Abstract
Although metastases from original (primary) tumors are highly studied, metastases from metastatic sites (secondary tumors) are far less studied. Here, using data from metastasis map (MetMap) project reported in a recent study (Jin et al. in Nature 588(7837): 331–336. 10.1038/s41586-020-2969-2, 2020), we found that human cancer cell lines isolated from metastatic sites have higher potential to metastasize to another site in mice, compared to human cancer cell lines isolated from primary sites, for certain types of cancer including liver, lung and pancreas cancer. In contrast, for cancer types such as ovarian and skin cancer, human cancer cell lines originated from primary tumors have increased metastatic potential in mice, compared to human cancer cell lines originated from metastatic sites. This preliminary analysis points that the potential of metastases to further metastasize compared to that of primary tumors might be cancer type-dependent, and further research is needed to understand why certain cancer cell lines isolated from metastatic sites are more likely to spread to other organs.
Keywords: Cancer, Metastasis, Metastatic potential, Liver cancer, Primary tumor, Cancer cell lines
Introduction
Metastasis of original (primary) tumors is highly studied in cancer research over the last century; however, studies on metastasis of metastases is rather limited and inconclusive (Sugarbaker et al. 1971; Hoover and Ketcham, 1975; Bethge et al. 2012; Tait et al. 2004; Hölzel et al. 2010; August et al. 1985). Therefore, experimental data on different cancer types regarding whether metastases can further metastasize to other body parts is needed. A better understanding of the dissemination of an established metastatic foci is of great importance to maximize the efficacy of current treatment options and to improve survival rates of cancer patients, since most deaths from cancer are due to metastasis.
Recently, Jin et al. studied the metastatic potential of 500 human cancer cell lines to specific organs in mice, namely bone, brain, kidney, liver and lung, using a novel in vivo barcoding strategy (2020). They identified organ-specific patterns of metastasis for human cancer cell lines using xenograft models (Jin et al. 2020). They found that cell lines originated from metastatic sites have increased metastatic potential in general compared to cell lines originated from primary tumors (p = 0.00028; Fig. 2b in their paper) (Jin et al. 2020). Here, by using publicly available data provided by the authors, we compared the metastatic potential of human cancer cells isolated from primary tumors and of those from metastatic sites, separately for each cancer type. We found that, for certain cancer types including liver, lung and pancreas cancer, human cancer cell lines isolated from metastatic sites have higher metastatic potential in mice, compared to those isolated from primary tumors. The opposite was observed for certain types of cancer such as oesophagus, ovarian and skin cancer. This analysis shows that the potential of metastases to metastasize compared to that of primary tumors might be cancer type- or tissue of origin-dependent and highlights the need for further research.
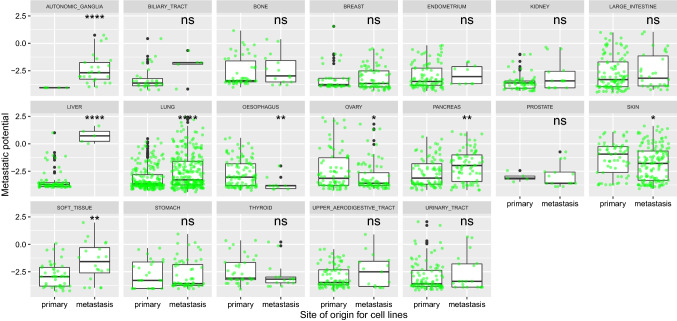
Fig. 2.
Mean metastatic potential of human cancer cell lines from different tissues in order, separately for cell lines isolated from primary (original) tumors and from metastases. non-significant (ns): p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001;****p ≤ 0.0001. Metastatic potential of ≤ − 4: non-metastatic; − 4 to − 2: (weakly) metastatic, but with low confidence; ≥ − 2: metastatic, with higher confidence. Data from Jin et al. (2020)
Materials and methods
Dataset
We used the metastasis map of human cancer cell lines (MetMap) dataset by Jin et al. (https://depmap.org/metmap/data/index.html) (2020). Here, metastatic potential of human cancer cell lines in mice following injection were given on a log10 scale as determined by the in vivo barcoding approach described in the paper, ranging from − 4 to + 4. Metastatic potential were defined as follows by the authors: less than or equal to − 4: non-metastatic; from − 4 to − 2: weakly metastatic, but with low confidence; higher or equal to − 2: metastatic, with higher confidence (Jin et al. 2020). We refer readers to the original paper for the details of the experimental approach to calculate metastatic potential of human cancer cell lines in xenograft models at scale (Jin et al. 2020).
Data analysis and visualization
Data analysis and visualization in this study were performed completely in R programming environment (R Core Team 2020), using following R packages: tidyverse (Wickham 2019), readxl (Wickham and Bryan 2019), ggpubr (Kassambara 2020), rmarkdown (Allaire et al. 2020) and knitr (Xie 2020). Statistical analysis was performed using Student's t-Test (Kassambara 2020). Following convention for star symbols indicating statistical significance was used in the analysis: non-significant (ns): p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001;****p ≤ 0.0001 (ggpubr package) (11). R code used in this analysis is available as a supplementary file to make the study reproducible.
Results
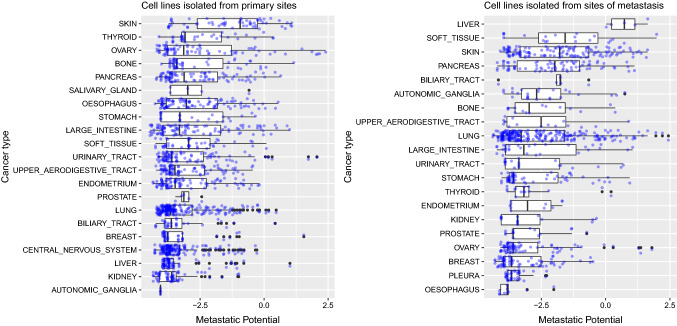
In the current study, we found that human cancer cell lines isolated from metastatic sites have higher metastatic potential when injected into mice compared to cell lines isolated from primary tumors, for cancers originating in following tissues: autonomic ganglia, liver, lung, pancreas and soft tissue (Fig. 1). Difference in metastatic potential between cell lines isolated from primary tumors and those isolated from metastases is especially large for liver, such that mean metastatic potential of cell lines from metastases is higher than 0, which indicates metastatic potential with higher confidence; however, cell lines from primary tumors are only weakly metastatic, since their mean metastatic potential is less than − 2 (Fig. 1). In contrast, human cancer cell lines isolated from metastases have decreased potential to metastasize in these xenograft models, relative to cell lines isolated from primary tumors, for certain tissues: oesophagus, ovary and skin (Fig. 1). However, in 10 out of 19 studied tissues, we did not observe any significant difference in the metastatic potential of cell lines originating from primary tumor tissue and of those originating from metastatic foci (biliary tract, bone, breast, endometrium, kidney, large intestine, prostate, stomach, thyroid, upper aerodigestive tract and urinary tract) (Fig. 1).
Fig. 1.
Relative metastatic potential of human cancer cell lines isolated from primary tumors and of those from metastases for various tissues, when injected into xenograft models. non-significant (ns): p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001;****p ≤ 0.0001. Metastatic potential of ≤ − 4: non-metastatic; − 4 to − 2: (weakly) metastatic, but with low confidence; ≥ − 2: metastatic, with higher confidence. Data from Jin et al. (2020)
We also ordered cancer types based on mean metastatic potential of cell lines originating from these cancers, separately for cell lines isolated from primary tumors and those isolated from metastases (Fig. 2). For human cancer cell lines originating from primary tumors, skin cancer cell lines have the highest mean metastatic potential, followed by cancer cell lines of thyroid and ovarian tissues (Fig. 2, first panel). However, for human cancer cell lines originating from metastatic sites, liver cancer cell lines have the highest mean metastatic potential, followed by cancer cell lines of soft tissue and skin (Fig. 2, second panel). Also, note that although cancer cell lines isolated from primary liver tumor have one of the lowest mean metastatic potential among other cells from primary tumors, cancer cell lines isolated from metastases of liver tumor have the highest metastatic potential (Fig. 2).
Discussion
It is still not clear if metastases have the potential to further metastasize to distinct sites in the body. In this preliminary analysis, we pointed that for certain tissues, metastases might have increased chance to disseminate to other sites in the body compared to primary tumors. In the present study, we used data from Jin et al. where they calculated metastatic potential of 500 human cancer cell lines when injected into mice, using a novel approach performed at a large scale (Jin et al. 2020). They used xenograft models to test the potential of human cancer cell lines to metastasize to various tissues; thus, it should be taken into account that these cell lines might have different metastatic potential in allografts, i.e. in human body. Number of cancer cell lines used in this study is relatively lower for certain tissues such as prostate for which we did not observe any difference in metastatic potential of primary tumors and metastases; therefore, a dataset with a larger sample size can provide novel insights. Nevertheless, the current study highlights that metastatic potential of metastases might even be higher compared to metastatic potential of primary tumors; thus, it demonstrates that a better understanding of further metastasis events from metastases is urgently needed.
Funding
CB is funded by TUBITAK (The Scientific and Technological Research Council of Turkey) 2211-E program.
Data availability
We used the metastasis map of human cancer cell lines (MetMap) dataset by Jin et al. (https://depmap.org/metmap/data/index.html) (2020).
Code availability
(software application or custom code): R code used in this analysis is available as a supplementary file to make the study reproducible.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Caglar Berkel, Email: caglar.berkel@gop.edu.tr.
Ercan Cacan, Email: ercan.cacan@gop.edu.tr.
References
- Allaire JJ, Xie Y, McPherson J, Luraschi J, Ushey K, Atkins A, Wickham H, Cheng J, Chang W, Iannone R (2020) rmarkdown: dynamic documents for R. R package version 2.6. URL https://rmarkdown.rstudio.com.
- August DA, Sugarbarker PH, Schneider PD. Lymphatic dissemination of hepatic metastases. Cancer. 1985;55:1490–1494. doi: 10.1002/1097-0142(19850401)55:7<1490::AID-CNCR2820550712>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Bethge A, Schumacher U, Wree A, Wedemann G. Are metastases from metastases clinical relevant? Computer modelling of cancer spread in a case of hepatocellular carcinoma. PLoS ONE. 2012;7(4):e35689. doi: 10.1371/journal.pone.0035689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel D, Eckel R, Emeny RT, Engel J. Distant metastases do not metastasize. Cancer Metastasis Rev. 2010;29(4):737–750. doi: 10.1007/s10555-010-9260-1. [DOI] [PubMed] [Google Scholar]
- Hoover HC, Ketcham AS. Metastasis of metastases. Am J Surg. 1975;130(4):405–411. doi: 10.1016/0002-9610(75)90473-0. [DOI] [PubMed] [Google Scholar]
- Jin X, Demere Z, Nair K, Ali A, Ferraro GB, Natoli T, Deik A, Petronio L, Tang AA, Zhu C, Wang L, Rosenberg D, Mangena V, Roth J, Chung K, Jain RK, Clish CB, Vander Heiden MG, Golub TR. A metastasis map of human cancer cell lines. Nature. 2020;588(7837):331–336. doi: 10.1038/s41586-020-2969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A (2020) ggpubr: 'ggplot2' based publication ready plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr
- R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- Sugarbaker EV, Cohen AM, Ketcham AS. Do metastases metastasize? Ann Surg. 1971;174(2):161–166. doi: 10.1097/00000658-197108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait CR, Dodwell D, Horgan K. Do metastases metastasize? J Pathol. 2004;203(1):515–518. doi: 10.1002/path.1544. [DOI] [PubMed] [Google Scholar]
- Wickham H, Bryan J (2019) readxl: read excel files. R package version 1.3.1. https://CRAN.R-project.org/package=readxl
- Wickham, et al. Welcome to the tidyverse. J Open Sour Softw. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- Xie Y. knitr: a general-purpose package for dynamic report generation in R. R package version. 2020;1:30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We used the metastasis map of human cancer cell lines (MetMap) dataset by Jin et al. (https://depmap.org/metmap/data/index.html) (2020).
(software application or custom code): R code used in this analysis is available as a supplementary file to make the study reproducible.