Abstract
Sedentary behavior (SB) has recently been recognized as a strong risk factor for cardiovascular disease, with new guidelines encouraging adults to „sit less, move more.‟ Yet, there are few randomized trials demonstrating that reducing SB improves cardiovascular health. The Effect of Reducing Sedentary Behavior on Blood Pressure (RESET BP) randomized clinical trial addresses this gap by testing the effect of a 3-month SB reduction intervention on resting systolic BP. Secondary outcomes include other BP measures, pulse wave velocity, plasma renin activity and aldosterone, and objectively-measured SB (via thigh-mounted activPAL) and physical activity (via waist-worn GT3X accelerometer). RESET BP has a targeted recruitment of 300 adults with desk jobs, along with elevated, non-medicated BP (systolic BP 120–159 mmHg or diastolic BP 80–99 mmHg) and physical inactivity (self-reported aerobic physical activity below recommended levels). The multi-component intervention promotes 2–4 fewer hours of SB per day by replacing sitting with standing and light-intensity movement breaks. Participants assigned to the intervention condition receive a sit-stand desk attachment, a wrist-worn activity prompter, behavioral counseling every two weeks (alternating in-person and phone), and twice-weekly automated text messages. Herein, we review the study rationale, describe and evaluate recruitment strategies based on enrollment to date, and detail the intervention and assessment protocols. We also document our mid-trial adaptations to participant recruitment, intervention deployment, and outcome assessments due to the intervening COVID-19 pandemic. Our research methods, experiences to date, and COVID-specific accommodations could inform other research studying BP and hypertension or targeting working populations, including those seeking remote methods.
1. Introduction
Hypertension is the most common modifiable risk factor for cardiovascular disease (CVD).[1] According to the 2017 Blood Pressure Guidelines,[2] nearly half of American adults have prevalent hypertension defined as systolic blood pressure (BP) ≥130 mmHg, diastolic BP ≥80 mmHg, or use of antihypertensive medications. An additional 12% have elevated BP defined as non-medicated systolic BP 120–129 mmHg with diastolic BP <80 mmHg.[3] For elevated BP and some patients with a new diagnosis of hypertension and low CVD risk, lifestyle treatment is recommended prior to prescription of antihypertensive medications.[2]
Sedentary behavior (SB), defined as low intensity behavior while awake in a seated, reclining, or lying posture,[4] has gained attention as a highly prevalent behavior, distinct from moderate-to-vigorous intensity physical activity (MVPA).[5] Accumulating epidemiological evidence suggests that higher levels of SB are associated with higher BP,[6–9] arterial stiffness,[10–12] CVD,[13] and mortality.[14, 15] The 2018 Physical Activity Guidelines Advisory Committee graded the evidence that SB was associated with mortality and CVD as ‘strong’[16] and added a nonquantitative recommendation to ‘sit less and move more’ to the 2018 federal Physical Activity Guidelines.[17] In 2020, Canada released 24-hour Movement Guidelines recommending that adults break up and limit SB to <8 hours per day.[18] Importantly, the adverse effects of SB appear to be more deleterious in populations who do not achieve recommended levels of MVPA.[19] These data, coupled with Americans spending 57% of the waking day in SB,[20] suggest that SB reduction – particularly among inactive adults – could be an additional lifestyle treatment target for high BP.
Yet, there is a dearth of sufficiently-powered, randomized clinical trials (RCT) examining whether SB reduction leads to health benefits, including reduced BP.[21–25] Laboratory crossover studies have shown that interrupting or replacing SB with light-intensity physical activity (i.e., standing or walking) acutely reduces BP.[26–30] Mechanistic studies have begun to explore how prolonged sitting impairs cardiovascular function including hemodynamic, hormonal and sympathetic effects.[28, 30–32] However, chronic effects of sustained SB reduction on BP remain unclear. In addition, whether SB reduction improves other markers of CVD risk, such as 24-hour ambulatory BP and carotid-femoral pulse wave velocity [cfPWV], is unknown [33–36].
Thus, the Effect of Reducing Sedentary Behavior on Blood Pressure (RESET BP) study, funded by the National Heart, Lung, and Blood Institute (R01HL134809), seeks to examine whether reducing SB can lower BP and improve cardiovascular health. RESET BP is a 3-month randomized clinical trial with a proposed sample of 300 inactive desk workers with non-medicated, elevated BP or hypertension that randomizes participants to either a multicomponent SB reduction intervention or a passive control group. The 3-month intervention, including behavioral counseling every 2 weeks, a sit-stand desk attachment, a wrist-worn activity prompter, and text messages, intends to reduce prolonged SB through standing and light intensity movement breaks. The primary outcome is resting systolic BP. Secondary outcomes include diastolic BP, 24-hour ambulatory BP, and cfPWV. The renin-angiotensin-aldosterone system (RAAS) will be evaluated as a potential mediating mechanism. Objective monitoring of SB, standing, and movement will allow for examination of dose-response associations with outcomes.
2. Objectives
The RESET BP study specific aims are to:
evaluate the efficacy of the intervention targeting decreased SB over 3 months on systolic BP (primary), diastolic BP, 24-hour ambulatory BP, and cfPWV;
explore whether RAAS activation (plasma renin activity (PRA) and aldosterone) partially mediates changes in BP elicited by SB reduction;
examine associations between achieved reductions in SB, increases in standing and light physical activity, and BP reduction; and
evaluate the effect of the SB reduction intervention on other cardiometabolic risk factors that may improve and are related to BP including body weight, glucose, and insulin in an exploratory manner.
3. Methods
3.1. Study design overview
Given the current scientific equipoise regarding the effect of reducing SB on BP, a 2-arm, 3-month randomized clinical trial was chosen as the most robust design to establish initial efficacy (see Figure 1). Assessments occur at 0 and 3 months and are conducted by blinded assessors. Because the intervention follow-up period is only 3 months, specific time windows were defined for assessment of eligibility criteria (≤30 days before randomization), time to intervention initiation (≤2 weeks after randomization), and to complete follow-up assessments (91–101 days after randomization). While intervention participants begin the protocol within 2 weeks of randomization; control participants do not receive any intervention during the 3-month follow-up interval. As remuneration, intervention participants are given the choice to keep their sit-stand desk attachment or return it and receive $200 after completing all follow-up assessments. Control participants are given the choice to receive a delayed intervention (sit-stand desk attachment + behavioral lessons) or receive $200 after completing all follow-up assessments. All participants are given a wrist-worn activity prompter to keep either during (intervention) or following (controls) completion of study follow-up assessments. RESET BP is registered on clinicaltrials.gov (NCT03307343).
Figure 1.
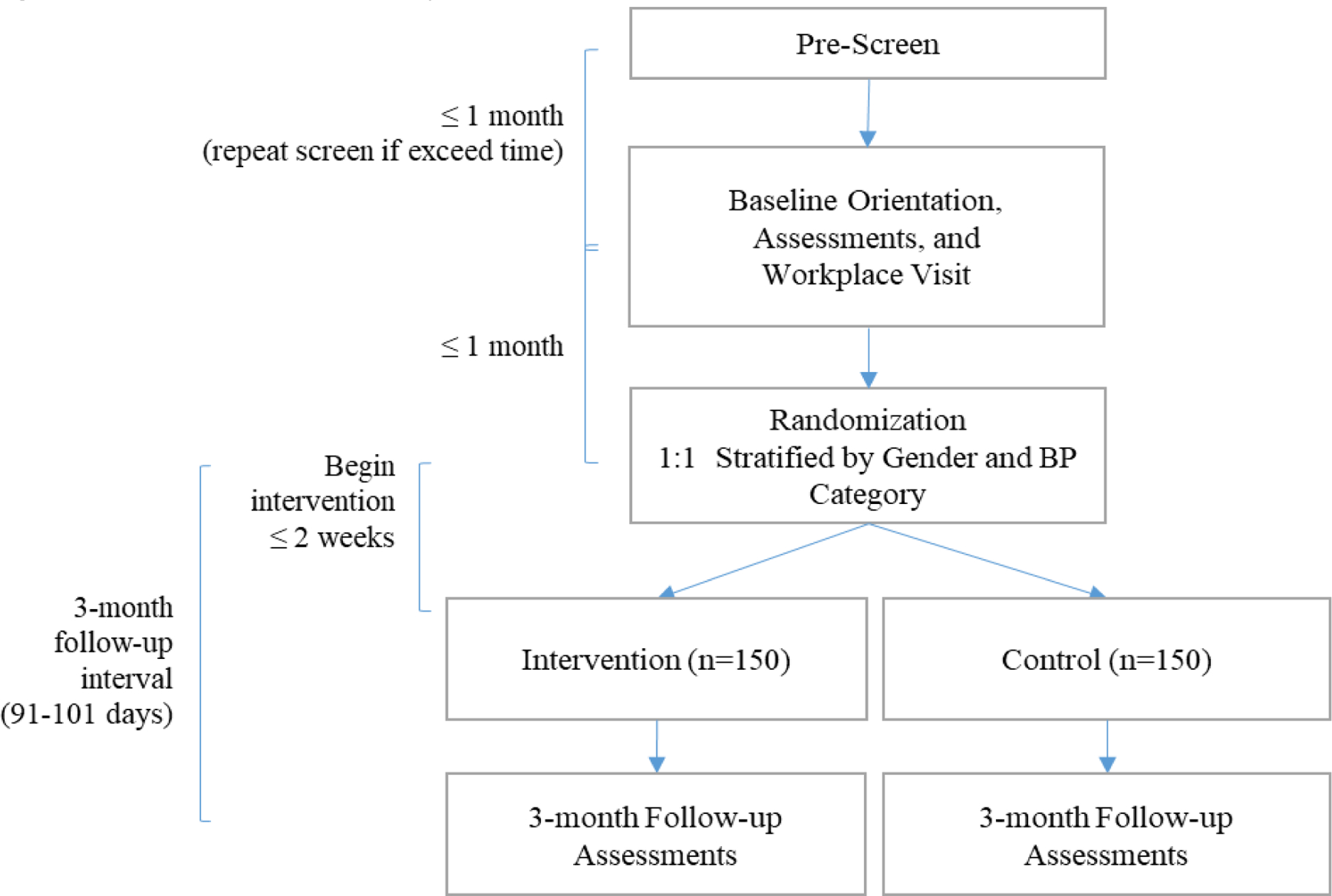
Flowchart of study visits
3.2. Inclusion/exclusion criteria
Eligibility criteria for RESET BP are listed in Table 1. We recruit individuals with elevated or high BP who would be recommended for treatment with lifestyle as first-line therapy.[2] Participants are required to obtain medical clearance from their primary care provider or physician to join the study during screening; the purpose of this clearance is to inform the physician of the participant’s screening BP readings and ensure appropriateness for their patient to spend the next three months without antihypertensive medication use. Co-investigator study physicians deemed the three-month follow-up as a reasonable amount of time to try lifestyle-only treatment without initiating pharmacotherapy in this low-risk population, based on clinical treatment guidelines and typical clinical follow-up intervals for elevated or stage 1 BP.[2] Any use of BP or diabetes medication is exclusionary due to the potential influence on study outcomes. Certain criteria were selected to provide a participant group with structured sedentary time that our intervention could effectively modify and for which preliminary recommendations on dose of SB reduction were available.[37] These include desk job with stable employment, supervisor consent to participate, no physical limitation to reducing SB, not already using a sit-stand workstation or activity prompter, and limited planned absences from work during the study period. Finally, we only include inactive participants with self-reported MVPA below current recommendations since the epidemiologic evidence suggests that the deleterious effects of SB are more apparent among inactive adults.[16, 19]
Table 1:
Eligibility criteria for study participation
| Inclusion criteria | |
| Age | 21–65 years |
| Elevated or high BP | Resting systolic 120–159 mmHg or diastolic 80–99 mmHg |
| Inactive lifestyle | Engages in less than 150 minutes per week of moderate + 2x vigorous intensity physical activity by self-report |
| Desk worker | Currently perform deskwork for ≥ 20 hours per week |
| Office location | Employment within an approximate 25-mile radius |
| Stable employment | ≥ 3 months in current job, plan to be in current job for the next 3 months |
| Supervisor approval | Supervisor permission to join the intervention on provided consent form |
| Cell phone | Possession of a cellular phone able to receive text messages |
| Exclusion criteria | |
| BP indicating need for medication | Resting systolic BP ≥ 160 mmHg or diastolic BP ≥ 100 mmHg |
| Medications | Antihypertensive or glucose-controlling medications |
| Comorbid conditions | Conditions that would limit ability to reduce SB (e.g., musculoskeletal condition, current chemotherapy) |
| Cardiovascular disease | History of ischemic heart disease, chronic heart failure, stroke, or chronic kidney disease |
| No medical provider clearance | Unable to provide written consent from primary care provider or physician to participate |
| Other exclusions | Current use of sit-stand or standing desk, SB prompting device, enrollment in a weight loss or exercise study program, recent (< 1 year) or planned bariatric surgery |
| Pregnancy status | Currently pregnant or pregnant in the last 6 months; breastfeeding currently or in the last 3 months |
| Inadequate availability | Plans to be away from desk for an extended period (>1 week) during the study period (e.g., for a prolonged vacation or planned surgery) |
3.3. Recruitment and screening
3.3.1. Recruitment methods
We target recruitment of a representative sample of participants working in the Greater Pittsburgh area, within an approximately 25-mile radius of the main campus of the University of Pittsburgh. We use a variety of referral sources to meet enrollment targets. Flyers are posted in public spaces (such as university buildings, hospitals, coffee shops, and libraries), on public transit (e.g., buses and trolleys), on electronic message boards (such as LinkedIn, Facebook, and Craig’s list), and in magazines and newspapers. We also use the University of Pittsburgh Clinical Translational Science Institute’s Pitt+Me recruitment registry, send electronic flyers through the University of Pittsburgh’s Read Green e-mail system, and the University of Pittsburgh Medical Center’s (UPMC) online newsletter. We send postcards or paper/electronic recruitment letters to members of other affiliated research registries, to local businesses and organizations willing to advertise our study to their employees, and to the general public within a 25-mile range of the university campus using a directed mailing strategy that targets households with members in the eligible age strata. We attend events such as health fairs and give lay research presentations for local businesses or groups in the Pittsburgh area to identify eligible candidates. In addition, we partner with individual UPMC primary care physician practices to recruit appropriate patients for our study. Following agreement of the practice physicians to partner with our study, patients can learn about participating in the study by i) seeing posted advertisements in the waiting room or exam room areas; ii) having their doctor directly refer them to the study; or iii) receiving a descriptive letter about the study, cosigned by the primary care practice. To facilitate the “letter” process, we additionally collaborate with the University of Pittsburgh Health Records Research Request (R3) to identify appropriate primary care patients who meet certain eligibility criteria (age, recent BP measurement in range, and not currently using antihypertensive and/or glucose lowering medications). Lastly, we encourage enrolled participants or even individuals that are ineligible during screening to share our study by providing electronic or paper flyers to distribute. Advertisement materials provide a phone number, e-mail, and link to our study-specific website (www.sit-less.pitt.edu). Referral inquiries are then received via e-mail, phone calls, from a Qualtrics online survey available through our website, or via the Pitt+Me Research Registry.
3.3.2. Screening and orientation
Initial study eligibility is determined by trained research staff after a referred candidate has completed a screening survey, either online or by phone. The screening survey includes a detailed summary description of the study, a request for consent to complete the screening survey, and specific questions about the candidate’s current medications, medical history, exercise habits, work environment, demographics, and contact information. Self-reported responses to eligibility questions are reviewed by study staff and in consultation with the study investigators, as needed. If a candidate reports information that deems them ineligible, they are told the reason at that time.
Candidates who are determined to be initially eligible by research staff during the self-report screening process are invited to attend an in-person orientation, including an informed consent process, review of self-reported eligibility criteria, and assessment of additional eligibility criteria (i.e., BP as described below). The average of two baseline BPs determines the participant’s final eligibility.
3.4. Assessment visits
Assessments occur at baseline and 3 months and are conducted by trained, blinded study personnel (Table 2).
Table 2.
Detailed summary of data collection at study visits
| Eligibility & Baseline | 3-Month Follow-Up | ||||
|---|---|---|---|---|---|
| Assessment Measures | Orientation & Baseline Assess 1 | Baseline Assess 2 | Follow-Up Assess 1 | Follow-up Assess 2 | |
| Written informed consent | x | Randomization – Intervention or Control | |||
| Resting BP (average of two readings) | x | x | x | x | |
| Demographics, medication use & history | x | ||||
| 24-hour ambulatory BP | x | x | |||
| cfPWV | x | x | |||
| Fasting bloodwork (plasma renin activity, aldosterone, glucose, insulin) | x | x | |||
| Self-reported and objective SB and physical activity | x | x | |||
| Anthropometry | x | x | |||
| Dietary intake | x | x | |||
| Adverse events | x | ||||
| Contamination | x | ||||
Resting BP
Resting BP is measured at baseline and 3-month follow-up using a protocol based on published recommendations for accurate BP measurement (Figure 2).[38–40] At each assessment timepoint, BP is measured twice (≥ 1 day apart) on each of two occasions (four total readings), as follows: following verbally-confirmed 8-hour abstention from food, caffeine, and nicotine and 24-hour abstention from MVPA and alcohol; between 6:00–11:00 AM; using a validated oscillometric device (HEM-907 XL Omron Healthcare, Lake Forest, IL)[41]; following a 10-minute quiet rest[42] with arm supported at chest level and feet supported; and using an appropriately sized cuff where the bladder encircles 80% of the arm circumference (as recommended by the American Heart Association).[38, 39] Initially, BP is taken on both arms and the arm with the higher systolic BP is used thereafter for the remainder of the trial.[40] Two measures are taken, with a 1-minute rest between, and averaged. If systolic BP differs by ≥10 mmHg or diastolic BP by ≥6 mmHg, a third measurement is taken and included in the average. Staff completed training, undergo regular quality assurance, and follow guided assessment checklists to facilitate per protocol measurements.
Figure 2. BP Measurement Protocol.
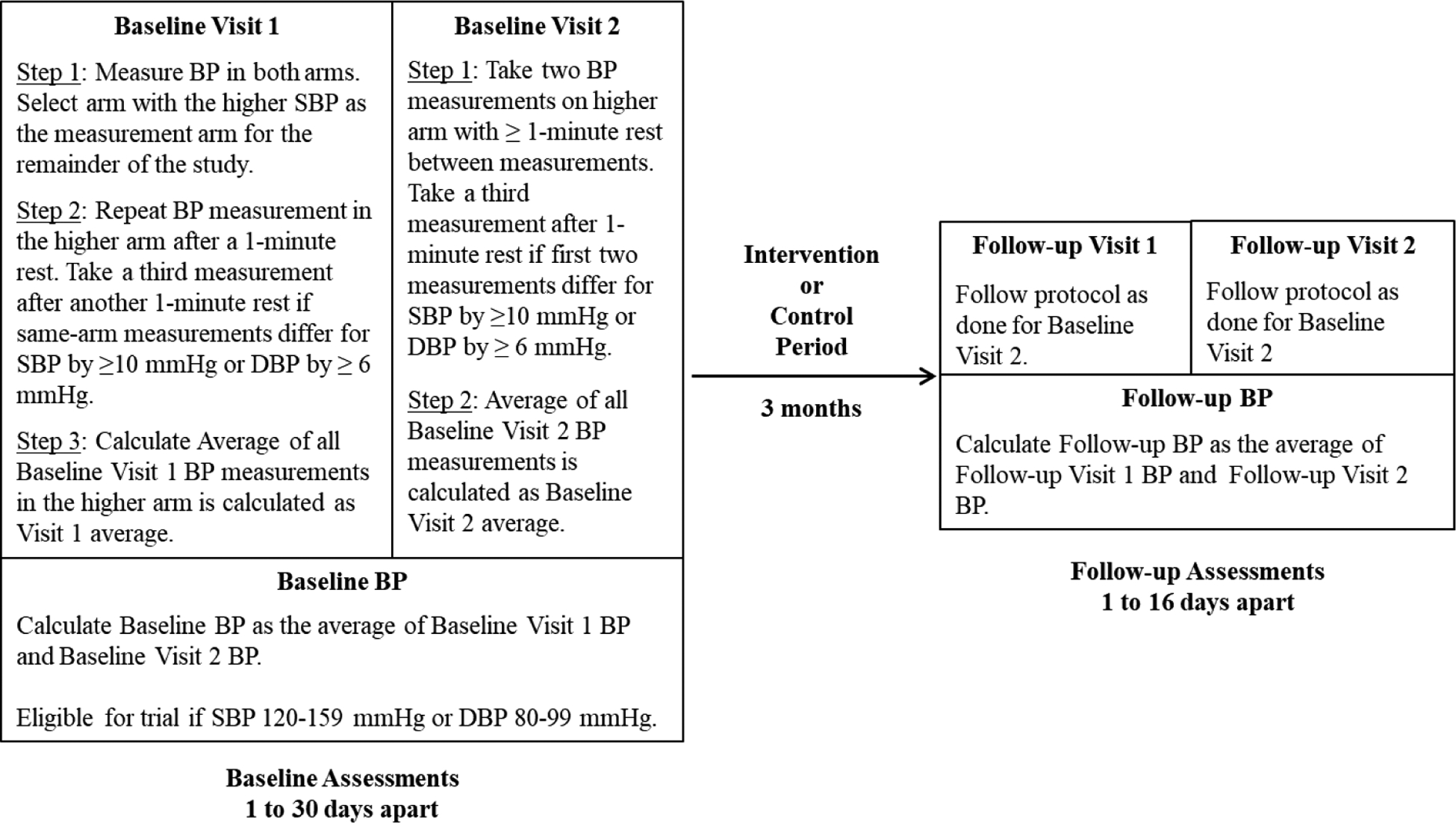
All measurements occur in the morning (6:00 AM – 11:00 AM), using an HEM-907 XL oscillometric BP monitor and an appropriately sized cuff, and following a 10-minute seated rest with proper arm positioning, back supported, and feet flat, a verbally confirmed 8-hour abstention from food, caffeine and nicotine, and a 24-hour abstention from MVPA and alcohol.
24-hour ambulatory
24-hour ambulatory BP is measured using the Oscar 2 24-hour BP monitor (Suntech Medical, Morrisville, NC) on the non-dominant arm with appropriate cuff size based on arm circumference. Participants are provided general instructions to wear the monitor continuously for 25 hours, including while sleeping.[43] Using each participant’s report of their anticipated bedtime and awakening, the monitor is programed to record a BP every 30 minutes while awake and every 60 minutes at night. Data editing follows notable error codes (e.g., “artifact/erratic oscillotertic signal”) for physiologic BP readings. Using daytime/awake and nocturnal/asleep periods from the participant diary, daytime and nocturnal BP are determined. Because objectively-measured activity from the 15 minutes prior and ambulatory BP are directly related,[44] concurrently-measured posture (activPAL) and activity (GT3X) in the 15 minutes prior to ambulatory BP will be used to further classify daytime ambulatory BP for analysis. These include i) seated ambulatory BP (prior 15 minutes all sitting with < 100 cpm) or ii) non-seated BP (any standing, stepping, or ≥ 100 cpm in the 15 minutes prior).
cfPWV
cfPWV is measured following the same pre-visit instruction for measurement of BP. Pulse pressure waveforms are captured from the right carotid and femoral arteries using tonometry after 10 minutes of supine rest. Sensor output is processed by the Complior Analyse® (ALAM Medical, France) based on current recommendations.[45] Three runs capturing 10 waveforms each are averaged. Using our protocol, our laboratory has excellent inter- and intra-technician ICCs of 0.91 and 0.94–0.98, respectively.
Fasting blood sample collection
Fasting blood sample collection occurs following an 8-hour fast and after participants complete ≥ 30 minutes of seated assessments (e.g., BP, questionnaires) to limit the influence of posture on plasma renin activity and aldosterone. One tube of plasma and serum are collected, processed in a centrifuge, and pipetted into 2 ml cryovials for storage in a −80°C freezer until future analysis for plasma renin activity, aldosterone, glucose, and insulin. Samples are stored to be run simultaneously to reduce inter-batch variability. Plasma and serum are also stored for potential future analyses.
SB and physical activity
SB and physical activity are self-reported using the Paffenbarger Physical Activity Questionnaire[46] and the Sedentary Behavior Questionnaire.[47]
Objective SB and MVPA are measured by two monitors, the activPAL3 micro (PAL Technologies, LTD, Glasgow, Scotland) and the Actigraph GT3X accelerometer (Actigraph, LLC, Pensacola, FL). Both are necessary as these devices are, respectively, best practice methodology for assessing SB (activPAL3) and MVPA (GT3X).[48, 49] Participants are instructed to wear both monitors for 9 days on the thigh (activPAL3, 24-hour wear protocol) and hip (GT3X, waking wear protocol). The first two days of monitoring are used in conjunction with the ambulatory BP data to account for concurrent posture and activity during waking 24-hour BP monitoring. Days 3–9 (7-day period after the ambulatory BP monitoring is completed) are used to measure usual SB and physical activity. During the wear period, participants complete a diary to report work, non-work, sleep, and non-wear periods that are used in data processing described below. SB and activity data are considered valid if ≥ 4 days with ≥ 10 hours of waking wear time are captured.[50, 51]
For activPAL3, 24-hour event data are downloaded, exported, and cleaned (removing non-wear and sleep) using established methods for quantifying time spent in SB, standing, or stepping, as well as steps per day, sit-stand transitions, and periods of prolonged sitting (e.g., ≥30 min).[52, 53] These outcomes are averaged across valid days,[51] both overall and during reported working hours.
GT3X data are reintegrated into 60-second epochs using ActiLife software. Periods of nonwear are removed using the Choi algorithm,[54] after which MVPA is quantified using Freedson vector magnitude cut points.[55] In addition, daily minutes of bouted MVPA (≥10 minutes with allowance for 2 minutes below the cut point) will be quantified.[50] Daily estimates of total and bouted MVPA are then averaged across valid days.[51]
Anthropometry:
Height is measured at baseline only by stadiometer as the average of two measures within 0.5 cm. Weight is assessed at baseline and follow-up using a calibrated, Tanita digital scale as the average of two measures within 0.1 kg.
Medical history and medication use
Medical history and medication use are assessed at baseline during screening using a standardized form. At the 3-month follow-up, an interval medical history form captures any changes to medical history or medication (start, stop, or change in dosage) that have occurred since the time of randomization.
Diet
Diet is measured as a covariate, as no dietary intervention is provided by the study. Dietary habits are assessed using the Diet Screener Questionnaire (DSQ).[56]
Adverse events
Adverse events are captured prospectively as well as systematically at 3-month follow-up during the interval medical history (see above). These two methods are used because the increased contact frequency with intervention participants could result in increased reporting in that group. Any new or worsening medical conditions reported by the participant trigger the completion of an adverse event form. Details of the adverse event are collected and then the adverse event is classified with respect to severity and relationship to the intervention by blinded study personnel.
Contamination
Contamination is measured in all participants during the follow-up assessment. Control participants are asked not to begin using a sit-stand desk or wrist prompter during the study, and these components are offered as remuneration at the end of the follow-up to discourage use by participants in the control group. The contamination questionnaire assesses whether participants are exposed to each component of the intervention, externally from the RESET BP research (e.g., co-worker or spouse participating in the study, purchase or receipt of an activity-prompting wearable device).
3.5. Intervention
Several considerations informed our intervention design. First, at the time we began, quantitative guidelines for reducing SB were not available. We synthesized the available evidence that greater SB, and in particular prolonged SB, was associated with adverse cardiovascular health [10–12, 16, 26–29] with an expert statement recommending that desk-based workers should avoid prolonged postures (either sitting or standing) and replace 2–4 hours of SB per day with standing and activity.[37] Combining these, we set behavioral targets to i) replace 2–4 hours of SB per day with standing and light-intensity activity, and ii) reduce periods of prolonged SB (i.e., >60 min).
To achieve this large reduction in SB, a behavior that is ubiquitous, habitual, and often environmentally-determined,[57] we use an evidence-based, multi-component intervention strategy across two levels of the socioecological model (see details in Table 3). The approach includes behavioral strategies (self-monitoring, goal setting, problem solving),[58] environment modification (sit-stand desk attachment),[59] and proximal (fitbit Flex 2) and distal (text messages) external prompts.[60] The overall behavioral target of reducing SB is separated into replacement of SB with standing (2–4 hours per day) and the addition of light-intensity movement breaks to interrupt periods of prolonged SB (4–8 per day). Participants are encouraged to accumulate these targets across the day so as to be consistent with ergonomic recommendations to alter posture frequently and to reduce prolonged SB.[37]
Table 3.
Intervention components and description
| Component | Socio-ecological level | Description |
|---|---|---|
Sit-stand desk
|
Environment | Research staff install a desktop sit-stand device, typically a Quickstand Eco (Humanscale, New York, NY) or a Work-Fit T (Ergotron, Saint Paul, MN). Other sit-stand options are provided based on physical limitations of individual workstations or company policies. This component supports the RESET BP standing goals to alter posture frequently and to stand for 2–4 hours per workday (i.e., 15–30 minutes per hour across an 8-hour workday). |
Wrist prompter
|
Individual | Participants are instructed to wear a wrist prompter (Flex 2, fitbit, San Francisco, CA) during waking hours. The prompter is set to vibrate hourly if the participant is inactive as a proximal, external prompt to break up prolonged SB. The inactivity alert is set to be active during a participant-defined period that includes the workday (e.g., 8:00 AM – 8:00 PM). Participants are instructed to respond to an inactivity prompt by taking a movement break, e.g., a 2- to 3-minute walking break or another light-intensity activity. This component supports the movement break goal of 4–8 per workday (i.e., a movement break every 1–2 hours). Participants are instructed that these movement breaks should be in addition to activity in their typical day. To limit the influence on other health behaviors from the fitbit smartphone interface (e.g., MVPA, diet, or sleep), the monitor is activated by study staff using a sham ID. Participants are instructed to limit use to the inactivity prompt and not to use the smartphone interface during the study. |
Behavioral counselling
|
Individual | Participants engage in 6 contacts over the 3-month study period. Lessons are delivered by an interventionist, trained by the Principal Investigator, and with expertise in exercise physiology and health behavior change. The interventionist uses motivational intervening-informed problem solving to choose strategies for SB reduction and self-monitoring, set goals for standing and movement breaks, and overcome barriers. See Table 4 for lesson schedule, content, and details. |
Self-monitoring
|
Individual | Participants are provided with a paper tracking diary for daily standing and movement. They are instructed to self-monitor to facilitate behavior change and for accurate reporting to the interventionist during contacts. Participants are also allowed to choose an alternative self-monitoring method, e.g., a smartphone app. |
Text messaging
|
Individual | Participants are sent a brief, automated text message twice per week as a distal prompt to encourage maintenance of their SB reduction behavior change. The short messages target motivation, education, engagement, and reinforcement. |
The schedule and content of the behavioral lessons is described in Table 4. Facilitated by the interventionist, participants set initial goals at the baseline visit (e.g., stand for 1–2 hours per day and take 2–3 additional movement breaks per day). Goals are advanced every other week at intervention contacts to reach the study targets. Fidelity of the intervention is assessed at each intervention contact by measuring delivery (provision of intervention components), receipt (self-report of components working properly and goal setting), and enactment (self-report of self-monitoring and goal achievement).[61]
Table 4.
Schedule and description of RESET BP behavioral intervention content
| Week | Delivery | Location | Duration | Behavioral lesson content |
|---|---|---|---|---|
| 0 | In-person | Participant’s office | 90 min |
|
| 2 | Phone | - | 15 min |
|
| 4 | In-person | Research center | 40 min |
|
| 6 | Phone | - | 15 min |
|
| 8 | In-person | Research center | 40 min |
|
| 10 | Phone | - | 15 min |
|
3.6. Randomization
Participants are randomized in a 1:1 ratio to intervention and control groups using random block sizes chosen from small even numbers. The exact block sizes and probability for each block size will be revealed at study completion. The randomization scheme is stratified by participant gender and BP stage (elevated/stage 1/stage 2) to ensure a balance between the two arms of these crucial factors by design rather than chance.
3.7. Statistical considerations
3.7.1. Analysis plan
Overview:
We will perform main analyses with an intention-to-treat basis. Participants who initiate antihypertensive medication after randomization will be treated as drop-outs for the assessments that take place after medication initiation. We believe this is a conservative approach based on the plausibility that, if any, the greater number of such participants would likely be in the control group. If we were to include their BP outcomes without medication, the intervention effects would even be larger than that observed under this strategy. We will compare the baseline measures between arms using independent samples t-, Wilcoxon rank sum, chi-square or Fisher’s exact tests, as appropriate. Any found to be different will be used as additional covariates in sensitivity analyses. We will use multiple imputation to account for missing data, including those treated as missing due to medication initiation.[62, 63]
Aim 1:
Hypotheses are about intervention efficacy, aimed at demonstrating greater 3-month improvements in the intervention group compared to the controls. We will fit a series of analysis of covariance models with baseline to 3-month change in each continuous outcome measured once per assessment (resting BP, cfPWV) as the dependent variable, intervention arm as the only factor of interest, and baseline value of the outcome as a covariate. For continuous outcomes measured multiple times per assessment (nocturnal and seated ambulatory BP), we will fit a series of linear mixed models with each 3-month measurement as dependent variable, intervention arm as the fixed effect of interest, average baseline measurement and time of day [63] as fixed effect covariates, and a banded correlation structure. Non-seated ambulatory BP will analyzed similarly, but will include measures of posture, physical activity [64], and proximity to activity as additional fixed effect covariates. Statistical significance of the between-arm comparisons at α=0.05 will serve as the formal tests of the Aim 1 hypotheses.
Aim 2:
We will perform an exploratory mediation analysis beginning with simpler crude approaches, employing increasingly complex approaches and basing findings on a model a sufficiently complex model to explain the phenomenon. We will add the change in RAAS measures as additional fixed effects in the Aim 1 statistical models, and note the absolute and relative reductions in the intervention effect to quantify the role that RAAS plays using the naïve causal steps approach. We will employ the Preacher and Hayes multiple mediation approach to more formally examine the said role as well as individual relative contributions of PRA and adelsterone to the mediating role [65]. For a more nuanced exploration incorporating covariates, interaction effects among covariates, treatment and mediators, and potential nonlinearities, we will employ VanderWeele’s counterfactual framework but with PRA and adelsterone individually as simple mediators [66]. While exposure-outcome confounding is mitigated due to randomization, any unbalanced covariates will be incorporated. We will be able to appropriately partition the total intervention effect to controlled/natural direct/indirect effects via each measure of RAAS activation, and any differences in mediating role based on assigned intervention. Mediator-outcome covariates are likely not known and/or measured and will be a limitation.
Aim 3:
To examine how changes in sedentary behavior and physical activity measures are associated with changes in outcomes, we will compute correlation coefficients between 3-month changes in those measures and changes in outcome variables measured once per time point. For those measured multiple times per time point (e.g., ambulatory BP), we will consider both averaging by time point before computing correlations and linear mixed modeling strategies similar to those in Aim 1, which can take into account both the correlation among multiple measurements and dependence on time of day, posture, physical activity and/or proximity to physical activity prior to measurement. We will analyze both with and without stratification by arm.
Aim 4: Exploratory continuous outcomes
Exploratory continuous outcomes (i.e., adiposity and HOMA) will be analyzed similarly using the methods described for Aim 1 and 3.
Sensitivity analyses
Sensitivity analyses will involve adjusting for additional baseline covariates significantly different between groups, repeating analyses excluding participants reporting contamination, and including those initiating antihypertensive medication (if any).
3.7.2. Sample size justification
We based our sample size on prior pilot data from our RiSE [67] and RiSE@Work studies and other sources,[68] and published statistical methods [69–72] implemented in commercially available statistical software (PASS 2012®, Number Cruncher Statistical Systems, LLC, Kaysville, UT). We estimated that baseline and change in the primary outcome systolic BP will have standard deviations of 10 and 11 mmHg, respectively. With 300 participants randomized in equal proportions, and with anticipated 240 completers based on an allowance for up to a 20% attrition rate over 3 months, we are able to detect statistical significance of a between-arm difference as small as 4 mmHg in systolic BP change with 80% statistical power in a two-tailed test at α=0.05. At approximately 60% recruitment, our current attrition rate is <2%. We chose 4 mmHg as the most conservative estimate from our prior pilot data, which ranged from 4–6 mmHg. We also note this is a clinically meaningful change in systolic BP, corresponding to a Cohen’s d=0.40 (small-moderate effect size), that studies of aerobic exercise training have observed similar effects [73, 74], and that lifestyle intervention trials achieving similar reductions in systolic BP among samples with elevated-to-stage 1 hypertension have reduced the incidence of progression to stage 2 hypertension by up to 50%.[75] Thus, the sample size affords adequate statistical sensitivity for the primary hypothesis and also will be able to detect similar small-to-moderate effect sizes for secondary outcomes. Among intervention participants, we will be able to detect a correlation as small as 0.25 between changes in activity measures and change in outcomes.
4. Current state of the study and lessons learned
4.1. Recruitment
4.1.1. Recruitment yield
Of recruitment sources reported in Figure 3, utilized from October 2017 – December 2020, e-mail advertisements (e.g., University of Pittsburgh employee e-mails, primary care physician-cosigned letters to patients, business partners), printed postcards mailed to home addresses, and advertisements sent through the university-sponsored research registry (CTSI Pitt+Me) yielded a consistent flow of candidates. Sources generating fewer referrals included community tabling eventslay presentations, advertisements on public transit, direct referrals from friends and colleagues, posted flyers in and around the university campus, and direct primary care physician referrals.
Figure 3.
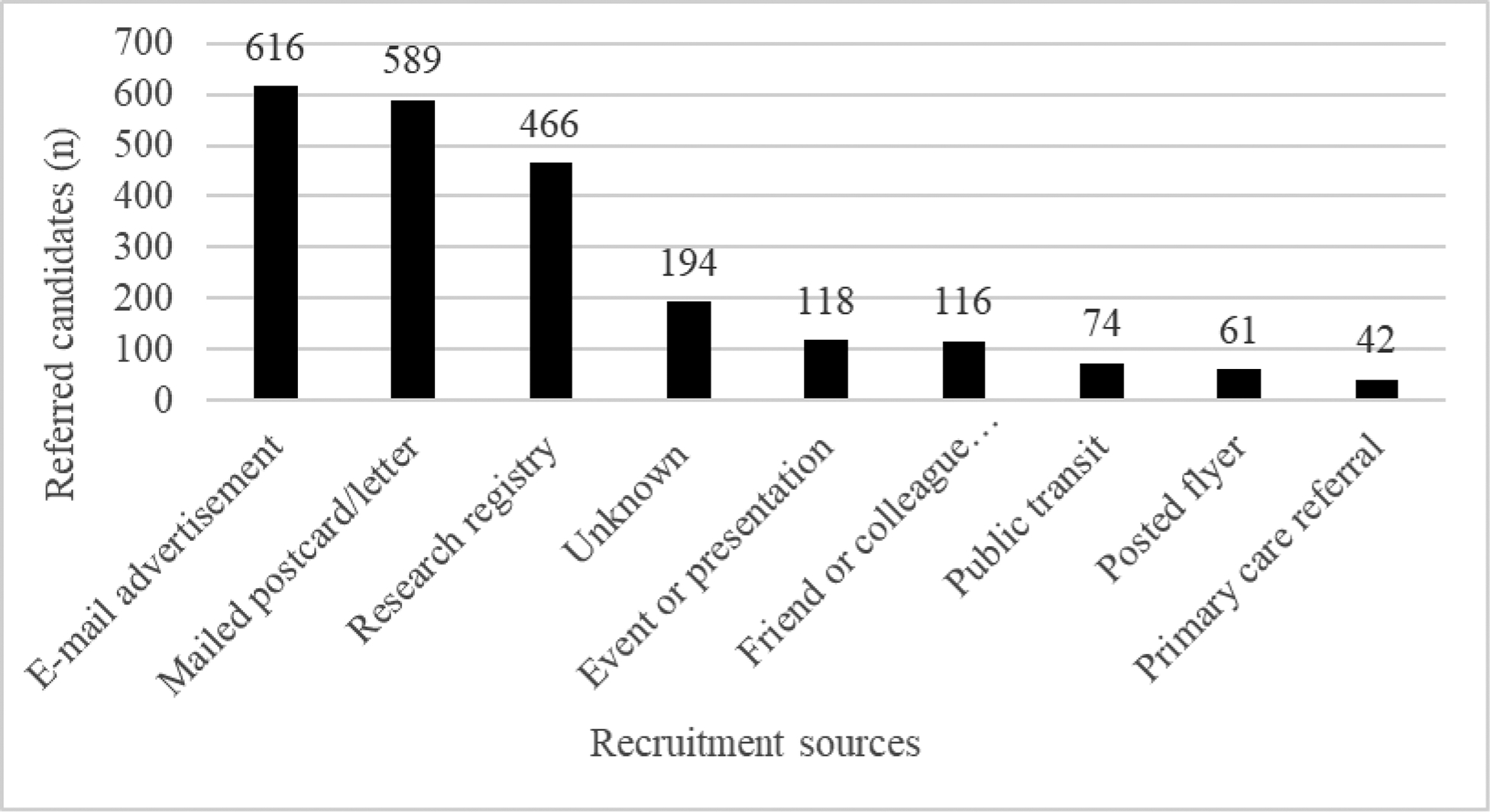
Recruitment sources for the first 2276 referrals
4.1.2. Reasons for ineligibility
From October 2017 – December 2020, we received a total of 2,276 referrals, of which 1,921 were excluded after the completion of an online/phone screening (Figure 4A). The largest group of ineligible candidates (N=350) were too physically active to participate in this trial (reported greater than or equal to 150 minutes per week of moderate + 2x vigorous intensity physical activity). Other common exclusions included BP ineligibility (n=190 self-reported as too low or too high) or current use of a BP and/or glucose lowering medication (n=134). Reflecting the growing trend in height-adjustable workstations, 67 referrals were excluded for current use of a sit/stand desk or active workstation. Unfortunately, we were unable to assess the reasons for screening ineligibility in the majority of referrals (N=963), even after multiple contact attempts. These candidates likely self-selected out of the study after reviewing the more extensive study description provided if they did not meet the BP or other criteria (self-determined) or they lacked the time or interest to participate.
Figure 4.
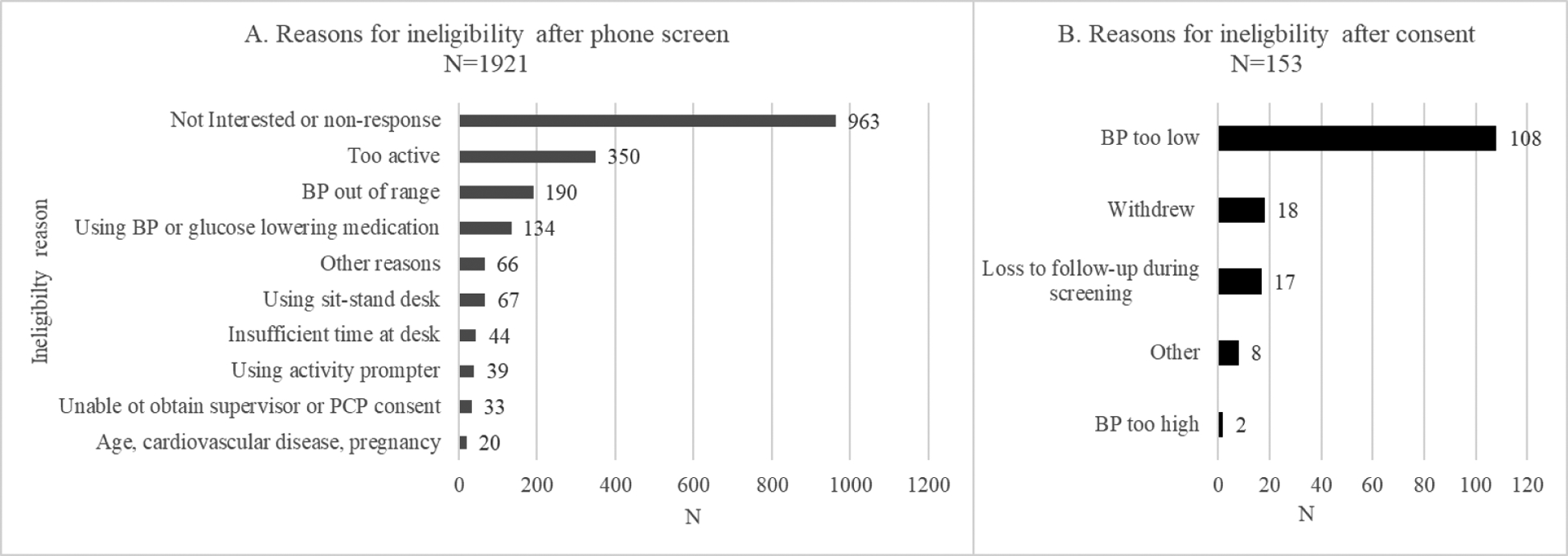
Reasons for ineligibility after phone screen (panel A; N=1921) and after informed consent (panel B; N=153)
Of these, 326 participants provided informed consent and at least one in-person visit. A total of 153 participants were additionally excluded, most frequently due to BP measurements that were too low (n=108) or too high (n=2), withdrawal (n=18), or loss to follow-up during screening (n=17). Less frequent ‘other’ reasons for exclusion after consent (n=8) included: unstable/loss of employment (n=3), starting BP medication (n=1), the disapproval of their supervisor (n=1), on disallowed medication (n=1), wants to be more active (n=1), and possible COVID-19 infection (n=1) (Figure 4B).
4.1.3. Nuances of assessing eligibility during recruitment
As systolic BP is our primary outcome and we have specific BP eligibility ranges, we have gained knowledge in practical methods to bring potentially eligible participants into the laboratory while minimizing screen failures due to BP below or above eligibility. This process is complicated by recent changes in BP diagnostic criteria,[2] where many participants perceive themselves as having low or normal BP even though they may be within the study-specified ranges. As such, we have developed the following screening protocol prior to scheduling an in-person visit: i) ask participants to recall a recent BP, ideally within the past 3 months but up to the past year; ii) ask participants to review their physical or electronic health records for the most recent BP reading; iii) request that participants get their BP measured, e.g. at a local drug or grocery store; and iv) if the earlier methods are not accessible, schedule an in-lab initial BP study visit. Participants reporting a previous BP near our ranges (e.g., within 5 mmHg below or 10 mmHg above) are invited to attend an initial visit due to the known variability in BP over time and expected differences with our highly standardized BP measurement protocol. Though low BP remains the leading reason for screen failure after providing informed consent (see Figure 3), these procedures minimize wasted time for participants and staff.
Evaluation of other eligibility criteria also require a nuanced approach. To find inactive participants, we ask detailed questions adapted from the Paffenbarger Physical Activity Questionnaire[46] regarding time spent active while brisk walking and in other travel or sports/leisure activities. Trained research staff code the intensity of activities using the 2011 Compendium of Physical Activities,[76] calculate usual weekly minutes of moderate and vigorous activity, and use these data to determine whether the research candidate meets our criteria of aerobic activity below current federal Guidelines.[17] Another exclusion criteria that requires careful evaluation is the current use of a sit-stand desk or activity prompter, which are increasingly popular in our target population. Many potential participants report having a sit-stand desk or a wearable activity monitor. To maximize inclusion and generalizability, we only exclude participants who report using their sit-stand desk and/or routinely responding to the automated activity prompting notifications on their wearable monitor over the past month.
4.2. Balancing practicality and rigor
Time for research procedures is a barrier for participating among many full-time desk workers. Yet, for scientific rigor of BP assessment, it was important that we prioritize control of all possible influences (including diurnal variation) and to obtain two measurements, on different days, at each assessment timepoint. Thus, assessments needed to occur in the morning (before 11:00 AM) and in a controlled environment (e.g., in the clinical environment of our research center). It can be difficult for some participants to attend these visits as they occur during typical business or commuting hours and our longer assessment visits lasts approximately 2 hours. To accommodate, we have instituted flexible scheduling – adhering to our protocols to ensure research integrity but improving our reach and optimizing participant involvement and satisfaction. In the instances when participants are unable to complete research assessments during our usual appointment times (e.g., 7:30–10:00 AM on weekdays), we offer earlier start times (6:00 AM – 7:00 AM), the opportunity to split up the assessments over two separate days, or (on rare occasions) the option for research staff to administer certain assessments at the participant’s office locations.
Study staff have encountered other challenges, but prioritize inclusiveness for appropriate candidates. For example, some participants have unusual office spaces where our typical sit-stand desk attachments are a poor fit. In these cases, we have worked with suppliers and participants to find solutions. For example, for several participants that work from home with limited or no dedicated desk space, we have provided an Ergotron Learn-Fit, a small, versatile, stand-alone workstation that provides a height-adjustable work option and does not required permanent placement on a desk or table.
4.3. Innovative adaptations during the COVID-19 pandemic
RESET BP was among the many ongoing clinical trials that were forced to demonstrate ‘creativity and persistence’ to continue amid government and institutional pandemic restrictions to reduce the spread of the COVID-19.[77] The University of Pittsburgh suspended non-life-sustaining, in-person research beginning in March of 2020, and allowed research to restart during the summer months using a multilevel approval process that included consultation with institutional administration, human subjects protection, and environmental health safety officers.
4.3.1. Modifications to the intervention
During the initial suspension of in-person research, our first priority was to sustain the intervention for active participants. A major strategy by which our intervention reduces SB is via replacement with standing at a study-provided sit-stand desk attachment in the workplace. The majority of our participants moved their place of work from in-office to their home, which introduced challenges as some participants were unable to relocate their desk attachment and/or had no formal workstation at home. For these participants, we provided additional or different height-adjustable workstations to be used in the home that research staff dropped off on doorsteps. Staff also supported correct assembly and installation remotely. At the same time, we carefully collected data about changes in work environments so that we will be able to consider these changes in post hoc sensitivity analyses. Lastly, though our initial intervention protocol had 3 in-person behavioral lessons, mode of delivery was modified to remote video or phone conference. At the time of this submission, we continue to deliver the intervention remotely by videoconference as the pandemic is still active in our community.
4.3.2. Remote assessments of BP and other outcomes
A second priority during the suspension of in-person research was to modify our assessment protocol to allow for a ‘remote exit’ of enrolled participants that had finished the 3-month trial. Certain outcomes could not be ascertained using remote-only procedures (i.e., blood sampling and PWV assessment). However, using doorstep drop-off of equipment by staff, conversion of all self-administered questionnaires to online surveys, and videoconferencing, we were able to continue to collect the majority of outcomes including our primary outcome using remote procedures including two measures of BP on separate days, ambulatory BP, 9-day activity monitoring, and all questionnaires.
For remote measurement of resting BP, we purchased additional HEM 907-XL automated devices and developed a protocol that could be completed by the participant during a videoconference with study personnel. This included: i) verbal confirmation of pre-measurement abstentions; ii) self-measurement of arm circumference using a flexible measuring tape that was secured to the arm using study-provided medical tape; iii) self-placement of the appropriate cuff with visual inspection for correct cuff and body positioning via videoconferencing; iv) video observation of the 10-minute seated rest; and v) automated measurement with the oscillometric BP monitor screen facing the video camera and non-visible to the participant during measurements (to reduce reactivity). We also added collection of BP measurement location (i.e., clinic, home, or other), again for future consideration in post hoc sensitivity analyses. Through the remarkable dedication and flexibility of the RESET BP staff and participants, we were able to complete remote follow-up assessments on all but one participant (who withdrew from the study) during the in-person research suspension.
4.3.3. Study protocols during re-initiation of in-person research
When designing our research restart plan, we prioritized maintenance of exposure-limiting, remote procedures where study integrity was not compromised. At the current time, this includes our study orientation and informed consent procedures (videoconferncing), self-administered questionnaires (REDCap), and all behavioral intervention contacts (videoconferencing). For our primary outcome of BP, we initially continued at-home assessments since we had developed an acceptable remote method (see 4.3.2). However, with approval by our institution for this low-contact measurement, we have reinitiated in-clinic BP for the following reasons. Firstly, for the scientific reason that home BP is well known to be lower than clinic BP.[78] Secondly, for the practical reasons that that remote BP measurement took considerably more participant time and created difficult logistics for delivering and retrieving our BP monitors. During the transition back to clinic BP assessments, we took care to match baseline and follow-up assessment locations when possible (i.e., home or clinic) to maximize the internal validity of outcome assessments.
Thus, our modified protocol currently includes only necessary in-person assessments due to the currently active pandemic. Our laboratory continues to practice stringent safety procedures aligned with CDC and University of Pittsburgh guidelines including: pre-visit screening with questions and temperature checks, social distancing, limited personnel and time spent in the same room (e.g., research personnel leave the room during resting periods), personal protective equipment including participant and research personnel masks, cleaning all surfaces before and after participant visits, and observing all government and institutional regulations for quarantine and travel restrictions.
5. Summary and Future Directions
The RESET BP trial will test the effect of a 3-month SB reduction intervention compared to control on BP and cardiovascular health among inactive desk workers with elevated, non-medicated BP. Though broad recruitment strategies have been needed to meet targets in this working population, the most successful strategies have been emailing or mailing study advertisements to registries, businesses, primary care patients, and targeted local zip codes. High activity levels and BP out of range are the most common reasons for screen failure, giving insight into interested population groups who might be included in broader dissemination efforts. With the continued recruitment and retention of participants in this trial, we are encouraged to brainstorm creative approaches and solutions to recruitment challenges, scheduling roadblocks, and a pandemic, while continuing to adhere to the study protocol, in hopes of maximizing generalizability.
Using the intervention and assessment methodology described herein, we hope to provide high quality data on the initial efficacy of SB reduction for reducing BP and improving cardiovascular health in working adults. Important future directions include designing interventions that are of longer duration and that address broader populations, e.g. non-desk workers and underserved populations that have disproportionately high rates of hypertension and cardiovascular risk, as well as rigorously accounting for sodium intake. We anticipate these data will inform future effectiveness trials, and eventually, more specific and quantitative SB recommendations.
ACKNOWLEDGEMENTS
This research is supported by the National Institutes of Health [R01 HL147610 and UL1TR001857].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation 139(10) (2019) e56–e528. [DOI] [PubMed] [Google Scholar]
- [2].Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Hypertension 71(6) (2018) e13–e115. [DOI] [PubMed] [Google Scholar]
- [3].Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr., Whelton PK, Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline, J. Am. Coll. Cardiol 71(2) (2018) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM, Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome, Int. J. Behav. Nutr. Phys. Act 14(1) (2017) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP, Amount of time spent in sedentary behaviors in the United States, 2003–2004, Am. J. Epidemiol 167(7) (2008) 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beunza JJ, Martínez-González MA, Ebrahim S, Bes-Rastrollo M, Núñez J, Martínez JA, Alonso A, Sedentary behaviors and the risk of incident hypertension: the SUN Cohort, Am. J. Hypertens 20(11) (2007) 1156–62. [DOI] [PubMed] [Google Scholar]
- [7].Lee PH, Wong FK, The association between time spent in sedentary behaviors and blood pressure: a systematic review and meta-analysis, Sports Med 45(6) (2015) 867–80. [DOI] [PubMed] [Google Scholar]
- [8].Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey ME, Gill JM, Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers, PLoS One 7(5) (2012) e36345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sohn MW, Manheim LM, Chang RW, Greenland P, Hochberg MC, Nevitt MC, Semanik PA, Dunlop DD, Sedentary behavior and blood pressure control among osteoarthritis initiative participants, Osteoarthritis Cartilage 22(9) (2014) 1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Horta BL, Schaan BD, Bielemann RM, Vianna C, Gigante DP, Barros FC, Ekelund U, Hallal PC, Objectively measured physical activity and sedentary-time are associated with arterial stiffness in Brazilian young adults, Atherosclerosis 243(1) (2015) 148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].García-Hermoso A, Notario-Pacheco B, Recio-Rodríguez JI, Martínez-Vizcaíno V, Rodrigo de Pablo E, Magdalena Belio JF, Gómez-Marcos MA, García-Ortiz L, Sedentary behaviour patterns and arterial stiffness in a Spanish adult population - The EVIDENT trial, Atherosclerosis 243(2) (2015) 516–22. [DOI] [PubMed] [Google Scholar]
- [12].Suboc TB, Knabel D, Strath SJ, Dharmashankar K, Coulliard A, Malik M, Haak K, Widlansky ME, Associations of Reducing Sedentary Time With Vascular Function and Insulin Sensitivity in Older Sedentary Adults, Am. J. Hypertens 29(1) (2016) 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pandey A, Salahuddin U, Garg S, Ayers C, Kulinski J, Anand V, Mayo H, Kumbhani DJ, de Lemos J, Berry JD, Continuous Dose-Response Association Between Sedentary Time and Risk for Cardiovascular Disease: A Meta-analysis, JAMA Cardiol 1(5) (2016) 575–83. [DOI] [PubMed] [Google Scholar]
- [14].Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA, Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis, Ann. Intern. Med 162(2) (2015) 123–32. [DOI] [PubMed] [Google Scholar]
- [15].Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagströmer M, Edwardson C, Yates T, Shiroma E, Anderssen SA, Lee IM, Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis, BMJ 366 (2019) l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Katzmarzyk PT, Powell KE, Jakicic JM, Troiano RP, Piercy K, Tennant B, Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee, Med Sci Sports Exerc 51(6) (2019) 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Piercy KL, Troiano RP, Physical Activity Guidelines for Americans From the US Department of Health and Human Services, Circ. Cardiovasc. Qual. Outcomes 11(11) (2018) e005263. [DOI] [PubMed] [Google Scholar]
- [18].Ross R, Chaput J-P, Giangregorio LM, Janssen I, Saunders TJ, Kho ME, Poitras VJ, Tomasone JR, El-Kotob R, McLaughlin EC, Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep, Appl. Physiol. Nutr. Metab 45(10) (2020) S57–S102. [DOI] [PubMed] [Google Scholar]
- [19].Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM, Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women, The Lancet 388(10051) (2016) 1302–1310. [DOI] [PubMed] [Google Scholar]
- [20].Buman MP, Winkler EA, Kurka JM, Hekler EB, Baldwin CM, Owen N, Ainsworth BE, Healy GN, Gardiner PA, Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006, Am. J. Epidemiol 179(3) (2014) 323–34. [DOI] [PubMed] [Google Scholar]
- [21].Kozey Keadle S, Lyden K, Staudenmayer J, Hickey A, Viskochil R, Braun B, Freedson PS, The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors, Appl Physiol Nutr Metab 39(7) (2014) 770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Biddle SJ, Edwardson CL, Wilmot EG, Yates T, Gorely T, Bodicoat DH, Ashra N, Khunti K, Nimmo MA, Davies MJ, A Randomised Controlled Trial to Reduce Sedentary Time in Young Adults at Risk of Type 2 Diabetes Mellitus: Project STAND (Sedentary Time ANd Diabetes), PLoS One 10(12) (2015) e0143398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carr LJ, Karvinen K, Peavler M, Smith R, Cangelosi K, Multicomponent intervention to reduce daily sedentary time: a randomised controlled trial, BMJ Open 3(10) (2013) e003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mailey EL, Rosenkranz SK, Casey K, Swank A, Comparing the effects of two different break strategies on occupational sedentary behavior in a real world setting: A randomized trial, Prev Med Rep 4 (2016) 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barone Gibbs B, Brach JS, Byard T, Creasy S, Davis KK, McCoy S, Peluso A, Rogers RJ, Rupp K, Jakicic JM, Reducing Sedentary Behavior Versus Increasing Moderate-to-Vigorous Intensity Physical Activity in Older Adults, J Aging Health 29(2) (2017) 247–267. [DOI] [PubMed] [Google Scholar]
- [26].Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW, Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults, Nutr. Metab. Cardiovasc. Dis 24(9) (2014) 976–82. [DOI] [PubMed] [Google Scholar]
- [27].Zeigler ZS, Mullane SL, Crespo NC, Buman MP, Gaesser GA, Effects of Standing and Light-Intensity Activity on Ambulatory Blood Pressure, Med Sci Sports Exerc 48(2) (2016) 175–81. [DOI] [PubMed] [Google Scholar]
- [28].Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW, Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes, J. Hypertens 34(12) (2016) 2376–2382. [DOI] [PubMed] [Google Scholar]
- [29].Barone Gibbs B, Kowalsky RJ, Perdomo SJ, Taormina JM, Balzer JR, Jakicic JM, Effect of alternating standing and sitting on blood pressure and pulse wave velocity during a simulated workday in adults with overweight/obesity, J. Hypertens 35(12) (2017) 2411–2418. [DOI] [PubMed] [Google Scholar]
- [30].Dempsey PC, Larsen RN, Dunstan DW, Owen N, Kingwell BA, Sitting less and moving more: implications for hypertension, Hypertension 72(5) (2018) 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shvartz E, Gaume JG, White RT, Reibold RC, Hemodynamic responses during prolonged sitting, J Appl Physiol Respir Environ Exerc Physiol 54(6) (1983) 1673–80. [DOI] [PubMed] [Google Scholar]
- [32].Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr., Vita JA, Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers, Arterioscler. Thromb. Vasc. Biol 27(12) (2007) 2650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Siu AL, Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement, Ann. Intern. Med 163(10) (2015) 778–86. [DOI] [PubMed] [Google Scholar]
- [34].Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study, Circulation 122(14) (2010) 1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J, Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population, Circulation 113(5) (2006) 664–70. [DOI] [PubMed] [Google Scholar]
- [36].Vlachopoulos C, Aznaouridis K, Stefanadis C, Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis, J. Am. Coll. Cardiol 55(13) (2010) 1318–27. [DOI] [PubMed] [Google Scholar]
- [37].Buckley JP, Hedge A, Yates T, Copeland RJ, Loosemore M, Hamer M, Bradley G, Dunstan DW, The sedentary office: an expert statement on the growing case for change towards better health and productivity, Br. J. Sports Med 49(21) (2015) 1357–1362. [DOI] [PubMed] [Google Scholar]
- [38].Whelton Paul K, Carey Robert M, Aronow Wilbert S, Casey Donald E, Collins Karen J, Dennison Himmelfarb C, DePalma Sondra M, Gidding S, Jamerson Kenneth A, Jones Daniel W, MacLaughlin Eric J, Muntner P, Ovbiagele B, Smith Sidney C, Spencer Crystal C, Stafford Randall S, Taler Sandra J, Thomas Randal J, Williams Kim A, Williamson Jeff D, Wright Jackson T, 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults, J. Am. Coll. Cardiol 71(19) (2018) e127–e248. [DOI] [PubMed] [Google Scholar]
- [39].Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ, Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research, Circulation 111(5) (2005) 697–716. [DOI] [PubMed] [Google Scholar]
- [40].Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT, Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association, Hypertension 73(5) (2019) e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].El Assaad MA, Topouchian JA, Darné BM, Asmar RG, Validation of the Omron HEM-907 device for blood pressure measurement, Blood Press. Monit 7(4) (2002) 237–41. [DOI] [PubMed] [Google Scholar]
- [42].Nikolic SB, Abhayaratna WP, Leano R, Stowasser M, Sharman JE, Waiting a few extra minutes before measuring blood pressure has potentially important clinical and research ramifications, J. Hum. Hypertens 28(1) (2014) 56–61. [DOI] [PubMed] [Google Scholar]
- [43].O’Brien E, Asmar R, Beilin L, Imai Y, Mallion J-M, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement, Journal of hypertension 21(5) (2003) 821–848. [DOI] [PubMed] [Google Scholar]
- [44].Leary AC, Donnan PT, MacDonald TM, Murphy MB, The influence of physical activity on the variability of ambulatory blood pressure, Am. J. Hypertens 13(10) (2000) 1067–73. [DOI] [PubMed] [Google Scholar]
- [45].Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank J, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity, Journal of hypertension 30(3) (2012) 445–448. [DOI] [PubMed] [Google Scholar]
- [46].Paffenbarger R, Wing A, Hyde R, Paffenbarger physical activity questionnaire, Am. J. Epidemiol 108(3) (1978) 161–175. [DOI] [PubMed] [Google Scholar]
- [47].Rosenberg DE, Norman GJ, Wagner N, Patrick K, Calfas KJ, Sallis JF, Reliability and validity of the Sedentary Behavior Questionnaire (SBQ) for adults, Journal of Physical Activity and Health 7(6) (2010) 697–705. [DOI] [PubMed] [Google Scholar]
- [48].Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS, Validation of wearable monitors for assessing sedentary behavior, Med. Sci. Sports Exerc 43(8) (2011) 1561–1567. [DOI] [PubMed] [Google Scholar]
- [49].Lee I-M, Shiroma EJ, Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges, Br. J. Sports Med 48(3) (2014) 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M, Physical activity in the United States measured by accelerometer, Med. Sci. Sports Exerc 40(1) (2008) 181–188. [DOI] [PubMed] [Google Scholar]
- [51].Matthews CE, Hagströmer M, Pober DM, Bowles HR, Best practices for using physical activity monitors in population-based research, Med. Sci. Sports Exerc 44(1 Suppl 1) (2012) S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Edwardson CL, Winkler EA, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, Healy GN, Considerations when using the activPAL monitor in field-based research with adult populations, Journal of sport and health science 6(2) (2017) 162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gibbs BB, Kline CE, When does sedentary behavior become sleep? A proposed framework for classifying activity during sleep-wake transitions, International Journal of Behavioral Nutrition and Physical Activity 15(1) (2018) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Choi L, Liu Z, Matthews CE, Buchowski MS, Validation of accelerometer wear and nonwear time classification algorithm, Med. Sci. Sports Exerc 43(2) (2011) 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sasaki JE, John D, Freedson PS, Validation and comparison of ActiGraph activity monitors, Journal of science and medicine in sport 14(5) (2011) 411–416. [DOI] [PubMed] [Google Scholar]
- [56].Thompson FE, Midthune D, Kahle L, Dodd KW, Development and evaluation of the National Cancer Institute’s Dietary Screener Questionnaire scoring algorithms, The Journal of nutrition 147(6) (2017) 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Manini TM, Carr LJ, King AC, Marshall S, Robinson TN, Rejeski WJ, Interventions to reduce sedentary behavior, Med. Sci. Sports Exerc 47(6) (2015) 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bandura A, The primacy of self-regulation in health promotion, Appl. Psychol 54(2) (2005) 245–254. [Google Scholar]
- [59].Owen N, Sugiyama T, Eakin EE, Gardiner PA, Tremblay MS, Sallis JF, Adults’ sedentary behavior: determinants and interventions, Am. J. Prev. Med 41(2) (2011) 189–196. [DOI] [PubMed] [Google Scholar]
- [60].Deci EL, Ryan RM, Handbook of self-determination research, University Rochester Press; 2004. [Google Scholar]
- [61].Gitlin LN, Parisi JM, Are treatment effects real? The role of fidelity, Behavioral intervention research: Designing, evaluating and implementing New York: Springer; (2016). [Google Scholar]
- [62].Rubin DB, Multiple imputation for nonresponse in surveys, John Wiley & Sons; 2004. [Google Scholar]
- [63].Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, The prevention and treatment of missing data in clinical trials, New England Journal of Medicine 367(14) (2012) 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Leary AC, Donnan PT, MacDonald TM, Murphy MB, The influence of physical activity on the variability of ambulatory blood pressure, Am. J. Hypertens 13(10) (2000) 1067–1073. [DOI] [PubMed] [Google Scholar]
- [65].Preacher KJ, Hayes AF, Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models, Behav. Res. Methods 40(3) (2008) 879–891. [DOI] [PubMed] [Google Scholar]
- [66].Valeri L, Vanderweele TJ, Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros, Psychol. Methods 18(2) (2013) 137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Barone Gibbs B, Brach JS, Byard T, Creasy S, Davis KK, McCoy S, Peluso A, Rogers RJ, Rupp K, Jakicic JM, Reducing sedentary behavior versus increasing moderate-to-vigorous intensity physical activity in older adults: a 12-week randomized, clinical trial, Journal of aging and health 29(2) (2017) 247–267. [DOI] [PubMed] [Google Scholar]
- [68].Kozey Keadle S, Lyden K, Staudenmayer J, Hickey A, Viskochil R, Braun B, Freedson PS, The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors, Appl. Physiol. Nutr. Metab 39(7) (2014) 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chow S-C, Shao J, Wang H, Lokhnygina Y, Sample size calculations in clinical research, CRC press; 2017. [Google Scholar]
- [70].Benner A, Sample Size Tables For Clinical Studies. (2nd edn). David Machin, Michael J. Campbell, Peter M. Fayers and Alain P. Y. Pinol, Blackwell Science Ltd., Oxford, 1997. No. of pages: x+315. Price: £45. ISBN 0-86542-870-0, Stat. Med 18(4) (1999) 494–495. [Google Scholar]
- [71].Zar J, Biostatistical Analysis 2nd edn Prentice-Hall: Englewood Cliffs, NJ, USA: (1984). [Google Scholar]
- [72].Julious SA, Sample sizes for clinical trials, CRC Press; 2009. [DOI] [PubMed] [Google Scholar]
- [73].Cornelissen VA, Fagard RH, Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors, Hypertension 46(4) (2005) 667–675. [DOI] [PubMed] [Google Scholar]
- [74].Cohen J, Statistical power analyses for the behavioral sciences (rev. ed.) New York, Academic Press. ACCOUNTABILITY IN APPRAISALS, 1977. [Google Scholar]
- [75].Svetkey LP, Management of Prehypertension, Hypertension 45(6) (2005) 1056–1061. [DOI] [PubMed] [Google Scholar]
- [76].Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS, 2011 Compendium of Physical Activities: a second update of codes and MET values, Med Sci Sports Exerc 43(8) (2011) 1575–81. [DOI] [PubMed] [Google Scholar]
- [77].McDermott MM, Newman AB, Preserving clinical trial integrity during the coronavirus pandemic, JAMA (2020). [DOI] [PubMed] [Google Scholar]
- [78].Jula A, Puukka P, Karanko H, Multiple Clinic and Home Blood Pressure Measurements Versus Ambulatory Blood Pressure Monitoring, Hypertension 34(2) (1999) 261–266. [DOI] [PubMed] [Google Scholar]


