SUMMARY
Microvilli are actin bundle-supported surface protrusions that play essential roles in diverse epithelial functions. To develop our understanding of microvilli biogenesis, we used live imaging to directly visualize protrusion growth at early stages of epithelial differentiation. Time-lapse data revealed that specific factors, including epidermal growth factor pathway substrate 8 (EPS8) and insulin-receptor tyrosine kinase substrate (IRTKS, also known as BAIAP2L1), appear in diffraction-limited puncta at the cell surface and mark future sites of microvillus growth. New core actin bundles elongate from these puncta in parallel with the arrival of ezrin and subsequent plasma membrane encapsulation. In addition to de novo growth, we also observed that new microvilli emerge from pre-existing protrusions. Moreover, we found that nascent microvilli can also collapse, characterized first by loss of membrane wrapping and ezrin enrichment, followed by a sharp decrease in distal tip EPS8 and IRTKS levels, and ultimately disassembly of the core actin bundle itself. These studies are the first to offer a temporally resolved microvillus growth mechanism and highlight factors that participate in this process; they also provide important insights on the growth of apical specializations that will likely apply to diverse epithelial contexts.
Keywords: brush border, actin, cytoskeleton, membrane, protrusion
Graphical Abstract
eTOC Blurb
Gaeta et al. show that microvillus biogenesis occurs de novo and from existing microvilli, with the actin binding proteins EPS8 and IRTKS marking new sites of growth. Nascent microvilli also undergo collapse, whereby the protrusion initially loses membrane wrapping and EPS8/IRTKS tip enrichment, ultimately leading to loss of the core actin bundle.
INTRODUCTION
Actin bundle-supported surface protrusions are one of the defining features of eukaryotic cells, conferring the ability to move and sense the surrounding environment. Examples include filopodia and microvilli, fingerlike protrusions that extend from the leading edge of crawling cells and the apical surface of polarized epithelial cells, respectively. In an evolutionary context, filopodia-like structures predate animal cells1, whereas the evolution of microvilli occurred later, emerging in animal cells and choanoflagellates2,3. In higher eukaryotes, microvilli are found on the surface of diverse epithelial cell types, including those from intestine and kidney, where they increase apical surface area for nutrient and filtrate absorption, respectively4. Additionally, in the sensory epithelium, exaggerated microvilli referred to as stereocilia respond to mechanosensory stimuli that allow for hearing5. Despite their essential roles in a range of epithelial functions, little is known about the temporal and mechanistic details that govern when or how microvilli grow from the apical surface.
Microvilli ultrastructure is well characterized, with data from transmission electron microscopy (TEM) and biochemical studies spanning nearly six decades. Moreover, proteomic analyses have revealed a comprehensive list of resident proteins, many of which interact directly with the actin cytoskeleton6,7. In transporting epithelia, microvilli extend microns from the cell surface, supported by a core of ~20–40 actin filaments8,9. Actin filaments are bundled in parallel by villin-1 (VIL1), plastin-1 (PLS1, also known as fimbrin), and espin (ESPN)10–12. This core actin bundle is physically linked to the encapsulating plasma membrane by membrane-cytoskeleton linkers including ezrin (EZR), myosin-1A (MYO1A), and myosin-6 (MYO6)13–16. Within core bundles, actin filament barbed ends, which represent favorable sites for monomer incorporation, are oriented towards the distal tips8. Core bundle barbed ends are embedded in an electron dense cap, which may be enriched in factors that control the microvilli growth8,17. Electron dense caps are also found at the tips of filopodia and stereocilia18,19 suggesting a universal function in protrusion formation.
Although our understanding of molecular factors that control actin polymerization has expanded significantly in recent years, how microvillar growth is initiated at the cell surface remains unclear. Due to its exquisitely specific enrichment at the tips of microvilli, stereocilia and filopodia20–23, epidermal growth factor pathway substrate 8 (EPS8) is of particular interest as a factor that may initiate and/or drive protrusion growth. EPS8 consists of an N-terminal phosphotyrosine binding (PTB) domain, a central SH3 domain, and C-terminal actin binding motifs24,25. Studies in mouse models showed that loss of EPS8 leads to shortening of both microvilli and stereocilia26,27, suggesting a role in elongating both of these structures. EPS8 is also required for proper apical morphogenesis in C. elegans intestine, where genetic ablation leads to gross defects in tissue morphology and lethality if both EPS8 isoforms are lost22. Consistent with these findings, our group recently determined that EPS8 and IRTKS work together to promote elongation of microvilli23 and directional persistence of motile microvilli early in differentiation28. Mechanistically, EPS8 has been assigned both actin capping and bundling functions24,25. While its localization at the tips of protrusions is consistent with a potential role in capping barbed ends, this function is at odds with shortened protrusions that result from EPS8 loss-of-function23,26,27. Additionally, tip enrichment is not entirely consistent with a canonical role as a filament bundler, as other bundling proteins that reside in actin cores such as VIL1, PLS1, and ESPN localize along the length of protrusions11,29.
Though our understanding of microvillus ultrastructure and resident proteins has expanded in recent years, little is known about how protrusion growth is initiated at the apical surface during epithelial differentiation. To address this knowledge gap, we developed a live imaging assay that enabled us to directly observe the de novo growth of individual microvilli on the surface of epithelial cells. Based on its ubiquitous appearance at the tips of microvilli in diverse epithelial tissues and cell culture models, we tested the idea that EPS8 is involved in the de novo growth of microvilli. Consistent with this hypothesis, we observed that EPS8 and its binding partner IRTKS both marked sites of future microvilli growth. EPS8 and IRTKS puncta also remained associated with the distal tips of core actin bundles as these structures elongated. Unexpectedly, we also found that new microvilli commonly emerge from the base of pre-existing protrusions. Finally, we observed that membrane-cytoskeleton linking by ezrin, membrane wrapping, and enrichment of EPS8 and IRTKS are all needed for the survival of nascent microvilli, and that loss of membrane wrapping or tip enriched factors leads to core actin bundle disassembly. These data offer a high resolution molecular and temporal framework for understanding the growth of new microvilli on the apical surface of differentiating epithelial cells.
RESULTS
An approach for visualizing the de novo growth of individual microvilli
To define the time-course of molecular events that drive microvillus growth, we first established a live imaging approach. Here, we turned to the LLC-PK1-CL4 (CL4) porcine cell culture model, which we recently used to dissect mechanisms of microvillar motility28. These cells provide a powerful model for studying apical morphogenesis, as early in differentiation the surface density of microvilli is sparse enough to allow for observations of individual protrusions. CL4 microvilli also extend from the surface at all angles, which enables their visualization in volume projections generated using spinning disk confocal microscopy and structured illumination microscopy (SIM) (Figure 1A, S1A, Video S1). To capture individual growth events, we imaged sub-confluent or recently confluent CL4 cells at 30 second intervals for 30 minutes. Even under these conditions, quantifying individual growth events was limited to a subset of cells and events. For example, some cells produced few or no de novo growth events during the acquisition; in other cells, overlapping structures precluded measurement or events did not meet temporal selection criteria (see Methods). Using mCherry-ESPN (herein referred to as ESPN) as a probe for core actin bundles, we measured maximum projected intensities in the distal half of the growing microvillus. Normalized ESPN intensity increased and then plateaued after ~5 min (Figs. 1B and C), demonstrating the core actin bundles that support microvilli are assembled on a timescale of minutes.
Figure 1. Microvilli emerge from discrete EPS8 puncta on the apical surface of CL4 cells.
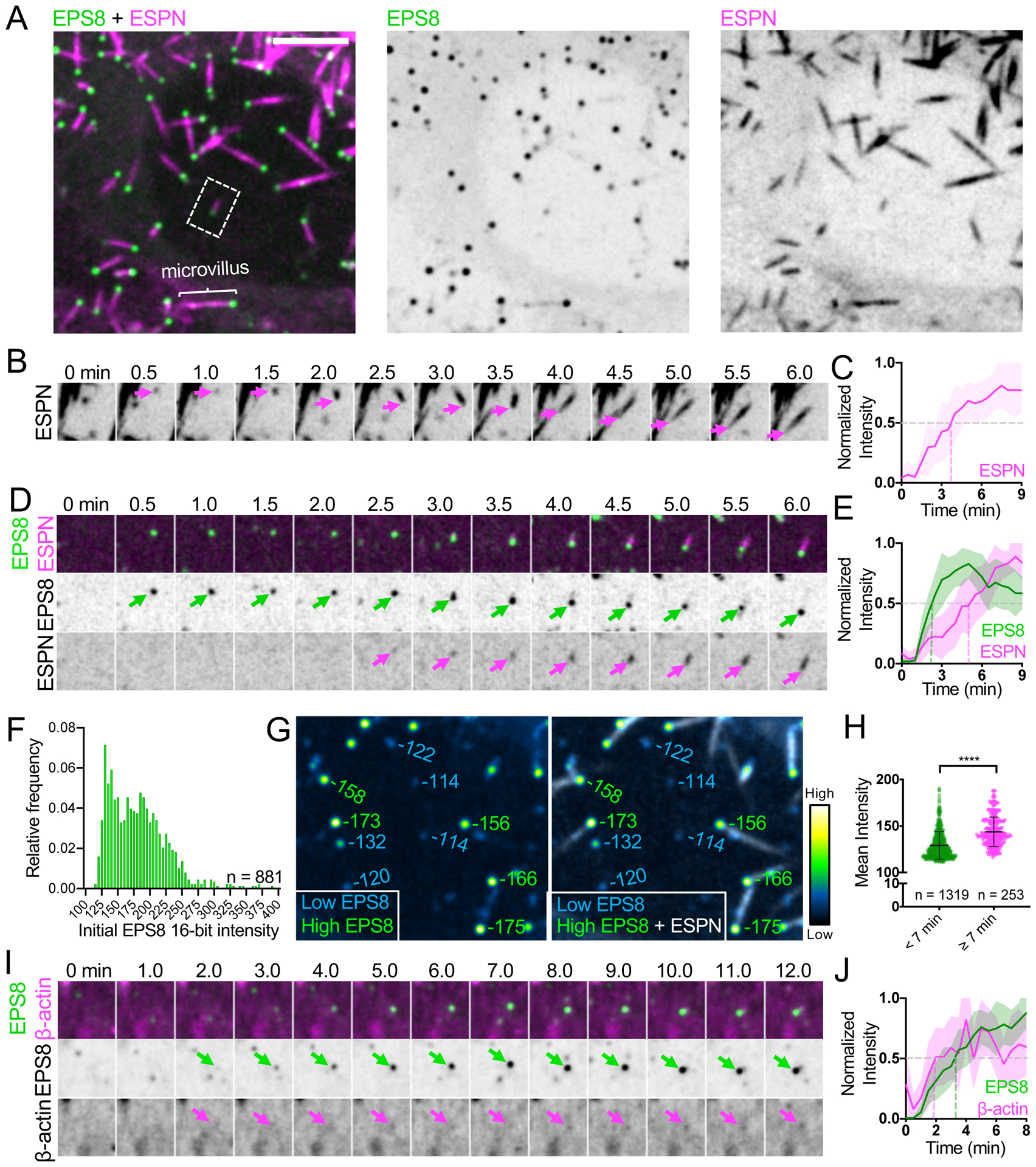
(A) Maximum intensity projection of a CL4 cell displaying microvilli. Core actin bundles are visualized by mCherry-ESPN (magenta) and EGFP-EPS8 (green). Merge (left), EGFP-EPS8 (middle), mCherry-ESPN (right). Scale bar = 5 μm. A single microvillus is denoted with the bracket. Dashed box represents microvillus shown in D. Related to Video S1. (B) Montage of a de novo microvillus growth event from a CL4 cell overexpressing mCherry-ESPN alone; magenta arrows mark the growing end of the core bundle. Box width = 4 μm. (C) Normalized intensity vs. time curve for microvillus growth events in CL4 cells expressing mCherry-ESPN alone; n = 13 events from 9 cells. T = 0 is defined as −3 frames (1.5 min) before the appearance of the mCherry-ESPN signal. (D) Montage of a de novo microvillus growth event from a CL4 cell expressing EGFP-EPS8 and mCherry-ESPN. Arrows denote the presence of EGFP-EPS8 (green) or mCherry-ESPN (magenta) at the growing end of the core bundle. Box width = 4 μm. Related to Video S2. (E) Normalized intensity vs. time curves for microvillus growth events in cells expressing EGFP-EPS8 (green) and mCherry-ESPN (magenta); n = 14 events from 7 cells. (F) EGFP-EPS8 puncta intensity histogram; n = number of puncta from a single representative cell. (G) Left, EGFP-EPS8 puncta pseudocolored by intensity, from the cell shown in A at a single time point. Right, merge of EGFP-EPS8 and mCherry-ESPN signals to differentiate bundle associated and non-bundle associated puncta. Values adjacent to each punctum represent raw deconvolved 16-bit intensity units. Box width = 14 μm. (H) EGFP-EPS8 puncta mean intensity plot with values derived from a single representative cell binned by lifetime (< 7 min or ≥ 7 min); n = number of puncta. ****p<0.0001, Mann-Whitney test. (I) Montage of a de novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 and mCherry-β-actin. Arrows denote the accumulation of EGFP-EPS8 (green) or mCherry-β-actin (magenta). Box width = 4 μm. Related to Video S3. (J) Normalized intensity vs. time curves for CL4 cells expressing EGFP-EPS8 (green) and mCherry-β-actin (magenta): n = 9 growth events from 5 cells. For E and J, t = 0 is defined as −3 frames (1.5 min) before the appearance of the EGFP-EPS8 signal. All images shown are maximum intensity projections. Dashed lines for all normalized intensity vs. time curves indicate point at which curves cross a normalized intensity of 0.5. For all curves, the solid line represents the mean and shading represents SD. See also Figures S1–S3.
Initiation of microvillus growth coincides with EPS8 enrichment in discrete puncta
EPS8 exhibits striking distal tip localization in diverse cell culture models and tissues, in every case examined by us and others (Figures 1A, S1, and S2)20,22,23,30. Importantly, EPS8 localizes to the tips of microvilli in intestinal crypt cells as well as crypt-like Ls174T-W4 (W4) cells (Figures S1B and S2A) suggesting this factor may be involved in early stages of microvilli growth. Moreover, we found that EPS8 distal tip enrichment is reduced in W4 cells treated with the barbed end blocking drug, cytochalasin D (Figures S1E–G). These data suggest that EPS8 binds directly to filament barbed ends and thus, is well positioned to regulate core actin bundle growth. With this in mind, we sought to determine when EPS8 arrives relative to the formation of core bundles as marked by ESPN accumulation. In CL4 cells co-expressing ESPN and EGFP-EPS8 (herein referred to as EPS8), analysis of 1011 ESPN positive bundles from 19 cells showed 88% were associated with EPS8 puncta, a value that is likely an underestimate due to limitations in core bundle segmentation. Time-lapse imaging revealed that future sites of microvillus growth are marked by the appearance of bright EPS8 puncta (Figure 1D, Video S2). Based on time series intensity plots, we found that on average ESPN begins to accumulate at the same time as EPS8 at these sites, albeit at a much slower rate (Figure 1E). Analysis of initial EPS8 puncta intensities revealed distributions that were at least bimodal (Figure 1F). In general, we found that the brightest EPS8 puncta were associated with ESPN positive actin bundles, whereas dimmer puncta were not bundle associated (Figure 1G, green vs. blue values, respectively). Moreover, analysis of 16,921 EPS8 puncta from 14 cells revealed that puncta with lifetimes ≥ 7 min — the approximate duration of microvillus maturation (Figure 1E) — had significantly higher mean intensities than puncta with duration times < 7 min (Figure 1H). Together these data suggest EPS8 puncta likely undergo maturation, accumulating to a threshold level of EPS8 before they can support the elongation of microvilli.
Although live cell probes that specifically report on the localization of G-actin are not available, co-expression of mCherry-β-actin (labeling G- and F-actin pools) with EPS8 revealed that microvillus growth events were preceded by the coalescence of β-actin signal, which coincided with the appearance of EPS8 puncta (Figure 1I and J, Video S3). In combination, these findings lead us to conclude that EPS8 puncta mark sites of microvillus growth and incorporate β-actin before actin core bundle elongation begins.
Mutation of actin binding residues impairs EPS8 puncta formation
As specific residues near the C-terminus of EPS8 (a.a. 649–822) have been implicated in actin binding25, we sought to determine if these motifs are important for EPS8 enrichment in puncta that associate with nascent microvilli (Figure 2). To this end, we examined the behavior of two EPS8 variants with mutations in these C-terminal motifs: V690D/L694D, which is expected to exhibit defective filament capping activity, and LNK758-760AAA (referred to herein as LNK:AAA), which is predicted to lack filament bundling activity25 (Figure S3). Using a tip targeting assay in W4 cells as we previously described23,31, both of these variants demonstrated distal tip enrichment relative to GFP only control, albeit reduced compared to WT EPS8 (Figures S3B,C). Additionally, we found that expression of an EPS8 mutant completely lacking the C-terminal actin binding region (EPS8 1–648) still retained some distal tip targeting activity, while expression of the C-terminal actin binding region alone (EPS8 649–822) did not. This suggests actin binding activity of EPS8 is necessary but not sufficient for robust distal tip targeting. To determine if mutations in the EPS8 actin binding motif impact the dynamics of microvilli growth, we co-expressed the EPS8-LNK:AAA or EPS8-V690D/L694D mutants with ESPN in CL4 cells (Figure 2). Although appearance of EPS8-LNK:AAA generally preceded ESPN accumulation, its signal exhibited pronounced intensity fluctuations during the imaging time course (Figures 2B,D). Moreover, while the appearance of EPS8-V690D/L694D coincided with a diffuse cloud of ESPN, coalescence of this signal into a linear structure was delayed, in some instances occurring several minutes after the initial appearance of ESPN (Figure 2C,E). Cells expressing EPS8-V690D/L694D exhibited significantly slower microvillus growth rates (0.07 ± 0.03 μm/min) compared to that of cells expressing ESPN alone (0.15± 0.07 μm/min). In contrast, growth rates in cells expressing WT EPS8 (0.12 ± 0.05 μm/min) and EPS8-LNK:AAA (0.21 ± 0.14 μm/min) were not statistically different from cells expressing ESPN alone (Figure S3D). Finally, EPS8-LNK:AAA exhibited significantly decreased initial mean intensities (measured in the first frame of detectable punctate EGFP signal) compared to EPS8 (Figure S3E). Together these fixed and live cell data suggest that the LNK758-760 and V690/L694 actin binding motifs promote the formation of EPS8 puncta and subsequent efficient assembly of core bundles.
Figure 2. EPS8 actin binding mutants disrupt EPS8 puncta formation and core bundle growth.
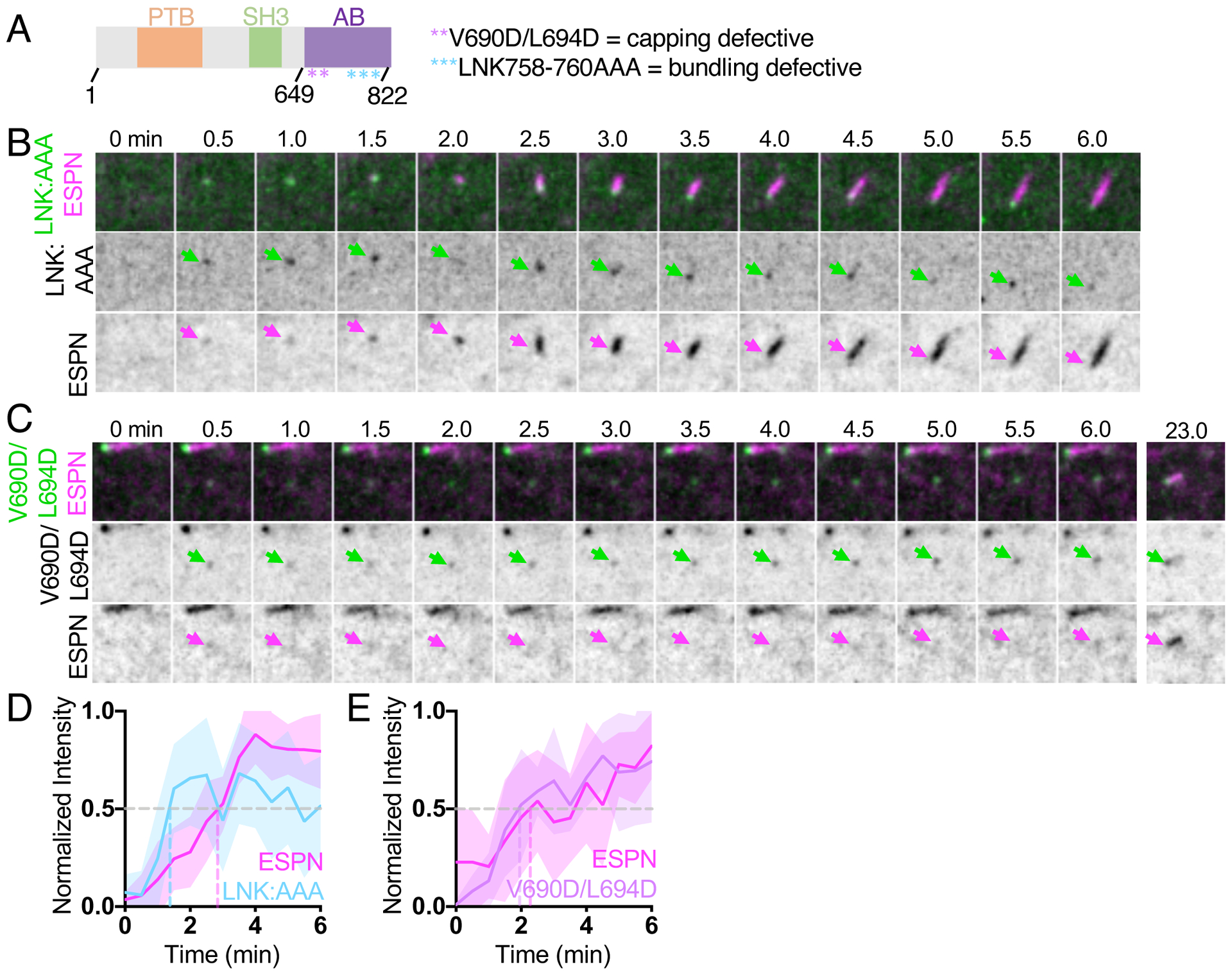
(A) Domain diagram of human EPS8. PTB = phosphotyrosine binding domain, SH3 = src-homology 3 domain, AB = actin binding domain. Asterisks represent point mutations in the residues shown. (B) Montage of a de novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 LNK758-760AAA (LNK:AAA) and mCherry-ESPN. Arrows denote the accumulation of EGFP-EPS8 LNK:AAA (green) or mCherry-ESPN (magenta). Box width = 4 μm. (C) Montage of a de novo microvillus growth event in cell expressing EGFP-EPS8 V690D/L694D and mCherry-ESPN. Arrows denote the accumulation of EGFP-EPS8 V690D/L694D (green) or mCherry-ESPN (magenta). Box width = 4 μm. (D) Normalized intensity vs. time curve for microvillus growth events in cells expressing EGFP-EPS8 LNK:AAA (light blue) and mCherry-ESPN (magenta); n = 11 events from 5 cells. (E) Normalized intensity vs. time curves for microvillus growth events in cells expressing EGFP-EPS8 V690D/L694D (lavender) and mCherry-ESPN (magenta); n = 9 events from 4 cells. For D and E, t = 0 is defined as −3 frames (1.5 min) before the appearance of the EGFP-EPS8 LNK:AAA or EGFP-EPS8 V690D L694D signal. All images shown are maximum intensity projections. For all normalized intensity vs. time curves, dashed lines indicate when curves cross a normalized intensity of 0.5. For all curves, the solid line represents the mean and shading represents SD. See also Figure S3.
Nascent microvilli also emerge from pre-existing protrusions
In contrast to de novo growth events, we unexpectedly observed that microvilli grow from pre-existing microvilli. In these cases, a nascent “mother” microvillus gave rise to one or more “daughter” microvilli (Figure 3A, M = mother, D = daughter). New daughter core bundles grew at angles to the pre-existing mother, giving rise to a structure with a branched or forked appearance (see 3 min time point, Figure 3A). Daughter microvilli typically emerged from the base or the side of an existing mother bundle, and 3D volume rendering of these structures confirmed that daughters are initially physically linked to their mothers (Figure S4A, Video S4). New daughter bundles eventually separated from mother bundles to become individualized protrusions (5 min time point, Figure 3A). Using SEM, we also observed branched microvilli on the apical surface of CL4 cells expressing ESPN (Figure 3B), suggesting that branched core bundles are in fact wrapped in plasma membrane. From SEM images, we estimated that ~25% of all microvilli appeared physically connected in a branched structure (Figure 3B, zoom 1) or oriented relative to a neighboring protrusion at an angle consistent with a branched structure (Figure 3B, zoom 2). Given that daughter microvilli separate from their mothers after their initial growth, 25% likely marks a lower bound for the fraction of total microvilli that are generated using this mechanism.
Figure 3. New microvilli can grow from pre-existing microvilli.
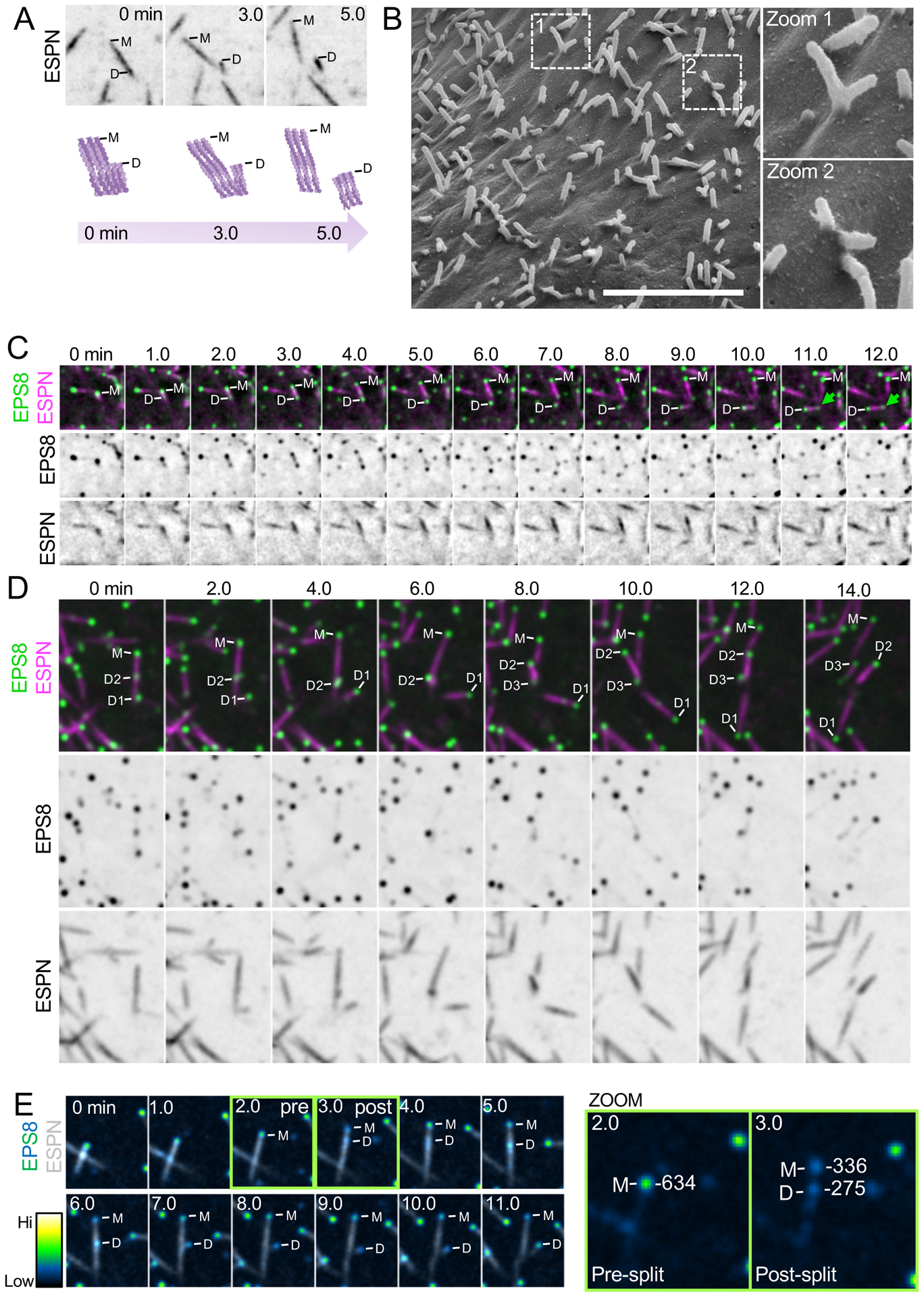
(A) Top, representative montage of a daughter microvillus growing from the base of an existing mother bundle in a CL4 cell expressing mCherry-ESPN. “M” denotes the mother microvillus and “D” denotes the growing daughter. Box width = 6 μm. Bottom, cartoon schematic of mother/daughter growth corresponding to montage above. Related to Figure S4 and Video S4. (B) SEM image of a CL4 cell expressing mCherry-ESPN at an early stage of differentiation. Zoom 1 highlights a branched mother/daughter structure whereas zoom 2 likely shows core bundles that may still be physically coupled beneath the plasma membrane. Scale bar = 4 μm. (C) Representative montage of a daughter microvillus growing from the base of an existing mother bundle in cells expressing EGFP-EPS8 and mCherry-Espin. “M” denotes EPS8 at the tip of the mother microvillus and “D” denotes EPS8 which will eventually mark the tip of a growing daughter microvillus. Green arrow highlights an EPS8 punctum forming at the base of the daughter bundle. Box width = 6 μm. (D) Representative montage of a mother microvillus in a CL4 cell expressing EGFP-EPS8 and mCherry-ESPN giving rise to multiple daughter microvilli from the base of the mother bundle. “M” denotes EPS8 at the tip of the mother microvillus and “D1–3” denote EPS8 puncta at the tips of growing daughters. Box width = 6.6 μm. (E) Montage of a daughter microvillus growth event (left) with EGFP-EPS8 pseudocolored by intensity. Green boxes highlight pre- and post-split frames. The corresponding quantification of EGFP-EPS8 integrated 16-bit intensity pre- and post-splitting of EPS8 tip punctum is highlighted in the zoom panels to the right. Note that the total post-split integrated intensity is nearly equal to the pre-split integrated intensity. Box width = 5.6 μm. All images shown are maximum intensity projections. See also Figure S4.
In cells co-expressing EPS8 and ESPN, the growth of daughter microvilli was marked by small dim puncta of EPS8 that traveled retrograde from the distal tip of an existing mother to a site near its base (Figure 3C). Consistent with this observation, we also noted dim EPS8 puncta localized to the base of microvilli in fixed CL4 cells stained for endogenous EPS8 (Figure S1A, top zoom, green arrows). By specifically tracking the fate of the EPS8 puncta that emerged during these events, we determined that daughter core bundles, in turn, serve as new mothers that give rise to new daughters, propagating the cycle of biogenesis (Figure 3D). Interestingly, the intensity of EPS8 at the distal tip of a microvillus dropped following the appearance of dim retrograde traveling puncta (compare pre vs. post, Figure 3E). Intensity analysis of distal tip vs. retrograde traveling EPS8 puncta revealed that the EPS8 signal that gives rise to daughter microvilli appears at the expense of EPS8 signal at the distal tip (Figure 3E, Zoom). Based on these data, we propose that formation of daughter microvilli from pre-existing mothers represents a distinct mechanism from de novo growth, which takes advantage of assembly factors such as EPS8 that are enriched in the vicinity of pre-existing structures.
IRTKS is also enriched in puncta that give rise to new microvilli
Previous work from our group implicated IRTKS as a binding partner of EPS8 and demonstrated that these proteins work together to promote the elongation of microvilli23. IRTKS possesses both membrane and actin binding motifs through its I-BAR and WH2 domains, respectively, and localizes to the tips of nascent microvilli in the crypts of intestinal organoids and W4 cells in culture23,32. Based on these properties, we sought to determine if IRTKS, like EPS8, accumulates in the puncta that give rise to microvilli. EGFP-IRTKS (herein referred to as IRTKS) exhibited enrichment in discrete puncta at the tips of existing microvilli, and also demonstrated some lateral localization (Figure 4A), as shown previously28. Like EPS8, IRTKS marked sites of microvilli growth (Figure 4B, Video S5). Based on time series intensity plots, we found that on average ESPN begins to accumulate at the same time as IRTKS at these sites, albeit at a slower rate (Figure 4D). The growth of daughter microvilli was also marked by small dim IRTKS puncta that were laterally associated with pre-existing mother core bundles (Figure 4C). Thus, EPS8 binding partner IRTKS also accumulates in diffraction limited puncta that eventually give rise to new microvilli.
Figure 4. IRTKS is enriched in puncta that give rise to new microvilli.
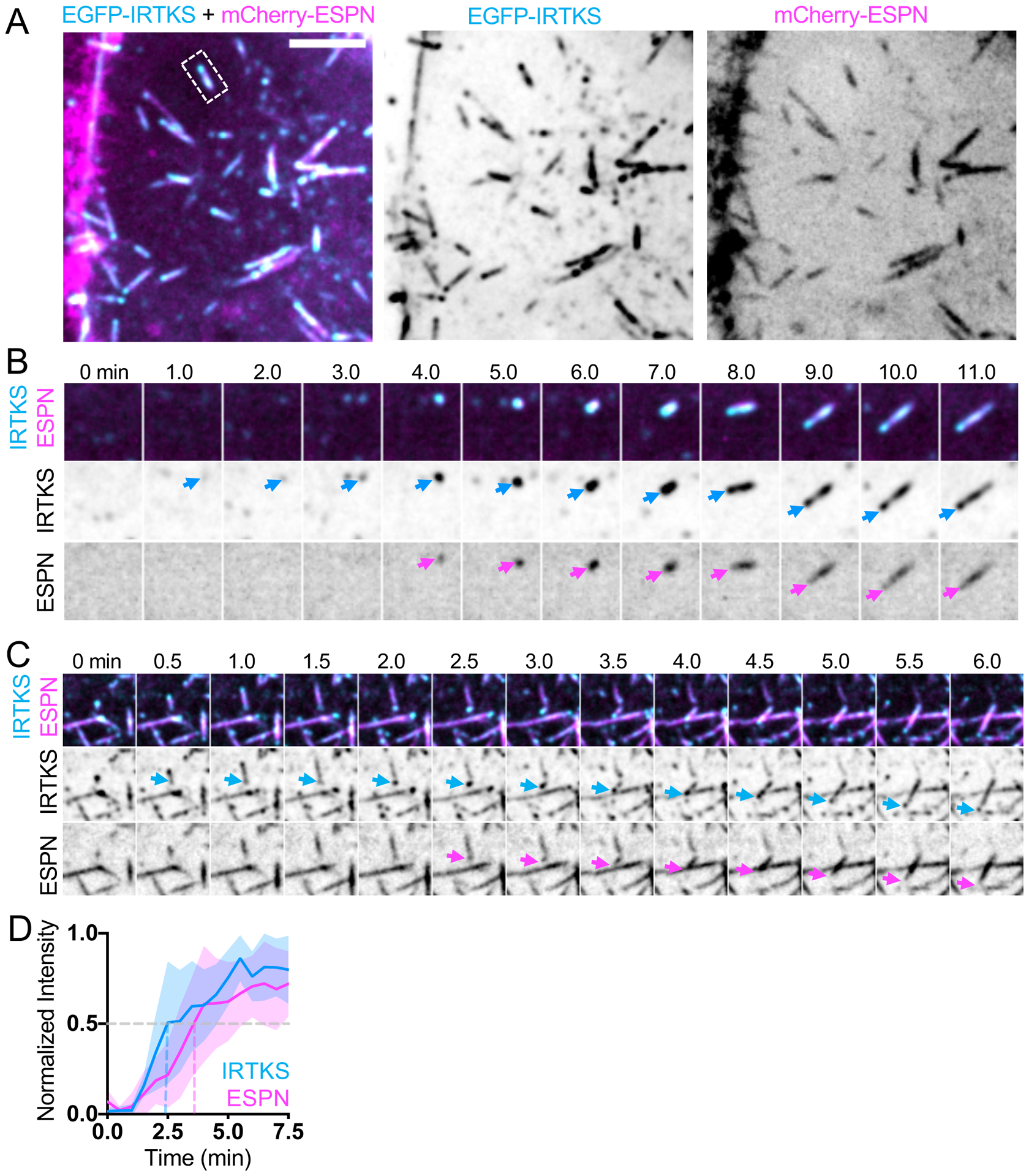
(A) Maximum intensity projection of a CL4 cell expressing mCherry-ESPN (magenta) and EGFP-IRTKS (cyan). Merge (left), EGFP-IRTKS (middle), mCherry-ESPN (right). Dashed box indicates microvillus shown in B. Scale bar = 5 μm. (B) Montage of a de novo microvillus growth event in a cell expressing EGFP-IRTKS and mCherry-ESPN. Arrows denote the presence of EGFP-IRTKS (cyan) or mCherry-ESPN (magenta). Box width = 4 μm. Related to Video S5 (C) Montage of daughter microvillus growing from pre-existing mother bundle in a cell expressing EGFP-IRTKS and mCherry-ESPN. Arrows denote EGFP-IRTKS (cyan) and mCherry-ESPN (magenta) of the daughter microvillus. Box width = 6 μm. (D) Normalized intensity vs. time curve of CL4 cells expressing EGFP-IRTKS and mCherry-ESPN; n = 9 events from 4 cells. T = 0 is defined as −3 frames (1.5 min) before the appearance of the EGFP-IRTKS signal. All images shown are maximum intensity projections. Dashed lines indicate point at which curves cross a normalized intensity of 0.5. For all curves, the solid line represents the mean and shading represents SD.
Core actin bundle elongation coincides with plasma membrane wrapping
As microvilli are plasma membrane wrapped protrusions, and because IRTKS directly interacts with the membrane through its I-BAR domain33, we sought to determine the timing of membrane wrapping relative to the formation of IRTKS puncta and the subsequent growth of core bundles. To test whether enrichment of IRTKS precedes membrane encapsulation, we incubated cells co-expressing IRTKS and ESPN with the live cell plasma membrane label CellMask-DeepRed (herein referred to as CellMask) (Figure 5A and 5D, Video S6). Individual microvilli typically exhibited CellMask enrichment at the distal end, which we interpreted as membrane encapsulation (Figure 5F). During de novo growth events, IRTKS enriched in puncta prior to the accumulation of ESPN and CellMask, which both increased in parallel (Figures 5A and 5D). Additionally, we found that daughter microvilli also become wrapped in membrane (Figure 5B), which is consistent with the branched microvilli observed in SEM images (Figure 3B). We next asked if membrane wrapping during microvillus growth was driven by the accumulation of membrane-actin linker proteins34. Here, we focused on the Ezrin-Radixin-Moesin (ERM) family protein EZR, which is known to stabilize microvilli34. Live imaging revealed that EZR-EGFP (herein referred to as EZR) and ESPN accumulated in parallel during microvillus growth (Figures 5C and 5E, Video S7). Together, these data suggest that core bundle formation and membrane wrapping driven by EZR are parallel events in microvillus growth, which follow the appearance of EPS8 and IRTKS puncta at the apical surface.
Figure 5. Membrane encapsulation occurs in parallel with core bundle formation.
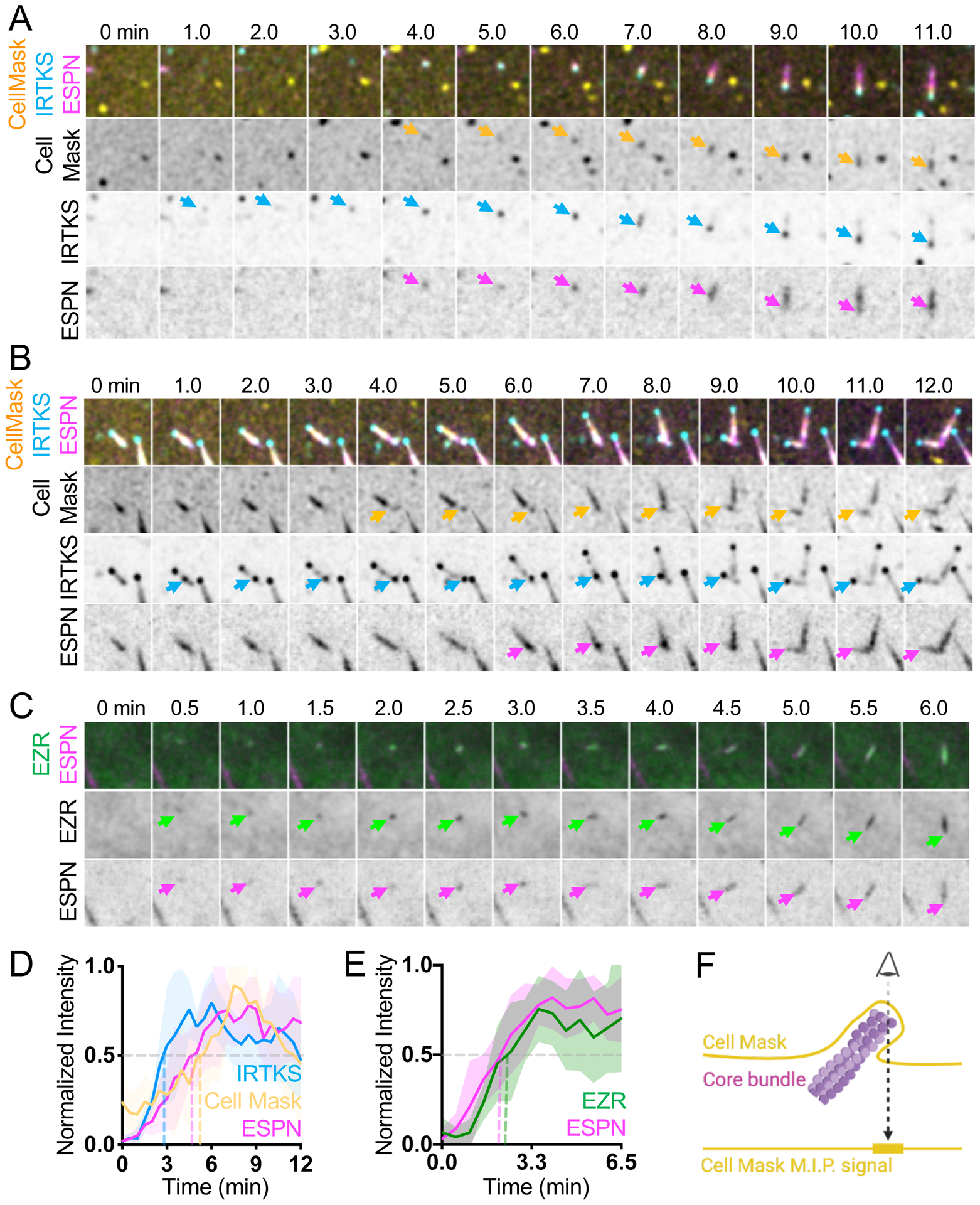
(A) Montage of de novo microvillus growth event in a CL4 cell expressing EGFP-IRTKS and mCherry-ESPN and stained with CellMask-DeepRed to mark the plasma membrane. Arrows denote the onset of membrane wrapping visualized by CellMask-DeepRed (yellow), or the presence of EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Box width = 4 μm. (B) Representative montage of daughter microvillus formation in a cell expressing EGFP-IRTKS and mCherry-ESPN and stained with CellMask-DeepRed. Arrows denote the onset of membrane wrapping as visualized by CellMask-DeepRed (yellow), or the presence of EGFP-IRTKS (cyan) and mCherry-ESPN (magenta) of the daughter microvillus. Related to Video S6. (C) Montage of a de novo microvillus growth event in a CL4 cell expressing EZR-GFP and mCherry-ESPN. Arrows denote the presence of EZR-GFP (green) or mCherry-ESPN (magenta). Box width = 4 μm. Related to Video S7. (D) Normalized intensity curves of cells expressing EGFP-IRTKS and mCherry-ESPN and stained with CellMask-DeepRed corresponding to montage in A; n = 6 growth events from 3 cells. (E) Normalized intensity curves of cells expressing EZR-GFP and mCherry-ESPN corresponding to montage in C; n = 11 growth events from 8 cells. (F) Schematic representation of a microvillus stained with cell mask and the resulting maximum intensity projected signal. All images shown are maximum intensity projections. Dashed lines for normalized intensity curves indicate point at which curves cross a normalized intensity of 0.5. For all curves, the solid line represents the mean and shading represents SD. For D and E, t = 0 is defined as −3 frames (1.5 min) before the appearance of the EGFP-IRTKS or EZR-EGFP signal.
Loss of membrane wrapping and tip targeted factors destabilizes core actin bundles
Analysis of our time-lapse data revealed that a subset of nascent microvilli collapsed, characterized by loss of ESPN signal and associated factors (Figure 6). Interestingly, collapse events were preceded by loss of EPS8 puncta from the distal tips (Figure 6A and 6C, Video S8), which was immediately followed by a decrease in core bundle length (Figure 6A and 6C). We also observed instances where core bundles appeared to “break”; in these cases, the core bundle remnant that maintained distal tip enriched EPS8 persisted, whereas the newer core bundle fragment that lacked EPS8 signal quickly collapsed (Figure S4B). Additionally, we found that remnants of collapsed bundles were occasionally used as substrate for growth of one or more daughter microvilli (Figure S4C). We observed the same general pattern with cells co-expressing IRTKS and ESPN, whereby loss of punctate IRTKS signal was followed by a decrease in microvillus length (Figures 6B and 6D). These results suggest that loss of EPS8 or IRTKS from the distal tip destabilizes nascent microvilli and leads to their collapse.
Figure 6. Microvilli cannot survive without EPS8 and IRTKS at the tips.
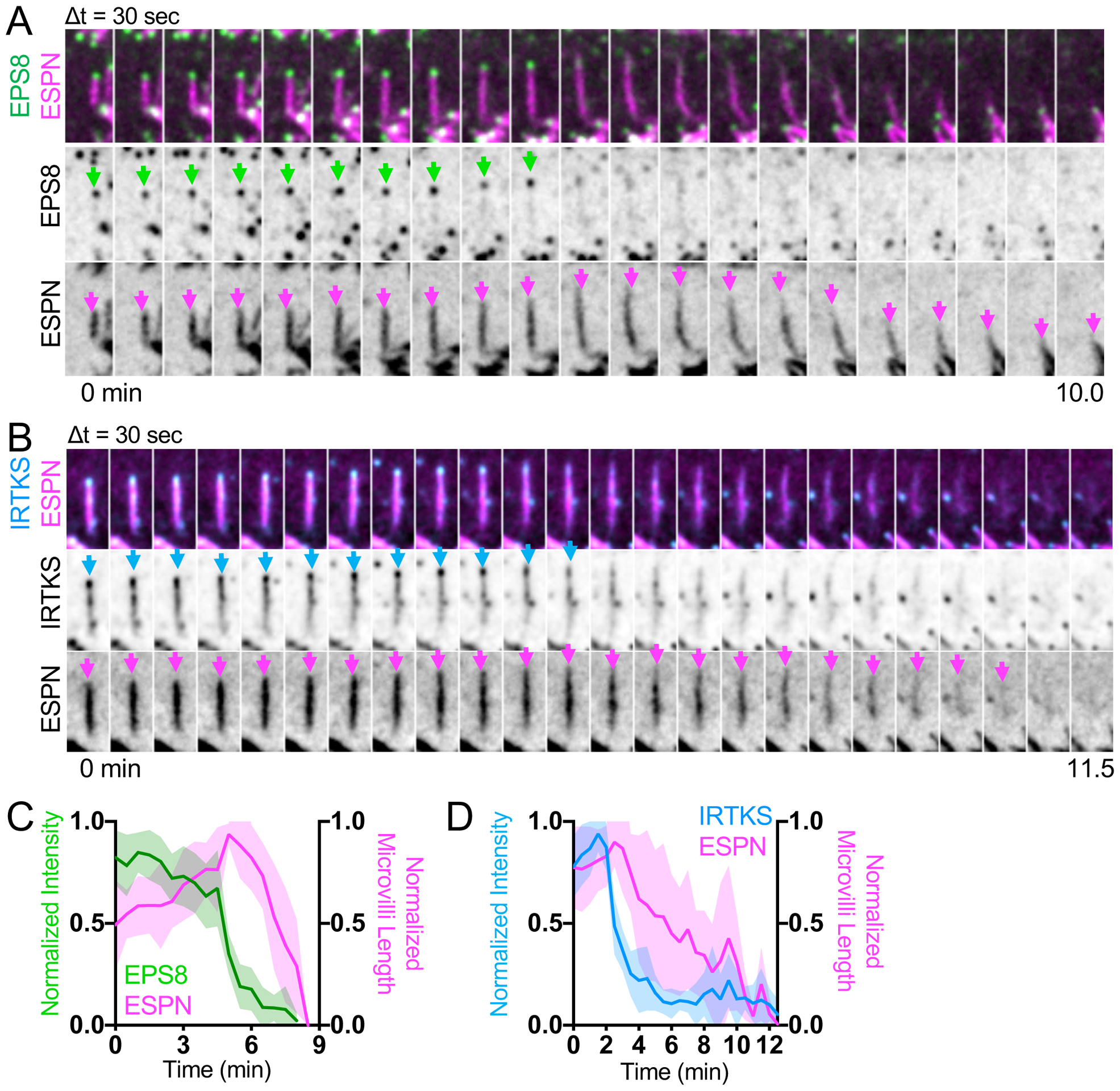
(A) Montage of a microvillus collapse event on the surface of a CL4 cell expressing EGFP-EPS8 and mCherry-ESPN. Green arrows denote tip targeted EGFP-EPS8 signal, whereas magenta arrows denote the core actin bundle. Box width = 2.5 μm. Related to Video S8. See also Figure S4. (B) Montage of microvillus collapse event in a cell expressing EGFP-IRTKS and mCherry-ESPN. Cyan arrows denote tip targeted EGFP-IRTKS signal. Magenta arrows denote the core actin bundle. Box width = 2.5 μm. (C) Quantification of EGFP-EPS8 intensity and microvilli length vs. time during microvillus collapse; n = 10 events from 6 cells. (D) Quantification of EGFP-IRTKS intensity and microvilli length vs. time during microvillus collapse; this plot was created by averaging traces of varying lengths ranging in duration from 4.5 – 12.5 min (IRTKS) and 4.5 – 12.5 min (ESPN); n = 12 events from 5 cells. For C, t = 0 is defined as −10 frames (5 min) before the drop in EGFP-EPS8 intensity from the tips of microvilli, whereas for D, t = 0 is defined as −5 frames (2.5 min) before the drop in EGFP-IRTKS intensity from the tips of microvilli. All images are represented as maximum intensity projections. For all curves, the solid line represents the mean and shading represents SD. See also Figure S4.
These observations prompted us to investigate upstream events that could potentially lead to loss of tip targeted factors and subsequent microvillus collapse. As IRTKS and EPS8 are enriched at the interface between membrane and core bundle, we again used CellMask to visualize the plasma membrane during collapse events. Interestingly, we found that CellMask enrichment, which we interpret as protruding membrane, is lost prior to collapse (Figure 7A–D). Loss of CellMask signal was followed by loss of punctate IRTKS signal and a subsequent decrease in microvillus length as marked by ESPN (Figures 7A, 7C, S4D, Video S9). Next, we determined if loss of membrane encapsulation was linked to a loss of EZR signal from nascent microvilli. Indeed, CellMask and EZR signals decreased in parallel, before a measurable decrease in microvillus length as marked by ESPN (Figures 7B, 7D, S4E, Video S10). These results suggest that microvilli cannot survive without membrane wrapping, loss of which is paralleled by loss of membrane-cytoskeleton linking, followed by loss of distal tip factors, and ultimately collapse of the core bundle. These findings also reveal that early in epithelial differentiation nascent microvilli undergo cycles of growth and collapse, which likely offer a degree of plasticity during the complex process of apical morphogenesis.
Figure 7. Membrane encapsulation is essential for microvillus survival.
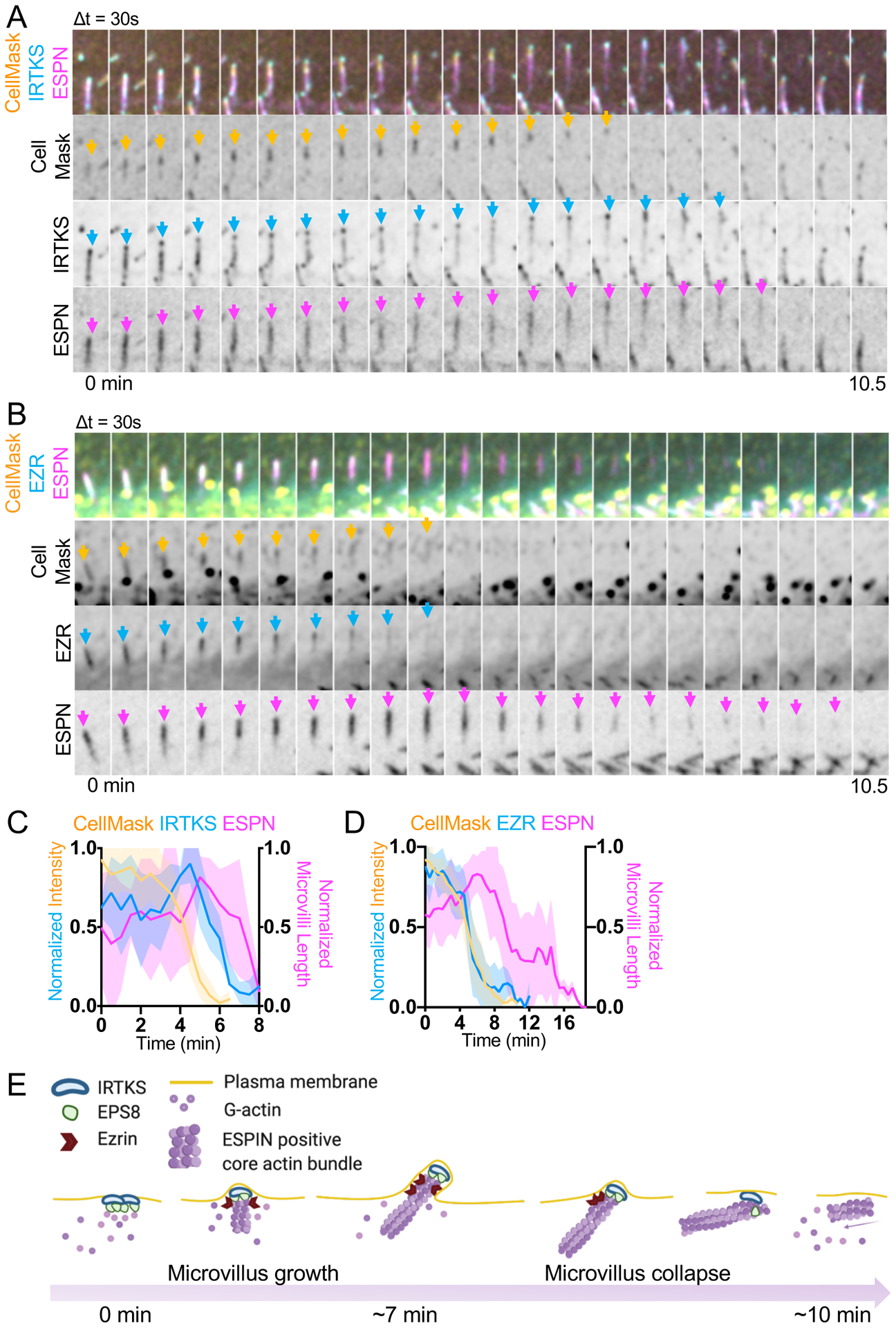
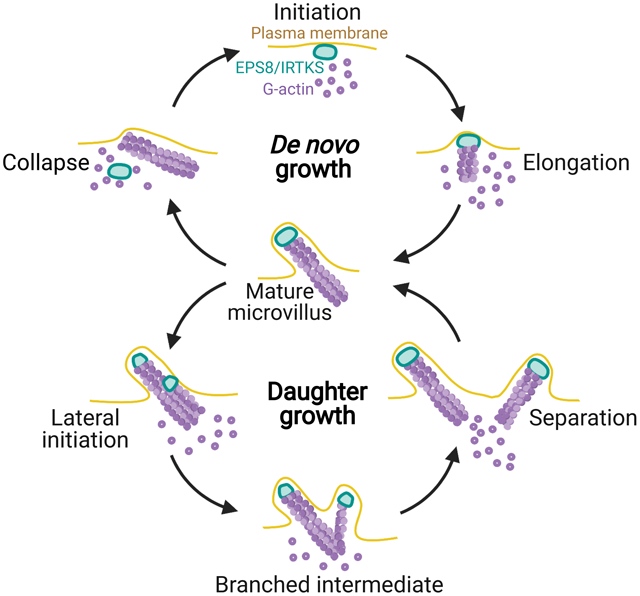
(A) Montage of a microvillus collapse event in a cell expressing EGFP-IRTKS and mCherry-ESPN, stained with CellMask-DeepRed. Box width = 2.5 μm. Related to Video S9. See also Figure S4D. (B) Montage of a microvillus collapse event in a cell expressing EZR-EGFP and mCherry-ESPN, stained with CellMask-DeepRed. Box width = 2.5 μm. Related to Video S10. See also Figure S4E. (C) Quantification of EGFP-IRTKS and CellMask-DeepRed intensity and microvilli length as represented in A; n = 4 events from 4 cells. (D) Quantification of EZR-EGFP and CellMask-DeepRed intensity and microvilli length vs. time. This plot was created by averaging traces of varying lengths ranging in duration from 6.5 – 12 min (EZR), 6 – 10.5 min (Cell Mask) and 7.5 – 18.5 min (ESPN); n = 15 events from 10 cells. For C and D, t = 0 is defined as −10 frames (5 min) before the drop in EGFP-IRTKS or EZR-EGFP intensity at the tips of microvilli. All images are represented as maximum intensity projections. For all curves, the solid line represents the mean and shading represents SD. (E) Model of de novo microvillus growth and collapse. Formation of EPS8 and IRTKS pucta coincide with local enrichment of G-actin followed by assembly of ESPN positive core actin bundles, recruitment of EZR, and plasma membrane protrusion. The process of de novo growth occurs on the scale of ~7 minutes. During microvillus collapse, nascent bundles lose membrane wrapping and enrichment of EZR in parallel, which in turn leads to loss of EPS8 and IRTKS, and subsequent collapse of the ESPN positive core actin bundle. The process of collapse also occurs on the scale of minutes. Model was created using BioRender. See also Figure S4.
DISCUSSION
Here we employed a live imaging approach to define a time-course for the recruitment of factors that drive the growth and stabilization of new microvilli. Using CL4 cells as an epithelial model system, our goal was to capture the earliest events underlying biogenesis of these protrusions. The resulting spatially and temporally resolved datasets allow us to stage the appearance of a core set of microvillar components including G-actin, tip enriched actin regulatory factors, filament bundling proteins, membrane-cytoskeleton linkers and the plasma membrane during this dynamic process (Figure 7E). The growth of a new microvillus takes place over the course of several minutes, similar to the timeframe reported for microvilli in Xenopus kidney epithelial A6 cells35. The earliest events that we were able to detect in this process are the enrichment of distal tip factors, EPS8 and IRTKS, in diffraction limited puncta at the apical surface. EPS8 enrichment is also accompanied by a moderate accumulation of G-actin. The tip enrichment of EPS8 is at least partially driven by binding to actin, as mutations in actin binding residues interfered with puncta formation and EPS8 puncta not associated with actin bundles typically exhibited lower intensities. Furthermore, mutation of the V690 and L694 residues resulted in delayed core bundle formation and decreased growth rates suggesting direct involvement of EPS8 in the growth of these structures. On average, ESPN begins to accumulate with the appearance of EPS8/IRTKS puncta, although at a slower rate. Simultaneous enrichment of ESPN with the CellMask membrane probe and membrane-actin linker EZR suggests that actin core bundles are assembled in close proximity to the apical surface, where they are quickly or immediately encapsulated in plasma membrane. The time course of molecular recruitment described here provides a framework for understanding how cells control the growth of surface features and may also apply to other finger-like protrusions such as stereocilia.
Because the appearance of EPS8 and IRTKS puncta preceded the formation of microvilli observed in our assays, it is tempting to speculate that these factors are components of the electron dense tip complex, which has long been proposed to function in initiating growth and thus, regulating protrusion numbers17. However, it also remains possible that other as-of-yet unidentified factors function upstream of EPS8 and IRTKS, to drive their accumulation at the plasma membrane and specify sites of growth. This latter possibility is consistent with previous studies showing that protrusion numbers are not significantly reduced in EPS8 loss-of-function models22,23,26.
In addition to de novo microvillus growth events, we also observed the formation of daughter microvilli from pre-existing mother protrusions. Formation of a new daughter core bundle was preceded by enrichment of EPS8 and IRTKS at the site of lateral growth from the mother. Relative to de novo assembly, such mother/daughter growth might be energetically favorable as materials for growing a new microvillus, such as distal tip factors and G-actin/F-actin, are enriched near pre-existing protrusions. In combination with de novo growth, mother/daughter growth might allow epithelial cells to quickly populate their apical surface with microvilli. This would be advantageous in the intestinal epithelium, where cells rapidly increase the number of microvilli as they migrate from stem cell containing crypts on to the villus36,37. The mother/daughter growth mechanism initially gives rise to structures with a forked appearance, and these are strikingly reminiscent of the “forked microvilli” captured during recovery from hydrostatic pressure effacement17. Remarkably, the authors noted electron dense plaques on the lateral surface of the newly formed core bundles, which were hypothesized to represent material that promotes the growth of new microvilli. One intriguing possibility is that the lateral plaques observed by Tilney and Cardell represent protein complexes that contain EPS8 and IRTKS, which would be consistent with our live time-lapse data. Moreover, given that Tilney and Cardell used intestinal epithelial samples from salamander and the observations we report here focused on cells derived from porcine kidney, the mother/daughter growth mechanism is likely conserved across species and tissue types.
Interestingly, not all nascent microvilli survive, and a notable fraction of the protrusions observed in our time-lapse datasets collapsed during the period of observation, consistent with previous observations using scanning ion conductance microscopy in Xenopus kidney epithelial A6 cells35 (Figure 7E). Collapse of a microvillus is predicted by loss of plasma membrane wrapping, which occurs in parallel with decreased membrane-cytoskeleton linking by EZR. Loss of membrane encapsulation is followed by loss of tip targeted EPS8 and IRTKS, which in turn is ultimately followed by collapse of the core actin bundle. This implies that in order to survive, core bundles must remain at least partially encapsulated by the plasma membrane. What leads to the loss of membrane-actin linking and membrane wrapping is not clear. As our time-lapse observations take place at the earliest stages of apical surface maturation, one possibility is that the cells are not yet expressing the factors needed to stabilize microvilli at high levels. This would be consistent with previous studies showing that a number of microvillus resident proteins gradually accumulate during the time course of differentiation38,39. An interesting example is provided by tip-targeted protocadherins, CDHR5 and CDHR2, which drive adherence between neighboring protrusions, and in turn might drive a mechanical capture mechanism that permits the long-term survival of new microvilli, as we proposed previously28.
While this study offers a temporally resolved molecular framework for understanding the growth of new microvilli, important questions remain unanswered. One question of longstanding interest pertains to mechanisms that control the number of actin filaments per core bundle. Microvilli in mature transporting epithelia exhibit a highly stereotyped number of actin filaments (~20–40) per core bundle8,9, suggesting that cells enlist mechanisms to count bundle components. One attractive hypothesis is that proteins in the distal tip complex control filament numbers17. If EPS8 does interact directly with filament barbed ends, the number of EPS8 molecules per tip puncta might be linked to the number of actin filaments per core bundle. Because the transfection-based studies described here rely on exogenous marker expression to label growing protrusions, our data do not allow us to define the absolute stoichiometry of EPS8 in tip targeted puncta. Future studies might address such questions by combining epithelial cell culture models expressing endogenously tagged molecules of interest with the methods for visualizing microvillus growth we introduce in this work.
Another important unanswered question asks how distal tip enriched factors are initially recruited to the plasma membrane. Epithelial cells are characterized by polarized separation of phosphatidylinositols, with phosphatidylinositol 4,5 bisphosphate (PI[4,5]P2) and phosphatidylinositol 3,4 bisphosphate (PI[3,4]P2) enriched at the apical surface and phosphatidyl inositol 3,4,5-trisphosphate (PI[3,4,5]P3) enriched basolaterally40–42. Although the emergence of EPS8 and IRTKS puncta appears to be stochastic in nature, the local enrichment of signaling lipids such as PI[4,5]P2, could be involved in recruitment of these factors. Both EPS8 and IRTKS contain structural motifs that make this a reasonable possibility. For instance, the EPS8 PTB domain shares sequence similarity with the PTB domain of Dab1, which has been shown to bind PI[4,5]P243. Similarly, other proteins of the I-BAR family such as MTSS1 (also known as MIM) and IRSP53 (also known as BAIAP2), have also been shown to directly bind PI[4,5]P233,44. Future time-resolved studies must focus on defining the nature of EPS8 interactions with membrane lipids and determining whether localization of EPS8 and IRTKS is controlled by specific lipid species.
In summary, this study is the first to capture the molecular details of microvilli biogenesis using a live imaging approach. The resulting time-lapse datasets allow us to construct a temporal framework for key factors involved in microvillus growth and offer insight into the nature of apical surface dynamics at early timepoints in epithelial differentiation. These results also hold implications for understanding the assembly and dynamics of related actin supported protrusions. For example, classic ultrastructural studies of early stage chick embryos revealed that the formation of stereocilia is preceded by the growth of microvilli45. A fraction of these microvilli are resorbed, and the remaining protrusions are believed to mature into stereocilia arranged in the characteristic staircase pattern. The collapse dynamics we report here may offer a mechanism to explain microvillar resorption during sensory epithelium differentiation.
STAR METHODS
LEAD CONTACT
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Matthew J. Tyska (matthew.tyska@vanderbilt.edu).
MATERIALS AVAILABILITY
Plasmids and cell lines generated during these studies will be made available upon request directed to the Lead Contact.
DATA AND CODE AVAILABILITY
No large-scale datasets or new codes were generated in this study.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture
LLC-PK1-CL4 (porcine kidney proximal tubule) and OK (opossum kidney, ATCC CRL-1849) cells were cultured in DMEM with high glucose and 2 mM L-glutamine supplemented with 10% fetal bovine serum (FBS) and 1% glutamine. CACO-2BBE cells (human colonic adenocarcinoma, ATCC CRL-2102) were cultured in DMEM with high glucose and 2 mM L-glutamine supplemented with 20% FBS and 1% glutamine. Ls174T-W4 cells (female human colon epithelial cells) were cultured in DMEM with high glucose and 2 mM L-glutamine supplemented with 10% tetracycline-free FBS, 1% glutamine, G-418 (1 mg/ml), blasticidin (10 μg/ml), and phleomycin (20 μg/ml). All cultures were maintained at 37°C with 5% CO2.
METHOD DETAILS
Cloning and constructs
pLVX-mCherry-ESPN encoding a tagged form of human espin-2B was used for all experiments except in Figures 1B–C and Figure 3B, which instead used mCherry-ESPN encoding a tagged form of rat espin-2B. EGFP-EPS8, and EGFP-IRTKS were described previously23,28, whereas EZR-EGFP was a gift from Dr. Stephen Shaw (NIH)46. The full length EGFP-EPS8 construct used for most experiments described herein contained two amino acid substitutions in the C-terminus (E818D and S820C) and made use of the stop codon in the plasmid backbone ~15 amino acids downstream of the native stop. All other EPS8 constructs are based on the sequence accession AAH30010.1 except for the indicated mutations or truncations. EGFP-EPS8 1–648 (primers 5’→3’ forward ATGAATGGTCATATTTCTAATCATCCC; 5’→3’ reverse TTATGACACAGGAACAGGTGCTGG) and EGFP-EPS8 649–822 (primers 5’→3’ forward AAGGTCCCAGCAAATATAACACGTCA; 5’→3’ reverse TTAGTGACTGCTTCCTTCATCAAAAGATTCC) constructs were generated by PCR, topo-cloned into the pCR8 entry vector (Invitrogen), and then shuttled into a gateway adapted EGFP-C1-GW vector via the LR recombination reaction. Point mutagenesis was performed on a pCR8-EPS8 template, and then shuttled into EGFP-C1-GW vector to create EGFP-EPS8 V690D L694D (primers 5’→3’ forward GAAATCTCAGATGGAGGAAGACCAAGATGAAGACATCCACAGACTGACCATTG; 5’→3’ reverse CAATGGTCAGTCTGTGGATGTCTTCATCTTGGTCTTCCTCCATCTGAGATTTC) and EGFP-EPS8 LNK758-760AAA (primers 5’→3’ forward GGTGCACAACTTTTCTCTGCCGCTGCGGATGAACTGAGGACAGTCTGCCC; 5’→3’ reverse GGGCAGACTGTCCTCAGTTCATCCGCAGCGGCAGAGAAAAGTTGTGCACC). To create pLVX-mCherry-βactin, mCherry-βactin was generated by PCR using primers (5’→3’ forward TAAGCAGAATTCGCCACCATGGTGAGCAAGG; 5’→3’ reverse TAAGCAGGGCCCCTAGAAGCATTTGCGGTGGACGA) and ligated into the pLVX-puro backbone (Takara) after digestion of the backbone and insert with EcoRI and ApaI. Constructs created for this study were confirmed via sequencing.
Stable cell line generation and transient transfection
Stable cell line generation:
To create cells stably expressing two fluorescent proteins, LLC-PK1-CL4 were first transiently transfected with EGFP tagged constructs and subsequently selected with 1 mg/mL G418 for approximately two to four weeks (EGFP-EPS8, EGFP-IRTKS, EGFP-EPS8LNKAAA, EGFP-EPS8 V690D L694D). Cells were then transduced with lentivirus expressing pLVX-mCherry-ESPN or pLVX-mCherry-β-actin to make the double stable cell line. Lentivirus was generated using either HEK293T or HEK293FT cells co-transfected with pLVX-mCherry-ESPN or pLVX-mCherry-β-actin, and psPAX2 and pMD2.G using Lipofectamine2000 (ThermoFisher), according to the manufacturer’s instructions. Media was changed 16 hrs after transfection, and virus was collected 72 and 96 hrs after transfection and concentrated using Lenti-X concentrator (Clontech). Cells were then transduced with virus after growing to 75% confluency and supplemented with 10 μg/mL polybrene. Media was changed one day after transduction and cells were placed under selection with puromycin (10 μg/mL concentration) 72 hrs after transduction. Established double stable cell lines were maintained in 1 mg/mL G418 and 10 μg/mL concentration puromycin. Cells expressing mCherry-ESPN were used previously28 and were created by transient transfection with pmCherry-ESPN selected with 1 mg/mL G418, sorted, and maintained in selection with 1 mg/mL G418.
Transient transfection:
W4 cells were seeded in 6 well plates and transfected at ~60% confluency, using Lipofectamine2000 according to manufacturer’s instructions. The following day, cells were re-plated on acid-washed glass coverslips and induced to form brush borders using 1 μg/ml doxycycline for 16 hr before fixation.
Immunofluorescent staining
Cultured cells:
Cells were rinsed 1x in prewarmed PBS and fixed by incubating in prewarmed 4% paraformaldehyde for 15 min at 37°C. Cells were then rinsed 3x for 5 min with PBS and permeabilized in 0.1% Triton X-100 for 15 min at RT. Cells were then rinsed with PBS 3x for 5 min and then blocked in 5% BSA for 1 hr at 37° C. Following blocking, cells were briefly rinsed 1x with PBS, and incubated with 1° antibodies for 1 hr at 37° C (mouse anti-EPS8, BD Transduction Laboratories 610144 1:400; Chicken IgY anti-GFP, GFP-1020 Aves Labs 1:200). Cells were then rinsed 4x for 5 min with PBS and incubated with 2° antibodies (Goat anti-mouse Alexa Fluor 488 F(ab’)2 fragment, Invitrogen A11017, 1:1000 dilution; Goat anti-mouse Alexa Fluor 568 F(ab’)2 fragment A11019, 1:1000 or Goat-anti chicken Alexa Fluor 488 IgG (H+L), Invitrogen A11039, 1:1000 dilution) and Alexa-Fluor 568 Phalloidin (Invitrogen A12380) or Alexa-Fluor 488 Phalloidin (Invitrogen Ref A12379) at 1:200 dilution for 30 min at RT. Following 2° antibody incubation, cells were rinsed 4x for 5 min in PBS and mounted on glass slides using ProLong Gold antifade reagent (Life Technologies).
Tissue:
Paraffin-embedded tissue sections of mouse WT small intestinal swiss rolls and kidney sections were deparaffinized using HistoClear solution (Fisher) and rehydrated in a descending graded ethanol series (100%, 95%, 90%, 70%, 50%) and rinsed with PBS 3x for 5 min. Slides were then subject to an antigen retrieval step consisting of boiling for 1 hr in a solution of 10 mM Tris (pH 9.0) and 0.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA). Slides were then washed in PBS 3x and blocked with 5% normal goat serum (NGS) ON at 4°C. After blocking, slides were briefly rinsed with PBS and stained ON at 4° C with antibodies (mouse anti-EPS8, BD Transduction Laboratories Cat#610144, 1:200; rabbit anti-Villin 1:50, Santa Cruz Cat# SC28283) in 1% NGS in PBS. After being washed with PBS four times, samples were incubated with secondary antibodies (Goat anti-mouse Alexa Fluor 488 F(ab’)2 fragment, 1:1000; anti-Rabbit H+L Alexa Fluor 568 1:1000 A11011) in 1% NGS in PBS for 2 hr at RT. Slides were then washed 4x with PBS, dehydrated in an ascending ethanol series (50%, 70%, 90%, 95%, 100%), and mounted using ProLong Gold Antifade reagent (Life Technologies).
Drug treatment
Wild-type W4 cells were treated with 1 μg/ml doxycycline for 16 hr prior to cytochalasin D treatment to induce brush border formation. Cells were then treated with 500 nM cytochalasin D or an equivalent volume of DMSO diluted in W4 media for 30 min. Cells were then fixed in 4% paraformaldehyde and stored at 4° C in 1x PBS prior to staining.
Live cell membrane labeling
CL4 cells were incubated with a 1.5x concentration of CellMask-DeepRed plasma membrane dye (Molecular Probes) in Fluorobrite Medium (Gibco) supplemented with 10% FBS and 1% L-glutamine for 10 min at 37° C. Cells were subsequently imaged in supplemented Fluoroborite Medium to minimize background fluorescence.
Microscopy
Light microscopy:
Laser scanning confocal microscopy was performed using a Nikon A1R or A1 equipped with 488 and 561 nm excitation lines, and 100x/1.49 NA TIRF, 40x/1.3 NA oil, and 25x/1.05 NA silicon immersion objectives. Structured Illumination Microscopy was performed using a Nikon N-SIM equipped with and Andor DU-897 EMCCD camera, four excitation lines (405, 488, 561, and 647 nm), and a 100x/1.49 NA TIRF objective. All images were reconstructed using Nikon Elements software with matching reconstruction parameters. Spinning disk confocal microscopy was performed using a Nikon Ti2 inverted light microscope equipped with a Yokogawa CSU-X1 spinning disk head, a Photometrics Prime 95B sCMOS or Hamamatsu Fusion BT sCMOS camera, 488 nm and 561 nm excitation lasers, and a 100x/1.49 NA TIRF objective. Cells were maintained in a stage top incubator at 37° C with 5% CO2 (Tokai Hit). In order to capture microvillus growth and collapse events, subconfluent to recently confluent CL4 cells plated on plasma cleaned 35 mm glass bottom dishes (CellVis) were imaged at 30 sec intervals for 30 min in CL4 media. CL4 cells expressing EGFP-EPS8 and mCherry-β-actin were plated on plasma cleaned 35-mm glass bottom dishes coated with 50 μg/mL laminin to minimize cell lifting during the acquisition. Z stacks of ~3 – 5μm were collected depending on cell thickness and sampled using a 0.18 μm step size with a triggered NIDAQ piezo Z stage. Image acquisition was performed using Nikon Elements software. Movies with intensities compared across conditions were collected using matching laser power and exposure times.
Electron microscopy:
All scanning electron microscopy (SEM) reagents were purchased from Electron Microscopy Sciences. To prepare samples for EM, cells were plated on glass coverslips and washed once with warm SEM buffer (0.1M HEPES, pH 7.3) supplemented with 2 mM CaCl2, then sequentially fixed for 1 hr at RT with 2.5% glutaraldehyde and 4% paraformaldehyde in SEM buffer supplemented with 2 mM CaCl2, washed with SEM buffer, incubated in 1% tannic acid in SEM buffer for 1 hr, washed with ddH2O, incubated with 1% OsO4 in ddH2O for 1 hr, washed with ddH2O, incubated with 1% uranyl acetate in ddH2O for 30 min, then washed with ddH2O. Samples were dehydrated in a graded ethanol series. After dehydration, SEM samples were then dried using critical point drying and mounted on aluminum stubs and coated with gold/palladium using a sputter coater. SEM imaging was performed using Quanta 250 Environmental-SEM operated in high vacuum mode with an accelerating voltage of 5–10 kV.
Quantification and Statistical Analysis
Time series analysis of microvillus growth:
Events were analyzed manually using tools in FIJI. All measurements were performed on z-projected images deconvolved using the Richardson-Lucy algorithm with 20 iterations in Nikon Elements Software. For EGFP and mCherry co-expressing cells, t = 0 was defined as −3 frames (1.5 min) before the appearance of the EGFP signal. A circular ROI was used to measure the mean EGFP signal, and the same ROI was then used to track the mCherry signal (ESPN or βactin) of the distal to middle region of the growing microvillus. ROIs of approximately the same size were used for all growth events. For cells expressing mCherry-ESPN only, t = 0 was defined as −3 frames (1.5 min) before the appearance of the mCherry signal, with a circular ROI used to measure the distal to middle region of the growing microvillus. For de novo growth, events were excluded if they held less than 3 frames (1.5 min) before the appearance of the EGFP signal, if the microvillus collapsed before reaching steady state growth, if a single bundle immediately gave rise to multiple microvilli, or if significant overlap with adjacent microvilli confounded our analysis. Using Prism 10 (GraphPad), mean intensity values were first normalized to account for intensity differences between microvilli, with the smallest value equal to 0 and the largest value equal to 1. Normalized values were then averaged and plotted with SD. Photobleaching corrections were not included in the analysis, as there was < 5–10% loss of signal over 10 min with EGFP and mCherry tagged probes.
Percent of ESPN positive bundles with EPS8 puncta:
Nikon Elements using “Define Threshold” to segment ESPN positive bundles and “Spot Detection” to detect puncta. The total numbers of ESPN bundles and EPS8 puncta were measured and the “Having” function was used to determine the fraction of ESPN positive bundles with overlapping with EPS8 signal.
EGFP-EPS8 puncta intensities (Figure 1F):
Nikon Elements using the “Spot Detection” function on the first frame of each time-lapse movie using a cell shaped ROI. Histograms of 16-bit intensities were generated using Prism 10 (Graphpad).
EGFP-EPS8 puncta lifetime (Figure 1H):
Nikon Elements using the “Spot Detection” to detect puncta and “Track Binaries” to track puncta intensities and durations. Mean puncta intensities were then binned into two categories - events lasting < 7 min vs. those lasting ≥ 7min - then plotted using Prism 10 (Graphpad).
Distal tip:cytosol ratios (Figure S1G and S3C):
Intensity measurements were performed in FIJI using SIM images. Images were acquired using matching laser power and exposure times within experiments. For EGFP-tagged proteins, images were excluded from analysis if raw EGFP intensities were < ~2000 gray values or > ~10,000 gray values – within this range EGFP-EPS8 recapitulated normal EPS8 localization. The EGFP or AlexaFluor488 signal was manually thresholded in each cell, and a rectangular ROI was drawn to measure the mean intensity at the tips of microvilli and in the cytoplasm, excluding the nuclear region. The same ROI area was used within a single cellular measurement, but not necessarily between cells, as to account for variation in cell size and shape.
Microvillus growth rate (Figure S3D):
The length of the microvillus was measured in FIJI starting at the first frame of detectable coalesced mCherry-ESPN signal (Lengthinitial) and then either the last frame of the movie, or the last frame with the microvillus mostly parallel to the cell surface (Lengthfinal). Events were excluded if the microvillus was mostly perpendicular to the cell surface, or if the growth event lasted less than 3.5 minutes. The growth rate was then calculated using (Lengthfinal − Lengthinitial)/(Timefinal−Timeinitial), with individual growth rates represented in the plot.
Initial mean growth intensities (Fig S3E):
Values were extracted from time series analysis of microvillus growth events. The initial mean intensity is defined as the background subtracted mean intensity of the first frame of detectable EGFP signal (t = 2 min for time series analysis).
Time series analysis of microvillus collapse:
Events were measured manually in FIJI. All measurements were performed on z-projected images deconvolved using the Richardson-Lucy algorithm in Nikon Elements software as described above. For intensity and length measurements defining microvillus collapse, t = 0 is defined as −10 frames before the characteristic drop in EGFP intensity (EGFP-EPS8, EGFP-IRTKS or EZR-GFP), unless specified in figure legends. A circular ROI was drawn to track the EGFP signal at the distal tips of microvilli. This same ROI was then used to measure CellMask-DeepRed intensity, when applicable. Circular ROIs of approximately the same size were used on all events. To measure lengths, a linear ROI was drawn to measure the change in microvilli length over time. Mean intensity values and lengths were first normalized and then averaged using Prism 10 (GraphPad), and plotted with SD. As individual collapse intensity and length traces varied on the timescale of a few minutes, some complementary montage examples may include timepoints outside of the plotted data range. However, plotted averages represent data normalized to the maximum time shown. Photobleaching corrections were not included in the analysis, as there was only ~5–10% loss of signal over 10 min with EGFP and mCherry tagged probes.
Mother/daughter growth event analysis:
SEM images were analyzed in FIJI by counting the total number of microvilli using the multipoint tool. Microvilli were scored as mother/daughter structures if they were physically connected or were in orientations consistent with recently separated microvilli (within ~100 nm), with each individual microvillus counted separately. 1,963 total microvilli from four cells were analyzed. The number of mother/daughter growth events was then calculated as a fraction of the total number of microvilli.
Statistical Analysis:
Statistical testing was performed in Prism 10 (GraphPad), by first running a D’Agostino and Pearson test to determine normality, and then a Mann-Whitney test or an unpaired t-test for pairwise comparison (Fig 1H, Fig S2G) or ANOVA with Kruskal-Wallis to compare more than two conditions (Figs. S3 C–E). For all figures, error bars are SD. Exact n values and their definitions are reported in figure legends.
Supplementary Material
Video S1. Visualization of microvilli on the surface of CL4 cells. Related to Figure 1. Dynamic microvilli on the surface of a CL4 cell expressing EGFP-EPS8 and mCherry-ESPN. Left: EGFP-EPS8 in green and mCherry-ESPN in magenta. Right: EGFP-EPS8 in white and mCherry-ESPN depth coded in z by warm colors (bottom of the z-stack) to cool colors (top of the z-stack). Scale bar = 5μm.
Video S2. De novo microvillus growth from an EPS8 puncta. Related to Figure 1. De novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S3. β-actin accumulates with EPS8 during de novo microvillus growth. Related to Figure 1. De novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-β-actin (magenta). Scale bar = 1μm.
Video S4. Daughter bundles are initially physically linked to mother bundles. Related to Figure 3. 3-dimensional rendering of a microvillus actin bundle (mCherry-ESPN), demonstrating daughter microvilli are derived from the mother microvillus. The rendering is rotated so the daughter bundles are growing to the right of the mother bundle.
Video S5. De novo microvillus growth from an IRTKS puncta. Related to Figure 4. De novo microvillus growth event in a CL4 cell expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S6. IRTKS enrichment occurs prior to microvillus membrane wrapping. Related to Figure 5. De novo microvillus growth event in a CL4 cell stained with CellMask-DeepRed (yellow), and expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S7. Core bundle formation and membrane wrapping occur in parallel. Related to Figure 5. De novo microvillus growth event in a CL4 cell expressing EZR-EGFP (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S8. EPS8 is lost at the tips of microvilli prior to collapse. Related to Figure 6. Microvillus collapse event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S9. Loss of membrane wrapping precedes IRTKS loss prior to microvillus collapse. Related to Figure 7. A microvillus collapse event in a CL4 cell stained with CellMask-DeepRed (yellow) and expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S10. Loss of membrane encapsulation and EZR signal occur in parallel during microvillus collapse. Related to Figure 7. A microvillus collapse event in a CL4 cell stained with CellMask-DeepRed (yellow) expressing EZR-EGFP (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-EPS8 (mouse) | BD Transduction Laboratories | Cat# 610144; RRID:AB_397545 |
| anti-GFP(chicken) | Aves Labs | Cat# GFP-1020; RRID:AB_10000240 |
| Goat anti-mouse Alexa Fluor 488 F(ab’)2 fragment | Molecular Probes | Cat# A-11017; RRID:AB_143160 |
| Goat anti-mouse Alexa Fluor 568 F(ab’)2 fragment | Molecular Probes | Cat# A11019; RRID:AB_143162 |
| Goat-anti chicken Alexa Fluor 488 IgG (H+L) | Molecular Probes | Cat# A-11039; RRID:AB_142924 |
| anti-Villin (rabbit) | Santa Cruz | Cat# sc28283; RRID: AB_2215971 |
| anti-Rabbit H+L Alexa Fluor 568 | Molecular Probes | Cat# A11011; RRID:AB_143157 |
| Bacterial and Virus Strains | ||
| E. coli DH5-Alpha competent cells | Molecular Cell Biology Resource Core, Vanderbilt Medical Center | Item# DH5 Alpha |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa-Fluor 568 Phalloidin | ThermoFisher | Cat# A12380 |
| Alexa-Fluor 488 Phalloidin | ThermoFisher | Cat# A12379 |
| ProLong Gold Antifade Reagent | Invitrogen | Cat# P36930 |
| 16% Paraformaldehyde solution | Electron Microscopy Sciences | Cat# 15710 |
| Triton X-100 | Sigma | Cat# T8787 |
| Lenti-X concentrator | Clontech | Cat# 631231 |
| FuGENE 6 | Promega | Cat# E2691 |
| Polybrene Infection Reagent | Sigma-Aldrich | Cat# TR-1003-G |
| Lipofectamine 2000 | ThermoFisher | Cat# 11668019 |
| Laminin | Corning | Cat#354232 |
| Phleomycin | InvivoGen | Cat# ant-ph-5 |
| Blasticidin | InvivoGen | Cat# ant-bl-1 |
| G418 Sulfate | Goldbio | Cat#G-418-25 |
| Doxycycline | RPI | Cat# 10592-13-9 |
| Cytochalasin D | Sigma | Cat# C2618 |
| CellMask Deep Red plasma membrane stain | Invitrogen | Cat# C10046 |
| Histoclear II | National Diagnostics | HS-202 |
| DMSO | Sigma | Cat# D8418 |
| Glutaraldehyde 25% | Electron Microscopy Sciences | Cat# 16220 |
| Tannic Acid | Electron Microscopy Sciences | Cat# 21700 |
| Osmium Tetroxide | Electron Microscopy Sciences | Cat# 19112 |
| Experimental Models: Cell Lines | ||
| Ls174T-W4 cells | Gift from Hans Clevers (Utrecht University, Netherlands) | RRID: CVCL J074 |
| HEK293FT | ATCC | CRL-3216 |
| HEK293T | ATCC | CRL-11268 |
| LLC-PK1-CL4 | Gift from Dr. Carolyn Slayman (Yale University) | N/A |
| CACO-2BBE | Gift from Dr. Mark Mooseker (Yale University) | N/A |
| OK | ATCC | CRL-1840 |
| Oligonucleotides | ||
| EGFP-EPS8 1-648 FORWARD: ATGAATGGTCATATTTCTAATCATCCC | This paper | N/A |
| EGFP-EPS8 1-648 REVERSE: TTATGACACAGGAACAGGTGCTGG | This paper | N/A |
| EGFP-EPS8 649-822 FORWARD: AAGGTCCCAGCAAATATAACACGTCA | This paper | N/A |
| EGFP-EPS8 649-822 REVERSE: TTAGTGACTGCTTCCTTCATCAAAAGATTCC | This paper | N/A |
| EGFP-EPS8 V690D L694D FORWARD: GAAATCTCAGATGGAGGAAGACCAAGATGAAGACATCCACAGACTGACCATTG | This paper | N/A |
| EGFP-EPS8 V690D L694D REVERSE: CAATGGTCAGTCTGTGGATGTCTTCATCTTGGTCTTCCTCCATCTGAGATTTC | This paper | N/A |
| EGFP-EPS8 LNK758-760AAA FORWARD: GGTGCACAACTTTTCTCTGCCGCTGCGGATGAACTGAGGACAGTCTGCCC | This paper | N/A |
| EGFP-EPS8 LNK758-760AAA REVERSE: GGGCAGACTGTCCTCAGTTCATCCGCAGCGGCAGAGAAAAGTTGTGCACC | This paper | N/A |
| MCHERRY-BACTIN FORWARD: TAAGCAGAATTCGCCACCATGGTGAGCAAGG | This paper | N/A |
| MCHERRY-BACTIN REVERSE: TAAGCAGGGCCCCTAGAAGCATTTGCGGTGGACGA | This paper | N/A |
| Recombinant DNA | ||
| pEGFP-C1-EPS8 | 23, 28 | N/A |
| pEGFP-EPS8 1-648 | 23 | N/A |
| pEGFP-EPS8 649-822 | This paper | N/A |
| pEGFP-EPS8 V690D L694D | This paper | N/A |
| pEGFP-EPS8 LNK758-760AAA | This paper | N/A |
| pLVX-mCherry-βactin | This paper | N/A |
| pLVX-mCherry-Espin | This paper | N/A |
| pmCherry-Espin | Gift from Dr. James Bartles, NWU | N/A |
| pEzrin-EGFP | 46 | Addgene #20680 |
| pEGFP-C1-IRTKS | 23, 28 | N/A |
| psPAX2 | Dr. Didier Trono | Addgene #12260 |
| pMD2.G envelope plasmid | Dr. Didier Trono | Addgene #12259 |
| Software and Algorithms | ||
| NIS Elements AR Analysis | Nikon Instruments | https://www.nikoninstruments.com/Products/Software |
| Prism10 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| FIJI/ ImageJ | Open source software | http://imagej.net/Fiji/Downloads |
| Other | ||
| 35 mm glass bottom dishes | CellVis | D35-20-1.5-N |
Highlights.
EPS8 and IRTKS puncta mark sites of new microvillus growth.
EPS8 and IRTKS puncta remain enriched at the distal tips of nearly all microvilli.
Existing microvilli also serve as mothers that give rise to daughter protrusions.
Microvilli collapse when membrane wrapping and EPS8/IRTKS tip enrichment are lost.
ACKNOWLEDGEMENTS
The authors would like to thank all members of the Tyska laboratory, members of the Vanderbilt Microtubules and Motors Club, the Epithelial Biology Center, and the Cellular, Biochemical, and Molecular Sciences Training program for feedback and guidance. Microscopy was performed in part by the Vanderbilt Cell Imaging Shared Resource. This work was supported by the Vanderbilt Cellular, Biochemical and Molecular Sciences Training Grant 5T32GM008554-25 (I.M.G.), the NIH NIDDK National Research Service Award F31DK122692 (I.M.G.), American Heart Association Predoctoral Fellowship (M.M.P.), Program in Developmental Biology Training Grant 5T32HD007502-20 (C.S.C), NIH grant T32-A1007474 (L.M.M.), Department of Veterans Affairs Career Development Award 1IK2-BX004885 (L.M.M.), and NIH grants R01-DK111949 and R01-DK095811 (M.J.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, et al. (2012). The revised classification of eukaryotes. J Eukaryot Microbiol 59, 429–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peña JF, Alié A, Richter DJ, Wang L, Funayama N, and Nichols SA (2016). Conserved expression of vertebrate microvillar gene homologs in choanocytes of freshwater sponges. EvoDevo 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karpov SA, and Leadbeater BSC (1998). Cytoskeleton Structure and Composition in Choanoflagellates. Journal of Eukaryotic Microbiology 45, 361–367. [Google Scholar]
- 4.Helander HF, and Fandriks L (2014). Surface area of the digestive tract - revisited. Scand J Gastroenterol 49, 681–689. [DOI] [PubMed] [Google Scholar]
- 5.Schwander M, Kachar B, and Muller U (2010). Review series: The cell biology of hearing. J Cell Biol 190, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell RE, Benesh AE, Mao S, Tabb DL, and Tyska MJ (2011). Proteomic analysis of the enterocyte brush border. American journal of physiology. Gastrointestinal and liver physiology 300, G914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, Loew D, Delacour D, Gilet J, Brot-Laroche E, Rivero F, et al. (2012). A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Molecular biology of the cell 23, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mooseker MS, and Tilney LG (1975). Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol 67, 725–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohta K, Higashi R, Sawaguchi A, and Nakamura K (2012). Helical arrangement of filaments in microvillar actin bundles. Journal of structural biology 177, 513–519. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher A, and Weber K (1979). Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci U S A 76, 2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartles JR, Zheng L, Li A, Wierda A, and Chen B (1998). Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol 143, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretscher A, and Weber K (1980). Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol 86, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bretscher A (1983). Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol 97, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berryman M, Franck Z, and Bretscher A (1993). Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105, 1025–1043. [DOI] [PubMed] [Google Scholar]
- 15.Howe CL, and Mooseker MS (1983). Characterization of the 110-kdalton actin-calmodulin-, and membrane-binding protein from microvilli of intestinal epithelial cells. J Cell Biol 97, 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegan PS, Giral H, Levi M, and Mooseker MS (2012). Myosin VI is required for maintenance of brush border structure, composition, and membrane trafficking functions in the intestinal epithelial cell. Cytoskeleton (Hoboken) 69, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilney LG, and Cardell RR (1970). Factors controlling the reassembly of the microvillous border of the small intestine of the salamander. J Cell Biol 47, 408–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, and Borisy GG (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 160, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, and Kachar B (2004). An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol 164, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, and Kachar B (2011). Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol 21, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, et al. (2006). Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol 8, 1337–1347. [DOI] [PubMed] [Google Scholar]
- 22.Croce A, Cassata G, Disanza A, Gagliani MC, Tacchetti C, Malabarba MG, Carlier MF, Scita G, Baumeister R, and Di Fiore PP (2004). A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat Cell Biol 6, 1173–1179. [DOI] [PubMed] [Google Scholar]
- 23.Postema MM, Grega-Larson NE, Neininger AC, and Tyska MJ (2018). IRTKS (BAIAP2L1) Elongates Epithelial Microvilli Using EPS8-Dependent and Independent Mechanisms. Curr Biol 28, 2876–2888 e2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, and Scita G (2004). Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 6, 1180–1188. [DOI] [PubMed] [Google Scholar]
- 25.Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, et al. (2010). Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS biology 8, e1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zampini V, Ruttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, et al. (2011). Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS biology 9, e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tocchetti A, Soppo CB, Zani F, Bianchi F, Gagliani MC, Pozzi B, Rozman J, Elvert R, Ehrhardt N, Rathkolb B, et al. (2010). Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One 5, e9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meenderink LM, Gaeta IM, Postema MM, Cencer CS, Chinowsky CR, Krystofiak ES, Millis BA, and Tyska MJ (2019). Actin Dynamics Drive Microvillar Motility and Clustering during Brush Border Assembly. Dev Cell 50, 545–556 e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretscher A, and Weber K (1979). Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci U S A 76, 2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwaenepoel I, Naba A, Da Cunha MM, Del Maestro L, Formstecher E, Louvard D, and Arpin M (2012). Ezrin regulates microvillus morphogenesis by promoting distinct activities of Eps8 proteins. Molecular biology of the cell 23, 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grega-Larson NE, Crawley SW, Erwin AL, and Tyska MJ (2015). Cordon bleu promotes the assembly of brush border microvilli. Molecular biology of the cell 26, 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Pykalainen A, and Lappalainen P (2011). I-BAR domain proteins: linking actin and plasma membrane dynamics. Curr Opin Cell Biol 23, 14–21. [DOI] [PubMed] [Google Scholar]
- 33.Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PK, Butcher SJ, and Lappalainen P (2009). Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol 19, 95–107. [DOI] [PubMed] [Google Scholar]
- 34.Pelaseyed T, and Bretscher A (2018). Regulation of actin-based apical structures on epithelial cells. J Cell Sci 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik J, Shevchuk AI, Frolenkov GI, Diakonov IA, Lab MJ, Kros CJ, Richardson GP, Vodyanoy I, Edwards CR, Klenerman D, et al. (2003). Dynamic assembly of surface structures in living cells. Proc Natl Acad Sci U S A 100, 5819–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dongen JM, Visser WJ, Daems WT, and Galjaard H (1976). The relation between cell proliferation, differentiation and ultrastructural development in rat intestinal epithelium. Cell Tissue Res 174, 183–199. [DOI] [PubMed] [Google Scholar]
- 37.Specian RD, and Neutra MR (1981). The surface topography of the colonic crypt in rabbit and monkey. Am J Anat 160, 461–472. [DOI] [PubMed] [Google Scholar]
- 38.Crawley SW, Shifrin DA Jr., Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, et al. (2014). Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heintzelman MB, and Mooseker MS (1990). Assembly of the brush border cytoskeleton: changes in the distribution of microvillar core proteins during enterocyte differentiation in adult chicken intestine. Cell motility and the cytoskeleton 15, 12–22. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, and Mostov K (2007). PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, and Mostov K (2006). Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8, 963–970. [DOI] [PubMed] [Google Scholar]
- 42.Roman-Fernandez A, Roignot J, Sandilands E, Nacke M, Mansour MA, McGarry L, Shanks E, Mostov KE, and Bryant DM (2018). The phospholipid PI(3,4)P2 is an apical identity determinant. Nat Commun 9, 5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, and Johnson GL (2005). Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol 345, 1–20. [DOI] [PubMed] [Google Scholar]
- 44.Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, and Lappalainen P (2007). Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol 176, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilney LG, Cotanche DA, and Tilney MS (1992). Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Development 116, 213–226. [DOI] [PubMed] [Google Scholar]
- 46.Hao JJ, Liu Y, Kruhlak M, Debell KE, Rellahan BL, and Shaw S (2009). Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol 184, 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Visualization of microvilli on the surface of CL4 cells. Related to Figure 1. Dynamic microvilli on the surface of a CL4 cell expressing EGFP-EPS8 and mCherry-ESPN. Left: EGFP-EPS8 in green and mCherry-ESPN in magenta. Right: EGFP-EPS8 in white and mCherry-ESPN depth coded in z by warm colors (bottom of the z-stack) to cool colors (top of the z-stack). Scale bar = 5μm.
Video S2. De novo microvillus growth from an EPS8 puncta. Related to Figure 1. De novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S3. β-actin accumulates with EPS8 during de novo microvillus growth. Related to Figure 1. De novo microvillus growth event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-β-actin (magenta). Scale bar = 1μm.
Video S4. Daughter bundles are initially physically linked to mother bundles. Related to Figure 3. 3-dimensional rendering of a microvillus actin bundle (mCherry-ESPN), demonstrating daughter microvilli are derived from the mother microvillus. The rendering is rotated so the daughter bundles are growing to the right of the mother bundle.
Video S5. De novo microvillus growth from an IRTKS puncta. Related to Figure 4. De novo microvillus growth event in a CL4 cell expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S6. IRTKS enrichment occurs prior to microvillus membrane wrapping. Related to Figure 5. De novo microvillus growth event in a CL4 cell stained with CellMask-DeepRed (yellow), and expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S7. Core bundle formation and membrane wrapping occur in parallel. Related to Figure 5. De novo microvillus growth event in a CL4 cell expressing EZR-EGFP (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S8. EPS8 is lost at the tips of microvilli prior to collapse. Related to Figure 6. Microvillus collapse event in a CL4 cell expressing EGFP-EPS8 (green) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S9. Loss of membrane wrapping precedes IRTKS loss prior to microvillus collapse. Related to Figure 7. A microvillus collapse event in a CL4 cell stained with CellMask-DeepRed (yellow) and expressing EGFP-IRTKS (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Video S10. Loss of membrane encapsulation and EZR signal occur in parallel during microvillus collapse. Related to Figure 7. A microvillus collapse event in a CL4 cell stained with CellMask-DeepRed (yellow) expressing EZR-EGFP (cyan) and mCherry-ESPN (magenta). Scale bar = 1μm.
Data Availability Statement
No large-scale datasets or new codes were generated in this study.


