Abstract
The earth’s climate is warming, accompanied by a consequently greater frequency and severity of extreme heat events. At the same time, lifespan is increasing and people of all ages are staying increasingly active. Age-related impairments in thermoregulatory function are well-documented, leading to increased heat-related health risks and reduced exercise/athletic performance for older adults in hot environmental conditions. High aerobic fitness improves body temperature regulation during exercise via augmented sweating and improved cardiovascular function, including cardiac output and skin blood flow, in humans of all ages. Consequently, the masters athlete is better suited for exercise/heat-stress compared to his or her less fit peers. However, while age and thermoregulation in general has been studied extensively, research on the most fit older adults including masters athletes is generally lacking.
The purpose of this review is to evaluate the currently-available literature regarding the impact of both primary aging and age-related fitness on thermoregulatory function during exercise in the heat. In so doing, we aim to (1) characterize the influence of fitness in mitigating age-related declines in thermoregulation, (2) address the limitations of prior experimental approaches for investigating age-related thermoregulatory impairments, (3) examine to what extent aerobic fitness can be maintained in the aging athlete, and (4) begin to address the specific environmental conditions in which age-related impairments in thermoregulatory function may place highly active older adults at increased risk for heat-related illness and injury and/or limited performance.
Keywords: Heat stress, sweating, skin blood flow, body temperature, exercise training
Introduction
The world’s aging population is growing rapidly, with one in six people in the world predicted to be over the age of 65 by 2050. In developed countries, a quarter of the adult life span is spent beyond the age of 651. In order to preserve the health span along the aging continuum, studies have focused on understanding the health-preserving effects of habitual physical activity, exercise training, and the successful phenotype of the older athlete.
Along with an aging population, the earth’s climate is warming, with an increase in frequency and severity of severe weather events, including heat waves2. There is a vast literature demonstrating impaired thermoregulatory and cardiovascular responses during heat exposure in aged adults, changes that contribute to increased morbidity and mortality of older adults during extreme heat exposure3. Although the health benefits of regular exercise training are known, there is a dearth of studies investigating thermoregulatory responses and the impact of extreme heat on the fittest of the aged – the older athlete.
This review will focus on characterizing thermoregulatory responses of older adults exercising in warm environments to give insights into thermoregulation in the older athlete in the context of healthy aging vs. the inevitable age-related decline in maximal aerobic capacity4. Accordingly, for the purpose of this review, age categories are broadly considered as young (18-35 years), middle aged (36-60 years), and older adulthood (>60 year). We rely primarily on three types of studies: (1) cross-sectional comparisons between young and older adults, (2) endurance training studies on older adults, and (3) multiple regression analyses to gain insights and draw our conclusions on a topic that has received relatively sparse attention over the years. Each of these experimental approaches has unique limitations with respect to the older athlete. Therefore, we carefully consider the impact of matching young and older exercising subjects for exercise workload (i.e., absolute, relative, or fixed rate of metabolic heat production), aerobic fitness and training status, biological sex, and anthropometric differences when evaluating cardiovascular responses and thermoregulatory effector mechanisms. We do so in sections related to sweating responses, cardiovascular responses, and body temperature responses to exercise in hot environments. Finally, we consider the question, “In what specific environments might these age-related impairments effect thermoregulatory function and put humans at a greater risk for heat-related illness and injury?”
Sweating
The capacity for humans to thermoregulate during exercise-heat stress conditions relies primarily on the ability to activate eccrine sweat glands via sympathetic cholinergic mechanisms. It is well-established that the sweating rate for a given increase in core temperature (Tc) decreases with advancing age5-8. This occurs in conjunction with a delayed onset threshold temperature and a reduced thermosensitivity of the sweating response9,10. Attenuated sweating capacity in older adults appears to be attributable to reduced sweat output per activated gland, rather than a reduced number of heat activated sweat glands11-13. Following intradermal injection of varying concentrations of the cholinergic analogue methylcholine (MCh) into the thighs of young and older men matched for aerobic fitness, there were no age-related differences in active gland density. However, there was a markedly lower sweat output per activated gland in the older group. A middle-aged group exhibited glandular sweat outputs that were intermediate to the young and older groups13, suggesting a progressive and continuous decline in sweat output per gland throughout adulthood.
Although as a population, older adults have an impaired sweat capacity compared to their younger counterparts, at least part of this reduction is due to declines in physical fitness and/or training status rather than chronological age. The sweating rate during exercise is strongly determined by both acclimation status and . Sweating rates are preserved in highly fit older men and women, albeit with a higher degree of variability10, 14-17. In older (58 years) and young (38 years) women matched for body surface area, sweating was correlated with , but not age, when resting in hot, dry conditions (40°C, 22.2 torr)15. These findings were extended in a study examining thermal transients, where again sweating rate was strongly determined by rather than age16. Further, multiple regression analysis of exercise (60 W for 1 h) in a warm environment (35°C, 80% relative humidity (RH)) in a wide range of subject ages (20-73 years) indicated a significant relation between sweat loss and , but not age (importantly, age and were not interrelated in this analysis17).
The relation between and sweating rate has recently been investigated comparing older highly trained cyclists (OHT; 56 years) with two younger groups. In this investigation one younger group was matched for training status (YHT; 26 years) and the other matched for (YMT; 27 years). All subject exercise at workloads equivalent to 70% of in both hot (35°C, 40% RH) and thermoneutral (20°C, 40% RH) conditions. Despite an elevated metabolic heat production in YHT, sweat loss (kg) relative to body surface area (m2) was similar to OHT. Combined data from the three groups yielded a significant correlation between sweat loss and , but not age18. Therefore, strategies aimed at improving aerobic fitness appear to augment sweating capacity, and thereby improve heat tolerance, in both young and older athletes19.
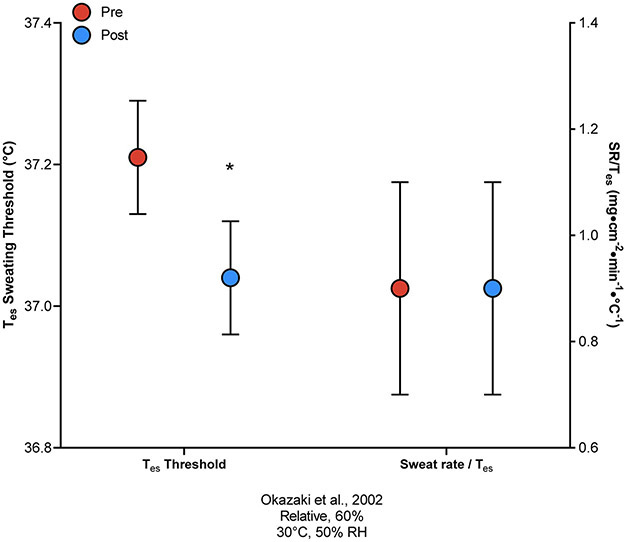
Exercise thermoregulatory responses in both moderate and hot conditions -- including sweat onset threshold, sweat gland output, and sweat rate -- can be improved in previously sedentary adults following aerobic exercise training20-23. In young adults, 10 days of aerobic training for 1 h·day−1 in a thermoneutral environment (25°C ) at 75% increased sweat rate and shifted the sweating onset threshold to a lower Tc23. Similarly, following 18 weeks of aerobic exercise training on a cycle ergometer at 50-80% (resulting in an approximate 20% increase in ), the core temperature onset threshold for increased sweat rate, but not the thermosensitivity (i.e., the slope of the increase in SR per °C rise in Tes), improved in previously sedentary older (64 years) men (Figure 1)21.
Figure 1.
Esophageal temperature (Tes) onset threshold for sweating responses (left y-axis) and the sweat rate (SR) thermosensitivity (i.e. slope of the increase in SR per °C rise in Tes; right y-axis) in older sedentary adults before (Pre) and after (Post) 18 weeks of exercise training (from Okazaki et al.21). * P < 0.05 compared to pre-training.
Habitual aerobic exercise also prevents age-related decrements in sweat production24. Best et al. observed no significant differences in sweating rates relative to either body surface area or power output between young (26 years) and middle-aged (56 years) highly trained male cyclists18. Similarly, others have reported that evaporative heat loss through sweating was impaired in untrained middle-aged and older men compared to both well-trained middle-aged and younger men during intermittent aerobic exercise in the heat, despite a similar sweating onset threshold25. Young (28 years) and middle-aged (54 years) competitive male runners matched for (Y: 58 mL·kg−1·min−1; MA: 61 mL·kg−1·min−1) displayed similar sweat rates and sweat sensitivity when exercising at a fixed workload (90% of 10-km race time) in the heat (40°C, 30% rh). While a lower sweat gland output was observed in the middle-aged men, this was compensated by an increased number of heat-activated sweat glands26, suggesting a possible compensatory mechanism by which total sweat capacity may be maintained in older athletes via continued training.
It is important to note that differences in regional sweat gland function may exist between young and older adults. Following MCh injection, older adults displayed a significantly blunted sweating rate compared to young adults, an impairment observed to a greater extent on the forehead and limbs compared to the trunk27. Furthermore, the reduced sweating response was more pronounced in older women compared to age-matched men27, indicating that sex may play a role in modulating regional sweat gland function and sweat rate. Smith and Havenith explored this possibility by detailed sweat mapping of the regional and local sweat patterns in elite young male and female runners exercising at two different intensities (60% and 75% ) in moderately warm (25°C, 45% RH) conditions28, 29. Though men experienced greater total sweat loss at both exercise intensities28, sweating rates were highest for both sexes on the central upper back and lowest toward the limbs; however, sweat distribution was skewed toward the arms and hands in women compared to men29.
With primary aging there is an attenuation in both regional sweating onset threshold and sweat rates during passive whole-body heating in older (64 years) versus young (23 years) adults30. However, in this same cohort, pharmacologically-induced regional sweating rates were similar between older and young subjects, suggesting an age-related decline in heat-activated sweat gland function but not cholinergic sensitivity30. George Havenith’s laboratory at Loughborough University has recently published detailed sweat maps of older adults during both rest and exercise in a hot environment (32°C, 50% RH)31. At rest, there were lower gross sweat losses and regional sweat rate in a majority of regions in older men (68 years), despite similar absolute heat production, compared to younger men (24 years). During exercise at a fixed heat production (200 W·m−2), reductions in gross sweat loss persisted in the older group, though reductions in regional sweat rate in the older group were relegated toward the extremities (hands, legs, ankles, and feet). However, no women were included in that study. It is possible that, as indicated previously27, the reduction in gross sweat loss and regional sweat rate, as well as the greater distribution of sweat toward the extremities, observed in older men during exercise at a fixed heat production may be exacerbated in older women. Future investigation is warranted to determine whether potential sex differences in regional sweat rate and onset threshold persist with age and are modified by sex.
Cardiovascular Responses to Heat Stress
In addition to evaporative heat loss, increases in cardiac output and blood flow redistribution from inactive visceral tissue to the cutaneous circulation allow for increased convective heat transfer32. Healthy human aging is associated with attenuated central and peripheral cardiovascular responses during exercise, including reduced cardiac output and less visceral blood flow redistribution to the skin. In thermoneutral conditions, age-related attenuations in sedentary older adults are attributable to reductions in stroke volume and maximal heart rate33, 34. Studies specifically investigating the impact of aging on cardiovascular responses during exercise in hyperthermic conditions are contradictory. Endurance trained older adults who exercised in the heat (≥60% 36°C, 20% RH) exhibited greater stroke volume, but attenuated cardiac output compared to young adults matched for 33. Cross-sectional studies differ on whether cardiac output is lower in aerobically fit older compared to young adults20, 35, 36. Interestingly, Ho et al.20 found that fit and sedentary older adults (age = 65 years, = 42 vs 28 ml·kg−1·min−1, respectively) did not differ in cardiac output when cycling at 60% (60 revolutions/min) in 36°C and 20% rh; however, four weeks of endurance exercise training in older sedentary adults sufficient to increase by 27% yielded a significant increase in cardiac output when exercising at a relative (60% ), but not absolute (60% pre-training ), exercise intensity in 36°C heat.
Increased cardiac output following training is likely due, at least in part, to a training-induced plasma volume expansion, although exercise studies are conflicting on whether the training-induced plasma volume expansion is attenuated with aging37-40. Importantly, most training studies lack a direct within-study age comparison, and cross-sectional comparisons between fit and sedentary older adults are equivocal regarding the role of fitness in the age-related declines in plasma volume41-43. Future studies should directly compare young and older adults and the impacts of aerobic fitness and/or exercise training on plasma volume expansion.
Redistribution of blood flow during exercise in the heat from the renal and splanchnic circulations to the skin is also attenuated in older adults35. When exercising in hyperthermic conditions (30-36°C, 20-60% RH), greater renal and splanchnic blood flow is observed in fitness-matched young vs. older adults20, 35, 44. For example, when exercising at 60% in 36°C, fit young adults (age = 26 years, = 43 ml·kg−1·min−1) redistributed ~37% more total blood flow away from the renal (Y: 1095 vs. 825 ml·min−1, O: 940 vs. 739 ml·min−1) and splanchnic (Y: 1,139 vs 630 ml·min−1, O: 1,137 vs. 770 ml·min−1) regions compared to fit older adults (age = 64 years, = 41.8 ml·kg−1·min−1)35. The degree to which fitness plays a role in mediating this age-related decrement is unknown. Ho et al. found that total redistribution of splanchnic plus renal blood flow (but neither independently) was greater in fit older adults compared to their sedentary counterparts (Δ568 vs Δ427 ml·min−1). Four weeks of endurance training in previously sedentary older adults had no impact on either total or regional blood flow redistribution20. Although speculative, fitness-mediated differences in blood flow redistribution may be due to increased sympathetic nerve activity, and/or increased end-organ responsiveness to a given rise in sympathetic nerve activity, in fit individuals. However, plasma norepinephrine concentrations were not different between fit and sedentary older adults, or after 4 weeks of training in previously sedentary older adults20, suggesting that the latter may be a more plausible explanation.
Adequate perfusion of the cutaneous circulation is critical for temperature regulation during exercise in the heat. Increases in skin blood flow are mediated via a sympathetic cholinergic active vasodilator system. During passive, supine heat stress, skin blood flow can rise as much as 8 L/min in young, healthy adults45. However, healthy aging is associated with impairments in heat- and exercise-induced increases in skin blood flow of, on average, 25-50% compared to young adults21, 35, 46-50. These impairments are largely mediated by an overall decreased sensitivity of the active vasodilator system, including decreased co-transmitter release and reduced nitric oxide bioavailability and signaling48, 51-53. Healthy aging is also associated with reductions in thermoregulation-induced skin sympathetic nerve activity (SSNA) responses. Older individuals exhibit reduced SSNA with heat stress and have a blunted vasodilator response for a given increase in SSNA compared to young adults, suggesting that age-related impairments in reflex cutaneous vasodilation are partially mediated by blunted efferent SSNA and/or end-organ responsiveness to SSNA during heat stress54. However, it is important to note these mechanistic skin blood flow and SSNA studies have been performed in non-exercising conditions due to experimental limitations with exercise. To date, no studies have investigated the impacts of fitness on these age-related declines future cross-sectional studies involving highly trained older adults and/or training studies are needed.
It is well-accepted that exercise training can improve central and peripheral cardiovascular function across all ages34. Thus, exercise training may be an effective strategy to mitigate age-related thermoregulatory cardiovascular impairments. However, the literature is equivocal regarding the effects of fitness on age-related cardiovascular function/impairment during exercise in the heat. Cross-sectional comparisons of young and older adults demonstrate age-related impairments in skin blood flow and other cardiac responses to exercise heat-stress even when subjects are matched for 47, 49, 50. Fitness status (as measured by ) and age are independently predictive of skin blood flow responses to exercise in warm humid environments (60W for 1hr in 35°C, 80% rh)17. In cross-sectional studies, fitness status in older cohorts is related to improved cardiovascular responses to exercise-heat stress10, 20. However, these responses are still generally lower than those of their younger cohorts. Thus, older elite athletes may still have decrements in skin blood flow responses to exercise-heat stress despite being highly trained.
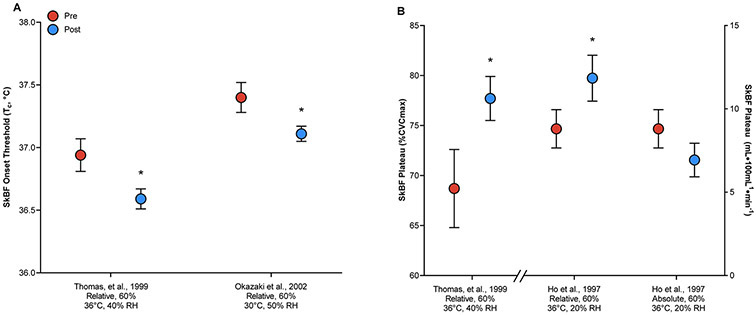
To our knowledge, only three studies have investigated the effects of exercise training on thermoregulatory cardiovascular responses in older adults, all finding improvements in skin blood flow during heat stress20-22. However, the mechanisms by which these improvements occur differ among the studies. Two investigations observed changes in the Tc onset threshold for cutaneous vasodilation, while the other saw improved thermosensitivity (i.e., increased slope of the relation between Tc and skin blood flow) (Figure 2). Ho et al. observed a leftward shift in the mean body temperature (Tb) onset threshold for increased skin blood flow in young adults after endurance training; however, older adults who underwent the same exercise training protocol had only an improved thermosensitivity, but not onset threshold20. In contrast, Thomas et al. and Okazaki et al. both observed significant leftward shifts in onset threshold in older adults after endurance training21, 22. Putative underlying mechanisms mediating these changes include improved sensitivity of the active vasodilator systems (i.e. increased SSNA)22 and increased endothelium-dependent cutaneous vasodilation55.
Figure 2.
The onset threshold for increased skin blood flow (SkBF; Panel A21, 22) and the magnitude of the SkBF response (Panel B20, 22) before (Pre) and after (Post) exercise training. For each study, the comparative workload and environmental conditions are provided on the x-axis. Relative = participants exercised at the same relative intensity before and after exercise training; Absolute = participants exercised at the same absolute intensity before and after exercise training; RH = relative humidity; Tc = core temperature; %CVCmax = percentage of maximal cutaneous vascular conductance. * P < 0.05 compared to pre-training.
Core and Skin Temperature Responses during Exercise in the Heat
Age-related impairments in sweating and skin blood flow responses can result in performance decrements during prolonged exercise-heat stress2. When comparing two groups exercising at the same absolute intensity, Tc responses are exaggerated in those with a lower 56. This has led researchers conducting cross-sectional comparisons between different age cohorts to match groups for relative exercise intensity (i.e., a percentage of ), often resulting in similar Tc and skin temperature (Tsk) responses10, 14, 28 despite disparate thermoeffector responses. Similar Tc and Tsk responses between young and older adults at different absolute exercise intensities suggest an impairment in the control of body temperature during exercise that may be at least partially mitigated with maintenance of fitness throughout aging.
The mitigation of age-related declines in thermoeffector responsiveness with maintenance of aerobic fitness results in improved temperature regulation in the heat. However, the extent to which aerobic fitness independently modulates the maintenance of body temperature during exercise-heat stress in older adults is difficult to fully elucidate. Studies in which young, middle-aged, and/or older adults are matched for most often demonstrate no differences in Tc or Tsk responses between age groups14, 25, 26, 33. In a cohort of 56 subjects ranging in age and , Havenith et al. determined the relative influence of age on cardiovascular and body temperature responses to low-intensity cycling exercise in 35°C, 80% RH conditions using multiple regression analysis17. The results of that study suggested that rectal temperature (Tre) responses were directly related to , but not age. However, due to age-related declines in even in trained individuals, cross-sectional studies generally compare highly trained older adults to recreationally active young adults in an effort to match for .
Direct comparisons between age groups become more difficult when comparing highly fit middle-aged or older adults to highly fit young adults. Age-related reductions in occur regardless of training state4, resulting in difficulties matching groups for metabolic heat production during exercise heat-stress. When thermoregulatory responses were compared during exercise heat-stress (30°C, 55% rh) at ~65% between 7 normally fit young (age = 29 years, = 44 ml·kg−1·min−1), 7 highly fit older (age = 64 years, = 46 ml·kg−1·min−1), and 6 normally fit older adults (age = 66 years, = 33 ml·kg−1·min−1), there were no differences in esophageal temperature (Tes) or Tsk between groups during exercise10. Importantly, absolute exercise intensity, and thus metabolic heat production, was similar between the normally fit young and highly fit older groups, but was lower in the normally fit older group. Similarly, Best et al.18 compared young highly-trained (YHT; 26 years), young moderately-trained (YMT; 27 years), and older highly-trained (OHT; 56 years) subjects during exercise at 70% of in 35°C, 40% RH conditions. Absolute and relative , and power output at 70% , were significantly higher in YHT compared to YMT and OHT, resulting in significantly higher metabolic heat production during exercise. Despite differences in heat production, however, neither Tre nor Tsk were different among the three groups. In another study, fit middle-aged (54 years; , 58 ml·kg−1·min−1) and young competitive male runners (28 years; , 61 ml·kg−1·min−1) demonstrated no differences in run performance or Tre responses in 40°C, 30% RH conditions26. Together, these studies provide strong evidence that age-related declines in body temperature regulation are primarily related to decrements in aerobic fitness, rather than age per se.
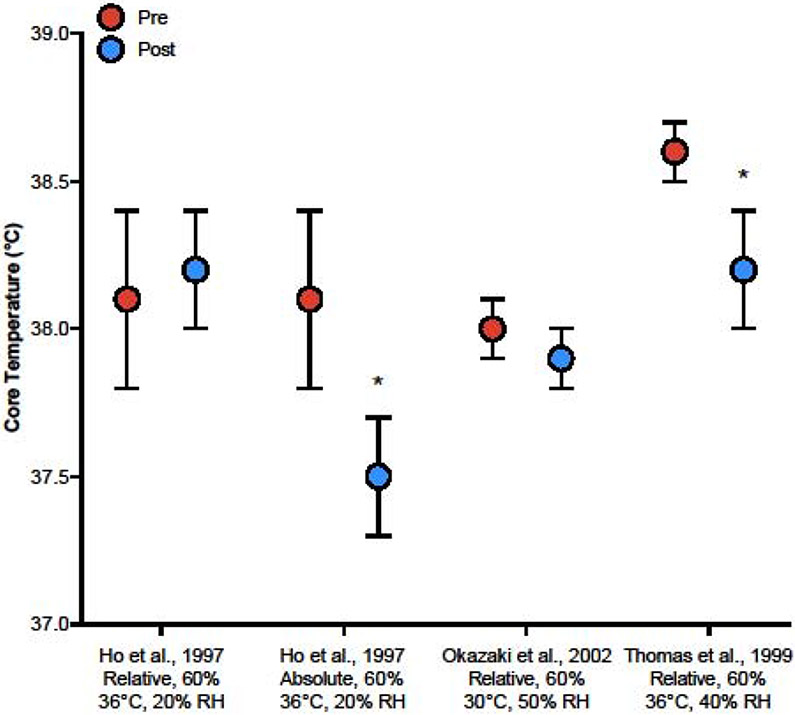
Training studies further indicate preserved body temperature control with improved aerobic fitness. In older (65 years), sedentary adults who exercised in the heat (36°C, 20% rh) at the same relative intensity (60% ) before and after 4 weeks of high-intensity aerobic training, there were no differences in Tes and Tsk responses20. When exercising at the same absolute intensity, however, both Tes and Tsk were lower at post- compared to pre-training. Similarly, 8 weeks and 18 weeks of exercise training resulted in progressive reductions in Tes and Tsk during exercise heat-stress21. In another study, older adults whose improved by a minimum of 5% after 16 weeks of aerobic training demonstrated a reduction in baseline and exercise Tb compared to pre-training22. In sum, these studies suggest that exercise training-induced improvements in heat dissipation in older adults (1) reduces the Tc responses at the same absolute workload, and (2) allows older adults to perform higher workloads compared to pre-training without further increases in Tc (Figure 3).
Figure 3.
Comparisons in core temperature responses during exercise-heat stress in older adults before (Pre) and after (Post) exercise training across studies20-22. For each study, the comparative workload and environmental conditions are provided on the x-axis. Relative = participants exercised at the same relative intensity before and after exercise training; Absolute = participants exercised at the same absolute intensity before and after exercise training. * P < 0.05 compared to pre-training.
Implications for Older Athletes
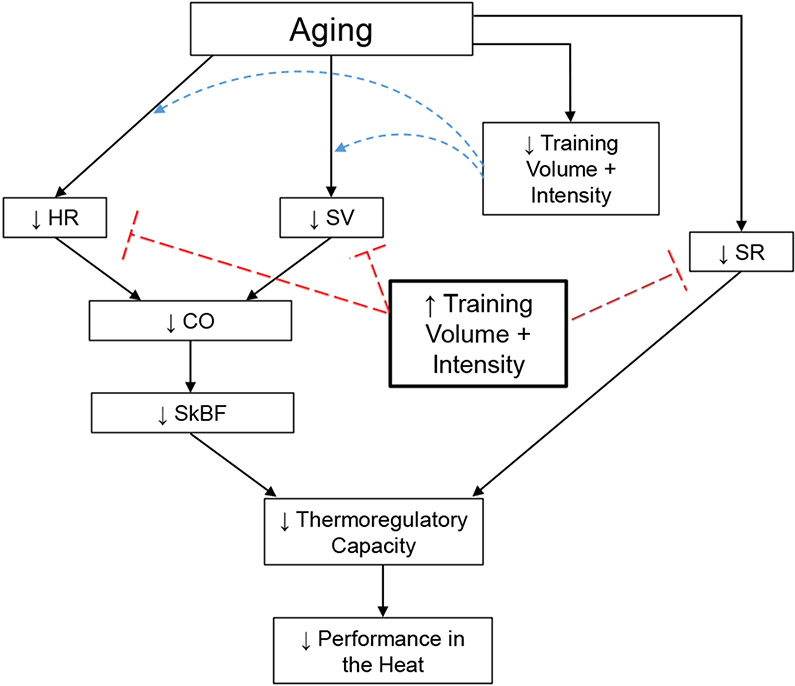
The mechanisms mediating age-related declines in thermoregulatory capacity are multidimensional, manifesting as impairments in sweat gland output and skin blood flow. Higher in older adults is related to improved sweating responses11, 15-18 and cardiovascular parameters including cardiac output and skin blood flow10, 20, 34, resulting in relatively preserved thermoregulatory function and better performance in the heat (Figure 4).
Figure 4.
The influences of aging and/or training volume and intensity on cardiovascular and thermoregulatory responses during exercise-heat stress. Aging may independently reduce heart rate (HR) and stroke volume (SV), and therefore cardiac output (CO), during exercise in athletes, resulting in impaired redistribution of blood to the cutaneous circulation during exercise-heat stress. Similarly, primary aging is associated with reduced sweat rates (SR). Reduced training volume and intensity in aging athletes likely contribute to such declines, although it is unclear to what magnitude. Although speculative, maintaining and/or increasing training volume and intensity throughout the aging process may prevent or slow the age-related declines in these responses in lifelong athletes, thereby improving thermoregulatory capacity and performance during exercise-heat stress.
Rates of decline in relative (mL · kg−1 · min−1) appear to be greater in endurance-trained men and women compared to their healthy, sedentary counterparts57. It’s unclear, however, how much of this decline in are directly related to primary aging vs. secondary to reduced training volume. Training volume (i.e. intensity and duration) is reduced with advancing age57, 58, thereby reducing the stimulus for maintenance of aerobic capacity. In a 30-year follow-up of 5 previously-trained men, remained lower in all subjects after a 6-month endurance training program compared to 30 years prior58. However, although the training dose in that study was similar to that which they completed 30 years earlier, in order to reduce risk of injury, the participants did not perform the same high-intensity training. Thus, although greater aerobic fitness is clearly associated with improved thermoregulation in older adults, it remains unclear whether maintaining a similar training volume and intensity throughout aging would effectively sustain , or to what extent that would translate to preventing age-related thermoregulatory impairments in older athletes.
When Does an Age Difference Matter?
There is a vast literature of cross-sectional studies published by ourselves9, 21, 24, 28, 45, 52 and others9, 10, 12, 13, 31 detailing age-related declines in physiological responses to environmental heat stress. However, these studies typically assess discrete physiological variables such as sweating rate, skin blood flow, hydration, cardiac responses, etc. Although it is important to understand age-related changes in those physiological variables, such changes may not always have meaningful impacts on tolerance to exercise in the heat (i.e., compensable vs. uncompensable exercise heat-stress). Indeed, greater whole-body heat storage in older compared to young adults does not always result in divergent Tc responses8, 59, 60. It is important, therefore, to consider the specific exercise intensities and environmental conditions in which age-related declines in thermoeffector responses may result in exaggerated exercise heat-stress in older adults.
Intensity.
Given its role in metabolic heat production, exercise intensity is an important driver of the overall heat load independent of environmental conditions. Direct and indirect calorimetry studies from Glen Kenny’s lab at the University of Ottawa have examined the exercise intensities at which aging impairs heat loss under fixed environmental conditions25, 60. Those investigations showed that age differences in evaporative heat loss became greater with increasing exercise intensities and concomitant increased heat loads. During exercise at incrementally increasing intensities in hot, dry conditions (40°C, 15% RH), older (58±5 years) women demonstrated an impaired capacity to dissipate heat compared to young, - matched women only at exercise-induced heat loads of ≥325 W60.
Environment.
While there is compelling evidence that aging is associated with decreased heat dissipation and elevated risk of heat illness in hot ambient conditions, the significant question remains: In what specific environments does this age disparity begin to occur? LaRose et al.59 demonstrated that heat dissipation is impaired in older men in both hot, dry (35°C, 20% rh) and warm, humid (35°C, 60% rh) environments due to reduced rates of evaporative heat loss. In that study, exercise was performed in four 15-min bouts, separated by 15-min recovery, at a rate of metabolic heat production of 400 W and external work output of ~80 W.
Our laboratory originally developed, and has a long history of using, a unique, rigorous, and reproducible approach to identify critical environmental limits to prolonged heat exposure61-63. This research paradigm combines physiological and biophysical approaches to delineate safe (compensable) from potentially unsafe (uncompensable) environmental conditions from a human heat balance standpoint. Our overarching goal is to identify those environments (combinations of ambient temperature and humidity) in which thermoregulatory health is compromised in healthy older adults.
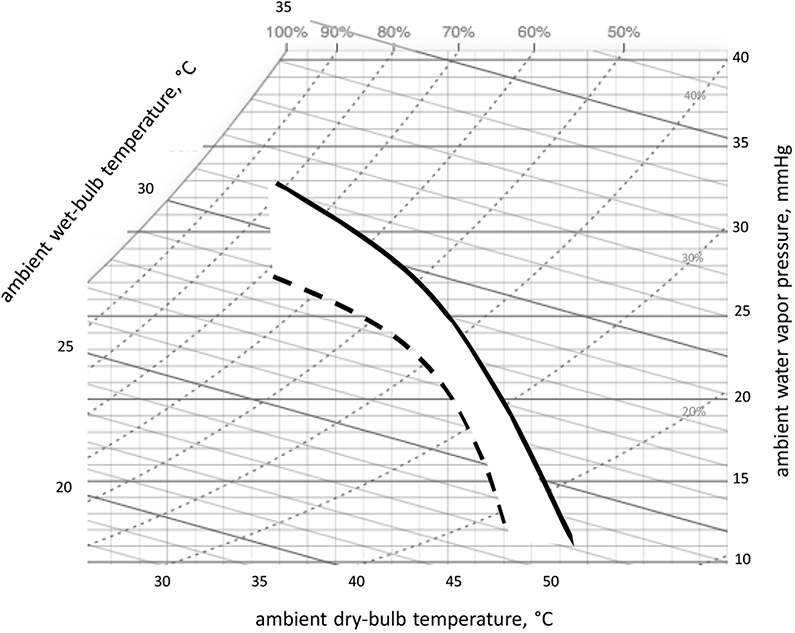
Toward that end, we recently described -- graphically and quantitatively – the critical environmental limits for exercising women between the ages of 62 and 80 yrs63. Rather than choosing a single environment (i.e., dry, humid) these psychrometric limit lines define the myriad combinations of ambient temperature and humidity above which human heat balance cannot be maintained for a given metabolic heat production. Figure 5 depicts environmental limit lines on a standard psychrometric chart for young and older women exercising at 30% . The white area between lines theoretically describes environments in which age makes a difference, i.e., older women are differentially adversely affected.
Figure 5.
Adapted from Kenney 202063, depicts psychrometric limit lines for unacclimated young (solid line) and older (dashed line) women exercising at 30% . The white area between lines theoretically describes environments in which the older women are differentially adversely affected.
These limits, and mathematically-derived critical evaporative coefficients (Ke'), can then be used to model low- to moderate-intensity exercise responses in hot environments but higher intensity exercise intensities have not been examined. In the studies performed to date, this intensity has historically been chosen because 1) it reasonably reflects light-to-moderate daily workloads typical of a healthy, normally active population; 2) it is the intensity associated with an 8-h work day in many industrial settings64; and 3) it reflects the intensity of many self-paced recreational activities. Values for older athletes exercising at higher intensities have not been determined using this protocol. Future studies should test the global hypothesis that aging will shift critical environmental limits to a narrower range of safe environments across the psychometric spectrum (encompassing warm-humid to hot-dry environments) in athletes exercising at high intensities.
Perspectives
Primary aging is associated with impaired thermoregulatory function, resulting in increased risk of heat-related illness and decreased athletic performance in the heat. Exercise training that increases or maintains throughout aging improves thermoregulatory function, including enhanced sweating and cardiovascular responses to exercise heat-stress. However, the extent to which can be maintained throughout the aging process, and how much training is requisite for maintained thermoregulatory function, remain unclear. Further, it is not evident to what degree age-related decrements in thermoregulatory function are mitigated by , per se, rather than lifelong engagement in exercise training independent of improvements in . Finally, although a great deal is known about the impact of aging and fitness on discrete physiological variables (such as sweating and skin blood flow responses) during heat stress, studies are only beginning to emerge detailing the specific exercise intensities and environmental conditions under which age-related changes likely result in greater body temperature responses in older compared to young adults. Future work is needed to identify those exercise intensities and/or environmental conditions under which age-related impairments in thermoregulatory function result in greater heat strain, risk of heat-related illness, and decrements in athletic performance in highly-fit and normally-fit older adults.
Table 1.
Summary of studies investigating age-related changes in thermoregulatory responses to exercise-heat stress.
| Comparative Workload |
Environmental Conditions |
Participants | SR | SkBF | Tsk | Tc | |
|---|---|---|---|---|---|---|---|
| Tankersley et al., 1991 | Relative 65% |
30°C, 55% RH | Vs. Young normally fit Older highly fit Older normally fit |
↔ ↓ |
↓ ↓↓ |
↔ ↔ |
↔ ↔ |
| Best et al., 2011 | Relative 70% |
35°C, 40% RH | Vs. Young highly-trained Young moderately-trained Older higltly-trained |
↔ ↔ |
↔ ↔ |
↔ ↔ |
↔ ↔ |
| Ho et al. 1997 | Relative 60% |
36°C, 20% RH | Vs. Young fit Young sedentary Older fit Older sedentary |
↔ ↔ ↔ |
↓ ↓↓ ↓↓ |
↔ ↔ ↔ |
↔ ↔ ↔ |
| de Paulo Viveiros 2011 | Relative/Absolute 90% 10-km time |
40°C, 30% RH | Vs. Young fit Middle-aged fit |
↔ | ↔ | ||
| Stapleton et al. 2015 | Absolute 300W 400W 500W |
40°C, 15% RH | Vs. Young normally fit Middle-aged trained Middle-aged untrained Older untrained |
↔ ↓ ↓ |
↔ ↔ ↔ |
↔ ↔ ↔ |
↔ ↑ ↑ |
| Larose et al. 2013 | Absolute 400 W |
35°C, 20% RH | Vs. 20-31 years 40-44 years 45-49 years 50-55 years 56-70 years |
↔ ↓ ↓ ↓ |
↔ ↔ ↔ ↔ |
||
| Kenney, 1988 | Relative ~40% |
37°C, 60% RH | Vs. Young Older |
↔ | ↓ | ↔ | ↔ |
| Kenney and Ho, 1995 | Relative/Absolute 60% |
36°C | Vs. Young fit Older fit |
↓ | ↓ | ↓ |
Arrows indicate direction of the difference compared to the reference group (Vs.). More than one arrow suggests a greater difference. Relative/absolute suggests that fitness-matched groups resulted in the same relative and absolute exercise intensity. SR = sweat rate; SkBF = skin blood flow; Tsk = skin temperature; Tc = core temperature; RH = relative humidity.
Acknowledgements
This work is supported by NIH Grant R01 AG067471 and the Marie Underhill Noll Endowment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United Nations DoE, Affairs S. World Population Ageing, 2019. 2019. [Google Scholar]
- 2.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc. 2014; 46(10):1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu R, Samet JM. Relation between Elevated Ambient Temperature and Mortality: A Review of the Epidemiologic Evidence. Epidemiologic Reviews. 2002; 24(2):190–202. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus NR, Lord JM, Harridge SDJTJop. The relationships and interactions between age, exercise and physiological function. 2019; 597(5):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. Journal of applied physiology. 1987; 63(3):1089–1094. [DOI] [PubMed] [Google Scholar]
- 6.Inbar O, Morris N, Epstein Y, Gass G. Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males. 2004; 89(6):691–700. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton JM, Poirier MP, Flouris AD, et al. Aging impairs heat loss, but when does it matter? Journal of applied physiology. 2015; 118(3):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larose J, Wright HE, Stapleton J, et al. Whole body heat loss is reduced in older males during short bouts of intermittent exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013; 305(6):R619–R629. [DOI] [PubMed] [Google Scholar]
- 9.Inbar O, Morris N, Epstein Y, Gass G. Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males. Exp Physiol. 2004; 89(6):691–700. [DOI] [PubMed] [Google Scholar]
- 10.Tankersley CG, Smolander J, Kenney WL, Fortney SM. Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake. Journal of applied physiology. 1991; 71(1):236–242. [DOI] [PubMed] [Google Scholar]
- 11.Ellis FP, Exton-Smith AN, Foster KG, Weiner JS. Eccrine sweating and mortality during heat waves in very young and very old persons. Isr J Med Sci. 1976; 12(8):815–817. [PubMed] [Google Scholar]
- 12.Inoue Y, Shibasaki M, Ueda H, Ishizashi H. Mechanisms underlying the age-related decrement in the human sweating response. Eur J Appl Physiol Occup Physiol. 1999; 79(2):121–126. [DOI] [PubMed] [Google Scholar]
- 13.Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol (1985). 1988; 65(3):1082–1086. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol (1985). 1993; 75(5):2162–2167. [DOI] [PubMed] [Google Scholar]
- 15.Drinkwater BL, Bedi JF, Loucks AB, Roche S, Horvath SM. Sweating sensitivity and capacity of women in relation to age. J Appl Physiol Respir Environ Exerc Physiol. 1982; 53(3):671–676. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez RR, Berglund LG, Stolwijk J. Thermoregulation in humans of different ages during thermal transients. Proceedings of the Satellite of 28th International Congress of Physiological Sciences2013:357–361. [Google Scholar]
- 17.Havenith G, Inoue Y, Luttikholt V, Kenney WL. Age predicts cardiovascular, but not thermoregulatory, responses to humid heat stress. European journal of applied physiology and occupational physiology. 1995; 70(1):88–96. [DOI] [PubMed] [Google Scholar]
- 18.Best S, Caillaud C, Thompson M. The effect of ageing and fitness on thermoregulatory response to high-intensity exercise. Scandinavian journal of medicine & science in sports. 2012; 22(4):e29–37. [DOI] [PubMed] [Google Scholar]
- 19.Alhadad SB, Tan PMS, Lee JKW. Efficacy of Heat Mitigation Strategies on Core Temperature and Endurance Exercise: A Meta-Analysis. Front Physiol. 2019; 10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol (1985). 1997; 82(4):1126–1135. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S, Nose H. Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol (1985). 2002; 93(5):1630–1637. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CM, Pierzga JM, Kenney WL. Aerobic training and cutaneous vasodilation in young and older men. J Appl Physiol (1985). 1999; 86(5):1676–1686. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol Respir Environ Exerc Physiol. 1977; 43(1):133–137. [DOI] [PubMed] [Google Scholar]
- 24.Buono MJ, McKenzie BK, Kasch FW. Effects of ageing and physical training on the peripheral sweat production of the human eccrine sweat gland. Age Ageing. 1991; 20(6):439–441. [DOI] [PubMed] [Google Scholar]
- 25.Stapleton JM, Poirier MP, Flouris AD, et al. Aging impairs heat loss, but when does it matter? J Appl Physiol. 2015; 118(3):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Paula Viveiros J, Amorim FT, Alves MN, Passos RL, Meyer F. Run performance of middle-aged and young adult runners in the heat. Int J Sports Med. 2012; 33(3):211–217. [DOI] [PubMed] [Google Scholar]
- 27.Foster KG, Ellis FP, Dore C, Exton-Smith AN, Weiner JS. Sweat responses in the aged. Age Ageing. 1976; 5(2):91–101. [DOI] [PubMed] [Google Scholar]
- 28.Smith CJ, Havenith G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur J Appl Physiol. 2011; 111(7):1391–1404. [DOI] [PubMed] [Google Scholar]
- 29.Smith CJ, Havenith G. Body mapping of sweating patterns in athletes: a sex comparison. Med Sci Sports Exerc. 2012; 44(12):2350–2361. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol. 2013; 305(8):R877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coull NA, West AM, Hodder SG, Wheeler P, Havenith G. Body mapping of regional sweat distribution in young and older males. Eur J Appl Physiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiological reviews. 1974; 54(1):75–159. [DOI] [PubMed] [Google Scholar]
- 33.Minson CT, Kenney WL. Age and cardiac output during cycle exercise in thermoneutral and warm environments. Med Sci Sports Exerc. 1997; 29(1):75–81. [DOI] [PubMed] [Google Scholar]
- 34.Kenney WL, Wilmore JH, Costill DL. Physiology of sport and exercise, Human kinetics; 2015. [Google Scholar]
- 35.Kenney WL, Ho CW. Age alters regional distribution of blood flow during moderate-intensity exercise. J Appl Physiol (1985). 1995; 79(4):1112–1119. [DOI] [PubMed] [Google Scholar]
- 36.Best S, Thompson M, Caillaud C, Holvik L, Fatseas G, Tammam A. Exercise-heat acclimation in young and older trained cyclists. Journal of science and medicine in sport. 2014; 17(6):677–682. [DOI] [PubMed] [Google Scholar]
- 37.Hagberg JM, Goldberg AP, Lakatta L, et al. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. 1998; 85(2):484–489. [DOI] [PubMed] [Google Scholar]
- 38.Pickering GP, Fellmann N, Morio B, et al. Effects of endurance training on the cardiovascular system and water compartments in elderly subjects. 1997; 83(4):1300–1306. [DOI] [PubMed] [Google Scholar]
- 39.Stachenfeld NS, Mack GW, DiPietro L, Morocco TS, Jozsi AC, Nadel ER. Regulation of blood volume during training in post-menopausal women. Med Sci Sports Exerc. 1998; 30(1):92–98. [DOI] [PubMed] [Google Scholar]
- 40.Zappe DH, Bell GW, Swartzentruber H, Wideman RF, Kenney WL. Age and regulation of fluid and electrolyte balance during repeated exercise sessions. The American journal of physiology. 1996; 270(1 Pt 2):R71–79. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson ET, Davy KP, Seals DR. Maximal aerobic capacity and total blood volume in highly trained middle-aged and older female endurance athletes. 1994; 77(4):1691–1696. [DOI] [PubMed] [Google Scholar]
- 42.Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. 1997; 82(4):1126–1135. [DOI] [PubMed] [Google Scholar]
- 43.Hagberg JM, Montain SJ, Martin WH 3rd, Ehsani AA. Effect of exercise training in 60- to 69-year-old persons with essential hypertension. The American journal of cardiology. 1989; 64(5):348–353. [DOI] [PubMed] [Google Scholar]
- 44.Kenney WL, Zappe DH. Effect of age on renal blood flow during exercise. Aging (Milano). 1994; 6(4):293–302. [DOI] [PubMed] [Google Scholar]
- 45.Rowell LB. 8. Thermal Stress. Human Circulation Regulation During Physical Stress. 1986:174–212. [Google Scholar]
- 46.Inoue Y, Shibasaki M, Hirata K, Araki T. Relationship between skin blood flow and sweating rate, and age related regional differences. European journal of applied physiology and occupational physiology. 1998; 79(1):17–23. [DOI] [PubMed] [Google Scholar]
- 47.Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol. 1988; 57(1):120–125. [DOI] [PubMed] [Google Scholar]
- 48.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. The American journal of physiology. 1997; 272(4 Pt 2):H1609–1614. [DOI] [PubMed] [Google Scholar]
- 49.Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol (1985). 1990; 68(5):1902–1908. [DOI] [PubMed] [Google Scholar]
- 50.Kenney WL, Tankersley CG, Newswanger DL, Puhl SM. Alpha 1-adrenergic blockade does not alter control of skin blood flow during exercise. Am J Physiol. 1991; 260(3 Pt 2):H855–861. [DOI] [PubMed] [Google Scholar]
- 51.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. AmJ Physiol Heart Clrc Physiol. 2006; 291(6):H2965–2970. [DOI] [PubMed] [Google Scholar]
- 52.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. Journal of applied physiology. 2002; 93(5):1644–1649. [DOI] [PubMed] [Google Scholar]
- 53.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life sciences. 1994; 55(24):1895–1902. [DOI] [PubMed] [Google Scholar]
- 54.Stanhewicz AE, Greaney JL, Alexander LM, Kenney WL. Blunted increases in skin sympathetic nerve activity are related to attenuated reflex vasodilation in aged human skin. J Appl Physiol (1985). 2016; 121(6):1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. The Journal of physiology. 2008; 586(14):3511–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gant N, Williams C, King J, Hodge BJ. Thermoregulatory responses to exercise: relative versus absolute intensity. Journal of sports sciences. 2004; 22(11-12):1083–1090. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. Journal of applied physiology. 1997; 83(6):1947–1953. [DOI] [PubMed] [Google Scholar]
- 58.McGuire DK, Levine BD, Williamson JW, et al. A 30-Year Follow-Up of the Dallas Bed Rest and Training Study. 2001; 104(12):1358–1366. [PubMed] [Google Scholar]
- 59.Larose J, Boulay P, Wright-Beatty HE, Sigal RJ, Hardcastle S, Kenny GP. Age-related differences in heat loss capacity occur under both dry and humid heat stress conditions. Journal of applied physiology. 2014; 117(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stapleton JM, Poirier MP, Flouris AD, et al. At what level of heat load are age-related impairments in the ability to dissipate heat evident in females? PloS one. 2015; 10(3):e0119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamon E, Avellini B. Physiologic limits to work in the heat and evaporative coefficient for women. J Appl Physiol. 1976; 41(1):71–76. [DOI] [PubMed] [Google Scholar]
- 62.Kenney WL, Zeman MJ. Psychrometric limits and critical evaporative coefficients for unacclimated men and women. J Appl Physiol (1985). 2002; 92(6):2256–2263. [DOI] [PubMed] [Google Scholar]
- 63.Kenney WL. Psychrometric limits and critical evaporative coefficients for exercising older women. J Appl Physiol (1985). 2020; 129(2):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonjer FJE. Actual energy expenditure in relation to the physical working capacity. 1962; 5(1):29–31. [Google Scholar]