Abstract
Podocytes are the key cells involved in protein filtration in the glomerulus. Once proteins appear in the urine when podocytes fail, patients will end with renal failure due to the progression of glomerular damage if no proper treatment is applied. The injury and loss of podocytes can be attributed to diverse factors, such as genetic, immunologic, toxic, or metabolic disorders. Recently, autophagy has emerged as a key mechanism to eliminate the unwanted cytoplasmic materials and to prolong the lifespan of podocytes by alleviating cell damage and stress. Typically, the fundamental function of extracellular vesicles (EVs) is to mediate the intercellular communication. Recent studies have suggested that, EVs, especially exosomes, play a certain role in information transfer by communicating proteins, mRNAs, and microRNAs with recipient cells. Under physiological and pathological conditions, EVs assist in the bioinformation interchange between kidneys and other organs. It is suggested that EVs are related to the pathogenesis of acute kidney injury and chronic kidney disease, including glomerular disease, diabetic nephropathy, renal fibrosis and end-stage renal disease. However, the role of EVs in podocyte autophagy remains unclear so far. Here, this study integrated the existing information about the relevancy, diagnostic value and therapeutic potential of EVs in a variety of podocytes-related diseases. The accumulating evidence highlighted that autophagy played a critical role in the homeostasis of podocytes in glomerular disease.
Keywords: Autophagy, Exosomes, Nephropathy, Podocytes
Introduction
Extracellular vesicles (EVs) are the membrane-bound vesicles produced and released by cells that contain various molecules, such as proteins, lipids, DNA, messenger RNA (mRNA), and microRNAs (miRNAs) (Camussi et al. 2010). EVs are originally regarded as the cellular dust, but there is accumulating evidence supporting their important role as messengers in diverse biological and physiological processes (Kourembanas 2015). Recent studies have confirmed that EVs play a vital role in cell-cell communication (Krause et al. 2015). In this regard, EVs may be related to the dysfunction in protein, lipid, and nucleic acid transfer, and serve as a noninvasive diagnostic biomarker for disease. In the kidney, renal EVs are produced and secreted by renal cells, which are often interrelated with the organic or functional diseases of the kidney.
Proteinuria may be caused by the damaged podocytes, while cell death or detachment is a critical step for podocyte loss in the progression of glomerular disease (Wiggins 2007). Analysis of the urinary extracellular vesicles (uEVs) may provide certain reference for the novel diagnostic approach to manage podocyte injury (Pisitkun et al. 2004). Autophagy has been increasingly identified to contribute to podocyte injury in glomerular disease under pathological condition. This review aimed to comprehensively characterize EVs and analyze the available information regarding the roles of EVs in podocyte autophagy in various renal diseases.
EVs
EVs were originally described as“ cellular dust” in the early 1960s, since then, they were assumed as the meaningless metabolic waste of cells (Wolf 1967). Recently, numerous studies suggest that EVs act as the “messengers” in cell survival, apoptosis, and autophagy, but the underlying mechanism of action remains unclear. EVs can be broadly classified as three types based on their mechanism of release and size: apoptotic bodies (larger than 1000 nm in diameter), microvesicles or microparticles (100–1000 nm), and exosomes (less than 150 nm) (van der Pol et al. 2012). Cytoplasmic contents (such as lipids, proteins, mRNA and/or miRNAs, and even DNA) are encapsulated by a lipid bilayer. All types of EVs are generated by their parent cells. With regard to the origin, exosomes originate from the endosomes, which fuse with the plasma membrane to release exosomes into the extracellular environment (Fig. 1). By contrast, microvesicles and apoptotic bodies are directly derived from the plasma membrane in living or dying cells (Tkach and Théry 2016).
Fig. 1.
Release of EVs and Intercellular communication. EVs biogenesis starts within the endosomal system; early endosomes mature into late endosomes or MVBs, and during this process the endosomal membrane invaginates to generate ILVs in the lumen of the organelles. There are several cellular steps for release of EVs: ① Formation of intraluminal vesicles (ILVs) in MVBs; ② Transport of MVBs to the plasma membrane; ③ Fusion of MVBs with the plasma membrane; ④ Release of EVs. EVs from different nephron segments cells can be uptaken by adjacent and distal cells, such as distal tubule, collecting duct cells, and etc
It has been extensively recognized that, plasma EVs can be found in any body fluid that can indicate the status of intrinsic cells, tissues and/or organs. In addition, these vesicles seem to serve as the “pigeons” that deliver massages in intercellular communication, and they are associated with numerous physiological and pathological functions (Record et al. 2014). At present, it is still unclear about the underlying mechanism concerning the formation and release of microparticles (MPs), but there is a consensus that the entire MP-releasing process is the vesiculation and outward budding of the plasma membrane after cytoskeletal reorganization and phosphatidylserine (PS) translocation. This process can be evoked by various stimuli and regulated by the physical or biochemical factors (Burger et al. 2013). Under physiological condition, the fusion of intracellular multivesicular bodies (MVBs) with cytomembrane and the release of exosomes is a continuous process, but it may also be a response to a variety of stimulus. Vesicles can not only transport the biological substances to neighboring cells, but also to the relatively long-raged cells. EVs can act on target cells through different ways, including genetic material transformation (mainly mRNA and miRNAs), cell stimulation, or proteins and lipids, even at the remote sites (Nawaz et al. 2016). EVs contain RNAs, such as mRNA and miRNA for MPs and exosomes; in the meantime, the apoptotic bodies are likely to contain DNA fragments. Many common proteins have been identified in EVs, including heat shock proteins (HSP), tetraspanins (CD9, CD63), tumor susceptibility gene 101 (TSG101), flotillin, annexins, Alix from MVB, and membrane fusion protein GTPases. In addition, EVs may also contain lipids, like cholesterol and sphingomyelin (Iraci et al. 2016). As a result, EVs may regulate target cells and reprogram cell morphologies and functions with a long lasting outcome (Bruno and Camussi 2013).
The existing studies on nephrology have detected the presence of EVs in various body fluids, including urine and blood. Some cell types in urinary system, such as glomerular, tubular, prostate, and bladder cells, can synthesize and secret EVs (Pisitkun et al. 2004). During the MP formation process, amino phospholipids can only be found on the inner plasma membrane under normal conditions, while outward blebbing may be dependent on different enzymes and calcium influx. Naturally, MP formation seems to occur selectively in the lipid-rich microdomains (lipid rafts/caveolae) within the membrane (Morel et al. 2011).Other than MPs, exosomes can also be identified by some exosome-specific markers, such as CD81, CD9, apoptosis-linked gene 2-interacting protein X and TSG 101 (Colombo et al. 2014). Both exosomes and MPs carry different surface markers depending on the parent cells and stimuli, as a result, they can be transplanted in vivo to better understand the function under biochemical conditions (Burger et al. 2013).
Autophagy and podocytes
Podocytes, the glomerular epithelial cells, are composed of cytoskeletal structure, joint connection, and branching foot processes that circle the glomerular basement membrane (GBM). In the glomerulus, podocytes are the most important cells to execute the filtration function. However, podocytes are the highly specialized and terminally differentiated cells, so they cannot regenerate after injury. Any deleterious factors, like mechanical stress, reactive oxygen species (ROS) and diverse cytokine receptors, can lead to loss of their function as the glomerular filtration barrier (Maezawa et al. 2015). It is suggested in existing studies that, podocyte injury induced by inflammatory and metabolic diseases mostly focuses on the autophagy and nucleotide-oligomerization domain-like receptor 3 (NLRP3) inflammasome pathways (Davis et al. 2011; Kawakami et al. 2015).
As an evolutionarily conserved intracellular catabolic pathway, autophagy occurs in most cells at a basal level to maintain cellular homeostasis (Kelekar 2005). According to the different types of degraded substrates, autophagy can be classified as selective or nonselective type. Of them, selective autophagy is involved in the degradation of some impaired organelles and lipophagy, whereas the nonselective type is thought to be caused by nutrient deficiency (Kraft et al. 2009). Moreover, based on the different ways of transporting intracellular constituents to lysosomes, autophagy can also be classified into macroautophagy, microautophagy, or chaperone-mediated type(Mizushima and Komatsu 2011).Among them, macroautophagy conveys the cell elements to lysosomes by autophagosomes, which is similar to that of chaperone-mediated autophagy by the chaperone complex (Kaushik and Cuervo 2012). By contrast, microautophagy allows to directly transport the cell constituents to lysosomes (Mizushima et al. 2010). Macroautophagy has been the most extensively investigated autophagy type. Therefore, this reviewed focused on autophagy (referred to macroautophagy).
As a complicated process, autophagy represents the cooperation of multiple autophagy-related gene (Atg) products. Atg proteins can be classified into five groups, which are Atg1 kinase complex [Atg1/Unc-51-like kinase (ULK) 1/2], Atg9, class III phosphoinositide 3-kinase complex (PI3KC3), and two ubiquitin-like conjugation systems (Atg12-Atg5 and Atg8 conjugation system) (Mizushima 2010). Besides, the nephrology experts have reached a consensus that two protein complexes are responsible for the initiation of autophagy. ①the 13 (Atg13)-FIP200-complex, which is related to the Unc-51-like kinase 1 (ULK1)-autophagy, can be activated by the AMP-activated protein kinase (AMPK). In addition, it can react to energy depletion and is down-regulated by the mammalian target of rapamycin complex 1 (mTORC1). ② the beclin 1-interacting complex, which comprises beclin 1, vascuolar protein sorting 34 and other interacting proteins, can produce the phosphatidylinositol-3 (PI3) phosphate and facilitate the autophagosomal membrane nucleation after it is activated (Ravikumar et al. 2010).
Up to now, many studies related to glomerular disease prove that autophagy plays a protective role in healthy podocytes. As reported by Hartleben et al., with the deletion of autophagy-related protein 5 (the podocyte-specific protein), the podocytes in mice were more likely to be damaged by the accumulation of oxidized and ubiquitinated proteins, together with the endoplasmic reticulum stress (ERS), finally showing up with the appearance of proteinuria (Hartleben et al. 2010). Zeng et al. reported that the higher Beclin1-mediated autophagic activity was observed in patients with minimal change disease (MCD) than those with focal segmental glomerulosclerosis (FSGS), and the autophagy activities decreased in the course of MCD progressing into FSGS. Further, some experiment is carried out to study the puromycin aminonucleoside-induced podocyte injury in Beclin1-knockout mice and mice treated with autophagy inhibitors, and it is discovered that the induction of autophagy by mTORC1 is reduced. This verifies that autophagy is needed by podocytes to maintain their physiological functions (Zeng et al. 2014).
Relationship between EVs and podocyte autophagy
Some studies have suggested that podocyte autophagy may be induced by pressure or hyperglycemia; meanwhile, EVs are found to be related to glomerular disease. Therefore, EVs produced due to pressure or other factors are speculated to play a role in the development of glomerular disease (Pisitkun et al. 2004). On the one hand, in endothelial cells, when autophagy and apoptosis are activated simultaneously, EVs will be released in response to cellular stress (Zhang et al. 2014b). In short, the complicated roles of EVs and autophagy in podocytes are still unclear so far, but it appears that there are mutual impacts among them.
Autophagy-related proteins in the biogenesis of exosomes
The biogenesis of EVs may be affected by the autophagy machinery in eukaryocytes, such as the formation and release of vesicles into the extracellular space (Murrow et al. 2015). Guo et al. illustrated that Atg5 played a certain role in the dissociation of vacuolar proton pumps (V1Vo-ATPase) from MVBs, which prevented the acidification of the MVB lumen, and allowed MVB to fuse with the plasma membrane and release EVs (Guo et al. 2017). Therefore, the knockout of Atg5 or ATG16L1 affects EVs release and reduces LC3B lipidation. In addition, cells with Atg5 knockout will increase EVs release after the treatment with lysosomal or ATPase inhibitors. However, the EVs release is not affected in the Atg7-knockout cells, indicating that Atg 7 is not required for autophagosome formation or LC3B lipidation. Moreover, Murrow et al. verified that the Atg12-Atg3 complex catalyzed the conjugation of LC3B, and that an ESCRT-associated protein called ALG-2-interacting protein X (ALIX/PDCD6IP) was crucial for exosome biogenesis. In the case of Atg12-Atg3 loss, the biogenesis of exosomes will decrease (Murrow et al. 2015). Besides, the starvation-related autophagy will also impact the biogenesis of exosomes, implying that different regulatory mechanisms are connected with autophagy and exosome biogenesis. Crosstalk between exosome and autophagy is the response to different forms of cellular stresses. Bader et al. implicated that Atg9, the unique transmembrane Atg, was related to the formation of intraluminal vesicles in amphisomes and autolysosomes of Drosophila, and its release mechanism might be similar to that of EVs (Bader et al. 2015).
The class III PI3K complex is needed for autophagy and endocytosis to produce PI (3)P for regulating membrane trafficking after the phosphorylation of phosphatidylinositide, which is also related to exosome biogenesis. Beclin1 is suppressed by the destabilization of the PI3K complex, in addition, Spautin-1 treatment or siRNA-mediated knockdown results in reduced exosome release and autophagy flux in chronic myeloid leukemia (CML) cells (Liu et al. 2016). Given the role of the PI3K complex in endocytosis and autophagy, it is necessary to further investigate the impacts of its various components on exosome biogenesis. It is not surprising that the same protein complexes are used as media in both autophagy and exosome biogenesis, but autophagy may also be influenced by other types of exosomes, which will be discussed below.
Different autophagy-related pathways in podocytes
The cytoplasm quality is partly controlled by autophagy via the degradation process of proteins, peroxidases, and damaged organelles, so as to maintain the homeostasis of intracellular environment(Suzuki 2013; Zhu et al. 2013). For the highly differentiated cells, such as podocytes, the self-repaired feature of autophagy is vital in cell differentiation and proliferation (Wang and Choi 2014). Some studies have examined that, the mechanisms of podocyte autophagy have an effect on podocytosis through mTOR, the Atg12-Atg5 conjugation system, adenosine 5’-monophosphate- (AMP-) activated protein kinase (AMPK), PI3K-1/Akt and SIRT1.
mTOR and EVs in autophagy
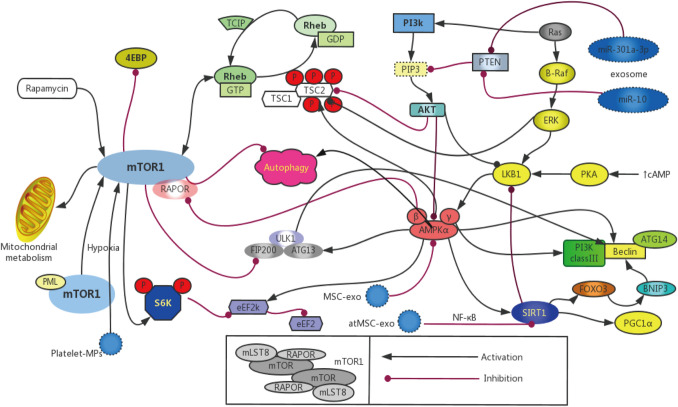
It is generally believed that the mTOR pathway is one of the most important autophagy regulators in diabetic kidneys, which is associated with two mTOR complexes, namely, the mTOR complex 1 (mTORC1) and complex 2 (mTORC2). mTORC1 is a rapamycin-sensitive protein kinase complex, which regulates cell growth, metabolism, proliferation and autophagy by the phosphorylation of p70 S6 kinase 1 (S6K1) and 4EBP1 (Zoncu et al. 2011). mTORC2 is much less sensitive to rapamycin than mTORC1, and it is composed of mTOR, GβL, rapamycin-insensitive companion of mTOR (RICTOR), protein related to RICTOR (PROTOR) and stress-activated protein kinase-interacting protein 1 (SIN1) (Inoki 2014). Under normal physiological status, mTORC1 negatively regulates autophagy by the phosphorylation of ULK1 and the inhibition of its activity. On the contrary, when the cells are exposed to various stress signals, such as hyperglycemia and hypoxemia mTORC1 will be suppressed by allowing for ULK1 and active autophagy under the AMPK pathway (Kim et al. 2011). Unlike mTORC1, the regulatory role of mTORC2 in autophagy remains poorly understood and less appreciated (Fig. 2).
Fig. 2.
Pathways of autophagy in podocyte for: There are four best known pathways of autophagy in podocyte, with the mechanistic target of rapamycin(mTOR), AMPK, SIRT and PI3K/Akt. Autophagy is suppressed by the activation of mTOR1, activation of AMPK can restrain activation of mTOR1 and activate the autophagy. PI3K/Akt can suppress the autophagy via Rheb to enhance the mTOR1 pathway, the suppression of AMPK and SIRT1 further impairs autophagy in various kidney disease. ① Circulating platelet microparticles was associated with endothelial cells autophagy by the activation of mTORC pathways; ② Mesenchymal stem cell (MSC)-derived exosomes reduced the expression of phosphorylated mTOR/mTOR and enhance the expression of AMPK; ③ Adipose tissue-derived mesenchymal stem cells (AtMSC) derived exosomes had renoprotective functions via the regulation of NF-κB activities through SIRT1; ④ Exosomal miR-30la-3p may play an important role in promoting cellar autophagy via inhibit the expression of PTEN and activate the PI3K/AKT pathway and exosomal miR-10b may targeting and inhibiting the PI3K/Akt/mTOR pathway
The release of EVs after platelet activation has been identified as a novel effector that plays a key role in the development of kidney diseases. Zhang et al. proved that the S6K1 phosphorylation level increased in the cytoplasm of CD31-positive cells of diabetic rats, which suggested the enhanced mTORC1 activity in the glomerular endothelium. At the same time, the elevated levels of p-mTOR, p-S6K1, and p-4EBP1 in the glomeruli of diabetic rats measured by Western blotting also confirmed the contribution of platelet EVs to mTORC1 activation (Zhang et al. 2018). Salem et al. confirmed that the circulating platelet MP level was associated with micro- and macro-angiopathy in young patients with type 1 diabetes, which was achieved by activating the mTORC pathways (Salem et al. 2015). Moreover, the activation of mTORC1 in non-diabetic mice is sufficient to induce renal damage, including GBM thickening, ECM expansion, podocyte loss, and proteinuria (Inoki et al. 2011). Therefore, it can be concluded that, EVs contribute to the progression of autophagy in podocytes.
The mTORC1 activity is indispensable to maintain the homeostasis in podocytes, but the inhibition of mTORC1 may has serious adverse effects on podocytes under non-diabetic conditions (Huber et al. 2011). Moreover, the lack of mTORC1 activity in podocyte-specific mice will lead to serious injuries to podocytes, such as glomerulosclerosis and proteinuria (Gödel et al. 2011). Taken together, these studies suggest that exosome biogenesis and autophagy are of crucial importance to maintain the tightly controlled mTOR signaling pathway, so as to ensure the normal function of podocytes (Table 1).
Table 1.
Roles of EVs in autophagy-related pathways
| Pathways | Species/models | Type of EV | Results | References |
|---|---|---|---|---|
| mTOR |
STZ-induced diabetic rat; Rat glomerular endothelial cells |
Microparticles from activated platelets | Platelets microparticles activated the mTORC1 pathway in glomerular endothelial cells | Zhang et al. (2018) |
| Children and adolescents with type 1 | Microparticles from platelets | Circulating platelet microparticles were associated with micro- and macro-angiopathy in young patients with type 1 diabetes | Salem et al. (2015) | |
| AMPK | Rat H9C2 cardiomyocytes | MSC-derived exosomes | MSC-exosomes reduced the expression of phosphorylated mTOR/mTOR and enhance the expression of AMPK in rat cardiomyocytes after ischemia reperfusion injury | Liu et al. (2017) |
| PI3K-1/Akt | Human with pancreatic cancer | Pancreatic cancer cell-derived exosomes | Exosomal miR-30la-3p activate the PI3K/AKT pathway for cell migration and EMT in vitro | Wang et al. (2018b) |
|
Human colorectal cancer cells; Human fibroblasts |
Exosomes from colorectal cancer cells | Exosomal miR-10b targeting and inhibiting the PI3K/Akt/mTOR pathway for cellar fibroblasts | Dai et al. (2018) | |
| SIRT1 |
HEK-293T cells; HK-2 cells |
EVs from HEK-293T and HK-2 cells | Exosome is increased in SIRT1-depleted cells by the disscociation of the V0 domain | Latifkar et al. (2019) |
| STZ-induced T2DM rat | Exosomes from human umbilical cord MSC | HucMSC-ex could protect pancreatic islets β-cell from damage in STZ-induced T2DM rats | Sun et al. (2018) | |
| Pigs with metabolic syndrome | EVs from adipose tissue-derived MSCs | EVs attenuated renal fibrosis, ultimately improving stenotic kidney function in chronic experimental MetS + RAS | Eirin et al. (2017) |
AMPK and EVs in autophagy
AMPK is a conserved heterotrimeric protein complex consisting of three proteins (α, β and γ), which can serve as an important regulator in a range of energy metabolism. The AMP/ATP ratio differs under different cell conditions, and it may be affected by the Ca2+ concentration and provoked by numerous hormones, adipokines and cytokines in the cytoplasm (Zhang et al. 2009). In some energy stress cases, liver kinase B1 (LKBl) is a major kinase that phosphorylates AMPK (Hardie et al. 2012). Compared with mTORCl, AMPK is a positive regulator of autophagy in the case of undernutrition or energy depletion. AMPK promotes cell autophagy by directly phosphorylating ULK1 (Lee et al. 2010). In the meantime, AMPK activates the tuberous sclerosis complex 1 and 2 (TSC1/2) and RAPTOR in the presence of ATP deficiency, inhibits Rheb and finally promotes autophagy by suppressing the activation of mTOR (Sanders et al. 2007). In addition, mechanical stress also causes damage to podocytes through the renin-angiotensin system and autophagy activation by the AMPK pathway (Fig. 2).
In some experimental models of glomerular disease, such as type 1 and 2 diabetes mellitus (T1DM and T2DM), AMPK in the kidney is restrained, and this can be reversed by some AMPK activators to alleviate renal injury (Cammisotto et al. 2008). Kim et al. (2013) verified that the phosphorylation level of AMPK in db/db mice might be promoted by resveratrol, which thus mitigated ECM expansion and proteinuria. Metformin, another AMPK stimulator, is a well-known antidiabetic drug that has been discovered to protect podocytes in many kinds of glomerular injuries (Lee et al. 2007; Sokolovska et al. 2010; Kim et al. 2012a, b). Besides, berberine can also inhibit podocyte apoptosis in mice through activating AMPK in the high-glucose environment (Jin et al. 2017). Fenofibrate, a well-recognized lipid-lowering agent, can activate AMPK by intensifying the LKB1 mRNA expression and reducing Oxidative stress (OS), so as to protect podocytes and endothelial cells from diabetic injury (Al-Rasheed et al. 2015). From the above, the above studies have provided some potential activators to activate the AMPK and suppress autophagy in podocytosis (Table 1).
The mesenchymal stem cell (MSC)-derived exosomes evidently down-regulate the expression of phosphorylated mTOR/mTOR but up-regulate that of AMPK in rat cardiomyocytes after ischemia reperfusion (I/R) injury, as verified by Liu and colleagues. Further, the authors also proved that the exosome-treated cells inhibited the mTOR pathway to partially enhance the autophagic activities (Liu et al. 2017).
PI3K-1/Akt and EVs in autophagy
There are three isoforms of phosphatidylinositol 3-kinases (PI3K), including class I, class II, and class III. It is believed that class I PI3K inhibits autophagy, and it binds to the catalytic subunit p110 with regulatory subunit p58 to promote phosphatidylinositol 3,4,5-triphosphate, eventually activating the Akt/mTOR signaling pathway (Sarbassov et al. 2005). Akt (also known as protein kinase B) is the major downstream effector of PI3K, which also plays a certain role in mediating cell autophagy. Notably, a consensus is currently reached that the PI3K/Akt pathway is a common autophagy-related signaling pathway (Heras-Sandoval et al. 2014). Wan G et al. discovered that ApoL1 overexpression might induce cell death through the process of autophagy, which was related to the PI3K inhibitors 3-methyladenine and wortmannin, and this might be observed in Atg5- or Atg7-knockout embryonic fibroblasts (Wan et al. 2008). Recent studies suggest that the PI3K/AKT signaling pathway may be correlated with the autophagy of podocytes. For instance, Xing and his colleagues supported that, incubating podocytes under the high-glucose environment activated the PI3K/AKT pathway, suppressed the protein expression of nephrin and podocalyxin, but promoted that of α-SMA and desmin. These results have verified the relevance between the PI3K/AKT signaling pathway and the epithelial−mesenchymal transition (EMT) in podocytes (Xing et al. 2015). The activation of PKD1 and Akt will suppress the expression of TSC1/2 and may inhibit the podocyte autophagy via the mTOR pathway.
Numerous existing studies have focused on the relationship between the autophagy-related pathways and DN or other glomerular diseases. In the DN model, the phosphorylation level of Akt/PKB may be blocked by insulin resistance. Rheb is up-regulated after the activation of mTOR, which thus suppresses the podocyte autophagy (Sarbassov et al. 2005). Wang et al. demonstrated that the exosomal miR-30la-3p might inhibit PTEN expression and activate the PI3K/AKT pathway to facilitate cell migration and EMT in vitro. Additionally, it was revealed in that study that, the exosomal miR-30la-3p played an important role in promoting cell autophagy by regulating the PI3K/AKT pathway (Wang et al. 2018b). By contrast, Dai et al. proved that the exosomal miR-10b might target and inhibit the PI3K/Akt/mTOR pathway to up-regulate the TGF-β and SM α-actin levels, which was critical for fibroblasts, indicating that exosomes might function as the active vesicles responsible for cell autophagy and cell differentiation induction (Dai et al. 2018). Nonetheless, the effects of exosomes and the PI3K/AKT pathway on the podocyte autophagy have not yet been addressed completely, so more related research is warranted (Table 1).
SIRT1 and EVs in autophagy
Sirtuins (SIRTs) are the NAD+-dependent class III histone deacetylases (HDACs). Of them, Sirtuin 1 (SIRT1) is a redox-sensitive enzyme of the sirtuin family, which regulates various cellular events, including autophagy, apoptosis, cell survival, endocrine signaling, chromatin remodeling, and gene transcription (Ng and Tang 2013). In addition, SIRT1 also inhibits the stress response-induced inflammation via the nonhistone targets, like peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), fork head box class O proteins (FoxOs), and nuclear factor kappa B (NF-κB) (Han et al. 2016).
SIRT1 is primarily expressed in various cells, especially in kidney, such as podocytes, mesangial cells, vascular endothelial cells, and renal tubular epithelial cells express SIRT1 at a high level. It exerts important renoprotective effects against oxidative stress (He et al. 2010). Similar to AMPK, the expression of SIRT1 tends to be down-regulated in the renal cells in human and animal models of glomerulopathy, besides, the activation of SIRT1 protects the renal podocyte from injury. In the streptozocin (STZ)-induced diabetic rats, the SIRT1 expression decreases in the proximal tubules, which protects podocytes from diabetic injury, and the subsequent proteinuria is significantly attenuated (Hasegawa et al. 2013). Many autophagy-related genes, like Atg5, Atg7 and Atg8 that are critical for the formation and elongation of the autophagosomal membrane, are the deacetylation substrates for SIRT1 (Kaufmann et al. 2014). Similar to AMPK, SIRT1 is activated by resveratrol and promotes LKB1 deacetylation to activate AMPK (Price et al. 2012). Ma et al. (2016) also verified that resveratrol protected the podocytes and proximal tubular epithelial cells (PTECs) through the reactivation of SIRT1 and the restoration of autophagy in T2DM rats and in the hypoxia-treated PTECs. Moreover, SIRT1 regulates the Starvation-induced autophagy by promoting the microtubule associated protein light chain 3 (LC3). All the above-mentioned evidence supports that SIRT1 shows renoprotective effect and inhibits autophagy to alleviate renal injury.
Exosomes are also related to the SIRT1 pathway. The ATP6V1A expression decreases in SIRT1-depleted cells, which may lead to the disassociation of the V0 domain and subsequently increase the release of exosomes (Latifkar et al. 2019). However, it is reported that, the MSC-derived exosomes can effectively reduce renal cell injury in patients with T2DM and AKI (Eirin et al. 2017). Fang Gao et al. confirmed that exosomes from adipose tissue-derived MSCs exerted renoprotective effects against the sepsis-induced AKI, which was possibly achieved through regulating the NF-κB activities via SIRT1 (Gao et al. 2020) (Table 1). From these experimental data above, it can be indicated that exosomes might cooperatively interact with SIRT1 to regulate autophagy in different podocyte diseases. Future studies with the role of EVs to regulate autophagy for kidney diseases by SIRT1 pathway are needed to settle this issue.
Intracellular stress of podocytes and autophagy
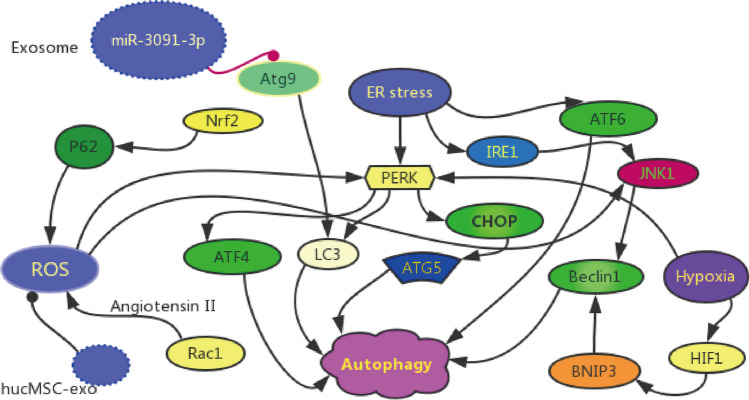
Apart from the different nutrient-sensing pathways, autophagy is also regulated by some intracellular stresses, such as ER Stress, oxidative stress and hypoxia (Fig. 3). The various intracellular stresses have diverse impacts on the biogenesis and secretion of EVs, which are the supplement for the autophagy-related pathways. Exosomes loaded with miRNAs may also be involved in the regulation of intracellular stress, and they may even play therapeutic role in podocytes.
Fig. 3.
Intracellular stress of autophagy in podocyte. Autophagy is regulated by several intracellular stress, such as ER stress, oxidative stress and hypoxia. ① Tumor-derived exosomes loaded with miR-3091-3p would suppress Atg9b expression and alleviate both LC3 and p62 related to autophagy-associated degradation; ② hucMSC-derived exosomes could reduce cisplatin-induced oxidative stress and autophagy
ER stress and EVs in autophagy
The endoplasmic reticulum (ER) regulates protein homeostasis and activates autophagy to protect cells via the unfolded protein response (UPR) pathway (Oakes and Papa 2015). ER Stress is one of the intrinsic stress signals corresponding with hypoxia and oxidative stress. It is a vicious cycle of oxidative stress in podocytes, since ER stress can be induced by ROS and mitochondrial damage after ROS generation. As demonstrated in some studies, ER stress is strongly linked with the autophagy of podocytes in glomerulopathy. Hyperglycemia can aggravate ER stress via a variety of ways and cause cellular damage in DN (Cao et al. 2014). Additionally, ER stress disables the function of ER and activates the downstream apoptotic signaling molecules to induce the autophagy process (Rong et al. 2015).
It is believed that cellular stresses, including oxidative stress and ER stress, will promote the apoptosis and detachment of podocytes from the GBM (Reidy et al. 2014). Besides, the ERS-dependent autophagy can protect podocytes by activating autophagy via the UPR pathway. The UPR system consists of three ERS transducers to monitor the ER status in mammalian cells, which are the activating transcription factor 6 (ATF6), inositol required enzyme 1 (IRE1) and protein kinase RNA-like ER kinase (PERK) (Walter and Ron 2011; Taniguchi and Yoshida 2015). It is postulated that autophagy is induced after ER stress. IRE1 can stimulate autophagy by the JNK1-mediated activation of BECN1 and the inhibition of autophagy via X-box binding protein 1 (XBP1) as the downstream effector (Kroemer et al. 2010). Moreover, PERK also activates LC3 and Atg5 by the ATF4 and C/EBP homologous protein (CHOP) to induce autophagy (Rouschop et al. 2010). ATF6 can also induce autophagy. As reported by Tagawa A et al., the loss of podocytes and histological glomerulosclerosis were observed in the kidneys of the aged mice, which was attributed to the accumulation of oxidized and ubiquitinated proteins, ER stress, and proteinuria (Tagawa et al. 2016).
It is well known that, most MVBs are destined to degrade after fusing with lysosomes, and some MVBs will fuse with the plasma membrane to be released as the exosomes. The formation of exosomes is encouraged by ER stress and the ER stress-dependent autophagy as the UPR pathways (Hanson and Cashikar 2012). Exosomal miRNAs are critical for regulating the structures and functions of podocytes under certain physiological conditions. Wang et al. proved that the tumor-derived exosomes loaded with miR-3091-3p suppressed Atg9b expression and subsequently alleviated the autophagy-associated degradation of LC3 and p62 (Wang et al. 2017b) (Fig. 3). The Atg9 protein can serve as a source of autophagosome, which is essential for autophagy (Tang et al. 2013). Wang et al. demonstrated that ER stress was significantly induced following reperfusion, and that the exosomes loaded with miR-199a-5p from bone marrow–derived mesenchymal stem cells (BMSCs) obviously attenuated the renal I/R injury (Wang et al. 2019) (Table 2). Collectively, these results provide acceptable evidence that the involvement of ER stress in the autophagy of renal cells. Also we can infer that exosomes may affect the autophagy of podocytes, one kind of renal cells, via ER stress exerting renoprotective effects.
Table 2.
Roles of EVs and autophagy with intracellular stress
| Intracellular stress | Species/models | Type of EVs | Results | References |
|---|---|---|---|---|
| ER stress | NAFLD mice | Exosomes from hepatic tumor | Exosomes loaded with miR-3091-3p would suppress Atg9b expression and subsequent alleviate both LC3 and p62 related to autophagy under ER stress | Hanson and Cashikar (2012) |
| BALB/c mice with renal I/R injury | Exosomes from BMSCs | Exosome loaded with miR-199a-5p from BMSCs would attenuate renal I/R injury and ER stress was induced next to reperfusion | Tang et al. (2013) | |
| Oxidative stress |
Sprague–Dawley rats with AKI; NRK-52E cell |
HucMSC exosomes | HucMSC exosome can repair cisplatin-induced AKI in rats and NRK-52E cell injury by ameliorating oxidative stress and cell apoptosis | Zhou et al. (2013b) |
| Hypoxia |
Human MM cell; HUVECs |
Exosomes from MM cells | Exosomes could enhance angiogenesis by miR-135b that may inhibit HIF-1 in chronic hypoxia | Haase (2006) |
| Human RPTC | Exosomes from RPTC | Exosome by hypoxia in renal tubular cells that are cytoprotective for renal tubular cells by alleviating cell apoptosis | Kume et al. (2010) | |
|
Human TECs; Mouse TECs |
Exosomes from injured TECs | Exosomal protein CD63 is increased during hypoxia of renal tubular cells | Dorayappan et al. (2018) |
Oxidative stress and EVs in autophagy
ROS are the oxygen-derived free radicals produced by the mitochondrial respiration and redox-mediating enzymes in metabolism. The kidney cells are rich in mitochondria and always running at a high respiration rate to support metabolism. As a highly energetic organ, the kidney is easier to be damaged by OS, OS is associated with many renal diseases (Daenen et al. 2018). Nicotine will accelerate the production of ROS and associate downstream MAPK signaling to cause podocyte apoptosis in human podocytes (Lan et al. 2016). In age mice, excessive oxidative stress is part of the reason for the decreased ability to handle ER stress and are susceptible to AKI (Liu et al. 2019). Rac1 is a well-known receptor that mediates ROS synthesis in podocytes under different kidney diseases states, including hypertensive nephropathy and DN (Nagase and Fujita 2013). The Rac1–induced nicotinamide adenine dinucleotide phosphate oxidase-ROS cascade can be activated by angiotensin II through the type I angiotensin II receptor, and the podocyte function is impaired by the dysregulation of cytoskeletal organization (Liu et al. 2013).
Autophagy can be activated by ROS in multiple podocytopathys through multiple mechanisms. For instance, Jiang XS et al. Discovered the ROS-mediated autophagy in the cultured podocytes treated with palmitic acid (Jiang et al. 2017). In addition, the content of EVs may be affected by a variety of cellular stresses, such as oxidative stress, hypoxia and inflammation (Bruno et al. 2016). Zhou and colleagues constructed a rat model of acute kidney toxicity induced by cisplatin treatment. Their results showed that exosomes secreted by human umbilical cord mesenchymal stromal cells reduced the cisplatin-induced oxidative stress and autophagy in vivo, while promoting the proliferation of renal epithelial cell line and renal tubular epithelial cells in vitro (Zhou et al. 2013b). Besides that, other studies offer better understanding of the possibility that the exosome may lighten cell damage caused by OS. Exosomal miR-21 derived from MSCs could be transported to C-kit + cardiac stem cells to functionally inhibit PTEN expression, activating PI3K/AKT signaling and leading to protection against oxidative stress-triggered cell death (Fan et al. 2018). Jiang et al. confirmed that down-regulation of exosomal miR-137 will alleviates oxidative stress injury in neurone by up-regulating OXR1 for Parkinson’s disease mouse model (Jiang et al. 2019).These experimental results make a challenge that is it possible the podocytes will benefit from certain special source of exosome? Nonetheless, more studies are needed to verify the different contents and functions of EVs in the oxidative stress-induced autophagy in podocytes for kidney disease (Fig. 3).
Hypoxia and EVs in autophagy
Hypoxia can be induced by the hyperglycemic injury to red blood cells, decreased nitric oxide (NO) activity, and renal vasoconstriction resulting from the hyperactivation of RAS (Ding and Choi 2015). Hypoxia can be attributed to chronic kidney disease by the deterioration of glomerular and peritubular capillaries in renal fibrosis (Tanaka et al. 2012). The hypoxia-inducible factor (HIF) family, including HIF1, HIF2 and HIF3, plays an important role in the regulation of hypoxia (Haase 2006). Exosomes can enhance angiogenesis via miR-135b, which may inhibit HIF-1 under the condition of chronic hypoxia (Umezu et al. 2014). In the case of chronic hypoxia, exosomes also enhance angiogenesis by targeting the factor that inhibits HIF1. In the aged kidneys, the declined activity of hypoxia-induced autophagy leads to the increases in damaged mitochondria and mitochondrial ROS; meanwhile, the Bnip3 expression is essential for inducing autophagy under hypoxic condition (Kume et al. 2010). Zhang et al. demonstrated that, in the case of hypoxia in renal tubular cells, the exosomes derived from hypoxic cells showed cytoprotective effects on renal tubular cells by alleviating cell apoptosis (Zhang et al. 2017). Besides, the activity of secretory Rab proteins is enhanced in hypoxic ovarian cancer cells, which increases the release of exosomes from the formed MVBs via the secretory pathway (Dorayappan et al. 2018). Also, Borges et al. proved that the exosomal protein CD63 was up-regulated during the hypoxia of renal tubular cells in mice with unilateral ureteral obstruction (Borges et al. 2013). Hypoxia has a certain impact on the biogenesis and content of EVs, both of which are engaged in the autophagy of podocytes.
EVs and autophagy for podocyte injury
The improved knowledge regarding the different autophagy-related pathways and cellular stress during glomerular diseases in podocytes indicates that, EVs are potentially related to the pathogenic mechanism. Under basal conditions, podocytes experience a high autophagy rate, which means that autophagy is a fundamental self-repair mechanism to maintain the homeostasis in podocytes (Hartleben et al. 2010). When podocytes are injured under certain circumstances, such as hypoxia and hyperglycemia, the structure of GBM that includes slit membrane molecules, actin cytoskeleton and cell adhesion molecules, may be destroyed, finally leading to proteinuria. So far, many studies have confirmed the central role of autophagy in podocytopathy, and the MSC-derived exosomes are beneficial to podocyte repair.
Autophagy and EVs in podocyte EMT
EMT is induced by the loss of podocyte polarity, the disappearance of cell contacts and the gain of typical mesenchymal markers, like vimentin, α-smooth muscle actin (α-SMA), and fibroblast-specific protein 1 (FSP1) (Liu 2004). In the occurrence of EMT, podocytes will lose their original features. To better understand how autophagy impacts the podocytes, Xing and his colleagues demonstrated that podocytes incubated at elevated glucose levels for over 48 h increased the expression of α-SMA and desmin by activating the PI3K/AKT pathway. This result proved that podocytes might be related to the PI3K/AKT signaling pathway (Xing et al. 2015). However, Wu et al. discovered that the TGF-β1-loaded exosomes derived from the endothelial-mesenchymal transition (EndoMT) induced the EMT and dysfunction of podocytes after high glucose treatment for 24 h. Therefore, inhibiting the release of exosomes loaded with TGF-β1 may be one way to prevent renal fibrosis in DN, which is achieved through protecting podocytes from EMT (Wu et al. 2017). As podocyte EMT is critical at the early stage of renal fibrosis in many glomerular diseases, it is important to figure out the therapeutic potential of EVs in podocyte autophagy.
Autophagy and EVs in podocyte hypertrophy
The highly differentiated podocytes will expand their size to compensate for the glomerular dilation and cover the exposed region of GBM (Inoki et al. 2011). It has been verified in many studies that; glomerular podocyte hypertrophy is associated with the development of glomerulopathy. The autophagy-related pathways are engaged in the process of podocyte hypertrophy (Herbach et al. 2009). As hypothesized in previous studies, the activation of podocyte hypertrophy is tightly correlated with mTORC1 in DN (Lu et al. 2011). The translationally controlled tumor protein (TCTP) is a kind of regulator that mediates cell growth, which can activate the mTORC1 signaling pathway and eventually cease the podocyte cycle and hypertrophy in the T1DM mouse model (Kim et al. 2012a). Besides, Kim et al. (2006) demonstrated that AngII was able to up-regulate the expression of ERK1/2 and Akt/PKB kinases, both of which induced the process of podocyte hypertrophy. EVs derived from renal parenchyma can identify the age-associated podocyte hypertrophy (Turco et al. 2016). Yet, there is no experimental evidence supporting the relationship between EVs and autophagy in the podocyte hypertrophy.
Both dead and alive podocytes are found in the urine of patients with kidney disease, and normal podocytes are also detected in healthy subjects. It is concluded that the podocyte detachment from GBM may be an earlier biomarker for glomerular disease (Yu et al. 2005). Moreover, podocyte apoptosis is also involved in numerous glomerular diseases, such as DN, hypoxia and IgA nephropathy. The expression levels of p38 protein kinase and caspase 3 are up-regulated by activating NADPH oxidase and ROS in podocytes of DN patients (Susztak et al. 2006). Meanwhile, ROS can also induce podocyte autophagy. No evidence in the existing studies confirms the association between EVs and autophagy in the process of podocyte detachment and apoptosis.
EVs and autophagy for podocytes in glomerular diseases
Various animal models of glomerular disease have indicated the increased susceptibility due to the loss of autophagy in podocytes (Hartleben et al. 2010). Studies of puromycin aminonucleoside-nephritis (PAN) and Heymann nephritis in rats show that the activity of autophagy is apparently enhanced during the podocyte recovery (Asanuma et al. 2003; Wu et al. 2013). As the early diagnostic biomarker and the possible novel therapy, EVs also have a certain impact on podocytes in glomerular disease. The autophagy of podocytes affects the content and release of EVs, and the latter also induces different autophagy-related pathways by intercellular interaction (Table 3).
Table 3.
EVs and autophagy for podocytes in glomerular diseases
| Glomerular diseases | Species/models | Type of EVs | Results | References |
|---|---|---|---|---|
| DN |
Rat GMCs; Human podocytes |
Exosomes from HG-induced MCs | Exosomes derived from GMCs induced by high level glucose, are able to activate PI3K/AKT pathway in podocytes via TGFβ1 | Wang et al. (2018c) |
| db/db mice with T2DM | Exosomes from ADSCs | ADSCs-Exo ameliorated DN symptom by enhancing the expression of miR-486 and inhibition of Smad1/mTOR signaling pathway in podocyte | Jin et al. (2019) | |
| FSGS |
Human podocyte cells; Double-transgenic mice with CG |
Exosomes from urine | Urinary exosomal WT-1 levels are higher in children with NS caused by FSGS than with SSNS not remission | Lee et al. (2012) |
| Children with FSGS and MCD | Exosomes from urine | Urinary exosomal miR-193a of children with PNS as the early diagnosis of primary FSGS by regulating autophagy in podocytes | Huang et al. (2017) | |
| IgAN | Adult with IgAN | Exosomes from urine | Exosome excretion was elevated in IgAN patients and exosomal CCL2 mRNA was markedly induced in IgAN patients | Feng et al. (2018) |
Diabetic nephropathy
DN is identified with the typical characteristics of podocyte injury, glomerular basement thickening, mesangial expansion, and proteinuria. Previous experiments have activated the mTOR signaling pathway in podocytes, which is characterized as a mile stone in the diabetic nephropathy mouse models (Gödel et al. 2011). In podocyte-specific conditional Tsc1 knockout diabetic mice (Tsc1-PcKO), podocyte hypertrophy is induced through activating the mTORC1 signaling, and the expression of slit diaphragm proteins is reduced under ER stress, which finally leads to podocyte detachment and glomerulosclerosis (Inoki et al. 2011). It has been discovered that the expression of beclin 1, LC3, and Atg5-Atg12 is down-regulated, which is associated with the suppression of podocyte autophagy in the STZ-induced T1DM mouse model and in the immortalized mouse podocytes incubated with high glucose for 48 h (Fang et al. 2013). Another study suggests that, high glucose incubation of the immortalized murine podocytes for a short term (24 h) induces the generation of ROS and leads to the increased levels of beclin 1, LC3-II and autolysosomes, which finally up-regulates autophagy (Ma et al. 2013).
A number of recent studies have provided accumulating data about the role of EVs in DN, which can be used as the early detection biomarker and novel therapy for podocytes in DN. The podocalyxin–positive and podoplanin–positive urinary MPs are detected in diabetic mice before the onset of albuminuria (Burger et al. 2014). Urinary exosomes loaded with the high expression of WT-1 (a transcription factor required for kidney development and podocyte injury) can be found in diabetic patients (Kalani et al. 2013). Huang G et al. proved that TGFβ1 was able to activate the PI3K/Akt signaling pathway, which was the typical autophagy pathway involved in podocyte injury and renal fibrosis (Huang et al. 2016). Additionally, exosomes derived from the glomerular mesangial cells (GMCs) treated with high glucose level can activate the PI3K/AKT pathway in podocytes via TGFβ1 (Wang et al. 2018c). Jin J et al. confirmed that miR-486 directly regulated Smad1 expression and then increased the mTOR-mediated autophagy flux. Besides, exosomes from adipose-derived stem cells were rich in miR-486 and they mediated the transport of miR-486 to podocytes, while the up-regulation of miR-486 suppressed renal injury by regulating the smad1/mTOR signaling pathway (Jin et al. 2019). Nonetheless, the interplay of EVs and the autophagy of podocytes in DN remain to be further elucidated.
Focal segmental glomerulosclerosis (FSGS)
FSGS is a process that begins with podocyte loss, glomerular capillary tuft adhering to the Bowman’s capsule, subsequent capillary obliteration and eventual total nephron degeneration (D’Agati 2012). Kawakami et al. verified that autophagy failure was a key mechanism in the pathogenesis of FSGS (Kawakami et al. 2015). Atg5 PcKO mouse is a typical model with podocyte degeneration, podocyte loss and glomerulosclerosis, suggesting that autophagy is a monitor for cellular stress of podocytes (Hartleben et al. 2010). The apolipoprotein L1 gene (ApoL1), a kind of lipid binding protein that can combine with phosphatidic acid, can serve as a positive regulator of mTOR and phosphatidylinositol phosphates (including PI3 phosphate) that are necessary for autophagy. It is currently unknown about the molecular mechanisms of ApoL1 in modulating the autophagic activity, but autophagy and the autophagy-associated cell death can be induced in cells incubated in vitro through the overexpression of ApoL1 (Wan et al. 2008). Giovinazzo et al. verified that propose a model wherein APOL1 channel activity is the upstream event causing cell death by active channels at the plasma membrane, which results in an influx of both Na+ and Ca2+ in human cells (Giovinazzo et al. 2020).
WT-1 is critical in podocyte damage, but the relationship of exosomal WT-1 with FSGS is still controversial. Zhou H et al. found that the urinary exosomal WT-1 levels were apparently higher in children with nephrotic syndrome caused by FSGS than those with the unrelieved steroid–sensitive nephrotic syndrome (SSNS) (Zhou et al. 2013a). On the contrary, Lee H and his colleagues demonstrated that WT-1 was only detected in 60% of the 40 children with FSGS and SSNS (Lee et al. 2012). Mcl-a is a target gene of miR-193a that participates in the regulation of cellular apoptosis and autophagy (Germain and Slack 2011). Huang Z et al. tested the urinary exosomal miR-193a levels in children with primary nephrotic syndrome, and their results indicated that the exosomal miR-193a levels might be used for the early diagnosis of primary FSGS by regulating podocyte autophagy (Huang et al. 2017). Exosomes derived from MSCs can be potentially adopted to treat FSGS, but more research is still warranted. EVs may participate in the process of podocyte autophagy in FSGS, which can provide the new target for curing the disease or delaying its progression to avoid end-stage renal disease (ESRD).
Membranous nephropathy (MN) and IgA nephropathy
Podocytes in Heymann nephritis, a classical rat model of MN, displays the up-regulated autophagy induced by ER stress and the complement C5b-9 membrane attack complex (Wang et al. 2012). Similarly, Wu L et al. discovered that mTORC1 was negatively correlated with autophagy in the passive Heymann nephritis model and the reduced autophagy, and that autophagy increased during the decubation (Wu et al. 2013). With the help of electron microscopy, autophagosomes are detected in IgA nephropathy (Sato et al. 2009). Four candidate urinary exosomal proteins can be identified by proteomic analysis from patients with early IgA nephropathy compared with those with thin basement membrane nephropathy. These candidate proteins are aminopeptidase N, vasorin precursor, a-1-antitrypsin and ceruloplasmin (Moon et al. 2011). Feng Y et al. detected the roles of urinary exosome protein and exosomal inflammatory response-related mRNA in the diagnosis of IgAN, as well as their correlations with renal histological damage. Their results showed that exosome excretion elevated in IgAN patients compared with healthy controls, and that the CCL2 mRNA was markedly induced in urinary exosomes from IgAN patients (Feng et al. 2018). From the above studies, there may be a special biomarker to distinguish IgAN from MN or other glomerular diseases.
However, more convincing experimental data are needed to resolve the role of glomerular autophagy in the course of glomerulonephritis. In addition, it is practical to reveal whether EVs have causal or protective effect on podocytopathy via autophagy.
Therapeutic strategy of EVs via autophagy in the treatment of kidney disease
According to the above studies, EVs can be used in the early diagnosis of kidney diseases and they can regulate the autophagy-related cellular signaling pathways in podocytes. In addition, in numerous animal models of kidney diseases, EVs derived from various cells, such as stem cells, glomerular mesangial cells or even podocytes, can induce autophagy to protect podocytes and promote the recovery of renal functions (Table 4).
Table 4.
EVs via autophagy in the treatment of kidney disease
| Kidney disease | Species/models | Type of EVs | Therapeutic strategy | References |
|---|---|---|---|---|
| AKI |
SD Rats with UKI Human UVEC |
Microvesicles from hWJMSCs | Decline expression of NOX2 and alleviate oxidative stress | Zhang et al. (2014a) |
| NRK-52E cells | Exosomes from hucMSC | Increase the expression of the autophagic marker protein LC3B and the ATG5 and ATG7 | Wang et al. (2017a) | |
|
HK-2 cells HEK293T |
Exosomes from hucMSC | HucMSC-ex-transported 14-3-3ζ can induce autophagy, which prevents HK-2 cells from the injury of cisplatin | Wang et al. (2018a) | |
| Mice with AKI | EVs from MSC | MSC-derived EVs can transfer miRNA to restrain the expression of pro-inflammatory genes and promote podocytes regeneration | Collino et al. (2015) | |
| CKD |
C57BL6/J mice HK-2 cells |
Microvesicles from MSC | MSC-derived EVs protect kidneys both in vivo and vitro with CKD | He et al. (2015) |
|
FVB/N mice HUVECs |
Microparticles from KMSCs | KMSC-derived microparticles alleviate renal fibrosis and restore EMT by modulating TGF-β and αSMA expression | Choi et al. (2015) | |
| Human with CKD | EVs from hucMSC | HucMSC-derived EVs can decrease TNF-α, increase IL-10 and ameliorate the inflammatory immune reaction | Nassar et al. (2016) | |
| DN | Adult male albino rats | Exosomes from MSC | MSC-derived exosomes increase of LC3 and Beclin-1 and decrease of mTOR and fibrotic marker expression | Ebrahim et al. (2018) |
| STZ-induced SD rat | Exosomes from USCs | USCs-Exosome contained growth factor, TGF-β1, angiogenin and BMP-7 related with renal cell survival | Jiang et al. (2016) |
Acute kidney injury (AKI) is generally considered as a typical symptom of the sudden loss of renal function. A variety of potential factors can cause AKI, such as renal ischemia due to low blood pressure, crush injury, inflammation, and urinary tract obstruction or infection. EVs derived from human Wharton-Jelly MSCs can alleviate the renal OS by modulating the expression of NADPH oxidase 2 (the pro-oxidant), which can thus enhance renal cell proliferation, increase autophagy and decrease apoptosis in the I/R rat models (Zhang et al. 2014a). Baixauli et al. proposed that exosomes alleviated the intracellular stress conditions in coordination with the autophagy-lysosomal pathway, which was necessary for preserving the intracellular protein and RNA homeostasis (Baixauli et al. 2014). Furthermore, it has been confirmed that the hucMSC-derived exosomes can induce autophagy as a renoprotective mechanism, including the inhibition of mitochondrial apoptosis and the release of inflammatory cytokines, in the experimental models of AKI induced by renal toxicants like cisplatin (Wang et al. 2017a, 2018a). Moreover, the MSC-derived EVs can transfer miRNA to the recipient cells to restrain the expression of pro-inflammatory genes and promote the regeneration of podocytes and renal tubular cells in the AKI models (Collino et al. 2015).
Chronic kidney disease (CKD) is characterized by the progressive loss of renal function, and it is associated with diverse causes and complex symptoms, finally leading to ESRD. Numerous studies have confirmed the efficacy of EVs in the treatment of CKD. For instance, He.J. and his colleagues carried out one experimental trial and demonstrated that MSC-derived EVs showed renoprotective efficacy in CKD patients; meanwhile, the eGFR, creatinine and BUN levels were significantly improved (He et al. 2015). After the administration of MSC-derived EVs in the unilateral ureteral obstruction (UUO) animal models, renal fibrosis is alleviated by restoring the EMT morphological changes and modulating the expression levels of TGF-β and α-SMA. Typically, the aim of improving renal function may be achieved through the autophagy of podocytes (Choi et al. 2015). The hucMSC-derived EVs can decrease TNF-α expression, increase IL-10 level and ameliorate the inflammatory immune reaction, thus promoting renal regeneration and improving the renal function in CKD (Nassar et al. 2016). Therefore, it can be concluded from these studies that, EVs have the potential therapeutic value via the autophagy in CKD.
DN is a common progress in diabetes mellitus and also a major cause of CKD. In a diabetic mouse model, the MSC-derived EV therapy has been demonstrated to prevent DN progression, as evidenced by the improved renal function and the repair of damaged kidney tissues through modulating autophagy (Ebrahim et al. 2018). Exosomes derived from the urine-derived stem cells can promote the proliferation of glomerular endothelial cells at the early stage of diabetic kidney impairment, and up-regulate the levels of VEGF, TGF-β1, angiogenin and BMP-7, which may thereby inhibit podocyte apoptosis and promote glomerular angiogenesis (Jiang et al. 2016). With the expression of miR-486, ADSCs-Exo can inhibit the Smad1/mTOR signaling pathway in podocytes and ameliorate the DN symptoms. This will provide a potential therapeutic strategy for DN (Jin et al. 2019).
Conclusions and perspectives
In the field of kidney, podocytopathy is a major cause of renal diseases, which can result in CKD and even ESRD despite of the application of multiple intensive therapies. The value of EVs as the diagnostic biomarker and potential therapy has been recognized in podocytopathy. This review examines the underlying mechanisms of EVs and podocyte autophagy in certain glomerular diseases. The investigation of EVs and autophagy in podocytes has embarked on the greatest venture. Nonetheless, the therapeutic efficacy of EVs via autophagy, which may be translated into clinical application in the near future, should be verified in further laboratory experiments. All in all, the better understanding of EVs and autophagy in podocytes will usher in a novel era for the diagnosis and treatment of various kidney diseases.
Acknowledgements
BCS thank Prof. Qingshan Ma, Prof. Ping Luo and Prof. Kaishu Zhao for their constant encouragement, suggestions and support throughout.
Abbreviations
- ADSCs
Adipose-derived stem cells.
- AKI
Acute kidney injury
- AMPK
AMP-activated protein kinase
- ATF
Activating transcription factor
- ATG
Autophagy-related gene
- BMP
Bone morphogenetic protein
- BMSCs
Bone marrow-derived mesenchymal stem cells
- CHOP
C/EBP homologous protein
- CKD
Chronic kidney disease
- DN
Diabetic nephropathy
- EMT
Epithelial–mesenchymal transition
- ERS
Endoplasmic reticulum stress
- EVs
Extracellular vesicles
- ESRD
End-stage renal disease
- FSGS
Focal segmental glomerulosclerosis
- GBM
Glomerular basement membrane
- HG
High glucose
- HIF1
Hypoxia-inducible factor 1
- IRE1
Inositol requiring enzyme 1
- I/R
Ischemia-reperfusion
- JNK1
c-junk N-terminal kinase 1
- LC3
Microtubule-associated protein light chain 3
- LKB1
Liver kinase B1
- MCD
Minimal change disease
- MN
Membranous nephropathy
- mTORC
Mammalian target of rapamycin complex
- MVBs
Multivesicular bodies
- MPs
Microparticles
- NAFLD
Non-alcoholic fatty liver disease
- NLRP3
Nucleotide-oligomerization domain-like receptor 3
- NS
Nephrotic syndrome
- PERK
Protein kinase RNA-like ER kinase
- RAPTOR
Regulatory associated protein of mTOR
- Rheb
Ras homolog enriched in brain
- ROS
Reactive oxygen species
- SIRT1
Sirtuin 1
- SSNS
Steroid-sensitive nephrotic syndrome
- TSC1/2
Tuberous sclerosis complex 1/2
- ULK1
Unc-51-like1
- UPR
Unfolded protein response
- USCs
Urine-derived stem cells
Authors’ contributions
BCS contributed in conceptualization and wrote the manuscript. SBZ and LZ equally contributed in reviewed the draft and made critical modifications. GDS was in charge of writing, and editing the manuscript. All authors had read and approved the final manuscript.
Funding
Not available.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Al-Rasheed NM, Al-Rasheed NM, Attia HA, Al-Amin MA, Al-Ajmi HN, Hasan IH, Mohamad RA, Sinjilawi NA. Renoprotective effects of fenofibrate via modulation of LKB1/AMPK mRNA expression and endothelial dysfunction in a rat model of diabetic nephropathy. Pharmacology. 2015;95(5–6):229–239. doi: 10.1159/000381190. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, Kominami E, Tomino Y. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. Faseb J. 2003;17(9):1165–1167. doi: 10.1096/fj.02-0580fje. [DOI] [PubMed] [Google Scholar]
- Bader CA, Shandala T, Ng YS, Johnson IR, Brooks DA. Atg9 is required for intraluminal vesicles in amphisomes and autolysosomes. Biol Open. 2015;4(11):1345–1355. doi: 10.1242/bio.013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixauli F, López-Otín C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH 2nd, LeBleu VS, Kalluri R (2013) TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24(3):385–392 [DOI] [PMC free article] [PubMed]
- Bruno S, Camussi G. Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatr Nephrol. 2013;28(12):2249–2254. doi: 10.1007/s00467-013-2413-z. [DOI] [PubMed] [Google Scholar]
- Bruno S, Porta S, Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol. 2016;790:83–91. doi: 10.1016/j.ejphar.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond) 2013;124(7):423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol. 2014;25(7):1401–1407. doi: 10.1681/ASN.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammisotto PG, Londono I, Gingras D, Bendayan M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol. 2008;294(4):F881–F889. doi: 10.1152/ajprenal.00373.2007. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W, Duan H. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med. 2014;33(4):809–816. doi: 10.3892/ijmm.2014.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HY, Lee HG, Kim BS, Ahn SH, Jung A, Lee M, Lee JE, Kim HJ, Ha SK, Park HC. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial–mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther. 2015;6(1):18. doi: 10.1186/s13287-015-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol. 2015;26(10):2349–2360. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatric Nephrol. 2018;34(6):975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- Dai G, Yao X, Zhang Y, Gu J, Geng Y, Xue F, Zhang J. Colorectal cancer cell-derived exosomes containing miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull Cancer. 2018;105(4):336–349. doi: 10.1016/j.bulcan.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agati VD. Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens. 2012;21(3):243–250. doi: 10.1097/MNH.0b013e32835200df. [DOI] [PubMed] [Google Scholar]
- Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224(1):R15–R30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA, Cohn DE, Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7(12):226. doi: 10.3390/cells7120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G-C, Shi B, Wang Y, Zhao R, Long X, Deng W, Wang Z. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit + cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS ONE. 2018;13(2):e0191616. doi: 10.1371/journal.pone.0191616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, Dai C, Yang J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE. 2013;8(4):e60546. doi: 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Lv LL, Wu WJ, Li ZL, Chen J, Ni HF, Zhou LT, Tang TT, Wang FM, Wang B, Chen PS, Crowley SD, Liu BC. Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. Am J Pathol. 2018;188(11):2542–2552. doi: 10.1016/j.ajpath.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719. [DOI] [PubMed] [Google Scholar]
- Germain M, Slack RS. MCL-1 regulates the balance between autophagy and apoptosis. Autophagy. 2011;7(5):549–551. doi: 10.4161/auto.7.5.15098. [DOI] [PubMed] [Google Scholar]
- Giovinazzo JA, Thomson RP, Khalizova N, Zager PJ, Malani N, Rodriguez-Boulan E, Raper J, Schreiner R. Apolipoprotein L-1 renal risk variants form active channels at the plasma membrane driving cytotoxicity. Elife. 2020;9:e51185. doi: 10.7554/eLife.51185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Chitiprolu M, Roncevic L, Javalet C, Hemming FJ, Trung MT, Meng L, Latreille E, Tanese de Souza C, McCulloch D, Baldwin RM, Auer R, Côté J, Russell RC, Sadoul R, Gibbings D. Atg5 disassociates the V(1)V(0)-ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43(6):716–730.e717. doi: 10.1016/j.devcel.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Investig. 2011;121(6):2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291(2):F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Huang W, Li X, Gao L, Su T, Li X, Ma S, Liu T, Li C, Chen J, Gao E, Cao F. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J Pineal Res. 2016;60(2):178–192. doi: 10.1111/jpi.12299. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120(4):1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, Hosoya K, Komatsu M, Kaneko Y, Kanda T, Kubota E, Tokuyama H, Hayashi K, Guarente L, Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120(4):1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wang Y, Lu X, Zhu B, Pei X, Wu J, Zhao W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 2015;20(9):591–600. doi: 10.1111/nep.12490. [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Herbach N, Schairer I, Blutke A, Kautz S, Siebert A, Göke B, Wolf E, Wanke R. Diabetic kidney lesions of GIPRdn transgenic mice: podocyte hypertrophy and thickening of the GBM precede glomerular hypertrophy and glomerulosclerosis. Am J Physiol Renal Physiol. 2009;296(4):F819–F829. doi: 10.1152/ajprenal.90665.2008. [DOI] [PubMed] [Google Scholar]
- Huang G, Lv J, Li T, Huai G, Li X, Xiang S, Wang L, Qin Z, Pang J, Zou B, Wang Y. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38(4):1179–1189. doi: 10.3892/ijmm.2016.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang Y, Zhou J, Zhang Y. Urinary exosomal miR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed Res Int. 2017;2017:7298160. doi: 10.1155/2017/7298160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int. 2011;79(5):502–511. doi: 10.1038/ki.2010.457. [DOI] [PubMed] [Google Scholar]
- Inoki K. mTOR signaling in autophagy regulation in the kidney. Semin Nephrol. 2014;34(1):2–8. doi: 10.1016/j.semnephrol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17(2):171. doi: 10.3390/ijms17020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XS, Chen XM, Wan JM, Gui HB, Ruan XZ, Du XG. Autophagy protects against palmitic acid-induced apoptosis in podocytes in vitro. Sci Rep. 2017;7:42764. doi: 10.1038/srep42764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu J, Chen L, Jin Y, Zhang G, Lin Z, Du S, Fu Z, Chen T, Qin Y, Sun X. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res. 2019;1722:146331. doi: 10.1016/j.brainres.2019.146331. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liu S, Ma Q, Xiao D, Chen L. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol. 2017;794:106–114. doi: 10.1016/j.ejphar.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. doi: 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, Tiwari S. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS ONE. 2013;8(3):e60177. doi: 10.1371/journal.pone.0060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Beier V, Franquelim HG, Wollert T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell. 2014;156(3):469–481. doi: 10.1016/j.cell.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22(8):407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Gomez IG, Ren S, Hudkins K, Roach A, Alpers CE, Shankland SJ, D’Agati VD, Duffield JS. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol. 2015;26(5):1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A. Autophagy. Ann N Y Acad Sci. 2005;1066:259–271. doi: 10.1196/annals.1363.015. [DOI] [PubMed] [Google Scholar]
- Kim NH, Rincon-Choles H, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Redox dependence of glomerular epithelial cell hypertrophy in response to glucose. Am J Physiol Renal Physiol. 2006;290(3):F741–F751. doi: 10.1152/ajprenal.00313.2005. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Nam BY, Li JJ, Park JT, Lee SH, Kim DH, Kim JY, Kang HY, Han SH, Yoo TH, Han DS, Kang SW. Translationally controlled tumour protein is associated with podocyte hypertrophy in a mouse model of type 1 diabetes. Diabetologia. 2012;55(4):1205–1217. doi: 10.1007/s00125-012-2467-7. [DOI] [PubMed] [Google Scholar]
- Kim J, Shon E, Kim CS, Kim JS. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res. 2012;2012:210821. doi: 10.1155/2012/210821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Lim JH, Youn HH, Hong YA, Yang KS, Park HS, Chung S, Ko SH, Shin SJ, Choi BS, Kim HW, Kim YS, Lee JH, Chang YS, Park CW. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56(1):204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- Kraft C, Reggiori F, Peter M. Selective types of autophagy in yeast. Biochim Biophys Acta. 2009;1793(9):1404–1412. doi: 10.1016/j.bbamcr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol. 2015;3:65. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Lederman R, Eng JM, Shoshtari SS, Saleem MA, Malhotra A, Singhal PC. Nicotine induces podocyte apoptosis through increasing oxidative stress. PLoS ONE. 2016;11(12):e0167071. doi: 10.1371/journal.pone.0167071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifkar A, Ling L, Hingorani A, Johansen E, Clement A, Zhang X, Hartman J, Fischbach C, Lin H, Cerione RA, Antonyak MA. Loss of sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell. 2019;49(3):393–408.e397. doi: 10.1016/j.devcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292(2):F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS ONE. 2010;5(11):e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Han KH, Lee SE, Kim SH, Kang HG, Cheong HI. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr Nephrol. 2012;27(2):317–320. doi: 10.1007/s00467-011-2035-2. [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15(1):1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hitomi H, Diah S, Deguchi K, Mori H, Masaki T, Nakano D, Kobori H, Nishiyama A. Roles of Na+/H+ exchanger type 1 and intracellular pH in angiotensin II-induced reactive oxygen species generation and podocyte apoptosis. J Pharmacol Sci. 2013;122(3):176–183. doi: 10.1254/jphs.12291fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang Y, Liu A, Wang J, Li L, Chen X, Gao X, Xue Y, Zhang X, Liu Y. Distinct dasatinib-induced mechanisms of apoptotic response and exosome release in imatinib-resistant human chronic myeloid leukemia cells. Int J Mol Sci. 2016;17(4):531. doi: 10.3390/ijms17040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy Via AMPK and Akt pathways. Cell Physiol Biochem. 2017;43(1):52–68. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang R, Huang L, Zheng Z, Vlassara H, Striker G, Zhang X, Guan Y, Zheng F. Excessive oxidative stress contributes to increased acute ER stress kidney injury in aged mice. Oxid Med Cell Longev. 2019;2019:1–15. doi: 10.1155/2019/2746521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MK, Gong XG, Guan KL. mTOR in podocyte function: is rapamycin good for diabetic nephropathy? Cell Cycle. 2011;10(20):3415–3416. doi: 10.4161/cc.10.20.17686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319(6):779–789. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, Lv Z, Chen Z, Han J, Jia L, Wang L. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract. 2016;212(4):310–318. doi: 10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6(1):3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22(2):132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon PG, Lee JE, You S, Kim TK, Cho JH, Kim IS, Kwon TH, Kim CD, Park SH, Hwang D, Kim YL, Baek MC. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics. 2011;11(12):2459–2475. doi: 10.1002/pmic.201000443. [DOI] [PubMed] [Google Scholar]
- Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- Murrow L, Malhotra R, Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17(3):300–310. doi: 10.1038/ncb3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol. 2013;9(2):86–98. doi: 10.1038/nrneph.2012.282. [DOI] [PubMed] [Google Scholar]
- Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekström K, Kholia S, Whitt JD, Fernandes JD, Pochampally R, Squire JA, Camussi G. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228(12):2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Investig. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong G, Tang X, Guo T, Duan N, Wang Y, Yang L, Zhang J, Liang X. Advanced oxidation protein products induce apoptosis in podocytes through induction of endoplasmic reticulum stress. J Physiol Biochem. 2015;71(3):455–470. doi: 10.1007/s13105-015-0424-x. [DOI] [PubMed] [Google Scholar]
- Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem MA, Adly AA, Ismail EA, Darwish YW, Kamel HA. Platelets microparticles as a link between micro- and macro-angiopathy in young patients with type 1 diabetes. Platelets. 2015;26(7):682–688. doi: 10.3109/09537104.2015.1018880. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sato S, Yanagihara T, Ghazizadeh M, Ishizaki M, Adachi A, Sasaki Y, Igarashi T, Fukunaga Y. Correlation of autophagy type in podocytes with histopathological diagnosis of IgA nephropathy. Pathobiology. 2009;76(5):221–226. doi: 10.1159/000228897. [DOI] [PubMed] [Google Scholar]
- Sokolovska J, Isajevs S, Sugoka O, Sharipova J, Lauberte L, Svirina D, Rostoka E, Sjakste T, Kalvinsh I, Sjakste N. Influence of metformin on GLUT1 gene and protein expression in rat streptozotocin diabetes mellitus model. Arch Physiol Biochem. 2010;116(3):137–145. doi: 10.3109/13813455.2010.494672. [DOI] [PubMed] [Google Scholar]
- Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- Suzuki K. Selective autophagy in budding yeast. Cell Death Differ. 2013;20(1):43–48. doi: 10.1038/cdd.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwara N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2016;65(3):755–767. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kume S, Kitada M, Kanasaki K, Uzu T, Maegawa H, Koya D. Autophagy as a therapeutic target in diabetic nephropathy. Exp Diabetes Res. 2012;2012:628978. doi: 10.1155/2012/628978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HW, Liao HM, Peng WH, Lin HR, Chen CH, Chen GC. Atg9 interacts with dTRAF2/TRAF6 to regulate oxidative stress-induced JNK activation and autophagy induction. Dev Cell. 2013;27(5):489–503. doi: 10.1016/j.devcel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yoshida H. Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens. 2015;24(4):345–350. doi: 10.1097/MNH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Turco AE, Lam W, Rule AD, Denic A, Lieske JC, Miller VM, Larson JJ, Kremers WK, Jayachandran M. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles. 2016;5:29642. doi: 10.3402/jev.v5.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283(31):21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20(3):519–537. doi: 10.1089/ars.2013.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hong Q, Lv Y, Feng Z, Zhang X, Wu L, Cui S, Hou K, Su H, Huang Z, Wu D, Chen X. Autophagy can repair endoplasmic reticulum stress damage of the passive Heymann nephritis model as revealed by proteomics analysis. J Proteomics. 2012;75(13):3866–3876. doi: 10.1016/j.jprot.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8(1):75. doi: 10.1186/s13287-016-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tan HY, Li S, Feng Y. Atg9b deficiency suppresses autophagy and potentiates endoplasmic reticulum stress-associated hepatocyte apoptosis in hepatocarcinogenesis. Theranostics. 2017;7(8):2325–2338. doi: 10.7150/thno.18225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jia H, Zhang B, Yin L, Mao F, Yu J, Ji C, Xu X, Yan Y, Xu W, Qian H. HucMSC exosome-transported 14-3-3ζ prevents the injury of cisplatin to HK-2 cells by inducing autophagy in vitro. Cytotherapy. 2018;20(1):29–44. doi: 10.1016/j.jcyt.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFβ1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185–192. doi: 10.1016/j.ejphar.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhu G, He W, Yin H, Lin F, Gou X, Li X. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. Faseb j. 2019;33(4):5440–5456. doi: 10.1096/fj.201801821R. [DOI] [PubMed] [Google Scholar]
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Wu L, Feng Z, Cui S, Hou K, Tang L, Zhou J, Cai G, Xie Y, Hong Q, Fu B, Chen X. Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS ONE. 2013;8(5):e63799. doi: 10.1371/journal.pone.0063799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D, Zhu Z, Luo L, Tian N, Wang X, Tong Y, Han Z. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial−mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7(1):9371. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Liu Q, Fu S, Li S, Yang L, Liu S, Hao J, Yu L, Duan H. PTEN inhibits high glucose-induced phenotypic transition in podocytes. J Cell Biochem. 2015;116(8):1776–1784. doi: 10.1002/jcb.25136. [DOI] [PubMed] [Google Scholar]
- Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16(6):1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong Y, Zhang C, Zen K, Liu Z. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J Pathol. 2014;234(2):203–213. doi: 10.1002/path.4382. [DOI] [PubMed] [Google Scholar]
- Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of micro-vesicles derived from human Wharton−Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS ONE. 2014;9(3):e92129. doi: 10.1371/journal.pone.0092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MZ, Wang Y, Paueksakon P, Harris RC. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes. 2014;63(6):2063–2072. doi: 10.2337/db13-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313(4):F906–Ff913. doi: 10.1152/ajprenal.00178.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma KL, Gong YX, Wang GH, Hu ZB, Liu L, Lu J, Chen PP, Lu CC, Ruan XZ, Liu BC. Platelet microparticles mediate glomerular endothelial injury in early diabetic nephropathy. J Am Soc Nephrol. 2018;29(11):2671–2695. doi: 10.1681/ASN.2018040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, Frank R, Kopp JB, Trachtman H, Star RA, Yuen PS. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol. 2013;305(4):F553–F559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9(11):1663–1676. doi: 10.4161/auto.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]