Abstract
Objective
We reviewed our institutional data to evaluate toxicity and efficacy outcomes of pembrolizumab/lenvatinib in recurrent endometrial cancer in a “real-world” clinical setting and to compare the impact of reduced lenvatinib starting dose on outcomes.
Methods
Retrospectively, we reviewed toxicity, treatment responses, and survival outcomes of patients with recurrent endometrial cancer who received ≥1 cycle of pembrolizumab/lenvatinib. We compared subgroups based on lenvatinib starting dose (recommended [20 mg] vs reduced [<20 mg]) and histologic type.
Results
We analyzed 70 patients (recommended dose cohort, n = 16; reduced dose cohort, n = 54). The most common starting dose was 14 mg daily. Compared to the reduced dose cohort, the recommended dose cohort had a significantly higher mean number of lenvatinib dose reductions due to side effects (1.1 vs. 0.4; p=0.003) and significantly shorter median time to treatment toxicity (1.3 vs. 3.7 days; p=0.0001). Response rates did not differ significantly between the recommended and reduced dose cohorts (28.6% vs. 38.3%, respectively; p=0.752). Two patients, both in the reduced dose cohort, had complete responses. Patients with carcinosarcoma histology had response and clinical benefit rates of 25% (3 of 12) and 58.3% (7 of 12), respectively. There were no differences between the 2 dose cohorts with respect to progression-free (p=0.245) or overall survival (p=0.858).
Conclusion
In clinical practice, a lower starting dose of lenvatinib (14 mg daily) in combination with pembrolizumab was safe and efficacious in recurrent endometrial cancer. The combination produced responses in endometrial carcinosarcomas. Larger studies are required to validate these findings.
Keywords: endometrial cancer, pembrolizumab, lenvatinib, immunotherapy, toxicity, clinical efficacy
Introduction
Management of recurrent endometrial cancer is challenging as increasing lines of systemic therapy result in poor response rates [2, 3]. Recently, immunotherapeutic regimens have garnered high interest as promising treatment alternatives [4, 5]. Inhibiting the PD-1/PD-L1 pathway, pembrolizumab (an anti-PD-1 monoclonal antibody) has significantly improved response rates in microsatellite instability high (MSI-H) or mismatch match repair deficient (dMMR) solid tumors [6, 7]. In MSI-H endometrial cancer, the objective response rate (ORR) to pembrolizumab monotherapy was 57.1% [4]. With these remarkable response rates, pembrolizumab was given accelerated FDA approval for use in unresectable or metastatic MSI-H or dMMR solid tumors following failed prior systemic treatment in May 2017; this represented the first tissue-agnostic approval.
In contrast, response to pembrolizumab monotherapy has been limited in microsatellite stable (MSS) endometrial cancer (ORR = 13%) [8]. This landscape was significantly changed with the publication of the KEYNOTE-146/Study 111 trial results, which demonstrated that the addition of lenvatinib (multiple tyrosine kinase inhibitor) dramatically increases therapeutic responses (ORR = 37.2%) [5, 9]. Furthermore, pembrolizumab and lenvatinib treatment was observed to also have significant response rates in high-risk histologic subtypes, such as endometrial serous carcinoma (ORR = 40%; 14 of 35) [5]. As a result, in September 2019, the combination of pembrolizumab (200 mg intravenously [IV] every 3 weeks) and lenvatinib (20 mg orally [po] daily) was given accelerated FDA approval for the treatment of non-MSI-H/dMMR advanced endometrial cancer that had progressed on prior systemic therapy. However, despite its clinical efficacy, the combination of pembrolizumab and lenvatinib at the recommended dosage is associated with significant treatment-related toxicity [5]. In KEYNOTE-146/Study 111, grade 3 or 4 treatment-related adverse events occurred in 66.9% of patients, with nearly 1 in 5 patients (22 of 124) discontinuing 1 or both drugs because of toxicity [5]. Of the 22, 19 discontinued because of lenvatinib toxicity. Additionally, there were treatment interruptions in 70.2% of patients and lenvatinib dose reductions in 62.9% [5]. Despite a starting dosage of lenvatinib of 20 mg po daily, the mean dose intensity was 14.4 mg/day, and only 8.9% of patients remained on lenvatinib for ≥6 months without any dose reductions [5].
Following the FDA accelerated approval, gynecologic oncologists at our institution were observing significant treatment-related toxicity in patients with recurrent endometrial cancer receiving the recommended starting lenvatinib dosage. Given this initial experience and the differences between a selected clinical trial population and the corresponding general population encountered in clinical practice, our group felt that starting with a lower dose of lenvatinib might improve the safety and tolerability of this regimen. Accordingly, consideration of starting lenvatinib doses of less than 20 mg was employed at our institution. In this study, we sought to review our institutional data to evaluate the toxicity and clinical efficacy of pembrolizumab and lenvatinib in recurrent endometrial cancer in “real-world” clinical practice. Furthermore, we sought to compare the toxicity and clinical efficacy of the recommended (20 mg) vs reduced (<20 mg) starting dosages of lenvatinib.
Methods
Patient population
In this retrospective cohort study design, all patients with recurrent endometrial cancer who underwent evaluation at The University of Texas MD Anderson Cancer Center from September 2019 to October 2020 for treatment planning with pembrolizumab and lenvatinib were reviewed for inclusion into the study. The study inclusion criteria were the following: age ≥18 years old, histologically confirmed recurrent endometrial cancer, and treatment with at least 1 cycle of pembrolizumab and lenvatinib combination therapy. Patients were excluded from the study if there was loss to follow-up without any documentation of treatment toxicity, treatment response, or survival outcomes. Although all patients underwent initial evaluation and treatment planning at MD Anderson, patients had the option of receiving treatment either at MD Anderson or with their local oncologist. Irrespective of the location of treatment administration, all patients maintained follow-up care with review of on-treatment imaging results and treatment toxicity at MD Anderson. Furthermore, treatment recommendations were formulated by attending MD Anderson gynecologic oncologists. This study was approved by the MD Anderson Institutional Review Board (Protocol 2020–0865) and a waiver of informed consent was obtained for this retrospective review.
Clinical data collection
Clinical data were extracted from electronic medical records and included the following demographic and clinicopathologic information: age, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance status, location of administered treatment, tumor histology, tumor grade, microsatellite instability status, and prior treatment history. Extracted treatment and associated toxicity and efficacy information included the following: number of cycles of pembrolizumab and lenvatinib, lenvatinib starting dosage, on-treatment hospitalizations, treatment discontinuations, lenvatinib dose reductions/interruptions, response to treatment, recurrence, and death. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at MD Anderson [10].
Statistical analysis
Demographic and clinicopathologic characteristics of the study population were summarized with standard descriptive statistics. The primary objective of the study was to evaluate treatment-related toxicity using the following endpoints: on-treatment hospitalization rate, treatment discontinuation rate, lenvatinib dose interruption rate, number of lenvatinib dose reductions, and time to treatment toxicity. Treatment discontinuation was defined as discontinuation of pembrolizumab and/or lenvatinib due to toxicity. Lenvatinib dose interruption was defined as interruption in lenvatinib treatment due to treatment-related toxicity; interruptions due to logistical or administrative reasons were not included. Number of lenvatinib dose reductions was defined as the number of reductions in lenvatinib dose from the initial prescribed dosage per patient. Time to treatment toxicity was defined from start of treatment until lenvatinib dose reduction or treatment discontinuation related to toxicity (whichever occurred sooner). Patients who were lost to follow-up, died, or had disease progression without any lenvatinib dose reduction or treatment discontinuation related to toxicity were censored at their date of last visit, death, or end of treatment (whichever was sooner).
The secondary objectives were treatment response and survival outcomes. Treatment response was evaluated by response rate and clinical benefit rate. Response rate was defined as the number of patients with radiologic response to treatment (best response as partial or complete) divided by the total number of patients with evaluable radiologic response. Clinical benefit rate was defined as the number of patients with radiologic response and stable disease divided by the total number of patients with evaluable radiologic response. Clinical benefit was measured at the first radiologic evaluation for treatment response. Survival outcomes were evaluated by calculating progression-free survival (PFS) and overall survival (OS). PFS was defined from start of treatment until date of disease progression or death. Those alive and without disease progression were censored at their date of last follow-up. OS was defined from start of treatment until date of death. Those alive were censored at their date of last follow-up. The product limit estimator of Kaplan-Meier was used to estimate PFS and OS.
Subgroup analyses were performed for the aforementioned toxicity, response, and survival endpoints based on initial lenvatinib dosage level: recommended (20 mg po daily) vs. reduced (<20 mg po daily). The Student t-test, X2 test, and Fisher exact test were performed where appropriate and a p-value <0.05 was considered statistically significant. All statistical analyses were performed using Stata/MP v16.0 (College Station, TX).
Results
From September 2019 to October 2020, 83 patients with recurrent endometrial cancer were evaluated at MD Anderson for treatment planning for combination therapy with pembrolizumab and lenvatinib (Figure 1). We excluded 13 patients from the study because either treatment was not initiated or insufficient outcome information was available. Thus, 70 patients with recurrent endometrial cancer who received at least 1 cycle of pembrolizumab and lenvatinib were included for study evaluation. The median follow-up was 7 months (range 0.9 – 14.5). Demographic and clinicopathologic characteristics are shown in Table 1. The median age and BMI were 65.5 years and 26.3 kg/m2, respectively. The majority of patients had an ECOG performance status of 0 or 1 (91.3%); 6 patients (8.7%) had a performance status of 2 or 3. The majority of patients had endometrioid (27.1%) or serous (27.1%) histologic subtypes followed by carcinosarcoma (18.6%) and mixed (14.3%) histologic subtypes. The majority of tumors were grade 3 (80%). Most tumors were microsatellite stable (95.6%), and 1 patient had an MSI-H tumor; 2 patients with serous histology did not have prior MSI testing. The patient with the MSI-H tumor had disease progression on 3 prior lines of systemic therapy, including pembrolizumab monotherapy. The median number of prior lines of systemic therapy was 2 (range 1 – 9). For the starting dosage of lenvatinib, 16 patients (22.9%) started at the recommended dosage (20 mg daily) and 54 patients (77.1%) started at a reduced dosage (<20 mg daily). Among the 54 patients with a reduced starting dosage, the most common dosage was 14 mg (n = 45; 83.3%), followed by 10 mg (n = 5; 9.3%), 16 mg (n = 3; 5.6%), and 12 mg (n = 1; 1.9%). There were no significant differences in baseline demographic and clinicopathologic characteristics between patients who received the recommended dosage and those who received the reduced dosage (Table 1). Among all patients, the median number of cycles of treatment with pembrolizumab and lenvatinib was 5 (range 1 – 15). The median numbers of cycles of treatment for the recommended and reduced dose cohorts were 4.5 (range 1 – 12) and 6 (range 1 – 15), respectively.
Figure 1 –
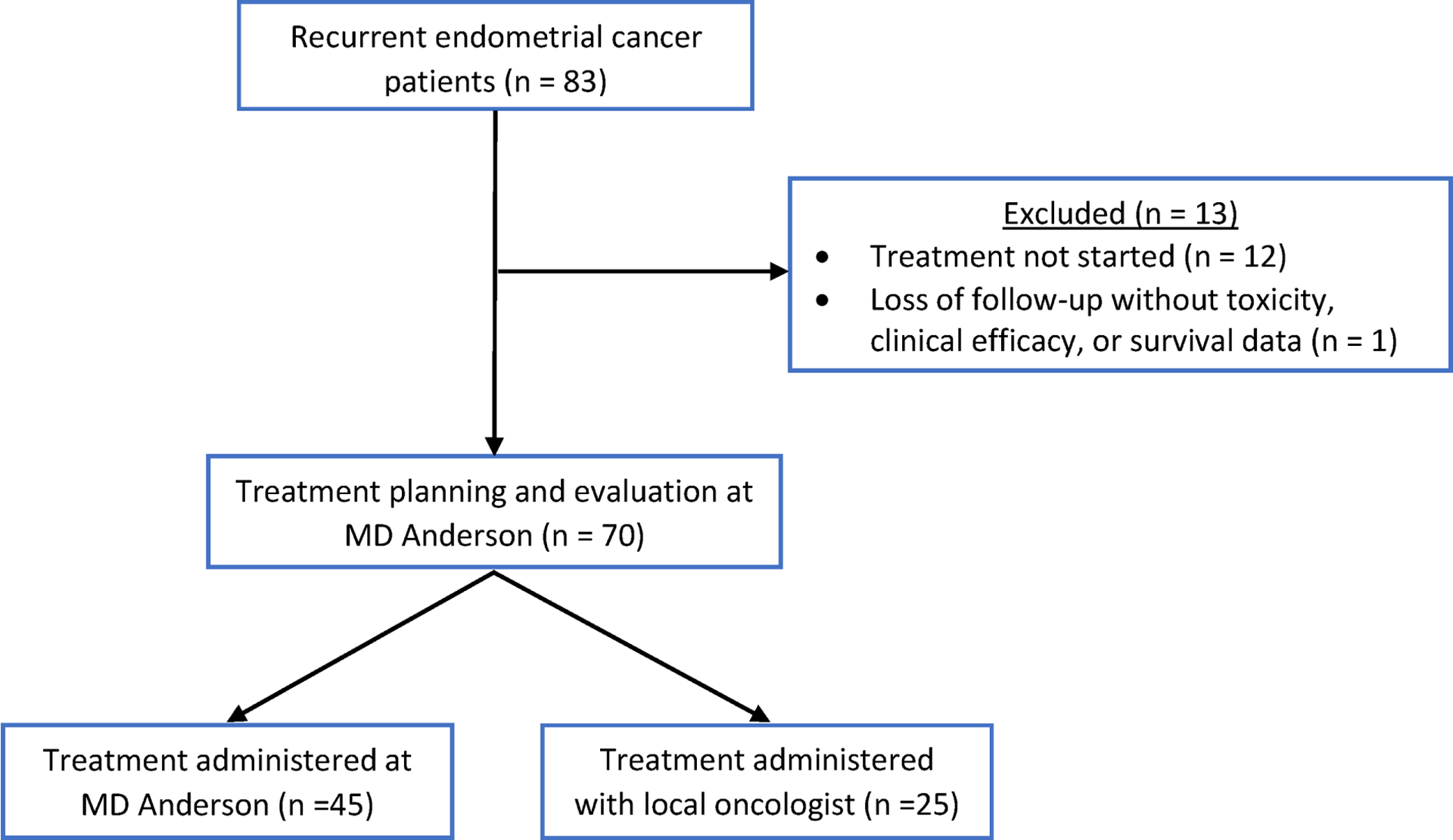
Study cohorts
Table 1 –
Demographic and clinicopathologic characteristics of the whole study population and subgroups based on lenvatinib starting dose level.
| Lenvatinib starting dose | |||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 70) | Recommended dose (n = 16) | Reduced dose (n = 54) | |||||
| Characteristic | N | % | N | % | N | % | p |
| Age (years) | 0.850 | ||||||
| Median (range) | 65.5 (31 – 77) | 64.5 (55 – 77) | 66 (31 – 77) | ||||
| BMI (kg/m2) | 0.261 | ||||||
| Median (range) | 26.3 (16.2 – 43.9) | 24.9 (18.6 – 37.6) | 27.1 (16.2 – 43.9) | ||||
| Location of treatment administration | 0.175 | ||||||
| MD Anderson | 45 | 64.3% | 8 | 50.0% | 37 | 68.5% | |
| Locally | 25 | 35.7% | 8 | 50.0% | 17 | 31.5% | |
| ECOG | 0.161 | ||||||
| 0 | 31 | 44.3% | 5 | 33.3% | 26 | 48.1% | |
| 1 | 32 | 45.7% | 9 | 60.0% | 23 | 42.6% | |
| 2 | 4 | 5.7% | 0 | 0.0% | 4 | 7.4% | |
| 3 | 2 | 2.9% | 1 | 6.7% | 1 | 1.9% | |
| Unknown | 1 | N/A | 1 | N/A | 0 | N/A | |
| Tumor histology | 0.634 | ||||||
| Endometrioid | 19 | 27.1% | 4 | 25.0% | 15 | 27.8% | |
| Serous | 19 | 27.1% | 3 | 18.8% | 16 | 29.6% | |
| Clear cell | 4 | 5.7% | 2 | 12.5% | 2 | 3.7% | |
| Carcinosarcoma | 13 | 18.6% | 3 | 18.8% | 10 | 18.5% | |
| Mixed | 10 | 14.3% | 2 | 12.5% | 8 | 14.8% | |
| Other | 5 | 7.1% | 2 | 12.5% | 3 | 5.6% | |
| Tumor grade | 0.857 | ||||||
| 1 | 4 | 6.2% | 1 | 6.7% | 3 | 6.0% | |
| 2 | 9 | 13.8% | 1 | 6.7% | 8 | 16.0% | |
| 3 | 52 | 80% | 13 | 86.7% | 39 | 78.0% | |
| Unknown | 5 | N/A | 1 | N/A | 4 | N/A | |
| MSI status | >0.999 | ||||||
| MSS | 65 | 95.6% | 16 | 100.0% | 49 | 94.2% | |
| MSI-L | 2 | 2.9% | 0 | 0.0% | 2 | 3.8% | |
| MSI-H | 1 | 1.5% | 0 | 0.0% | 1 | 1.9% | |
| Unknown | 2 | N/A | 0 | N/A | 2 | N/A | |
| Prior lines of systemic therapy | 0.830 | ||||||
| Median (range) | 2 (1 – 9) | 2 (1 – 6) | 2 (1 – 9) | ||||
| Prior immune checkpoint inhibitors | 0.582 | ||||||
| No | 65 | 92.9% | 16 | 100.0% | 49 | 90.7% | |
| Yes | 5 | 7.1% | 0 | 0.0% | 5 | 9.3% | |
BMI = body mass index. ECOG = Eastern Cooperative Oncology Group. MSI = microsatellite instability. MSI-H = microsatellite instability high. MSI-L = microsatellite instability low. MSS = microsatellite stable.
Toxicity
Details of toxicity associated with pembrolizumab and lenvatinib therapy are shown in Table 2. Overall, more than half (54.3%) of patients experienced at least 1 hospitalization while on treatment, with 32.9% of patients hospitalized because of treatment-related toxicity. The rates of treatment discontinuation due to toxicity and of lenvatinib dose interruption were 38.6% and 82.9% of patients, respectively. Comparing the recommended and reduced dose cohorts, there were no significant differences in rates of hospitalization due to any cause (50.0% vs. 55.6%, respectively; p = 0.695) or due to treatment-related toxicity (25.0% vs. 35.2%, p = 0.553). The rates of treatment discontinuation due to toxicity (50.0% vs. 35.2%, respectively; p = 0.285) and lenvatinib dose interruption (93.8% vs. 79.6%; respectively; p = 0.272) were similar between cohorts. Compared to the reduced dose cohort, the recommended dose cohort had significantly more dose reductions (p = 0.016) and higher mean number of lenvatinib dose reductions (1.1 vs. 0.4 dose reductions, p = 0.003). Evaluating the indications for lenvatinib dose reductions, patients in the recommended dose cohort had significantly more intolerable fatigue (37.5% vs. 14.8%, p=0.046), anorexia (25.0% vs. 5.6%, p=0.043), gastrointestinal toxicity (43.8% vs. 11.1%, p=0.003), and hematologic toxicity (18.8% vs. 1.9%, p=0.035) compared to the reduced dose cohort. Furthermore, the median time to treatment toxicity was significantly shorter in the recommended dose cohort (1.3 months vs. 3.7 months, p = 0.0001) (Figure 2).
Table 2 –
Treatment toxicity during pembrolizumab and lenvatinib therapy for the whole study population and by lenvatinib dose subgroups.
| Lenvatinib starting dose | |||||||
|---|---|---|---|---|---|---|---|
| Recommended dose (n = 16) | Reduced dose (n = 54) | ||||||
| Overall (n = 70) | |||||||
| Toxicity endpoint | N | % | N | % | N | % | p |
| Any hospitalization | 0.695 | ||||||
| No | 32 | 45.7% | 8 | 50.0% | 24 | 44.4% | |
| Yes | 38 | 54.3% | 8 | 50.0% | 30 | 55.6% | |
| Hospitalization related to toxicity | 0.553 | ||||||
| No | 47 | 67.1% | 12 | 75.0% | 35 | 64.8% | |
| Yes | 23 | 32.9% | 4 | 25.0% | 19 | 35.2% | |
| Treatment discontinuation due to toxicity | 0.285 | ||||||
| No | 43 | 61.4% | 8 | 50.0% | 35 | 64.8% | |
| Yes | 27 | 38.6% | 8 | 50.0% | 19 | 35.2% | |
| Lenvatinib dose interruption | 0.272 | ||||||
| No | 12 | 17.1% | 1 | 6.2% | 11 | 20.4% | |
| Yes | 58 | 82.9% | 15 | 93.8% | 43 | 79.6% | |
| Number of lenvatinib dose reductions | 0.016 | ||||||
| 0 | 40 | 57.1% | 6 | 37.5% | 34 | 63.0% | |
| 1 | 22 | 31.4% | 5 | 31.3% | 17 | 31.4% | |
| 2 | 6 | 8.6% | 3 | 18.8% | 3 | 5.6% | |
| 3 | 2 | 2.9% | 2 | 12.5% | 0 | 0.0% | |
| Cause of lenvatinib dose reduction* | |||||||
| Fatigue | 14 | 20.0% | 6 | 37.5% | 8 | 14.8% | 0.046 |
| Anorexia | 7 | 10.0% | 4 | 25.0% | 3 | 5.6% | 0.043 |
| Gastrointestinal | 13 | 18.6% | 7 | 43.8% | 6 | 11.1% | 0.003 |
| Hematologic | 4 | 5.7% | 3 | 18.8% | 1 | 1.9% | 0.035 |
| Hypertension | 4 | 5.7% | 1 | 6.3% | 3 | 5.6% | NP |
| Cardiac | 1 | 1.4% | 0 | 0.0% | 1 | 1.9% | NP |
| Renal | 1 | 1.4% | 0 | 0.0% | 1 | 1.9% | NP |
| Musculoskeletal | 2 | 2.9% | 0 | 0.0% | 2 | 3.7% | NP |
| Dermatologic | 2 | 2.9% | 0 | 0.0% | 2 | 3.7% | NP |
Causes of dose reductions may be multifactorial, and the reported percentages are out of each cohort. NP = not performed due to small numbers.
Figure 2 –
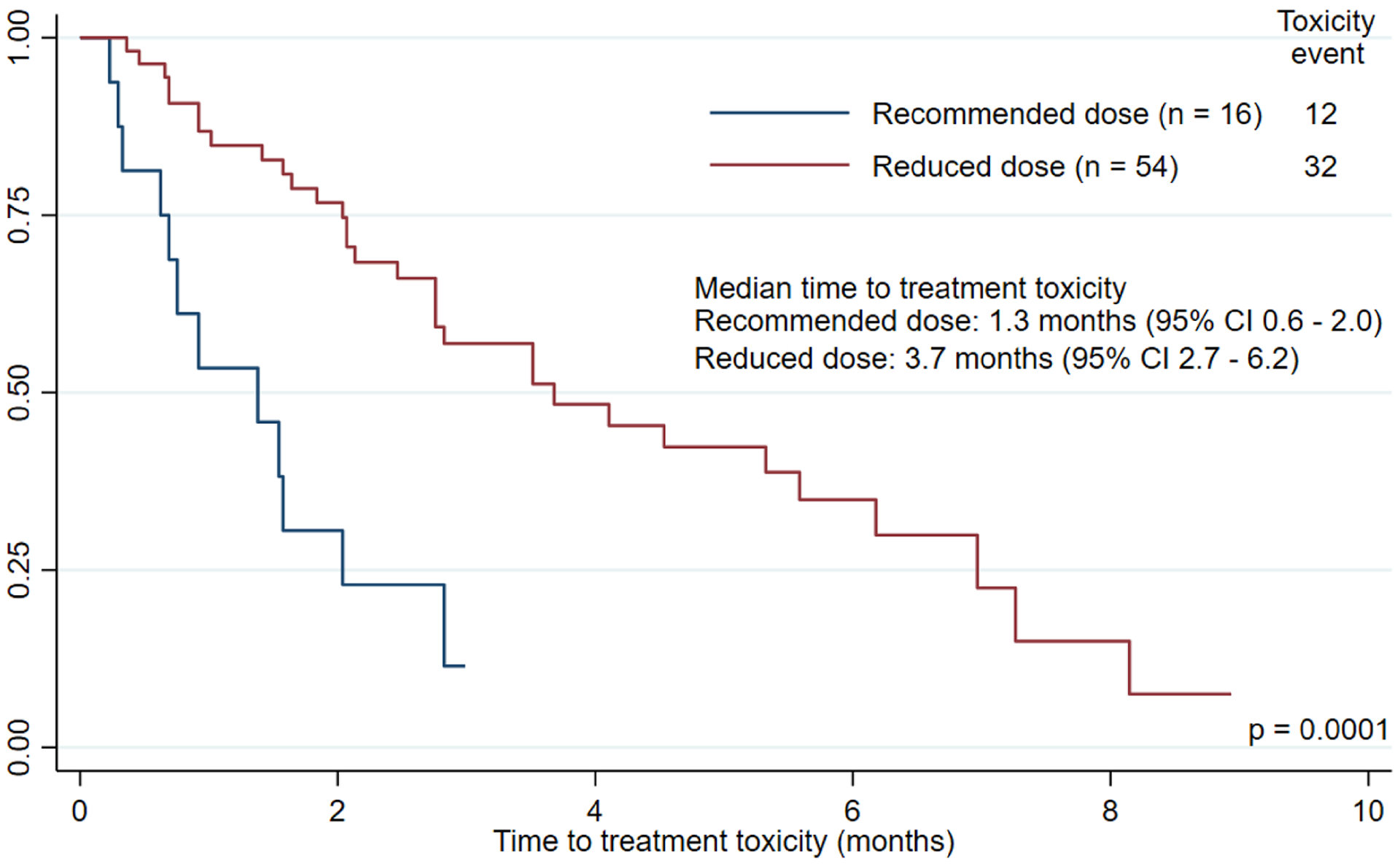
Kaplan-Meier curve for time to treatment toxicity by lenvatinib starting dose level
Clinical efficacy and survival
Of the 70 patients, 61 were evaluable for treatment response (Table 3). Overall, 20 patients had a partial response, 2 patients had a complete response, and 20 patients had stable disease; this resulted in a response rate of 36.1% (22 of 61) and clinical benefit rate of 68.9% (42 of 61). The recommended and reduced dosage cohorts did not significantly differ in response (28.6% vs. 38.3%, respectively; p = 0.752) or clinical benefit (57.1% vs. 72.3%, respectively; p = 0.224) rates (Table 3A). There was no difference in duration of response to treatment between cohorts (p = 0.280). The median durations of response in the recommended and reduced dose cohorts were 2.1 months (95% CI 1.5 – not reached) and 6.3 months (95% CI 2.8 – not reached), respectively. There was no difference in duration of clinical benefit between cohorts (p = 0.520). The median durations of clinical benefit in the recommended and reduced dose cohorts were 3.6 months (95% CI 0.7 – not reached) and 4.4 months (95% CI 3.4 – 7.8), respectively. Response to treatment by histologic subtype is shown in Table 3B. There was no significance difference in response rates (p = 0.476) nor clinical benefit rates (p = 0.451) between histologic subtypes. All 5 of 17 (29.4%) patients with serous histology who responded to treatment were in the reduced dose cohort; this also included 2 patients with MSS tumors who had complete responses. Of note among patients with endometrioid histology in the reduced dose cohort, one patient with a grade 2 MSS tumor had a near complete response and one patient with an MSI-H tumor (previously progressed on single-agent pembrolizumab) had a partial response to therapy. There were 12 patients with carcinosarcoma histology with evaluable responses. Of these 12 patients, 3 had partial responses, 4 had stable disease, and 5 had disease progression, leading to an overall response and clinical benefit rates of 25.0% and 58.3%, respectively.
Table 3 –
Response and clinical benefit rates among the 61 patients with evaluable responses by lenvatinib starting dose level and by histologic type.
| A) | |||||||
|---|---|---|---|---|---|---|---|
| Lenvatinib starting dose | |||||||
| Overall (n = 61) | Recommended dose (n = 14) | Reduced dose (n = 47) | |||||
| Outcome | N | % | N | % | N | % | p |
| Best response | 0.816 | ||||||
| Partial response | 20 | 32.8% | 4 | 28.6% | 16 | 34.0% | |
| Complete response | 2 | 3.3% | 0 | 0.0% | 2 | 4.3% | |
| Stable disease | 20 | 32.8% | 4 | 28.6% | 16 | 34.0% | |
| Progressive disease | 19 | 31.1% | 6 | 42.9% | 13 | 27.7% | |
| Response rate | 22 | 36.1% | 4 | 28.6% | 18 | 38.3% | 0.752 |
| Clinical benefit rate | 42 | 68.9% | 8 | 57.1% | 34 | 72.3% | 0.224 |
| B) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histologic type* | |||||||||||
| Endometrioid (n = 18) | Serous (n = 17) | Clear cell (n = 3) | Carcinosarcoma (n = 12) | Mixed (n = 7) | |||||||
| Outcome | N | % | N | % | N | % | N | % | N | % | p |
| Best response | 0.519 | ||||||||||
| Partial response | 8 | 44.4% | 3 | 17.6% | 0 | 0.0% | 3 | 25.0% | 4 | 57.1% | |
| Complete response | 0 | 0.0% | 2 | 11.8% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Stable disease | 4 | 22.2% | 7 | 41.2% | 1 | 33.3% | 4 | 33.3% | 2 | 28.6% | |
| Progressive disease | 6 | 33.3% | 5 | 29.4% | 2 | 66.7% | 5 | 41.7% | 1 | 14.3% | |
| Response rate | 8 | 44.4% | 5 | 29.4% | 0 | 0.0% | 3 | 25.0% | 4 | 57.1% | 0.476 |
| Clinical benefit rate | 12 | 66.7% | 12 | 70.6% | 1 | 33.3% | 7 | 58.3% | 6 | 85.7% | 0.451 |
Response rates for “other” histologic subtype are not shown.
Overall, there were 41 disease progressions and 33 deaths, with a median follow-up of 7 months (range 0.9 – 14.5). The median PFS was 4.6 months (95% CI 3.7 – 6.4) and median OS was 8.6 months (95% CI 7.6 – 12.2). Figure 3 demonstrates the Kaplan-Meier curves for PFS and OS based on the dosage cohorts. PFS did not significantly differ between cohorts (p = 0.245); median PFS durations were 3.2 months (95% CI 2.3 – 6.4) in the recommended dose cohort and 5.5 months (95% CI 3.8 – 6.6) in the reduced dose cohort. Similarly, OS did not significantly differ between cohorts (p = 0.858), with median OS durations were 8.6 months (95% CI 5.0 – NR) in the recommended dose cohort and 9.4 months (95% CI 7.6 – 12.2) in the reduced dose cohort.
Figure 3.
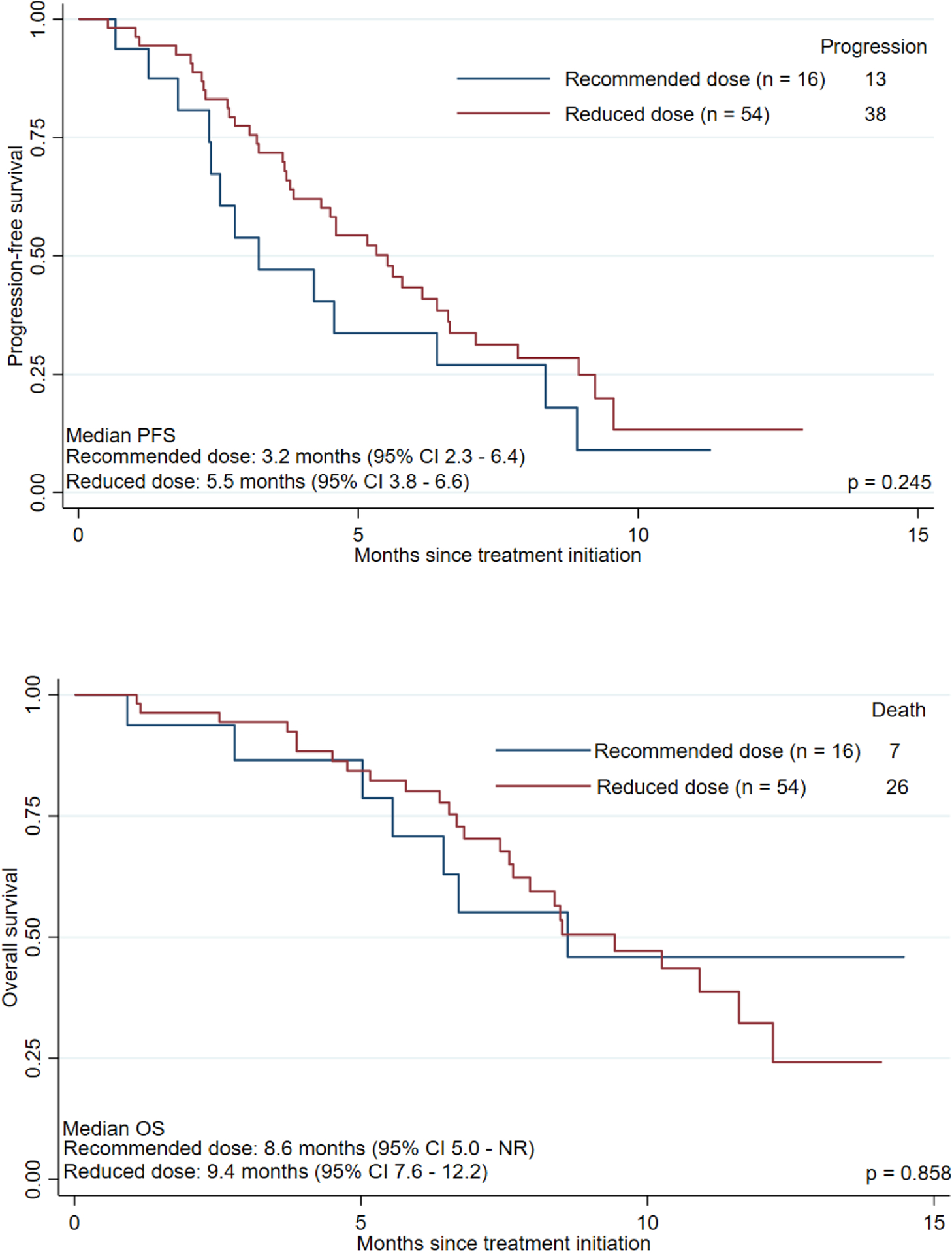
Kaplan-Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS) based on lenvatinib starting dosage. NR = not reached. 95% CI = 95% confidence interval.
Discussion
KEYNOTE-146/Study 111 was a landmark study that demonstrated promising results for pembrolizumab and lenvatinib as an alternative treatment option for advanced and recurrent endometrial cancer in a predominantly MSS tumor population, the combination was associated with significant toxicity [5, 9]. Not surprisingly, therapy-related toxicities can be even more significant in clinical practice, where patient selection is not as uniform or stringent as in a clinical trial setting. Our experience with the recommended 20 mg daily lenvatinib dose led us to empirically reduce the starting dose of lenvatinib (most commonly to 14 mg). In this retrospective review, we did not observe differences in hospitalization, treatment discontinuation, or lenvatinib interruption rates between dosage cohorts, but the recommended dose group had a significantly higher mean number of lenvatinib dose reductions. Specifically, the recommended dose cohort had significantly more lenvatinib dose reductions attributable to intolerable fatigue-related, anorexia-related, gastrointestinal, and hematologic toxicity. Additionally, the recommended dose cohort had a significantly shorter median time to treatment toxicity (1.3 months vs. 3.7 months, p = 0.0001).
The more favorable treatment-associated toxicity profile observed in the reduced dose cohort did not appear to come at the expense of clinical efficacy and survival outcomes. Response and clinical benefit rates were similar between the 2 cohorts. Furthermore, the 2 patients who had a complete response had MSS serous tumors and were treated with a starting lenvatinib dose of 14 mg. Overall, there was no difference in treatment response between histologic subtypes. PFS and OS were similar between both dosage cohorts.
Optimal management of patients with advanced/recurrent endometrial cancer who have progressed on a platinum-taxane regimen is challenging and requires careful consideration of risks and benefits. In this setting, single-agent pegylated liposomal doxorubicin had limited efficacy (ORR 9.5% and median duration of response 2.7 months) [11]. Single-agent bevacizumab had an ORR of 13.5% and median PFS of 4.2 months [12]. In combination with temsirolimus, bevacizumab had an ORR of 24.5% with a median PFS of 5.6 months but had significant toxicity, including a 5.7% (3 of 53) rate of mortality possibly related to treatment [13]. As a second-line option, single-agent paclitaxel had a response rate of 25% and median duration of response of 4.2 months in paclitaxel-naïve patients [14]. However, in a trial in which a majority of patients (94%) had received prior paclitaxel therapy, ixabepilone (which, like paclitaxel, is a mitotic microtubule stabilizer) had an ORR of 12% and median PFS of 2.9 months in the second-line setting [15]. Although single-agent pembrolizumab or lenvatinib produce limited response rates (13% and 14.3%, respectively), KEYNOTE-146/Study 111 showed that combining these drugs could have a synergistic effect on response rate (ORR 37.2%) [5, 8, 9, 16].
Comparing our present study to KEYNOTE-146/Study 111, there are notable differences in treatment and study population [5, 9]. First, only 22.9% of patients in the present study were started on a lenvatinib starting dosage of 20 mg, compared to 100% of patients in KEYNOTE-146/Study 111. As discussed earlier, this difference reflects a rapid shift in physician prescribing patterns to a reduced dose given observed treatment-related toxicity with the 20 mg dosage at our institution. Second, our study had a lower proportion of patients with endometrioid tumors (27.1% vs. 50.9%). Third, the present study included a higher proportion of patients with ≥2 prior lines of systemic therapy (62.9% vs. 47.2%) and grade 3 tumors (74.3% vs. 61.1%). Lastly, the present study included patients with ECOG performance status ≥2 (8.6%) and carcinosarcoma histology (18.6%), who would have been excluded in the KEYNOTE-146/Study 111 trial. To our knowledge, this is the first report of responses in patients with endometrial carcinosarcoma from combination therapy (response rate 25% and clinical benefit rate 58.3%). Thus, this regimen offers another potential therapeutic option for patients with this aggressive and poor prognostic endometrial cancer subtype.
Despite the differences above, it is notable that response rates in the reduced dose cohort in the present study and the previously treated population in KEYNOTE-146/Study 111 were quite similar (38.3% and 37.2%, respectively). Notably, the lenvatinib 14 mg dosage level was the predominant starting dosage in the reduced dose cohort (45 of 54; 83.3%) in our study. Interestingly, the investigators of the KEYNOTE-146/Study 111 trial reported a lenvatinib mean dose intensity of 14.4 mg/day in that trial [5]. Furthermore, in our study, the 2 patients with complete responses and 1 patient with a near complete response from treatment were started on lenvatinib 14 mg. It should be noted that none of the patients in this study had dose escalation of lenvatinib, even if starting at a reduced dosage level. Clinicians should weigh the potential risks and benefits of the intensity of starting lenvatinib dosage levels on clinical outcomes. First, there is worry that starting at a reduced dosage of lenvatinib may have lower anti-tumor efficacy. Additionally, worse prognostic features in the current study population (e.g. smaller proportion of endometrioid histology, higher proportion of grade 3 tumors, higher proportion of ≥2 lines of systemic therapy, and inclusion of ECOG ≥2 and carcinosarcoma histology) may not fully explain differences in overall median PFS (4.6 vs 7.4 months, respectively) and OS (8.6 vs 16.7 months, respectively) observed between the current study and KEYNOTE-146/Study 111[5]. This is a legitimate concern that may not be fully answered without prospective, randomized control data. It should be noted that lenvatinib has demonstrated anti-tumor activity in other solid tumors at dosage levels lower than 14 mg (e.g. FDA approved in hepatocellular carcinoma for 8 – 12 mg daily) [17]. Although some clinicians may choose to begin patients on the recommended lenvatinib dosage level then subsequently dose reduce if necessary, there should be awareness of the considerable rates of dose reduction and interruption rates reported in prospective trials of this regimen in a highly selected clinical trial eligible population with a good performance status. Patients who discontinue this therapy or who experience multiple or prolonged interruptions, will lose the opportunity to potentially benefit from the synergistic anti-tumor activity of pembrolizumab and lenvatinib. Thus, the decision regarding the starting dose has to be individualized by patient and practitioner, and our study suggests that starting lenvatinib at 14 mg may be an acceptable alternative until further prospective data is obtained.
Determining effective strategies of implementing the regimen of pembrolizumab and lenvatinib is important given the preliminary findings of Study 309/KEYNOTE-775 presented by Makker and colleagues at the Society of Gynecologic Oncology 2021 Annual Meeting on Women’s Cancer[18]. In this phase III randomized control trial, advanced/recurrent endometrial cancer patients with progression on prior platinum-based systemic therapy were randomized to either pembrolizumab and lenvatinib (n=411) vs. physician’s choice chemotherapy (single-agent doxorubicin or paclitaxel) (n=416)[18]. Compared to physician’s choice chemotherapy, pembrolizumab and lenvatinb had improved PFS (HR 0.56, 95% CI 0.48–0.66, p<0.0001) and OS (HR 0.62, 95% CI 0.51 – 0.75, p<0.0001)[18]. As we await the publication of Study 309/KEYNOTE-775, the preliminary results support pembrolizumab and lenvatinib as the next standard of care regimen for recurrent endometrial cancer that has progressed on prior systemic therapy[18]. However, toxicity of the recommended 20 mg starting dose of lenvatinib may still pose challenges for tolerability as the preliminary results of the trial demonstrated grade ≥3 treatment-related adverse events in 88.9% of patients and lenvatinib dose reductions in 66.5% [18].
Our study has several limitations. Due to the retrospective nature of this study design, there is a higher chance that the full spectrum of toxicity data may not be captured. Given this limitation, we placed emphasis on more objective, clinically significant toxicity outcomes (e.g. hospitalization, treatment discontinuation due to toxicity, lenvatinib dose interruption/reductions, and time to treatment toxicity). Additionally, this retrospective study design may increase risk for selection bias. Although selection bias is possible, this risk is low given that the prescribing patterns shifted heavily towards reduced lenvatinib starting doses (regardless of baseline patient characteristics) following the observation that the recommended 20 mg dosage level may be intolerable to patients. This shift in prescribing pattern explains the relatively small number of patients treated with the recommended dose compared to the reduced dose. Furthermore, there were no significant differences in baseline characteristics between the dose cohorts. Lastly, the small sample size may not have been powered to detect a significant difference in rates of hospitalization, treatment discontinuation due to toxicity, or lenvatinib dose interruption. Nonetheless, the significantly higher number of dose reductions and shorter time to treatment toxicity illustrates the severity of side effects experienced by patients receiving recommended doses of lenvatinib compared to reduced doses.
In conclusion, this study is the first to evaluate the real-world clinical application of the pembrolizumab and lenvatinib regimen for the treatment of recurrent endometrial cancer, including the impact of a reduced starting lenvatinib dosage level on toxicity, response rates, and survival. A reduced starting dosage of lenvatinib was associated with better toxicity profiles and tolerability compared to the FDA approved 20 mg dosage level while maintaining similar efficacy outcomes. Furthermore, patients with carcinosarcoma tumors appeared to benefit from pembrolizumab and lenvatinib treatment. Although our study findings highlight the use of a starting dosage of lenvatinib 14 mg daily as a potential combination pembrolizumab/lenvatinib strategy for endometrial cancer in clinical practice, larger prospective studies are needed to validate these safety and efficacy findings.
Highlights.
Recommended 20 mg lenvatinib dosage may be challenging for endometrial cancer patients to tolerate in clinical practice.
Compared to the recommended dose, a reduced lenvatinib starting dose had similar response rates in endometrial cancer.
Compared to the recommended dose, a reduced lenvatinib starting dose had less toxicity with similar survival outcomes.
For endometrial carcinosarcoma (not included in KEYNOTE-146/Study 111), the overall response rate was 25%.
Acknowledgements
The preliminary report of this research was presented at a scientific plenary session at the Society of Gynecologic Oncology 2021 Annual Meeting on Women’s Cancer [1]. This research was supported in part by the National Institutes of Health T32 training grant (T32 CA101642) (JAH) and The University of Texas MD Anderson Cancer Center Support Grant (P30CA016672), which supports the Biostatistics Resource Group, Tissue Biospecimen and Pathology Resource, and Clinical Trials Office. Furthermore, this study was supported by the uterine SPORE grant (CA098258) (SNW, PTS, KHL, and AAJ) and the Dr. Henry R. Shibata Fellowship Award/Cedars Cancer Foundation (JAH). These aforementioned funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. We thank Sunita Patterson, Senior Scientific Editor, Research Medical Library at MD Anderson, for editing this article.
Conflicts of interest statement
Jeffrey A. How reports grants from the NIH (T32CA101642) and Cedars Cancer Foundation during the conduct of the study.
Bryan Fellman reports grants from the NIH (P30CA016672).
Patrick Hwu reports conflicts of interest outside submitted work (scientific advisory board member on Immatics and Dragonfly).
Shannon N. Westin reports grants from NIH, GOG Foundation, Continga Pharmaceuticals, Bayer, and ArQule during the conduct of the study. She also reports grants and personal fees from AstraZeneca, Clovis Oncology, GlaxoSmithKline/Tesaro, Roche/Genentech, and Novartis. She reports personal fees from Merck, Pfizer, Eisai, personal CIrculogene, Zentalis, and Agenus outside the submitted work.
Nicole D. Fleming reports personal fees from Tesaro, Pfizer, Bristol Myers Squibb, and GlaxoSmithKline outside the submitted work.
Karen H. Lu, Lois M. Ramondetta, Pamela T. Soliman, and Shrina Patel report no conflicts of interest.
Amir A. Jazaeri reports personal fees from Gerson and Lehrman Group, Guidepoint, Iovance Advisory Board Meeting, NuProbe, Simcere, Pact Pharma, Genentech-Roche, Eisai, Agenus, and Macrogenics. He also reports grants from AstraZeneca, Bristol Myers Squibb, Iovance, Aravive, Pfizer, Immatics USA, Eli Lilly, Merck, and stock/stock options from AvengeBio outside submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].How JA, Patel S, Fellman B, Lu KH, Ramondetta LM, Westin SN, et al. The use of pembrolizumab and lenvatinib combination therapy in endometrial cancer: an examination of toxicity and treatment efficacy in clinical practice Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. Virtual: Gynecologic Oncology; March 19 – 25, 2021. [Google Scholar]
- [2].Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: a review of North American trials. Expert Rev Anticancer Ther. 2009;9:905–16. [DOI] [PubMed] [Google Scholar]
- [3].Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117:373–81. [DOI] [PubMed] [Google Scholar]
- [4].Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of Clinical Oncology. 2019;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020:Jco1902627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY). 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35:2535–41. [DOI] [PubMed] [Google Scholar]
- [9].Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360–4. [DOI] [PubMed] [Google Scholar]
- [12].Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:22–7. [DOI] [PubMed] [Google Scholar]
- [14].Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:277–81. [DOI] [PubMed] [Google Scholar]
- [15].Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol. 2009;27:3104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vergote I, Powell MA, Teneriello MG, Miller DS, Garcia AA, Mikheeva ON, et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol. 2020;156:575–82. [DOI] [PubMed] [Google Scholar]
- [17].Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. [DOI] [PubMed] [Google Scholar]
- [18].Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. A multicenter, open label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer: Study 309/KEYNOTE 775. Society of Gynecology Annual Conference on Women’s Cancer. Virtual: Gynecologic Oncology; 2021. [Google Scholar]


