Abstract
Endothelial nitric oxide synthase (eNOS) and receptor-type vascular endothelial protein tyrosine phosphatase (VE-PTP) are one of the majors signaling pathways related to endothelial health in diabetes. Several reports have shown that the inhibition of VE-PTP can lead the nitric oxide production, although repeated studies showed that VE-PTP regulated the eNOS exclusive at Ser1177 in indirect-manner. A recent, exciting paper (Siragusa et al. in Cardiovasc Res, 2020. https://doi.org/10.1093/cvr/cvaa213), showing that VE-PTP regulates eNOS in a direct-manner, dephosphorylating eNOS at Tyr81 and indirect at Ser1177 and the effects of a VE-PTP inhibitor, AKB-9778, in the blood pressure from diabetic patients.
Keywords: AKB-9778, VE-PTP, eNOS, eNOS tyrosine phosphorylation, eNOS serine phosphorylation
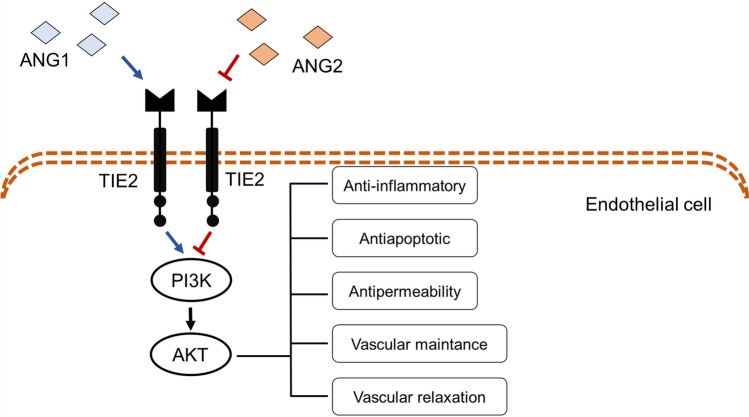
Receptor tyrosine kinase (RTK) signalling is an important pathway which play a role in wide range of complex physiological functions, such as, regulates vascular permeability, inflammation and angiogenesis and its dysregulation is closely related to cancer and neonatal death during gestation, in consequence of undevelopment blood and lymphatic in gestation (Du and Lovly 2018; Navia-Pelaez et al. 2018). Among the RTK, endothelial-enriched tunica interna endothelial cell kinase 2 (Tie2) is a cell-surface receptor and one of the major regulator kinase on vascular maintenance (Ghosh et al. 2016). Its regulation is mediated by angiopoietins, angiopoietin 1 (ANG1) acts as an agonist and angiopoietin 2 (ANG2), as an antagonist. The physiological functions mediated by Tie2 is mediated by the ligand ANG1, and consequently activation of phosphoinositide 3-kinase (PI3K) and AKT, as shown in Fig. 1.
Fig. 1.
Tie2 is regulated by angiopoietin 1 (ANG1) and 2 (ANG2). ANG1 acts as an agonist inducing the Tie2 signaling, which consists in the activation of phosphoinositide 3-kinase (PI3K) and AKT leading to the physiological responses related to this receptor. Although, ANG2 acts as an antagonist, inhibiting Tie2 signaling
Besides the ligands, vascular endothelial protein tyrosine phosphatase (VE-PTP) is crucial protein for regulation of Tie2, acting dephosphorylating the receptor, consequently inactivating Tie2 (Souma et al. 2018). Previous studies have shown that VE-PTP is upregulated in some pathological states, such as, renal microvasculature (Carota et al. 2019) and heart (Siragusa et al. 2020) of diabetic rodents. VE-PTP downregulation and knockdown increases the endothelial permeability, leukocyte extravasation (Juettner et al. 2019) and it is lethal to newborns in consequence of defects in angiogenesis (Dominguez et al. 2007).
Besides the well described role of VE-PTP on angiogenesis and vascular maintenance though the Tie2 pathway, VE-PTP is an important protein that regulate the endothelial in several pathways, in this context, this manuscript discusses the main advances made in VE-PTP, focusing on role of regulation of eNOS and its role on vascular function, well described by Siragusa et al. (2020).
Nitric oxide (NO) synthases (NOS) catalyze the conversion of L-arginine and oxygen to L-citrulline and NO. The eNOS is expressed in endothelial cells and is a fundamental modulator of endothelial homeostasis (Siragusa and Fleming 2016; Ruviaro et al. 2020). Impairment in eNOS signaling result in decreased NO bioavailability and endothelial dysfunction, which is characteristic feature of cardiovascular and metabolic diseases, such as hypertension, atherosclerosis, diabetes and metabolic syndrome (Brandes et al. 2005; Napoli et al. 2006; Parreira et al. 2018).
The activity of the eNOS enzyme depends on the availability of its substrate L-arginine, cofactors and interacting proteins, intracellular levels of Ca2+ as well as post-translational modifications, including phosphorylation (Förstermann and Sessa 2012). Phosphorylation has been demonstrated at specific serine (Ser) (Fisslthaler et al. 2008), threonine (Thr) (Chattopadhyay et al. 2014) and tyrosine (Tyr) (Fulton et al. 2008).
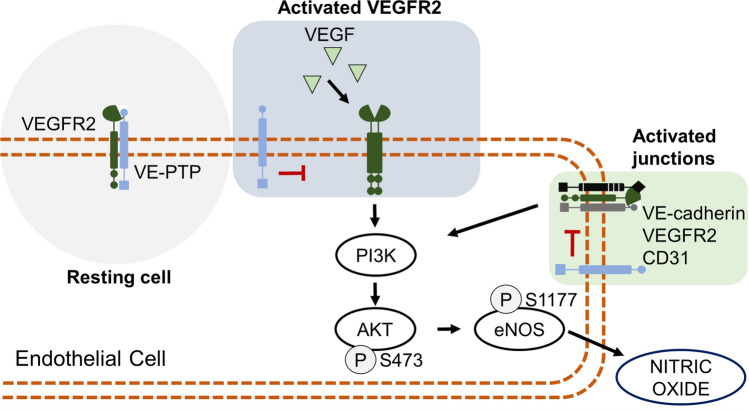
eNOS can be activated by several substances, one important signaling pathway is the vascular endothelial growth factor (VEGF) that activates vascular endothelial growth factor receptor 2 (VEGFR2) (Wang et al. 2004). The VEGFR2 activation leads to phosphorylation of AKT on residue S473 and consequently, eNOS on Ser1177, which induces the synthesis of nitric oxide (Di Lorenzo et al. 2013; Goel et al. 2013). In resting endothelial cells, VE-PTP and VEGFR2 form a complex, the VE-PTP acts as an inactivator of VEGFR2, although, this complex is disrupted by activation of VEGF on VEGFR2, and PI3K is activated leading to phosphorylation on AKT at Ser473 and consequently eNOS at Ser1177, finally, inducing the release of NO through this pathway, as shown in Fig. 2.
Fig. 2.
The role of VE-PTP in phosphorylation of eNOS at Ser1177. VE-PTP and VEGFR2 (vascular endothelial growth factor receptor 2) form a complex in resting cells, which VE-PTP acts inhibiting the VEGFR2 pathway. Although, when VEGFR2 is activated by VEGF (vascular endothelial growth factor) the complex is interrupted, which induces the VEGFR2 dimerization and activation, leading to the AKT phosphorylation at Ser473 and consequently on eNOS at Ser1177. On endothelial junctions, the activated junctions complex formed by VE-cadherin, VEGFR2, and CD31 acts in the same signaling pathway of VEGR2, although, VE-PTP acts inactivating this pathway
Another important pathway that VE-PTP regulates the eNOS is through the junction complex, which consists of VE-cadherin, VEGFR2, and CD31, its complex is activated mainly by shear stress and consequently the purinergic receptor P2Y2, which leads to the phosphorylation of Ser1177, similar to the phosphorylation through the activation of VEGFR2 (Albarrán-Juárez et al. 2018; Iring et al. 2019).
While the regulation of eNOS by alterations in serine phosphorylation is well studied, relatively little attention has been paid to the role of tyrosine phosphorylation. Tyr81 has emerged as the major phosphorylation site of tyrosine of eNOS and it is mediated by Src kinase (Fulton et al. 2008). Several substances were described to phosphorylate eNOS on this residue, such as, bradykinin, ATP, estrogen, angiopoietin that consequently induces the production of nitric oxide. Thus, the knockdown of Tyr81 on aortae from mice strongly impairs the relaxation induced by endothelium-dependent agonists (Fulton et al. 2005, 2008).
A recent report (Siragusa et al. 2020), the authors found that in human umbilical vein endothelial cell (HUVEC) stimulated with Yoda1, an agonist that mimic shear stress, the phosphorylation on eNOS at Tyr81 is partially abolished with the inhibition of Src and ABL1, and the release of NO is compromised by the inhibition of this tyrosine kinases.
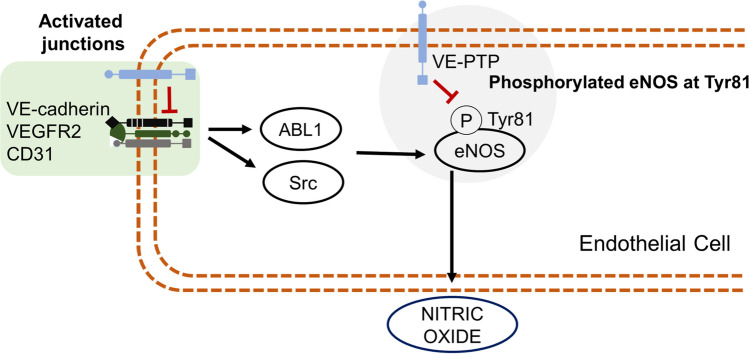
Similar to the activation of the junctions in the endothelial cells and phosphorylation of eNOS at Ser1177, the activation of this complex also activates Src and ABL1 pathway inducing the phosphorylation of Tyr81 and consequently the nitric oxide production. Besides, the VE-PTP can act directly dephosphorylating eNOS on Tyr81 when this residue is phosphorylated (Siragusa et al. 2020), as illustrated in Fig. 3. To find this result, the authors managed a phosphatase assay, using the immunoprecipitated of HUVECs stimulated HUVECs with Yoda1, in a cell-free in vitro reaction, the immunoprecipitated was incubated or not with the VE-PTP inhibitor, AKB-9778, interesting in non-incubated reactions the Tyr81 of eNOS was dephosphorylating in a time-dependent manner and AKB-9778 abolished the dephosphorylation of eNOS at Tyr81.
Fig. 3.
The role of VE-PTP in phosphorylation of eNOS at Tyr81. Activated endothelial junctions stimulate tyrosine kinases, ABL1 and Src, which leads to the phosphorylation of eNOS at Tyr81. VE-PTP acts inhibiting the tyrosine kinases, ABL1 and Src, and also dephosphorylates eNOS at Tyr81 in a direct manner
The authors found important role of inhibition of VE-PTP, in isolated aortae from healthy mice, the inhibition of VE-PTP induced relaxation in endothelium intact situation and the prior treatment of the vessels of diabetic aortae with the inhibitor of VE-PTP (AKB-9778) restored the endothelial function. In phase 2 clinical trial with diabetic patients, AKB-9778 was administrated subcutaneously, after 30 and 90 min the systolic and diastolic blood pressure consistently was reduced (Siragusa et al. 2020).
The endothelial dysfunction and impaired angiogenesis related to diabetes play a crucial role in the pathophysiology of this disease. Tie2 and VE-PTP are one of the majors signaling pathways related to endothelial health in diabetes (Campochiaro and Peters 2016; Hussain et al. 2019). A previous report (Carota et al. 2019) showed that VE-PTP is overexpressed in human endothelial cells stimulated with a high concentration of glucose or hypoxia and in the animal model of diabetes and hypertension. The higher expression of VE-PTP in these different models was associated with the lower expression of eNOS, that compromise the endothelial health.
It is important to highlight, AKB-9778 has emerged as a pharmacological tool to inhibit VE-PTP, and it is a potential candidate in the treatment of diabetic retinopathy (Campochiaro and Peters 2016) diabetic macular edema (Campochiaro et al. 2015). The action mechanism to improve these effects are major through the inhibition of VE-PTP and consequently increasing the activity of Tie2, which decreases the cellular permeability and restores the vascular maintenance(Goel et al. 2013; Miller and Fortun 2018).
Although, there is evidence of the use of VE-PTP inhibitor in diabetic retinopathy and diabetic macular edema reporting its effects on the Tie2 pathway, very few reports have been studying its effects on the nitric oxide pathway, in particular, the effects on the endothelial function. Since diabetes remains a major public health concern with important disease burden, and its physiopathology is closely related to the damage of the vascular/endothelial health and its damage can lead to the failure of different organs, such as eyes, kidneys, limbs, and, in men, penis. Siragusa et al. (2020) assessed partially the complex effect of VE-PTP on eNOS from a molecular and functional point of view. The expanding knowledge of the effects of VE-PTP, in particular, the endothelial dysfunction in diabetes, in particular nitric oxide pathway, allows for exciting new horizons endothelial diabetes related-diseases treatment care.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, Wettschureck N, Althoff TF, Offermanns S. Piezo1 and G q/G 11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215:2655–2672. doi: 10.1084/jem.20180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Peters KG. Targeting Tie2 for treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2016 doi: 10.1007/s11892-016-0816-5. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Sophie R, Tolentino M, Miller DM, Browning D, Boyer DS, Heier JS, Gambino L, Withers B, Brigell M, Peters K. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122:545–554. doi: 10.1016/j.ophtha.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Carota IA, Kenig-Kozlovsky Y, Onay T, Scott R, Thomson BR, Souma T, Bartlett CS, Li Y, Procissi D, Ramirez V, Yamaguchi S, Tarjus A, Tanna CE, Li C, Eremina V, Vestweber D, Oladipupo SS, Breyer MD, Quaggin SE. Targeting VE-PTP phosphatase protects the kidney from diabetic injury. J Exp Med. 2019;216:936–949. doi: 10.1084/jem.20180009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay R, Dyukova E, Singh NK, Ohba M, Mobley JA, Rao GN. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C e-mediated zona occludens-1 phosphorylation at threonine 770/772. J Biol Chem. 2014;289:3148–3163. doi: 10.1074/jbc.M113.528190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci. 2013;126:5541–5552. doi: 10.1242/jcs.153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, Noguera-Troise I, Murphy AJ, Valenzuela DM, Davis S, Thurston G, Yancopoulos GD, Gale NW. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embyronically because of defects in angiogenesis. Proc Natl Acad Sci U S A. 2007;104:3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:1–13. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-stimulated endothelial nitric oxid synthase activation and vascular relaxation role of eNOS phosphorylation at Tyr83. Circ Res. 2008;102:497–504. doi: 10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- Ghosh CC, David S, Zhang R, Berghelli A, Milam K, Higgins SJ, Hunter J, Mukherjee A, Wei Y, Tran M, Suber F, Kobzik L, Kain KC, Lu S, Santel A, Yano K, Guha P, Dumont DJ, Christiani DC, Parikh SM. Gene control of tyrosine kinase Tie2 and vascular manifestations of infections. Proc Natl Acad Sci U S A. 2016;113:2472–2477. doi: 10.1073/pnas.1519467113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, Kirkpatrick ND, Heishi T, Huang Y, Martin JD, Ager E, Samuel R, Wang S, Yazbek J, Vakoc BJ, Peterson RT, Padera TP, Duda DG, Fukumura D, Jain RK. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain RM, Neiweem AE, Kansara V, Harris A, Ciulla TA. Tie-2/angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs. 2019;28:861–869. doi: 10.1080/13543784.2019.1667333. [DOI] [PubMed] [Google Scholar]
- Iring A, Weinstein LS, Offermanns S. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 2019;129:2775–2791. doi: 10.1172/JCI123825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juettner VV, Kruse K, Dan A, Vu VH, Khan Y, Le J, Leckband D, Komarova Y, Malik AB. VE-PTP stabilizes VE-cadherin junctions and the endothelial barrier via a phosphatase-independent mechanism. J Cell Biol. 2019;218:1725–1742. doi: 10.1083/jcb.201807210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Fortun JA. Diabetic macular edema: current understanding, pharmacologic treatment options, and developing therapies. Asia Pac J Ophthalmol. 2018;7:28–35. doi: 10.22608/APO.2017529. [DOI] [PubMed] [Google Scholar]
- Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Navia-Pelaez JM, Campos GP, Araujo-Souza JC, Stergiopulos N, Capettini LSA. Modulation of nNOSser852 phosphorylation and translocation by PKA/PP1 pathway in endothelial cells. Nitric Oxide. 2018;72:52–58. doi: 10.1016/j.niox.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Parreira LB, de Oliveira Vitorino PV, Jardim PCBV, Sousa ALL, Jardim TV, de Moura SW, Justo AFO, Barroso WKS. Comparison between supervised and partly supervised cardiac rehabilitation protocols in hypertensive patients: a randomized controlled trial. Curr Hypertens Rev. 2018;14:161–169. doi: 10.2174/1573402114666180413121016. [DOI] [PubMed] [Google Scholar]
- Ruviaro AR, de Barbosa P, PM, Alexandre EC, Justo AF, Antunes E, Macedo GM, Aglycone-rich extracts from citrus by-products induced endothelium-independent relaxation in isolated arteries. Biocatal Agric Biotechnol. 2020;23:101481. doi: 10.1016/j.bcab.2019.101481. [DOI] [Google Scholar]
- Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch Eur J Physiol. 2016;468:1125–1137. doi: 10.1007/s00424-016-1839-0. [DOI] [PubMed] [Google Scholar]
- Siragusa M, Justo AFO, Malacarne PF, Strano A, Buch A, Withers B, Peters KG, Fleming I. VE-PTP inhibition elicits eNOS phosphorylation to blunt endothelial dysfunction and hypertension in diabetes. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa213. [DOI] [PubMed] [Google Scholar]
- Souma T, Thomson BR, Heinen S, Carota IA, Yamaguchi S, Onay T, Liu P, Ghosh AK, Li C, Eremina V, Hong YK, Economides AN, Vestweber D, Peters KG, Jin J, Quaggin SE. Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc Natl Acad Sci U S A. 2018;115:1298–1303. doi: 10.1073/pnas.1714446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nagase S, Koyama A. Stimulatory effect of IGF-I and VEGF on eNOS message, protein expression, eNOS phosphorylation and nitric oxide production in rat glomeruli, and the involvement of PI3-K signaling pathway. Nitric Oxide. 2004;10:25–35. doi: 10.1016/j.niox.2004.02.001. [DOI] [PubMed] [Google Scholar]