role of the Genta stain. J Clin Pathol 1997;50:867–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong FJ, Leong AS, Swift J. Signet-ring carcinoma of the prostate. Pathol Res Pract 1996;192:1232–8; discussion 9-41. [DOI] [PubMed] [Google Scholar]
- 14.Burnett AL, Epstein JI, Marshall FF. Adenocarcinoma of urinary bladder: classification and management. Urology 1991;37:315–21. [DOI] [PubMed] [Google Scholar]
- 15.Karabulut Z, Yildirim Y, Abaci I, Ilgici D, Ozyilkan O. Signet-ring cell carcinoma of the gallbladder: a case report. Adv Ther 2008;25:520–3. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Ma X, Li Y, et al. The characteristics and prognostic effect of E-cadherin expression in colorectal signet ring cell carcinoma. PLoS One 2016;11:e0160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou SH, Ziogas A, Zell JA. Primary signet-ring carcinoma (SRC) of the lung: a population-based epidemiologic study of 262 cases with comparison to adenocarcinoma of the lung. J Thorac Oncol 2010;5:420–7. [DOI] [PubMed] [Google Scholar]
- 18.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637–41; discussion 41-2. [PubMed] [Google Scholar]
- 19.Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 1991;48:28–33. [DOI] [PubMed] [Google Scholar]
- 20.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev 2002;11:601–7. [PubMed] [Google Scholar]
- 21.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 2003;163:49–56. [DOI] [PubMed] [Google Scholar]
- 22.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res Treat 2002;73:45–59. [DOI] [PubMed] [Google Scholar]
- 23.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-Receptor Status and Outcomes of Modern Chemotherapy for Patients With Node-Positive Breast Cancer. JAMA 2006;295:1658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Güth U, Myrick ME, Kilic N, Eppenberger-Castori S, Schmid SM. Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res Treat 2012;131:491–9. [DOI] [PubMed] [Google Scholar]
- 25.Cortina CS, Agarwal S, Mulder LL, et al. Are Providers and Patients Following Hormonal Therapy Guidelines for Patients Over the Age of 70? The Influence of CALGB 9343. Clin Breast Cancer 2018;18:e1289–e92. [DOI] [PubMed] [Google Scholar]
- 26.Anurag M, Ellis MJ, Haricharan S. DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget 2018;9:36252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morena AC, Lin YH, Bedrosian I, et al. Outcomes after Treatmetn of Metaplastic Versus Other Breast Cancer Subtypes. J Cancer 2020; 11(6):1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker-Smith TL, Peck J. Genetic and Genomic Advances in Breast Cancer Diagnosis and Treatment. Nursing for Women’s Health 2019;23:518–25. [DOI] [PubMed] [Google Scholar]
- 29.Natrajan R, Tutt ANJ, Lord CJ. Driver Oncogenes but Not as We Know Them: Targetable Fusion Genes in Breast Cancer. Cancer Discovery 2018;8:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


