Abstract
The search for cannabinoid receptors other than CB1R and CB2R has been ongoing for over a decade. A number of orphan receptors have been proposed as potential cannabinoid receptors primarily based on phylogenic arguments and reactivity towards known endocannabinoids and phytocannabinoids. Seven putative cannabinoid receptors are described and discussed, and evidence for and against their inclusion in this category are presented.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12079-021-00622-6.
Keywords: Anandamide, 2-Acyl glycerol, Endocannabinoid, Phytocannabinoid, Cannabinoid
Introduction
Cannabinoid receptors modulate numerous biological processes and are intimately involved in many disease states. Cannabinoid receptors are found in most tissues and are the subject of numerous reviews (Mackie and Stella 2006; Soderstrom et al. 2017; Zou and Kumar 2018; Ligresti et al. 2016; Pertwee et al. 2010). CB1R receptors are expressed primarily in the central nervous system (CNS), but are found in moderate amounts in endocrine, adipose, female, and lymphoid tissues and lower amounts in other tissues (http://proteinatlas.org, Uhlén et al. 2015). CB2R, on the other hand is primarily expressed in leukocytes (Uhlén et al. 2015) but is also found in the brainstem (Van Sickle et al. 2005) and other tissues in low amounts. The classic endocannabinoid agonists for these receptors are anandamide and 2-arachadonylglycerol (2-AG) and there are numerous phytocannabinoid agonists such as cannabidiol and D9-THC as well (Fig. 1). Experiments utilizing CB1R−/− and CB2R−/− mice have shown that there at least five distinct cannabinoid receptors, leaving at least three to be identified (Mackie and Stella 2006). Although a number of potential cannabinoid receptors have been proposed, none completely fulfill the criteria proposed by Pertwee et al. (2010). This manuscript presents the seven most likely candidates in terms of biological function, structure, expression, agonist binding, and regulation, and provides an analysis of their potential as one of the unidentified cannabinoid receptors.
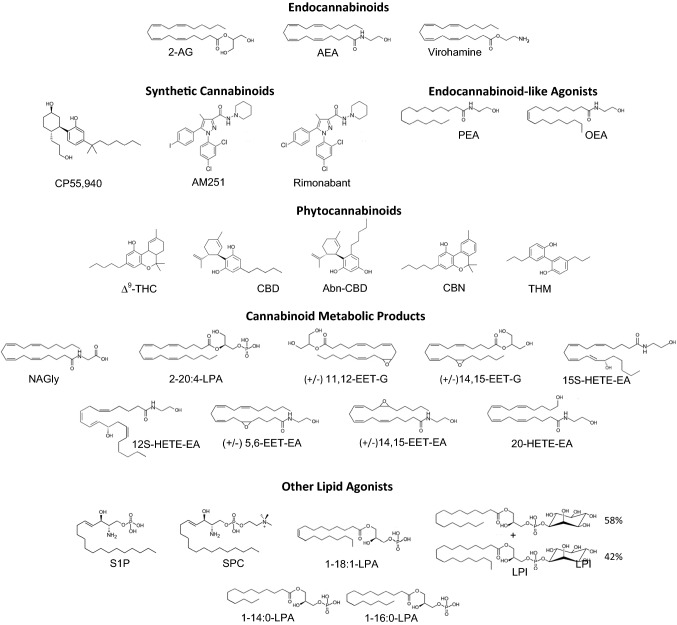
Fig. 1.
Structures for ligands discussed in the text. 1-14:0-LPA, 1-myristoyl lysophosphatidic acid; 1-16:0-LPA, 1-palmitoyl lysophosphatidic acid; 1-18:1-LPA, 1-oleoyl lysophosphatidic acid; 2-11,12-EET-G, 2-(11,12-epoxyeicosatrienoyl) glycerol; 2-14,15-EET-G, 2-(14,15-epoxyeicosatrienoyl) glycerol; 2-(14,15-epoxyeicosatrienoyl) glycerol; 2-20:4-LPA, 2-arachadonyl lysophosphatidic acid; 2-AG, 2-arachadonylglycerol; 5,6-EET-EA, 12S-HETE-EA, 12S-hydroxyeicosatetraenoic acid ethanolamide; 14,15-epoxyeicosatrienoyletahnolamide; 15S-HETE-EA, 15S-hydroxyeicosatetraenoic acid ethanolamide; 20-HETE-EA, 20-hydroxyeicosatetraenoic acid ethanolamide; Abn-CBD, abnormal cannabidiol; AEA, anandamide; AM251, synthetic cannabinoid agonist; CBD, cannabidiol; CBN, cannabinol; CP55,940, synthetic cannabinoid agonist; Δ9-THC, Δ9-tetrahydrocannibanol; LPI, mixture of 1-palmitoyl-sn-glycero-3-phosphatidylinositol (58%) and 1-steroyl-sn-glycero-3-phosphatidylinositol (42%); NAGly, N-arachidonyl glycine; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; S1P, sphingosine-1-phosphate; SPC, sphingosine-1-phosphocholine; THM, tetrahydromagnolol
GPR55
Introduction
Human GPR55 (hGPR55) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. This receptor has been reported to be activated by lysophosphatidylinositol (LPI) and various cannabinoid ligands, leading to the designation as a putative cannabinoid receptor. The lack of complete data as well as inconsistent data makes this cannabinoid receptor designation rather controversial (see review, Mackie and Stella 2006).
Expression and characterization
hGPR55 is expressed in bone marrow and lymphoid tissue with slightly lower expression in the brain, primarily in the basal ganglia, GI tissue, male tissues, and blood (Uhlén et al. 2015). The primary cellular location is the plasma membrane but is also found in membranes of intracellular vesicles following agonist-induced internalization (Henstridge et al. 2009).
The biological function of this receptor varies as a function of tissue. In the brain, stimulation appears to have a pro-inflammatory effect, as antagonists of GPR55 significantly reduce the release of pro-inflammatory PGE2 in primary microglia (Saliba et al. 2018). In rodent GI tracts, stimulation reduces intestinal contractions and activates immunocytes (Tudurí et al. 2017). In the blood, stimulation of GPR55 expressed in neutrophils limits the inflammatory response mediated by CB2R (Balenga et al. 2011). In the pancreas, stimulation of beta and alpha cells increases insulin secretion (Tudurí et al. 2017). Lastly, GPR55 expression in adipose tissue is positively associated with human obesity (Tudurí et al. 2017).
Activation of GPR55 by endocannabinoids, phytocannabinoids or synthetic cannabinoid agonists proceeds through multiple pathways. GPR55 expressed in HEK293 cells exhibits EC50 values for anandamide, virodhamine, and 2-arachadonylglycerol (2-AG) agonists in the low nM range as measured by GTPγS binding assays (Table 1). This is considerably lower than observed for CB1R and CB2R receptors (Ryberg et al. 2007; Felder et al. 1995). Further, the structurally related endogenous palmitoylethanolamide is also a significant agonist for initiating GTPγS binding (EC50 = 4 nM) but is not considered to be a true cannabinoid as it does not bind to either CB1R or CB2R. This high efficacy for GTPγS binding contrasts markedly with the results obtained from measurement of Ca2+ reported by Brown et al. (2011) but is in reasonable agreement with the calcium results reported by Lauckner et al. (2008). The phytocannabinoid Δ9-THC activates with EC50 values similar to that exhibited by CB1R and like CB1R and CB2R, GPR55 is not stimulated by cannabinol (CBN), or cannabidiol (CBD) to any significant degree (Table 1) (MacLennan et al. 1998). In fact, CBN acts as a GPR55 antagonist (Ryberg et al. 2007). GPR55 does bind abnormal cannabidiol (Abn-CBD), although the EC50 values vary widely (Ryberg et al. 2007; Johns et al. 2007).
Table 1.
Endocannabinoid, phytocannabinoid and synthetic cannabinoid ligand binding
| Receptor | Parameter measured | EC50/IC50 (nM) | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AEA | VH | 2-AG | PEA | OEA | Δ9-THC | Abn-CBD | CBN | CBD | THM | CP55940 | AM251 | RA | |||
| hCB1/HEK293a | GTPγS | 31 | 2920 | 519 | > 30E3 | > 30E3 | 6 | > 30E3 | > 30E3 | > 30E3 | – | – | A/IA | A/IA | Ryberg et al. (2007 |
| hCB1/CHOb | cAMP¯ | 322 | – | – | – | – | – | – | > 1000 | – | – | – | A/IA | A/IA | Felder et al. (1995) |
| hCB1/CHOc | cAMP¯ | – | – | – | – | – | 6.76 | – | – | – | 9010 | 2.28 | A/IA | A/IA | Fuchs et al. (2013) |
| hCB2/HEK293a | GTPγS | 27 | 381 | 618 | 20E3 | > 30E3 | 0.4 | > 30E3 | > 30E3 | > 30E3 | – | – | – | – | Ryberg et al. (2007) |
| hCB2/CHOb | cAMP¯ | 957 | – | – | – | – | – | – | > 1000 | – | – | – | – | – | Felder et al. (1995) |
| hCB2/CHO-K1a | GTPγS | – | – | – | – | – | – | – | 575 | – | – | – | – | – | MacLennan et al. (1998) |
| hCB2/CHOc | cAMP | – | – | – | – | – | 14 | – | – | – | 170 | 1 | – | – | Fuchs et al. (2013) |
| hGPR18/HEK293a | MAPK-P | 3830 | 44.5 | – | – | – | 960 | 835.7 | – | 5.10E4 | – | – | 9.60E + 04 | – | McHugh et al. (2012) |
| hGPR18/CHO | β-arrestin | – | – | – | – | – | 4610 | – | – | – | – | 5990d | – | 10E3d | Fuchs et al. (2013) |
| hGPR55/HEK293a | GTPγS | 18 | 13 | 3 | 4 | 440 | 8 | 2523 | Antag | > 30E3 | – | 5 | 39 | – | Ryberg et al. (2007) |
| hGPR55/HEK293a | GTPγS | – | – | – | – | – | – | 2.5 | – | – | – | – | – | – | Johns et al. (2007) |
| hGPR55/CHO | β-arrestin | – | – | – | – | – | 1420e | – | – | – | – | 1610e | – | 2010 | Fuchs et al. (2013) |
| hGPR55/HEK293f | Ca2+↑ | > 32E3 | > 32E3 | > 32E3 | > 32E3 | > 32E3 | – | – | – | – | – | – | 3200 | 7900 | Brown et al. (2011) |
| hGPR92/CV-1 g | SRE-luc | – | – | 1780 | – | – | – | – | – | – | – | – | – | – | Oh et al. (2008) |
| hGPR119/RIN-5Fh | cAMP↑ | – | – | – | – | 4400 | – | – | – | – | – | – | – | – | Chu et al. (2010) |
| hGPR119/U2OSi | β-arrestin | – | – | – | – | 502 | – | – | – | – | – | – | – | – | Southern et al. (2013) |
| hGPR119/COS-7 h | cAMP↑ | – | – | – | – | 200 | – | – | – | – | – | – | – | – | Hansen et al. (2011) |
| hGPR119/COS-7 h | cAMP↑ | – | – | – | – | 200 | – | – | – | – | – | – | – | – | Hassing et al. (2016) |
| hGPR119/HEK293h | Ca2+↑ | – | – | – | – | 1000 | – | – | – | – | – | – | – | – | Hassing et al. (2016) |
Agonist parameters given for human (h) receptors in the indicated cell line. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, subclone from the parental CHO cell line; CV-1, African green monkey kidney cell line;HEK293, Human embryonic kidney 293 cells; RIN-5F, secondary clone of the rat islet tumour cell line RIN-m; U2OS, Human bone osteosarcoma epithelial cells. 2-AG, 2-arachadonylglycerol; A/IA, antagonist/inverse agonist; Abn-CBD,Abnormal cannabidiol; AEA, anandamide; AM251, synthetic cannabinoid agonist; Antag., antagonist; Ca2+, Ca2+ mobilization; cAMP-A, cAMP accumulation assay; β-arrestin, β-arrestin binding assay; Ca2+↑, increase inintracellular calcium, CBD, canabidiol; CBN, canabinol; cAMP↓, decrease in intracellular cAMP; cAMP↑, decrease in intracellular cAMP;CP55940, synthetic cannabinoid agonist; Δ9-THC, Δ9-tetrahydrocannabinol; E3, × 1000; GTPγS, guanosine 5'-O-[gamma-thio]triphosphate; MAPK-P, MAP kinase phosphotylation; OEA, oleylethanolamide; PEA, palmitoylethanolamide; RA, rimonabant, synthetic cannabinoid agonist; THM, tetrahydromagnolol; VH, virodhamine
aEC50 determined from GTPγS binding assay
bEC50 measured as inhibition of forskolin-stimulated cAMP accumulation in CHO-CB2 and CHO-CB1 cells
cCompetition cAMP binding assay
dIC50 for antagonist for Δ9-THC induced b-arrestin recruitment
eIC50 for antagonist for LPI induced b-arrestin recruitment
fEC50 for calcium mobilization using aequorin assay
gEC50 for induction of SRE-luc activity
hEC50 for cAMP accumulation
iEC50 for β-arrestin binding using the Pathfinder™ assay
LPI is the primary non-cannabinoid agonist for GPR55 with an EC50 of 49 nM with respect to affecting an increase in [Ca2+]i (Table 2) (Henstridge et al. 2009, 2010). LPI stimulation leads to the activation of ERK (extracellular signal regulated kinases also known as MAPK, mitogen activate protein kinases) and release of intracellular calcium ([Ca2+]i) (Oka et al. 2007, 2009; Waldeck-Weiermair et al. 2008), which is known to modulate the activity of ERK through activation (Chuderland and Seger 2008). A subsequent report shows that LPI signaling is mediated though Gα13 and proceeds through a Ras homolog family member A/Rho-associated protein kinase (RhoA/ROCK) pathway where activated ROCK activates phospholipase C (PLC), initiating the release of the secondary messenger inositol-1,4,5-trisphosphate (IP3) that in turn initiates the release of calcium ion from the endoplasmic reticulum (Henstridge et al. 2009). Further, the increase in [Ca2+]i initiates the calcinurin-mediated dephosphorylation of NFAT (nuclear factor of activated T cells) leading to its activation as a transcription factor (Henstridge et al. 2009).
Table 2.
CBD inverse agonist and lipids agonists that are structurally-related to endocannabinoids and their products
| Receptor | Parameter measured | EC50/IC50 (nM) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CBD | S1P | SPC | LPI | FPP | 1–14:0-LPA | 1–16:0-LPA | 2–18:1-LPA | |||
| hGPR3/CHOa | β-arrestin | 1600 | – | – | – | – | – | – | – | Laun and Song (2017) and Laun (2018) |
| hGPR3/HEK293b | Ca2+↑ | – | 50 | – | – | – | – | – | – | Uhlenbrock et al. (2002) |
| hGPR6/CHOa | β-arrestin | 75 | – | – | – | – | – | – | – | Laun et al. (2018) |
| hGPR6/HEK293b | Ca2+↑ | – | 56 | – | – | – | – | – | – | Uhlenbrock et al. (2002) |
| mGPR6/CHOc | Ca2+↑ | – | 39 | – | – | – | – | – | – | Ignatov et al. (2003a) |
| mGPR6/XOd | GIRK currents | – | 22 | – | – | – | – | – | – | Ignatov et al. (2003a, b a) |
| hGPR6/CHOa | β-arrestin | 200 | – | – | – | – | – | – | Laun and Song (2017) and Laun (2018) | |
| hGPR12/HEK293b | Ca2+↑ | – | 106 | – | – | – | – | – | – | Uhlenbrock et al. (2002) |
| mGPR12/XOc | Ca2+↑ | – | 1200 | 66 | – | – | – | – | – | Ignatov et al. (2003b) |
| mGPR12/XOd | GIRK currents | – | 3100 | 32 | – | – | – | – | – | Ignatov et al. (2003b) |
| hGPR12/HEK293e | cAMP↓ | 10E3–100E3 | – | – | – | – | – | – | – | Brown et al. (2017) |
| hGPR55/HEK293f | Ca2+↑ | – | – | – | 49 | – | – | – | – | Henstridge et al. (2010) |
| hGPR55/HEK293g | NFAT | – | – | – | 1100 | – | – | – | – | Henstridge et al. (2010) |
| hGPR55/HEK293g | ERK | – | – | – | 74 | – | – | – | – | Henstridge et al. (2010) |
| hGPR55/HEK293g | CREB | – | – | – | 93 | – | – | – | – | Henstridge et al. (2010) |
| hGPR55/HEK293g | NF-kB | – | – | – | 1900 | – | – | – | – | Henstridge et al. (2010) |
| hGPR92/CV-1 h | SRE-luc | – | – | – | – | 260 | 1820 | – | – | Oh et al. (2008) |
| hGPR92/CV-1i | IP3↑ | – | – | – | – | 380 | 1030 | – | – | Oh et al. (2008) |
| hGPR92/CV-1j | cAMP | – | – | – | – | 1460 | > 50E3 | – | – | Oh et al. (2008) |
| hGPR92/HEK293-BAEAa | β-arrestin | – | – | – | – | 1.40E + 04 | – | 1.40E + 04 | 1400 | Yin et al. (2009) |
| hGPR92/CHOc | Ca2+↑ | – | – | – | – | 543 | – | – | 5 | Yin et al. (2009) |
| hGPR92/HEK293-BAEAk | β-gal | – | – | – | – | – | – | 5.8 | 339 | Yin et al. (2009) |
| hGPR92/HF1l | cAMP↑ | – | – | – | – | – | 830 | 400 | 9300 | Kotarsky et al. (2006) |
| hGPR92/RH7777h | Ca2+↑ | – | – | – | – | 40 | – | – | 8.9 | Williams et al. (2009) |
| hGPR119/RH7777m | cAMP↑ | – | – | – | 5700 | – | – | – | – | Soga et al. (2005) |
Agonist/inverse agonist parameters given for human (h) and murine (m) receptors in the indicated cell line. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, subclone from the parental CHO cell line; CV-1, African green monkey kidney cell line; HEK293, Human embryonic kidney 293 cells; HEK-293-BAEA, thiotriphosphate; HEK293, human embryonic kidney cells 293; HEK293-BAEA, HEK293 cells stably express -arrestin2-EA fusion protein; RH7777, rat hepatoma cell line; XO, xenopus oocytes; RH7777, rat hepatoma cell line; 2-18:1-LPA, 2-oleoyl lysophosphatdic acid; 2-18:2-LPA, 2-linoleoyl lysophosphatdic acid; β-arrestin, β-arrestin binding assay; β-gal, β-galactosidase assay that measures translocation of β-arrestin to GPCRs; Ca2+↑, increase n intracellular calcium; cAMP, cyclic AMP; cAMP↓, decrease of forskolin stimulated cAMP; cAMP↑, increase in intracellular cAMP; CBD, canabidiol; CREB, cAMP response element-binding protein (CREB) activation; ERK, Extracellular signal-regulated kinase (ERK) activation; FPP, farnesyl pyrophosphate; GIRK currents, outward current through GIRK channels; GTPγS, guanosine 5'-O-[gamma-thio]triphosphate; HF1, hybrid cells resulting from the fusion of H5 and Fao; LPI, mixture of 1-palmitoyl-sn-glycero-3-phosphatidylinositol (58%) and 1-steroyl-sn-glycero-3-phosphatidylinositol (42%); NFAT, nuclear factor of activated T cell activation; NF-kB, NF-kB transcription factor activation; S1P, sphingosine-1-phosphate; SPC, sphingosine-1-phosphocholine
aEC50 estimated from a plot using the PathHunter™ assay
bEC50 for Ca2+ increase measured with calcium indicator dye fluo-4
cEC50 for Ca2+ increase measured for aequorin-based bioluminescence assay
dEC50 for rectifying K+ channel GIRK1 measured by whole cell clamping
ecAMP production was reduced at both 10 µM and 100 µM CBD
fEC50 for Ca2+ increase measured with calcium indicator dye Fura-2-AM
gEC50 determined by immunoblot analysis for the given protein
hEC50 for induction of SRE-luc activity
iEC50 for of IP3 production from radiolabeled myo-[3H]inositol
jEC50 determined for cAMP using a homogeneous time-resolved fluorescence kit (CIS Bio International)
kEC50 determined using β-galactosidase enzyme fragment complementation technology to measure β-arrestin binding
lEC50 determined using a cAMP immunoassay
mEC50 determined for cAMP using a homogeneous time-resolved fluorescence kit (CIS Bio International)
As noted above, GPR55 signaling is known to be coupled through Gα13 which mediates the activation of RhoA but is also involved in the activation of the cell division control protein 42 homolog (cdc42) known to be involved in cell cycle regulation, and Ras-related C3 botulinum toxin substrate 1 (rac1) known to be involved in a number of cellular processes including activation of kinases, cytoskeletal management, and cell growth (Henstridge et al. 2009). A later study utilizing the increase in [Ca2+]i as a measure of GPR55 activation shows that of the aforementioned agonists, only activation with Δ9-THC and anandamide result in a significant increase in [Ca2+]i whereas 2-AG, virodhamine, PEA, cannabidiol, and Abn-CBD only increase [Ca2+]i to a minor degree (Lauckner et al. 2008). Further they report that the increase in [Ca2+]i involves Gαq or Gα12 signaling, and similar to LPI, excitation proceeds through RhoA/PLC pathway leading to a release of intracellular Ca2+. In addition, they show that actin cytoskeleton is necessary to initiate transient increases in [Ca2+]i. Waldeck-Weiermair et al. (2008) provide further detail on the GPR55 signaling pathway. Here they show that in the absence of extracellular calcium, anandamide stimulated of GPR55 interacts with clustered integrins ανβ3 and α5β1 to produce an increase in [Ca2+]i via a phosphoinositol 3 kinase/phospholipase C/inositol trisphosphate (PI3K/PLC/IP3) pathway, requiring active ROCK for the clustering of the integrins. The identity of the G-protein involved is unclear from the data but is likely one of the Gαq family. In addition, the bone marrow kinase, X-linked/epithelial and endothelial tyrosine kinase (Bmx/Etk) is the mediator between PI3K and PLC. Further, the resulting increase in [Ca2+]i stimulates the activation of both ERK and NFAT as observed for LPI signaling.
Structure
hGPR55 (hGPR55, UniprotKB- Q9Y2T6) is translated as a 319 amino acid polypeptide with a calculated molecular weight of 36.6 kDa. No additional isoforms have been reported. Four coding SNP variants have been reported where three (T215N, G195V, and T314I) have no known associated pathologies, and one, V103I, has been found in malignant prostate tumors, but relationship to the disease has not been noted (Stelzer et al. 2016; https://genecards.org; https://www.ncbi.nlm.nih.gov/clinvar/; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 21 residues and a cytosolic C-terminal with 26 residues. There are no X-ray structures reported, however, the SWISS-Model site suggests that the Lysophosphatidic acid receptor 6a structure (31.7% sequence homology; template 5xsz.1.A, PDB entry 5XSZ) can serve as a working template (https://swissmodel.expasy.org/, Waterhouse et al. 2018).
There are numerous potential N-glycosylations and potential O-glycosylations predicted using NetNGlyc and NetOGlyc webservers (http://www.cbs.dtu.dk/services/NetNGlyc/, Blom et al. 2004) and (http://www.cbs.dtu.dk/services/NetOGlyc/, Steentoft et al. 2013) respectively. Experimental confirmation of glycosylation is mixed. Rapino et al. (2019) report that N-deglycosylation with PNGase F does not alter the molecular weight as observed on SDS-PAGE and that treatment of cells expressing hGPR55 with tunicamucin, a known inhibitor of N-glycosylation, does not affect the expression of hGPR55. These data suggest that N-glycosylation is not necessary for and does not occur after expression. On the other hand, Mangini et al. (2017) show that treatment of hGPR55 containing membranes with a mixture of N-deglycosylation and O-deglycosylation enzymes significantly reduces the molecular weight of GPR55 on SDS-Page gels, indicating the presence of either or both N- and O-glycosylation. Phosphorylations are predicted using the NetPhos server (http://www.cbs.dtu.dk/services/NetPhos/, Blom et al. 1999) and additional GRK phosphorylation predicted using the GPS server (http://gps.biocuckoo.cn/) (Xue et al. 2011). None of these phosphorylation sites have been confirmed experimentally. All posttranslational modifications predicted by the algorithms noted above are given for all receptors discussed in this manuscript age given in Table S1.
Regulation
There are several known modalities for the regulation of GPR55. One is the classic mode for reducing GPCR signaling though agonist-induced internalization via b-arrestins. Utilization of the β-arrestin Pathfinder assay™ (Yin et al. 2009) and β-arr2-GFP protein binding (Kapur et al. 2009) reveals that only LPI and strong cannabinoid agonists (e.g., AM251) initiate β-arrestin binding and internalization. Other endocannabinoids such as AEA show no effect while virodhamine shows only a weak effect and phytocannabinoids show no effect under assay conditions. Similar results are reported by others (Henstridge et al. 2009). Intresingly, these results are quite different from those reported by Ryberg et al. (2007) for GTPγS binding where AEA, virodhamine, and Δ9-THC are found to be strong agonists. Clearly, actuation of G-protein binding and β-arrestin binding are two different events facilitated separately by the particular ligands. These results also warn against using any one assay to classify a ligand as an agonist.
Both LPI and the strong CB1R antagonist SR141716A stimulate internalization of GPR55. However, the presence of AEA or virodhamine, the internalization of GPR55 is inhibited, presumably through competition for the binding site on the receptor (Sharir et al. 2012). Interestingly, AEA was found to enhance the recruitment of both LPI and SR141716A at low concentrations but antagonizes internalization at higher concentration. Once internalized, vesicles containing GPR55 are directed to lysosomes through binding to the GPCR-associated sorting protein (GASP-1) or recycled back to the plasma membrane in the absence of GASP-1 (Kargl et al. 2012).
In the presence of extracellular Ca2+, CB1R couples with the β1 integrins associated with GPR55 and acts as a modulator of GPR55 activity (Waldeck-Weiermair et al. 2008). Stimulation of CB1R with anandamide activates spleen tyrosine kinase (SYK) via Gαi which in turn inhibits PI3K, thus diminishing all downstream anandamide-induced GPR55-mediated Ca2+ signaling. Upon removal of extracellular Ca2+, CB1R uncouples from the β1 integrins and the inhibition is removed. Of note is the fact that ERK1/2 signaling is active under either of these circumstances, as activation of CB1R also initiates ERK1/2 signaling, the physiological consequences of which require further exploration. Direct interaction of GPR55 and CB1R has also been confirmed in HEK293 cells transfected with both human CB1R-Rluc fusion protein and human GPR55-YFP fusion protein through BRET energy transfer studies and in situ proximity ligation assays (Martínez-Pinilla et al. 2014). In addition, it was shown with the same system that the addition the GPR55 specific agonist CID1792197 induces the activation of NFAT and that this is blocked by the CB1R specific antagonist SR141716. This experiment repeated with rat brain slices -following confirmation of the presence of both receptors—yield the same results (Martínez-Pinilla et al. 2014).
CB2R and GPR55 also form heteromers, a union that affects both receptors signaling. Direct interaction between the two was confirmed though BRET energy transfer assays utilizing GPR55-Tluc and CB2R-YFP transfected into HEK293 cells (Moreno et al. 2014). Co-transfected cells stimulated with the specific GPR55 agonist LPI results in a DMR (dynamic mass distribution) signal somewhat higher than observed from cells transfected with GPR55 alone. Stimulation with the CB2R-specific agonist HU-308 produces a DMR signal similar to that observed from cells transfected with CB2R alone (Moreno et al. 2014). Unlike the CB1R/GPR55 heterodimer, these results suggest little or no modulation of activities of either monomer by the presence of the other. However, the LPI signal is completely blocked in the presence of the CB2R antagonists AM360 and HU-308. Utilizing more specific CB2R assays such as reduction of forskolin-induced cAMP levels and the increase in ERK1/2 phosphorylation, it was found that HU-308 stimulates both processes but addition of the GPR55 antagonist HB prevents this signaling. Stimulation of GPR55 also produces an increase in ERK1/2 phosphorylation, but co-stimulation of both receptors results in reduced EKR1/2 phosphorylation. Thus, negative cross-talk and cross-antagonism is a feature of this heteromer.
GPR119
Introduction
Human GPR119 (hGPR119) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. This receptor is only weakly activated by AEA, but instead is activated by lesser unsaturated ethanolamides such as oleoylamide (OEA) and linoleyl ethanolamide (LEA) as well as palmitoyl ethanolamide (PEA) and the 2-AG analog 2-oleolylglycerol (Southern et al. 2013; Hansen et al. 2011; Chu et al. 2010; Hassing et al. 2016). Other agonists include a variety of lysophosphatidylcholines (Soga et al. 2005). Although phylogenetically related to CB1R/CB2R, this receptor is not activated by traditional endocannabinoids and phytocannabinoids such a Δ9-THC (Joffre et al. 2020) and thus, for this reason, is not always considered as a member of the endocannabinoid receptor family (Pertwee et al. 2010). However, the strong structural relationships between known GPR119 agonists and cannabinoid agonists and phylogenetic relationship should not be ignored.
Expression and characterization
hGPR119 is expressed primarily in the pancreas and gastrointestinal tract with low expression in both male and female tissues as well as in the blood (Uhlén et al. 2015). It is not expressed in the human brain but is found in small amounts in pig and mouse brain.
The primary biological function of this receptor is glucose homeostasis. GPR119 is expressed both in pancreatic β-cells and the L-cells located primarily in the colon and distal ileum. Stimulation with both synthetic agonists and dietary-derived monosaturated fatty acids with long chains (e.g., oleic acid) results in the enhancement of insulin release directly from the β-cells and indirectly through the release of glucagon-like peptide 1 (GLP-1) from L-cells which also increases insulin levels (Hansen et al. 2011; Chu et al. 2008, 2007; Cox et al. 2010; Lauffer et al. 2008, 2009). Although palmitoyl ethanolamide (PEA) and oleoyl ethanolamide (OEA) are strong GPR119 agonists, they are minor dietary components and thus are not likely to be involved in GLP-1 release (Table 1). However, dietary 2-monoacylglycerols are abundant and 2-oleoyl glycerol (2-OG) in particular is a strong agonist for GPR119 (Hansen et al. 2011) and thus a likely participant in GLP-1 release. GLP-1 is co-packaged with peptide YY (PYY) and co-released from L-cells upon stimulation (Böttcher et al. 1984). The release of PYY results in reduced gastric emptying and promotion of satiety (Chu et al. 2007). Recently it has been shown that GPR119 is also expressed in the murine eye and activation by 2-OG serves to reduce intraocular pressure (IOP) with a higher response in female than male (Miller et al. 2017). Interestingly, the endocannabinoid receptors GPR18 and CB1R are also found in mice eye and stimulation with Δ9-THC also results in the reduction of IOC, but to a higher degree in males than females (Miller et al. 2018).
It is well documented that GPR119 signaling proceeds through Gαs, resulting in an intracellular increase in cAMP (Hassing et al. 2016; Lauffer et al. 2009). The recruitment of β-arrestin has also been documented but agonist-induced internalization or possible G-protein independent signaling have not been reported (Hassing et al. 2016; Southern et al. 2013). More recently, coupling to Gαq and Gαi has been reported where IP3 serves as the second messenger (Hassing et al. 2016). OEA signal induction is biased and signaling through the different G proteins varies, where 30–70% is driven by Gαs, 10–30% is driven by Gαq, and 1–10% is driven by Gαi. All signaling ultimately results in an increase in [Ca2+]i that signals fusion of the insulin-containing vesicles with the plasma membrane (see also reviews Tengholm 2012; Fu et al. 2013).
Activation of GPR119 by short chain ethanolamides occur with EC50 values in the sub-micromolar to micromolar range where OEA exhibits the smallest EC50 of 200 nM (Table 1) (Hansen et al. 2011). The classic endocannabinoids AEA and 2AG produce very weak or no activation. In keeping with its function as a lipid sensor, the EC50 values for lysophosphatidylcholines and 2-OG are in the low micromolar range (Hansen et al. 2011).
Structure
hGPR119 (Glucose-dependent insulinotropic receptor, UniProtKB-Q8TDV5) is translated as a 335 amino acid polypeptide with a calculated molecular weight of 36.9 kDa. No additional isoforms have been reported. Two coding SNP variants have been reported (S309L and L236V) but associated pathologies are unknown (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 12 residues and a cytosolic C-terminal of 52 residues. There are no reported X-ray structures, however, the SWISS-Model site suggests a that the M5 muscarinic acetylcholine receptor (M5-T4L) bound to tiotropium (21.5% sequence homology; template 5cxv.1.A, PDB entry 5cxv) should serve as a working template (Waterhouse et al. 2018). Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1).
Regulation
To date, there are few reports on the regulation of GPR119. Although there are numerous potential phosphorylation sites on this receptor, including many on the C-terminus, there are no reports involving regulation through phosphorylation. β-arrestin binding has been observed (Southern et al. 2013; Hassing et al. 2016) but the effect on plasma membrane expression or overall activity remains unknown. Altered expression of GPR119 has been observed in tissues involved in inflammatory bowel disease (Grill et al. 2019) and pancreatic cancer (Odori et al. 2013) but the mechanism of action is not known.
GPR18
Introduction
Human GPR18 (hGPR18) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. The primary agonists for this receptor are reported to be N-arachidonyl glycine (NAGly) (McHugh et al. 2012; Kohno et al. 2006) and resolvin D2 (RvD2) (Chiang et al. 2015, 2017) as well as various cannabinoid ligands such as Δ9-THC, CBD, and Abn-CBD (McHugh et al. 2012), the latter agonists establishing it as at least a potential cannabinoid receptor. Not all reports confirm these findings (Finlay et al. 2016).
Expression and characterization
hGPR18 is highly expressed in the blood, with highest amounts in T-cells, B-cells and NK-cells, bone marrow and lymphoid tissue, testis, and with lower expression in the brain, GI tract, and minor amounts in most other tissues (Uhlén et al. 2015). Expression is higher in the peripheral lymphocyte subsets (CD45RO+, CD45RA+, CD19+, CD8+, CD4+, CD4+45RA+) than in lymphoid cell lines and monocytes (Kohno et al. 2006). Primary cellular location is the plasma membrane but is also found in intracellular membranes (Console-Bram et al. 2014).
GPR18 is expressed throughout the immune system. In murine lymphoid cells it is found in CD8αα and CD8αβ intraepithelial cells (IELs) where it functions to establish and maintain the effector T cell compartment (Sumida and Cyster 2018; Wang et al. 2014). It is also required for reconstitution of murine thymus-derived IELs following bone marrow transplantation (Becker et al. 2015). GPR18 is also expressed in neutrophils (PMN) and macrophages (MF) where stimulation with RvD2 serves to limit PMN infiltration and enhance macrophage phagocytosis and efferocytosis, thus acting in a pro-resolving manner (Zhang et al. 2019). RvD2 stimulation of GPR18 also suppresses the expression of pro-IL-1β and reduces the secretion of IL-1β in bone marrow-derived macrophages as well as apoptosis-associated speck-like protein (ASC) oligomerization, inflammasome assembly and caspase-1 activity (Lopategi et al. 2019). NAGly stimulation has shown mixed results (Table 3). NAGly stimulation of GPR18 initiates pro-resolving effects as shown by its enhancement of PMN apoptosis in induced murine peritonitis and through increasing the pro-resolving lipoxin A4 (LXA4) when transfected into HEK293 cells (Recchiuti and Serhan 2012). In another study using the same system, NAGly was shown to increase the amount of free arachidonic acid and the oxidized products 15-deoxy-delta-13,14-PGJ2 and LXA4, both implicated in pro-resolving activities (Burstein et al. 2011). However, in murine RAW 246.7 macrophages, stimulation with NAGly increases apoptosis and caspase-3 expression (Takenouchi et al. 2012). These results suggest that the pro- or anti-inflammatory response is cell type dependent. In humans with sepsis, patients had a lower percentage of GPR18 expressed in PMNs than healthy controls (Zhang et al. 2019). Further, the higher the percentage of PMNs expressing GPR18, the better the survival rate, again supporting a pro-resolving role.
Table 3.
Endocannabinoid metabolic product ligand binding
| Receptor | Parameter measured | EC50/IC50 (nM) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAGly | 12S-HETE-EA | 15S-HETE-EA | 20-HETE-EA | 14,15-EET-EA | 5,6-EET-EA | 2–11,12-EG | 2–14,15-EG | |||
| hCB1 membranesa | disp | – | – | – | – | – | 4000 | – | – | Snider et al. (2009) |
| rCB1 membranesa | disp | – | – | – | 1600 | 2500 | – | – | – | Sridar et al. (2011) |
| rCB1 membranesb | disp | – | 1240 | 4420 | 620 | – | – | – | – | Edgemond et al. (1998) |
| rCB1 membranesb | disp | – | 225 | 900 | – | – | – | – | – | van der Stelt et al. (2002) |
| hCB1 membranesa | disp | – | – | – | – | – | – | 122 | 225 | Chen et al. (2008) |
| hCB2 membranes | disp | – | – | – | – | – | 16 | – | – | Snider et al. (2009) |
| hCB2/CHO-K1c | cAMP¯ | – | – | – | – | – | 9.8 | – | – | Snider et al. (2009) |
| rCB2 membranesa | disp | – | 560 | > 6000 | 2700 | – | – | – | – | Edgemond et al. (1998) |
| rCB2 membranesa | disp | – | 750 | > 1500 | – | – | – | 38 | 65 | Chen et al. (2008) |
| hGPR18/HEK293d | MAPK-P | 44.5 | – | – | – | – | – | – | – | McHugh et al. (2012) |
| hGPR18/CHOc | cAMP¯ | 20 | – | – | – | – | – | – | – | Kohno et al. (2006) |
| hGPR92/CHOe | Ca2+↑ | > 6500 | – | – | – | – | – | – | – | Yin et al. (2009) |
| hGPR92/HEK293f | β-arrestin | ≈32E3 | – | – | – | – | – | – | – | Yin et al. (2009) |
| hGPR92/CV-1 g | SRE-luc | 4470 | – | – | – | – | – | – | – | Oh et al. (2008) |
| hGPR92/CV-1 h | IP3↑ | > 50,000 | – | – | – | – | – | –` | – | Oh et al. (2008) |
| hGPR119/FLPi | cAMP↑ | – | – | – | – | 14,000 | 14,000 | – | – | Soga et al. (2005) |
Agonist parameters given for human (h) and rat (r) receptors in the indicated cell line. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, Agonist parameters given for human (h) and rat (r) receptors in the indicated cell line. Cell type abbreviations: CHO, Chinese hamster ovary cells; CHO-K1, subclone from the parental CHO cell line; CV-1, African green monkey kidney cells; FLP, Flp-In 293 T-REx cells; HEK293, Human embryonic kidney 293 cells; RH7777, rat hepatoma cell line. 2-20:4-LPA, 2-arachadonyl lysophosphatidic acid; 2-11,12-EG, 2-(11,12-epoxyeicosatrienoyl) glycerol; 2-14,15-EG, 2-(14,15-epoxyeicosatrienoyl) glycerol; 12S-HETE-EA, 12S-hydroxyeicosatetraenoic ethanolamide; 5,6-EET-EA, 5,6-epoxyeicosatrienoyl ethanolamide; 14,15-EET-EA, 14,15-epoxyeicosatrienoyl ethanolamide; 15S-HETE-EA, 15S-hydroxyeicosatetraenoic ethanolamide; 20-HETE-EA, 20-hydroxyeicosatetraenoic ethanolamide; aequorin-Ca2+, coexpressed calcium activated photoprotein; β-arrestin, β-arrestin binding assay; Ca2+↑, increase in intracellular calcium; cAMP↑, increase inintracellular cAMP; cAMP↓, decrease of forskolin stimulated cAMP; disp, radiolabel displacement assay; disp; displacement of radiolabeled agonist; IP3, inositol trisphosphate production; LPA, lysophosphatidic acid; MAPK-P, MAP kinase phosphorylation; NAGly, N-arachidonyl glycine; SRE-luc, luciferase reporter assay
aIC50 for displacement of radiolabeled CP-55940 estimated from displacement graph
bIC50 back calculated from Ki using the Cheng–Prussoff equation with the same Kd and [CP55940] used by the authors
cEC50 for decrease in forskolin/IBMX-stimulated cAMP
dEC50 for MAPK phosphorylation as measured by western blotting
eEC50 for Ca2+ release measured by aqueorin assay as estimated from graph
fEC50 for β-arrestin binding measured with the PathHunter™ assay as estimated from graph
gEC50 for induction of SRE-luc activity
hEC50 for of IP3 production from radiolabeled myo-[3H]inositol
iEC50 determined for cAMP using a homogeneous time-resolved fluorescence kit (CIS Bio International)
GPR18 is also expressed in neural tissue. Treatment of endogenous GPR18 through direct application of Abn-CBD to the rostral ventrolateral medulla (RVLM) of conscious rats results in the release of nitric oxide (NO) and adiponectin (ADN) resulting in reduced blood pressure and oxidative stress (Penumarti and Abdel-Rahman 2014a; b). NAGly also increases NO and ADN, albeit to a lesser extent than Abn-CBD due to partial activation of co-localized CB1R, known to activate sympathoexcitation/pressor response that counteracts the response (Penumarti and Abdel-Rahman 2014b). GPR18 has also been proposed to be involved in microglial-neuronal communication (McHugh et al. 2014). Treatment of a BV-2 microglia model system with either NAGly or Δ9-THC results in a shift in microglial morphology from ameboid-like to branched and at the same time induces alterations in the secretion of five different cytokines (Axl, CD40, IGF-I, OPN, and Pro-MMP-9) known to be involved in cell survival, inflammatory response, cell growth, and cell adhesion. In contrast, treatment of murine GPR18 heterologously expressed in rat superior cervical ganglion with NAGly, anandamide, AbnCBD failed to elicit changes observed in other systems, suggesting that noncanonical signaling pathways are involved in this system (Lu et al. 2013).
The primary agonist for GPR18 is NAGly (Table 3) where stimulation of transfected GPR18 is known to increase [Ca2+]i in the recipient cell and inhibit forskolin-induced cAMP production (Kohno et al. 2006). The fact that these functions are inhibited by pertussin toxin (PTX) indicates that the signal transduction proceeds via a Gαi. NAGly stimulation of GPR18 in murine RAW264.7 macrophages also leads to signaling via a Gαi, resulting in activation of ERK1/2, p38 MAPK, and c-Jun N-terminal kinases (JNKs), all of which stimulate apoptosis (Takenouchi et al. 2012). More recently it has been shown that in addition to NAGly stimulation, both Δ9-THC and Abn-CBD also increase [Ca2+]i, activate MAPK, and signal through a Gαi pathway (Console-Bram et al. 2014) (Table 1). Interestingly, this same report shows that Δ9-THC also signals through a Gαq pathway and is the only one of these three agonists that initiates β-arrestin binding. More recently, RvD2 has been added to the list of GPR18 agonists. Like Δ9-THC, RvD2 also inititates β-arrestin binding, and cholera toxin (CTX) and not PTX inhibits signaling. This indicates that signaling proceeds through a Gαs pathway which is confirmed by the increase in cAMP upon stimulation (Davenport et al. 2013). Also, like Δ9-THC, RvD2 agonism proceed via multiple pathways, a cAMP-response element binding protein (cAMP/PKA/CREB) path and an alternate signal transducer and activator of transcription protein (STAT) path where STAT1, STAT3 and STAT5 are activated as well as pathways leading to the phosphorylation of protein kinase B (PKB or Akt), p38 MAPK, and ERK1/2 (Chiang et al. 2017).
Structure
hGPR18 (N-arachidonyl glycine receptor, UniProtKB- Q14330) is translated as a 331 amino acid polypeptide with a calculated molecular weight of 38.1 kDa. No additional isoforms have been reported. One coding SNP variants has been reported (L187M) but associated pathologies are unknown (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 26 residues and a cytosolic C-terminal of 42 residues. There are no X-ray structures reported, however, SWISS-Model site has a precalculated model available based on the zebrafish lysophosphatidic acid receptor LPA6 (26.7% sequence homology; template 5cxv.1.A, PDB entry 5CXV) to serve as a working template (Waterhouse et al. 2018). Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1). However, S322 is a likely phosphorylation candidate based on the known phosphorylation of murine GPR18 (S322, 85.8% sequence homology, UniProtKB-Q8K1Z6).
Regulation
hGPR18 is reported to undergo constitutive trafficking from the plasma membrane to internal vesicles, reducing surface expression in the absence of agonist (Finlay et al. 2016). As reported for GPR55 and both CB1R and CB2R (see above), GPR18 also forms heterodimers with CB2R but not CB1R when co-transfected into HEK293 cells (Reyes-Resina et al. 2018). The signaling pathway for this heterodimer does produce an increase cAMP, ERK1/2 phosphorylation and DMR as expected. However, when both receptors are activated, negative cross-talk is observed. This heteromer was found to be expressed in primary microglia cultures and upon activation with lipopolysaccharide (LPS) and interferon gamma (INF-γ), the expression of both receptors is upregulated.
GPR92
Introduction
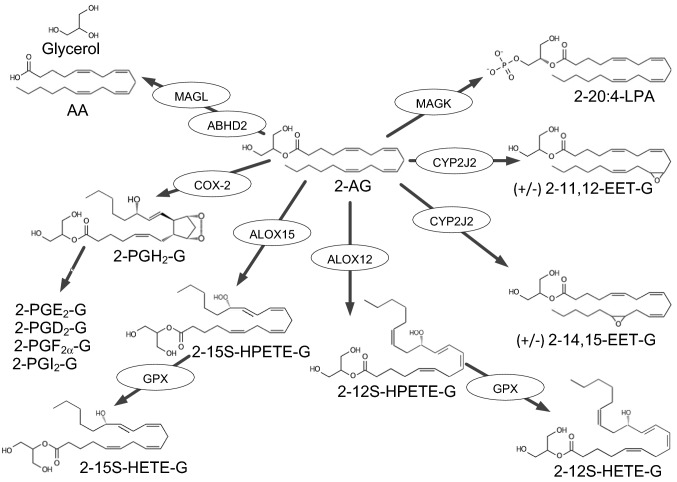
Human GPR92 (hGPR92) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family. The primary agonists for this receptor are reported to be lysophosphatidic acid (LPA) and farnesyl pyrophosphate (FPP) with weaker binding to both 2-arachadonyl glycine (NAG) and NAGly (Table 2) (Oh et al. 2008; Kotarsky et al. 2006; Williams et al. 2009; Yin et al. 2009). The inclusion of GPR92 in a list of potential cannabinoid or cannabinoid-like receptors is controversial, but not unfounded. Most of the known major agonists for this receptor are not agonists for either CB1R or CB2R, however, there are no reports of binding experiments for GPR92 with anandamide or any of the classic phytocannabinoids to exclude GPR92 from the list. However, there is one report of GPR92 stimulation by 2-AG (Oh et al. 2008), a known agonist for both CB1R and CB2R, albeit with a threefold higher EC50 (Table 1). The activation of GPR92 by NAGly, a known agonist for GPR18 and GPR55, both clearly strong candidates for the list, also support the inclusion of GPR92 as at least a cannabinoid-like receptor. Further, the upregulation of GPR92 in GPR55−/− mice and the elevated insulin secretory response to cannabinoid ligands (AM251 and SR141716A) in these mice suggest that GPR92 may be responsible for the response much like GPR55 and GPR119 (Ruz-Maldonado et al. 2020).
Expression and characterization
hGPR92 is highly expressed in the brain and blood, with highest amounts in T-cells, B-cells, bone marrow and lymphoid tissue, testis, with lower expression in the brain, proximal digestive tract, GI tract, bone marrow and lymph and minor amounts in most other tissues (Uhlén et al. 2015). Expression of GPR92 in the brain and peripheral neural tissue serves to regulate a number of different processes. Many dorsal root ganglia cells (DRG) co-express TRPV1 (Oh et al. 2008), an anandamide receptor known to be involved in pain sensing (Caterina et al. 1999), suggesting that GPR92 may also be involved in neuropathic pain, a clear defining characteristic of cannabinoid receptors. Another study reveals that GPR92 expression in DRG and spinal cord dorsal horn are involved in neuropathic pain and that activation only occurs during injury and through central pCREB (phosphorylated CREB) activation (Lin et al. 2012). In addition, GPR92−/− mice show decreased sensitivity to acute pain stimuli (Callaerts-Vegh et al. 2012), supporting its involvement on neuropathic pain. These GPR92−/− mice also show changes in anxiety-related and motivational behavior, consistent with the expression of GPR92 throughout the brain. Inhibition of microglial GPR92 with the receptor-specific antagonist TCLPA5 blunts all LPA-induced proinflammatory signals indicating that it is involved in the M1 polarization of microglia and hence the proinflammatory response to neural injury (Plastira et al. 2016).
GPR92 in an important player in the immune system involving both myeloid and lymphoid branches and is abundantly expressed in human mast cells (Lundequist and Boyce 2011). Reduction of expression levels with shRNA reduces the normal [Ca2+]i response to LPA activation and abolishes macrophage inflammatory protein 1β (MIP-1β or CCL4) secretion, a key function for activated mast cells. Another myeloid cell function for GPR92 is activation of human platelets. A variety of GPR92 agonists are known to induce human platelet shape change, an event consistent with platelet activation (Williams et al. 2009). Studies also show the involvement of GPR92 in the regulation of lymphoid cell types. GPR92 is also abundantly expressed in the CD8+ T cells obtained from mouse colon and mouse intestinal lymphocytes from all cell populations (Kotarsky et al. 2006). CD8+ T cell expression of GPR92 in mouse melanoma model tissue has been reported and found to be involved in tumor growth (Oda et al. 2013). Specifically, activation of GPR92 in these cells negatively regulates T cell antigen receptor (TCR) -induced calcium mobilization and antigen-mediated proliferation, leading to suppressed adaptive immunity and enhanced tumorigenesis. GPR92 is also expressed in mature murine B cells and LPA activation serves to inhibit B cell receptor signaling (BCR) through impairment of the normal calcium ion release from intracellular stores, as observed for T cells, as well as limiting the induction of CD69 and CD86 expression, proteins involved in cell proliferation (Hu et al. 2014).
The primary agonists for GPR92 are LPA and farnesyl pyrophosphate (FPP) (Table 2). The GPCR signaling pathway for hGPR92 is agonist dependent and proceeds though Gαq/11, Gαs, or Gα12/13-mediated pathways leading to IP3/Ca2+/PKC, cAMP/PKA, and Rho/MAPK mediated signaling respectively. LPA signals through all three pathways whereas FPP signals through both Gαq/11 and Gαs and NAG only signals through Gαq/11 (Oh et al. 2008). LPA/GPR92 signaling through Gαq/11 and Gα12/13 have been reported by others (Hu et al. 2014; Paugh et al. 2006).
Structure
hGPR92 (Lysophosphatidic acid receptor 5, LPA-5, UniProtKB-Q9H1C0) is translated as a 372 amino acid polypeptide with a calculated molecular weight of 41.3 kDa. No additional isoforms have been reported. No coding SNP variants have been reported (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 26 residues and a cytosolic C-terminal of 75 residues. There are no reported X-ray structures, however, SWISS-Model site hosts a model (5xsz.1.A) based on the zebrafish lysophosphatidic acid receptor LPA (38.0% sequence homology, PDB entry 5XSZ) to serve as a working template (Waterhouse et al. 2018). Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1).
Regulation
hGPR92 transfected into B103 cells (rat neuroblastoma cell line) is reported to undergo LPA induced internalization from the plasma membrane to internal vesicles (Lundequist and Boyce 2011; Lee et al. 2006). The specific mechanism for internalization is unknown. However, LPA induced β-arrestin binding has been confirmed (Yin et al. 2009).
GPR3
Introduction
Human GPR3 (hGPR3) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family and has been shown to exhibit constitutive activation of adenylate cyclase (AC) (Laun et al. 2019). However, sphingosine 1-phosphate (S1P) has been observed to be an hGPR3 agonist (Uhlenbrock et al. 2002; Zhang et al. 2012; Hinckley et al. 2005). A novel GPR3-specific agonist diphenyleneiodonium chloride (DPI) has also been reported (Ye et al. 2014; Capaldi et al. 2018), a claim disputed by others (Yin et al. 2009; Ye et al. 2014; Valverde et al. 2009). GPR3 is not activated by the classic cannabinoids (Laun and Song 2017). However, CBD has been shown to be an inverse agonist (Laun and Song 2017; Laun 2018; Laun et al. 2018).
Expression and characterization
hGPR3 is highly expressed in the brain, moderate amounts in endocrine and muscle tissue, and lower expression in most other tissues (Uhlén et al. 2015). Expression of GPR3 in the brain serves multiple purposes. Tanaka et al. (2007) have shown that GPR3 is highly expressed in developing rat cerebral granule neurons at all developmental stages examined (P1, P4, P7, P14). A siRNA knockdown of GPR3 on P7 granule neurons significantly inhibits neurite growth, suggesting a pro-neurite growth function. Transfection of GPR3 into Neuro2a neuroblastoma cells results in a transformation into neuron-like cells and extension of their neurites. Further, the upregulation of cAMP by GPR3 serves to block myelin inhibition. Clearly, GPR3 serves an active role in developing neurons where it stimulates neurite outgrowth and facilitates neuron protection by counteracting myelin inhibition. Tanaka et al. (2014) extended the role of GPR3 to include antiapoptotic activity under both hypoxic and reactive oxygen species (ROS) apoptotic conditions.
In contrast to the neuroprotective function, Thathiah et al. (2009) report that GPR3 is actively involved in amyloid-β (Aβ) production from the β-amyloid precursor protein (APP), a major step in the progression in Alzheimer’s disease (AD), by aiding the formation and cell surface localization of γ-secretase, one of two enzymes required for Aβ production from APP. More recently, this initial finding was expanded to show that it is the interaction of the C-terminal domain of GPR3 with β-arrestin-2 that is required for Aβ production (Thathiah et al. 2013). Further, the bound β-arrestin-2 directly interacts with the Aph-1α subunit of g-secretase complex to facilitate the redistribution of the complex to detergent-resistant membranes where the catalytic activity of the complex is enhanced. Huang et al. (2015) provide further support for these data where they have shown that genetic deletion of GPR3 in four different AD transgenic mouse models results in a reduction in amyloid pathology in all models. In addition, upon examination of the GPR3 production in human brain tissue they found that there is no significant correlation between GPR3 expression levels and age for non-AD individuals whereas GPR3 expression levels are elevated in AD-patients and the expression level correlates with progression of the disease.
GPR3 is also involved in the expression and development of neuropathic pain. Ruiz-Median et al. (2011) examined Gpr3−/− and Gpr3+/+ mice and found that in the absence of GPR3, mice exposed to nerve ligature experience hypersensitivity to thermal non-noxious and noxious stimuli without altering the spinal inflammation response associated with sciatic nerve injury. Such results are consistent with GPR3 modulation of nociceptive response to pain.
Sphingosine 1-phosphate (S1P) is the only known natural hGPR3 agonist, inducing an increase in [Ca2+]i associated with an increase in cAMP levels with a reported EC50 for Ca2+ mobilization of 50 nM for hGPR3 transfected into HEK293 cells (Table 2) (Uhlenbrock et al. 2002). S1P activation of GPR3 resulting in cAMP accumulation have also been reported for porcine (Zhang et al. 2012) and rodent GPR3 (Hinckley et al. 2005). Dihydrosphingosine 1-phosphate is also an agonist with EC50 values similar to S1P (Uhlenbrock et al. 2002). However, there are a number of reports that dispute this claim (Yin et al. 2009; Ye et al. 2014; Valverde et al. 2009) and thus assigning S1P as a native agonist still remains unclear. Interesting, S1P has been shown to be an antagonist for CB1R but not CB2R, suggesting similarity in the agonist binding pockets for CB1R and GPR3 (Paugh et al. 2006). A novel GPR3-specific agonist diphenyleneiodonium chloride (DPI) has been reported with EC50 values in the low micromolar range (Ye et al. 2014; Capaldi et al. 2018). Utilizing β-arresin-2 recruitment, activation with the classic cannabinoids, anandamide, 2-AG and well as virodhamine and noladin ether proved fruitless (Laun and Song 2017). Treatment with phytocannabinoids Δ9-THC, cannabinol, cannabigerol, and cannabichromene also has no effect. However, treatment with CBD results in a reduction in β-arrestin-2 recruitment, indicating that CBD is an inverse agonist of GPR3 with an EC50 in the low micromolar region (Table 2) (Laun and Song 2017; Laun 2018). These results offer only modest support for listing GPR3 as a cannabinoid-like receptor. However, it should be noted that the effect of either endocannabinoids or phytocannabinoids on the accumulation of cAMP or [Ca2+]i or GTPγS binding have not been reported and thus the true potential for cannabinoid agonism remains unknown.
GPR3 is known to possess both constitutive signaling as well as agonist-induced signaling. Uhlenbrock et al. (2002) observed that hGPR3 transfected into HEK293 cells exhibits constitutive activity via Gαs and Gαi signaling, leading to activation of adenylate cyclase (AC) or an increase in [Ca2+]i respectively. However, activated charcoal stripping of the media to remove potential lipid-like materials did reduce the constitutive activity somewhat, indicating that at least a portion of the constitutive activity was initiated by lipids of unknown identity. In the presence of S1P (or DHS1P), hGPR3 signals through both Gαi leading to release of Ca2+ from thapsigarin-sensitive ER stores and Gαs leading to a modest increase in AC activity. In the presence of PTX, which knocks out all Gαi activity, the AC activity is enhanced. In contrast, Ye et al. (2014) was unable to reproduce the aforementioned S1P experimental data but confirmed the constitutive Gαs activity. Further, they show that the synthetic agonist DPI stimulates an increase in intracellular cAMP via Gαs. Interestingly, when Gα16 is overexpressed in the same cells the signaling results in Ca2+ mobilization. Constitutive Gαs/cAMP signaling has also been reported in rodent systems (Hinckley et al. 2005; Tanaka et al. 2007). Although the data for constitutive signaling is quite solid, ligand induced signaling requires further exploration.
Structure
hGPR3 (G-protein coupled receptor 3, ACCA orphan receptor, UniProtKB-P46089) is translated as a 330 amino acid polypeptide with a calculated molecular weight of 35.0 kDa. No additional isoforms have been reported. No coding SNP variants have been reported (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 42 residues and a cytosolic C-terminal of 31 residues. There are no reported X-ray structures, however, SWISS-Model site hosts a model (6pt0.1.A) based on the cryo-EM structure of human cannabinoid receptor 2 -Gi protein in complex with agonist WIN 55,212–2 (25.9% sequence homology, PDB entry 6PTO) to serve as a working template (Waterhouse et al. 2018); see also (Morales et al. 2017). Capaldi et al. (2018) have described generating a model of their own. Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1). Only the C-terminus phosphorylations have experimental support, where phosphorylation of S237, S242, S316, S317, S318, S324, S326, and S328 have been identified (Lowther et al. 2013).
Regulation
Agonist-induced internalization of GPR3 has been documented by several groups. Lowether et al. (2013) show that unstimulated, constitutively active hGPR3 internalizes in the presence of overexpressed β-arrestin-2 and GRK2. Internalization also requires phosphorylation of S237, S242 on an intracellular loop and all six phosphorylation sites on the C-terminus. However, site specific mutation of S237 and S242 to alanine results in a species that produces twofold higher cAMP levels than wild type whereas mutation of all C-terminal serines has no effect on cAMP production. Clearly, phosphorylation of the loop serines serves to reduce cAMP signaling but is also required along with C-terminal serine phosphorylation for β-arrestin-2-mediated internalization. Further, utilizing catalytically inactive GRK2 (GRK2-K220R) they show that the binding is necessary for a reduction in cAMP levels, albeit not to the same levels as wild type GRK2, suggesting that phosphorylation has some importance in cAMP regulation. However, without the ability to phosphorylate, GRK2-K220R does not initiate internalization even in the presence of β-arrestin-2, confirming that phosphorylation is paramount to internalization. In another study DPI-stimulated hGPR3 also shows agonist-induced β-arrestin-2 receptor internalization and concomitant reduction in cAMP production (Ye et al. 2014). CBD has also been shown to recruit β-arrestin-2, but its stimulation of receptor internalization has not been evaluated (Laun et al. 2019).
Allosteric regulation of class A GPCRs by Na+ is a common feature where Na+ negatively regulates agonist binding whereas upon agonist binding, the Na+ binding site experiences a conformational collapse (review (Katritch et al. 2014). For some class A receptors, the bound Na+ appears to stabilize the inactive state and reduces the constitutive activity. Murine GPR3 is allosterically regulated by sodium ion binding through binding to the pocket formed by C267, F120, and D86. Capaldi et al. (2018) examined the DPI-induced cAMP production for the site specific mutants C267A, F120A, and D86A and found that while the first two mutants exhibited a 2 and eightfold reduction in activity respectively, the D86A mutant was completely inactive. Clearly the integrity of the Na+ binding site is paramount for normal activity of this receptor and D86 is involved in not only agonist-induced but also the constitutive activity of GPR3.
GPR6
Introduction
Human GPR6 (hGPR6) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family and like GPR3 it has been shown to exhibit constitutive activity in the production of cAMP (Laun et al. 2018; Tanaka et al. 2007). S1P is the only known natural agonist (Uhlenbrock et al. 2002; Ignatov et al. 2003a). Dihydrosphingosine 1-phosphate is also an agonist (Uhlenbrock et al. 2002) and CBD is a biased inverse agonist of GPR6 (Laun et al. 2018).
Expression and characterization
hGPR6 is highly expressed in the brain, the basal ganglia in particular, and in small amounts in other tissues (Uhlén et al. 2015). It has also been shown that GPR6 is primarily located in intracellular compartments (Padmanabhan et al. 2009).
Expression of GPR6 in the brain involves neuroprotection. Tanaka et al. (2007) investigated GPR6 in rat cerebral granule neurons and found that like GPR3 it is also expressed at all developmental stages examined (P1, P4, P7, P14), but at lower levels than GPR3, from 6 to 40% of GPR3 levels depending on the developmental stage. They also note that GPR6 is also involved in the promotion of neurite growth and myelin inhibition but provide no supporting data. Tanaka et al. (2014) also show that GPR6 is involved in antiapoptotic activity under both hypoxic and reactive oxygen species (ROS) apoptotic conditions. In contrast to GPR3, complement protein C1q-induced expression of GPR6 protects neurons from Aβ-induced neurotoxicity in murine Alzheimer Disease models, presumably by sustaining pCREB activation through maintaining intracellular cAMP levels (Benoit et al. 2013).
As observed for GPR3, GPR6 is known to possess both constitutive signaling and well as agonist-induced signaling. Constitutive activity proceeds through a Gαs and a competing Gαi signaling pathway leading to activation of AC or inhibition of AC and release of calcium from the ER respectively (Uhlenbrock et al. 2002). S1P stimulation of GPR6 results in the release of Ca2+ from intracellular stores via a sphingosine-kinase-mediated pathway (Uhlenbrock et al. 2002; Ignatov et al. 2003a) a kinase known to be activated through a Gαi-mediated pathway (Meyer zu et al. 2001). Activation of sphingosine kinase is also accompanied by an increase in MAPK activity under conditions of oxidative stress.
Sphingosine 1-phosphate (S1P) has been observed to be an agonist for hGPR6 with a reported EC50 of 106 nM (Uhlenbrock et al. 2002) as measured by an increase in intracellular Ca2+ or G-Protein-Coupled Inwardly Rectifying Potassium channel (GIRK) currents (Table2) (Ignatov et al. 2003a). Dihydrosphingosine 1-phosphate is also an agonist with EC50 values similar to S1P (Uhlenbrock et al. 2002). However, there is one report that disputes this claim (Yin et al. 2009) but it should be pointed out that the dissenting data is based on β-arrestin recruitment and the supporting data based on Ca2+ or GIRK currents and it is conceivable that the spingosine derivatives intitate non-β-arrestin signaling events. Treatment with CBD results in a reduction in β-arrestin-2 recruitment and [Ca2+]i production but not on cAMP production, indicating that CBD is a biased inverse agonist of GPR6. Laun et al. (2018) also report CBD inverse agonism and indicate that constitutive cAMP production is affected as well. The reported CBD EC50 values of 75 nM and 200 nM (Laun and Song 2017; Laun et al. 2018) (Table 2) offer modest support for listing GPR3 as a cannabinoid-like receptor. Further support for this is found in the binding of SR114528 (EC50 = 620 nM) and SR141716A (EC50 = 2770 nM) which are strong synthetic activators for CB2R and CB1R respectively (Laun et al. 2018).
Structure
hGPR6 (G-protein coupled receptor 6, Sphingosine 1-phosphate receptor GPR6, UniProtKB-P46095) is translated as a 362 amino acid polypeptide with a calculated molecular weight of 37.9 kDa. One additional isoform has been reported, differing only by a slightly elongated N-terminus (hGPR6-2, 377 amino acids, 39.4 kDa). No coding SNP variants have been reported (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 74 residues (90 residues for isoform 2) and a cytosolic C-terminal of 31 residues. There are no reported X-ray structures, however, SWISS-Model site hosts a model (6pt0.1.A) based on the cryo-EM structure of human cannabinoid receptor 2 -Gi protein in complex with agonist WIN 55,212-2 (28.1% sequence homology, PDB entry 6PTO) to serve as a working template (Waterhouse et al. 2018). Isawi et al. (2020) describe a model of their own based on the S1P1 receptor. Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1).
Regulation
Little is known about the regulation of GPR6. Regulation by agonist-induced internalization has been observed for GPR6 utilizing S1P as the ligand (Uhlenbrock et al. 2002). CBD and SR114528, a synthetic agonist of CB2R, are reasonably potent inverse agonists of GPR6, serving to recruit β-arrestin-2. Although only recruitment has been reported, the fact that β-arrestin binding is often involved in receptor-induced internalization suggests that these inverse agonists may potentially induce internalization. GPR6 has been shown to be upregulated by the innate immune system complement component 1q (C1q) in Aβ challenged rat neurons (Benoit et al. 2013) giving further support for the involvement of GPR6 in the protection of neurons from Aβ-induced neurotoxicity.
GPR12
Introduction
Human GPR12 (hGPR12) is a member of the seven transmembrane receptor G-protein coupled receptor 1 family, and like GPR3 and GPR6, has been shown to exhibit constitutive activity producing cAMP (Uhlenbrock et al. 2002; Tanaka et al. 2007). Sphingosylphosphorylcholine (SPC) is the primary endogenous agonist (Ignatov et al. 2003b) and S1P is a weaker agonist (Table 2). As observed for GPR 3 and 6, CBD acts as an inverse agonist for GPR12 (Brown et al. 2017). In contrast, dietary-derived tyrosol increases the constitutive cAMP accumulation (Lin et al. 2008).
Expression and characterization
hGPR12 is highly expressed in the brain, the highest amounts in the basal ganglia followed by the cerebral cortex and cerebellum and with lesser amounts found in the proximal digestive system, female tissues, and in granulocytes (Uhlén et al. 2015). hGPR12 is ubiquitously expressed in extremely low amounts in most other tissues.
Expression of GPR12 supports neurite growth and neuronal protection and is involved in brain development. Tanaka et al. (2007) investigated GPR12 in rat cerebral granule neurons and found that like GPR3 and GPR6 it is also expressed at all developmental stages examined (P1, P4, P7, P14), but at much lower levels than GPR3 and slightly lower levels than GPR6 depending on the developmental stage. They also note that GPR12, like GPR3 and GPR6, is involved in the promotion of neurite growth and was the most prominent of the three. GPR12 also reverses myelin inhibition through a Gαs/cAMP/PKA driven inhibition of the small GTPase RhoA which in turn serves to inhibit the action of myelin-associated glycoprotein (MAG). Tanaka et al. (2014) also show that GPR12 is involved in antiapoptotic activity under both hypoxic and reactive oxygen species (ROS) apoptotic conditions. Ignatov et al. (2003b) report that GPR12 is expressed in the murine CNS and within the areas of differentiation in particular. This is consistent with the observation that treatment of cultures of embryonal cerebral cortical neurons with SPC increases synaptic contacts. Further, Bédard et al. (2007) show that GPR12 is upregulated in microglia after treatment with cuprizone, a demyelinating toxin, suggesting a role in response to neuroinflammation. Utilizing HEK293 cells overexpressing hGPR12, Lu et al. (2012) show that GPR12 plays an important role in cell proliferation and cell survival, suggesting a similar role in neural cells.
Mammalian oocytes are maintained in the ovary in prophase I of meiosis until a surge of luteinizing hormone initiates the resumption of meiosis. This meiotic arrest depends on a high level of cAMP. Hinckley et al. (2005) report that the activity of both GPR3 and GPR12 is required for maintenance of the cAMP levels in rodent oocytes. On the other hand, Vaccari et al. (2008) report that only GPR3 is responsible of cAMP levels in rodent oocytes. Diluigi et al. (2008) explored this meiotic arrest in human oocytes and found that GPR12 is not expressed in human oocytes and thus cannot be directly involved in cAMP-induced meiotic arrest. They do suggest, however, that GPR12 expressed in the human ovary could provide cAMP to the oocytes through gap junctions, a possibility dismissed by Vaccari et al. (2008) as a possibility in mice.
Sphingosylphosphorylcholine (SPC) is the primary endogenous agonist for GPR12 with a reported EC50 between 32 and 66 nM for murine GPR12 as measured by the increase in GIRK currents and intracellular Ca2+ currents respectively (Ignatov et al. 2003b) (Table 2). S1P is a weaker binder with EC50 values of 106 nM for hGPR12 and 1–3 μM for murine GPR12 as measured by the increase in Ca2+ and GIRK currents (Uhlenbrock et al. 2002; Ignatov et al. 2003b) (Table 2). There is one report that disputes the claim that S1P is an agonist (Yin et al. 2009), however β-arrestin binding was the factor measured rather than Ca2+ or an increase on cAMP production. There is one report indicating that CBD acts as an inverse agonist for GPR12 with an EC50 in the 10–100 μM range for reduction of constitutive cAMP production (Brown et al. 2017) (Table 2). In contrast, various endocannabinoids and other phytocannabinoids show no effect on cAMP accumulation. This CBD signaling offers only modest support for listing GPR3 as a cannabinoid-like receptor. The addition of tyrosol, a common metabolite found in the plasma after ingestion of extra virgin olive oil, to the cell culture medium containing either hGPR12 transfected into CHO or HEK293 cells increases the constitutive cAMP accumulation (Lin et al. 2008). For transfected CHO cells the effect begins at 10 nM and is maximal at 100 nM and higher amounts reduce the increase in cAMP accumulation incrementally up to 50 μM tyrosol where cAMP production is still above basal levels. For transfected HEK293 cells a similar pattern was observed with the maximal induction of cAMP occurring at 1 μM.
As observed for both GPR3 and GPR6, GPR12 also possesses both constitutive signaling as well as agonist-induced signaling. Constitutive activity proceeds through a Gαs or a competing Gαi signaling pathway that leads to activation of AC or inhibition of AC and release of calcium from the ER respectively (Uhlenbrock et al. 2002; Tanaka et al. 2007; Ignatov et al. 2003b). S1P stimulation of GPR12 results in the release of Ca2+ from intracellular stores via a sphingosine-kinase-mediated pathway (Uhlenbrock et al. 2002; Ignatov et al. 2003b), a kinase known to be activated through a Gαi-mediated pathway (Meyer zu et al. 2001). CBD, on the other hand, acts as an inverse agonist that reduces constitutive cAMP production normally conducted via a constitutive Gαs-mediated pathway (Brown et al. 2017).
Structure
hGPR12 (G-protein coupled receptor 12, UniProtKB-P47775) is translated as a 334 amino acid polypeptide with a calculated molecular weight of 36.7 kDa. No additional isoforms have been reported. No coding SNP variants have been reported (Stelzer et al. 2016; Landrum et al. 2016). The basic structure consists of seven transmembrane helices with a N-terminal extracellular domain of 48 residues and a cytosolic C-terminal of 31 residues. There are no reported X-ray structures, however, SWISS-Model site hosts a model (6pt0.1.A) based on the cryo-EM structure of human cannabinoid receptor 2 -Gi protein in complex with agonist WIN 55,212-2 (28.5% sequence homology, PDB entry 6PTO) to serve as a working template (Waterhouse et al. 2018). Posttranslational modifications are predicted, but none have been confirmed experimentally (Table S1).
Regulation
Very little is known about the regulation of GPR12. It is known that GPR12 is upregulated in some areas of the brain over others and in the brain itself over other tissues (Uhlén et al. 2015; Ignatov et al. 2003b). In addition, phosphodiesterase 3A (PDE3A; a cAMP hydrolase) null murine oocytes were shown to exhibit chronically elevated cAMP and significant downregulation of GPR12 mRNA, indicating that high cAMP levels control GPR12 expression through an unknown pathway (Hinckley et al. 2005).
Discussion
Although much is known about the biological activity of the putative cannabinoid receptors discussed above, the dearth missing information leads to a number of unanswered questions. A few of which are addressed below.
Do these receptors fulfill the criteria required to be designated as cannabinoid receptors?
Experimental data confirm the existence of at least five distinct cannabinoid receptors (Mackie and Stella 2006; Ruz-Maldonado et al. 2020). The signature receptors are the CB1R and CB2R cannabinoid receptors and experiments utilizing CB1R−/− and CB2R−/− mice reveal the existence of at least three additional cannabinoid receptors. The receptors discussed above represent orphan receptors that fulfill at least some of the criteria defined by IUPHAR for receptors to be placed within this classification. The criteria for additions to the cannabinoid receptor classification published by Pertwee et al. (2010) are as follows:
It should be activated at its orthosteric site and with significant potency by an established CB1/CB2 receptor ligand.
It should be activated by at least one established endogenous CB1/CB2 receptor agonist at “physiologically relevant” concentrations.
If it is a GPCR, it should display significant amino acid sequence similarity with the CB1 or the CB2 receptor, which are members of the group of rhodopsin-type GPCRs.
It should not be a “well established” non-CB1/CB2 receptor or channel, especially if there is already strong evidence that (1) this is activated endogenously by a non-CB1/CB2 receptor ligand with appropriate potency and relative intrinsic activity and (2) this is not activated endogenously by any endocannabinoid with appropriate potency and relative intrinsic activity.
It should be expressed by mammalian cells that are known to be exposed to concentrations of endogenously released endocannabinoid molecules capable of eliciting a response.
Criterion 1 is the most stringent of the five and requires data that confirms that cannabinoids activate the receptor at reasonably low concentrations but must not activate the receptor in an allosteric manner. Ryberg et al. (2007) report that a number of endocannabinoids and phytocannabinoids are strong agonists for GPR55, fulfilling the potency requirement. However, others have failed to reproduce this data (Oka et al.2007; Kapur et al. 2009). Lauckner et al. (2008) report that Δ9-THC and anandamide at 5 µM concentrations both stimulate the increase in [Ca2+]i but do so in a gradual manner. Other cannabinoids and cannabimimetrics tested did not increase [Ca2+]i in statistically significant amounts. The fulfillment of the potency requirement is thus in doubt due to the inconsistency of cannabinoid binding results. What is well established is that the non-cannabinoid LPI is a potent GPR55 agonist (Henstridge et al. 2010). Anavi-Goffer et al. (2012) examined the effect of LPI and various cannabinoids and cannabimimetrics on GPR55 signaling in terms of ERK1/2 phosphorylation. They found that both the CB2 agonist GW405833 and the CB1R antagonist AM251 alone are weak GPR55 agonists. Experiments in the presence of LPI reveal that GW405833 is an allosteric enhancer and both Δ9-THC and AM251 are allosteric inhibitors of LPI signaling. These results indicate that cannabinoid binding to GPR55 does not occur at a site orthostatic to the LPI binding site and that any activation of GPR55 by cannabinoids likely occurs through this allosteric site. Although one could argue that LPI is an allosteric enhancer for GW405833, these results are clearly not consistent with the intent of criterion 1.
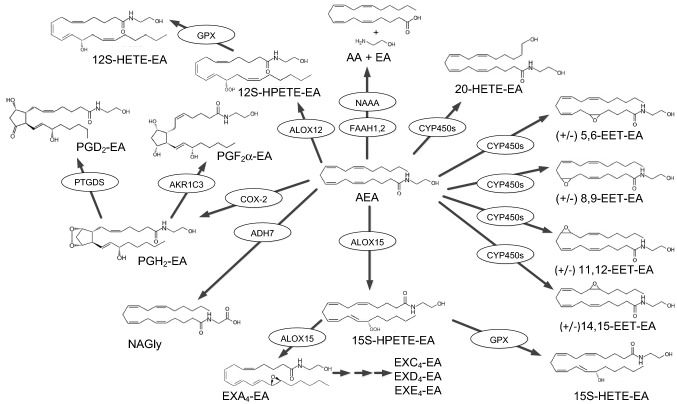
Virodhamine, anandamide and Δ9-THC are GPR18 agonists, albeit only virodhamine is clearly a tight binder (McHugh et al. 2010, 2012, 2014; Fuchs et al. 2013), potentially fulfilling the potency requirement. NAGly, has also been reported to be a potent agonist for GPR18, with EC50 values similar to virhodamine (McHugh et al. 2012; Kohno et al. 2006), however, not all reports completely agree with the findings (Finlay et al. 2016; Lu et al. 2013). Although similar in structure to anandamide, NAGly is not considered to be an endocannabinoid (Sheskin et al. 1997) and thus these activation results cannot be applied to fulfill criterion 1. Experiments examining potential allosteric interactions involving these agonists or binding site displacement experiments have yet to be reported. For these reasons orthostatic binding cannot yet be confirmed and fulfillment of criterion 1 remains undetermined.
There is one report indicating a micromolar efficacy for 2-AG with GPR92 (Oh et al. 2008), suggesting that GPR92 could be considered to be a cannabinoid receptor. However, based on EC50 values, the primary agonists for this receptor appear to be FPP and various LPAs. Again, the lack of competition experiments and those examining potential allosteric effects leaves criterion 1 unconfirmed for this receptor.
GPR119 is not activated by any of the accepted endocannabinoids or phytocannabinoids and thus fails to meet criterion 1. GPR119 is activated by the cannabinoid-like OEA with EC50 values in the nM range (Southern et al. 2013; Hansen et al. 2011; Hassing et al. 2016) whereas cannabinoid receptors do not respond to OEA, strongly suggesting that cannabinoid-like ligands will not fulfil criterion 1.
GPR3, GPR6, and GPR12 are constitutively active receptors that are further activated by S1P (Uhlenbrock et al. 2002; Zhang et al. 2012; Hinckley et al. 2005). CBD and several synthetic cannabinoid receptor agonists serve as inverse agonists for this receptor (Laun and Song 2017; Luan et al. 2018; Brown et al. 2017). Although the orthostatic nature of the binding sites for these ligands is unknown, the very fact that the phytocannabinoid CBD and synthetic cannabimimetrics serve as inverse agonists would necessarily disqualify these receptors from fulfilling criterion 1.
Criterion 2 requires more data to ensure compliance, as little is known about localized concentrations of endocannabinoids and thus plasma or measured tissue levels are not necessarily correct criterion for judging compliance. Further, different measurements of efficacy usually present with different EC50 values depending on the location of the metabolite in the signaling pathway and thus a high EC50 value for a downstream measurement may be just as viable as a low EC50 for an upstream event. That being said, if the results reported by Ryberg et al. (2007) are valid, the GPR55 would clearly fulfill this criterion, however, these results do require independent validation. Lauckner et al. (2008) report that Δ9-THC and anandamide increase in [Ca2+]I at 5 µM concentrations suggests that this criterion is reasonably well satisfied for GPR55. The nM agonism of GPR18 by virodhamine, anandamide and Δ9-THC clearly validate this receptor for compliance with criterion 2. Support for GPR92 comes only from a single report indicating an EC50 of 1.78 μM for 2-AG signaling. Although this is an unlikely plasma concentration, paracrine and autocrine signaling could potentially reach the necessary threshold, providing tepid support for criterion 2. If inverse agonism is considered, the EC50 values for GPR3, GPR6, and GPR12 gives some support for fulfilling criterion 2, however, this does not appear to be the intent of criterion 2.
Criterion 3 is met by all of the GPCRs noted here. Each of these receptors show a reasonable homology to either or both CB1R and CB2R (Table 4). Further, GPR3, GPR6, and GPR12 are closely phylogenetically related to both CB1R and CB2R (Morales and Reggio 2017).
Table 4.
Percent sequence identity of putative cannabinoid receptors compared to CB1R and CB2R
| % Sequence identity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniprotKB | Q9Y2T6 | Q8TDV5 | Q14330 | Q9H1C0 | P46089 | P46095 | P47775 | P21554 | P34972 |
| GPR55 | GPR119 | GPR18 | GPR92 | GPR3 | GPR6 | GPR12 | CB1R | CB2R | |
| Similarity to CB1 | 28.6 | 23.5 | 23 | 0 | 28 | 26.6 | 27.3 | 100 | 47.7 |
| Similarity to CB2 | 28.6 | 22.5 | 0 | 22.1 | 29.3 | 33 | 31.8 | 47.7 | 100 |
Sequence similarity determined using BLAST searches
Criterion 4 is also met by all of these GPCRs if the term “well established” is the primary defining term. However, GPR55, GPR18 and GPR92 are the only receptors discussed here that are known to be activated by cannabinoids thus are the only ones considered to fulfill this criterion. The inverse agonism associated with GPR3, GPR6, and GPR12 likely disqualifies these receptors from consideration.
Criterion 5 is also met by all GPCRs discussed here. CB1R and CB2R are highly expressed in the brain followed by endocrine tissue, adipose tissue, female tissue, lymphoid tissue, and the GI tract (Uhlén et al. 2015). GPR55, GPR3, GPR6, GPR12, and GPR92 are all highly expressed in the brain and thus would be exposed to the same concentrations of cannabinoids experienced by CB1R and CB2R. The argument for GPR18 is not as strong, as its expression is primarily within the lymphoid system. Support for GPR119 is the weakest, as it is primarily expressed in the pancreas and GI tract and is not found in the brain.
Should heteromers be included in the discussion?
Since the early 2000s, data has been accumulating which indicates that GPCRs have the ability to form dimers and higher order oligomers when expressed in the same cell. Additionally, the accumulating data also indicate that such unions have physiological consequences including alteration of ligand binding, negative cross-talk, and cross-antagonism (Ferré et al. 2009; González et al. 2012). Further, both homomers and heteromers have been identified in both transfected cells and living tissue. It is well established that both CB1R and CB2R form both homodimers and heterodimers with each other as well as heterodimers with numerous other receptors (review Morales and Reggio 2017).
GPR55 has been shown to form heterodimers with both CB1R and CB2R. HEK293 cells transfected with both hCB1R and hGPR55 show that each influences the signaling properties of the other. In one study (Kargl et al. 2012) co-immunoprecipitation experiments show that the receptors do indeed from heteromers. They further show that the presence of unstimulated CB1R inhibits the signaling of GPR55, as measured by MAP kinase activity and NFAT activation, and that the signaling is restored when CB1R is activated with strong agonists (e.g., GSK319197A). In addition, the CB1R signaling is enhanced in the presence of stimulated GPR55 (Kargl et al. 2012). Both CB1R/GPR55 and CB2R/GPR55 heteromers have been identified in parkinsonian macaque models and their expression are found to be increased in the basal ganglia of MPTP treated animals and reduced when the animals are treated with levodopa (Martínez-Pinilla et al. 2020). CB2R/GPR55 heteromers have also been detected in neurons and astrocytes of the human dorsolateral prefrontal cortex (García-Gutiérrez et al. 2018).
HEK293 cells transfected both CB1R and GPR18 or CB2R and GPR18 show that only CB2R forms heterodimers with GPR18 (Reyes-Resina et al. 2018). Upon simultaneous stimulation of both receptors, the net result in signaling (cAMP accumulation, ERK1/2 phosphorylation and dynamic mass distribution assays) is that the signals become reduced rather than additive (i.e., negative cross-talk) and that activation of GPR18 reduces the signaling of activated CB2R.
Although only two of the orphan receptors have thus far been shown to form heterodimers with cannabinoid receptors, it is worth considering the ramifications of such systems. Clearly one has to view the heterodimer as a complex with a particular biological function one might liken to a multi-subunit complex where one of the receptors is acting as a regulatory subunit. In this light it is worthwhile to consider cannabinoid complexes as a different class of receptors and thus non-cannabinoid ligands as regulatory molecules.
Is there an alternative to the traditional cannabinoid signaling system?
It is becoming increasingly clear that non-traditional endocannabinoids (e.g., not AEA of 2-AG) that do not bind or stimulate CB1R or CB2R elicit the same biological affects as cannabinoids do against these receptors and thus act through another pathway. This has led a number of researchers to speculate that there exists a parallel system that serves as a back-up to or augmentation for CB1R and CB2R signaling (Mackie and Stella 2006; Selley et al. 2013; McHugh et al. 2010; Rajaraman et al. 2016). For example, NAGly, an agonist for GPR18 reduces both allodynia and hyperalgesia associated with Freund’s complete adjuvant-induced inflammation and allodynia resulting from nerve ligation and inflammation in rats, a response typically associated with CB1R and CB2R response to classical cannabinoids (Succar et al. 2007). Stimulation of CB1R by traditional cannabinoids is known to result in hypotension through vasodilation. However, the non-traditional cannabinoid Abd-CBD does not stimulate signaling in CB1R or CB2R receptors but does induce hypotension in rat mesenteric arteries (Járai et al. 1999). Similarly, intrathecal application of S1P reduces nociceptive behavior in rats subjected to a formalin assay (Compton et al. 1993). On the other hand, there are reports indicating the LPI and its primary receptor GPR55 are pro-nociceptive (Deliu et al. 2015) and others that indicate no involvement in nociception at all (Carey et al. 2017).
Should endocannabinoid metabolic products be part of the equation?
It is becoming increasingly clear that non-traditional endocannabinoids (e.g., not AEA of 2-AG) also stimulate cannabinoid receptors (Figs. 1, 2, 3, Table 2). One such class of ligand is represented by the metabolic products of traditional endocannabinoids (Figs. 1, 2, 3). These ligands can thus be considered to be a continuation of the original AEA or 2-AG signal, enhancing the signal if it exhibits either tighter binding or longer half-life, or diminish the signal if it exhibits weaker binding or a shorter half-life,
Fig. 2.
Anandamide metabolism. (±)-5,6-EET-EA, racemic 5,6-epoxyeicosatrienoyl ethanolamide; (±)-8,9-EET-EA, racemic 8,9-epoxyeicosatrienoyl ethanolamide; (±)-11,12-EET-EA, racemic 11,12-epoxyeicosatrienoyl ethanolamide; 12S-HETE-EA, 12S-hydroxyeicosatetraenoic acid ethanolamide; 12S-HPETE-EA, 12S-hydrpertoxyeicosatetraenoic acid ethanolamide; (±)-14,15-EET-EA, racemic 14,15-epoxyeicosatrienoyl ethanolamide; 15S-HETE-EA, 15-hydroxyeicosatetraenoic acid ethanolamide; 15S-HPETE-EA, 15S-hydrpertoxyeicosatetraenoic acid ethanolamide; 20-HETE-EA, 20-hydroxyeicosatetraenoic acid ethanolamide; AA, arachidonic acid; ADH7, alcohol dehydrogenase 7; AEA, anandamide; ALOX12, arachidonate (12S)-lipoxygenase; ALOX15, arachidonate (15S)-lipoxygenase; AKR1C3, aldo-keto reductase family 1 member C3; COX2, cyclooxygenase 2; CYP450s, cytochrome P450 enzymes; EA, ethanolamide; EXA4-EA, eoxin A4 ethanolamide; EXC4-EA, eoxin C4 ethanolamide; EXD4-EA, eoxin D4 ethanolamide; EXE4-EA, eoxin E4 ethanolamide; FAAH1,2, Fatty acid amide hydrolase isozymes 1 and 2; GPX, glutathione peroxidase; NAAA, N-acylethanolamine acid amide hydrolase; NAGly, N-arachidonyl glycine; PGD2-EA, prostaglandin D2 ethanolamide; PGF2α-EA, prostaglandin F2α ethanolamide; PGH2-EA, prostaglandin H2 ethanolamide; PTGDS, prostaglandin D synthase
Fig. 3.
2-Arachadonyl glycerol metabolism. 2-AG, 2-arachadonyl glycerol; 2-20:4-LPA, 2-arachidonyl lysophosphatidic acid; (±)-11,12-EET-G, racemic 11,12-epoxyeicosatrienoyl glycerol; 12S-HETE-G, 12S-hydroxyeicosatetraenoyl glycerol; 12S-HPETE-G, 12S-hydroperoxyeicosatetraenoyl glycerol; (±)-14,15-EET-G, racemic 14,15-epoxyeicosatrienoyl glycerol; 15S-HETE-G, 15S-hydroxyeicosatetraenoyl glycerol; 15S-HPETE-G, 15S-hydroperoxyeicosatetraenoyl glycerol; AA, arachidonic acid; ABHD2, monoacylglycerol lipase ABHD2; ALOX12, arachidonate (12S)-lipoxygenase; ALOX15, arachidonate (15S)-lipoxygenase; COX2, cyclooxygenase 2; CYP2J2, cytochrome P450 2J2; GPX, glutathione peroxidase; MAGK, monoacylglycerol kinase; MAGL, monoacylglycerol lipase; PGD2-G, prostaglandin D2 glycerol; PGF2α-G, prostaglandin F2α glycerol; PGH2-G, prostaglandin H2 glycerol; PGI2-G, prostaglandin I2 glycerol
Cytochrome P450 enzymes (CYP450) convert AEA into a variety of epoxide products and the alcohol 20-hydroxyeicosatetraenoic acid ethanolamide (20-HETE-EA). One of these, 5,6-epoxyeicosatrienoyl ethanolamide (5,6-EET-EA) is a potent agonist for CB2R with comparable binding efficiency to AEA as well as being more stable than AEA (Snider et al. 2009), the production of which thus increases the potency of the original anandamide signaling. In addition, 5,6-EET-EA is also an agonist for CB1R, albeit with > 1000-fold weaker binding. 20-HETE-EA and 14,15-epoxyeicosatrienoyl ethanolamide (14,15-EET-EA) also bind to CB1R with 3.6-fold and 5.7-fold lower efficiency that anandamide respectively and are more rapidly degraded in the brain than anandamide (Sridar et al. 2011) and hence diminish, but not eliminate the anandamide signal. Both 5,6-EET-EA and 14,15-EET-EA bind to GPR119 albeit with IC50 values in the micromolar range (Syed et al. 2012), considerably higher IC50 values than endocannabinoids for cannabinoid receptors. This may represent diminishment of the original signal; however, half-lives of these products must be considered, well as the efficacy and half-lives of their metabolic products.
Lipoxygenase enzymes convert AEA to enzyme specific hydroperoxy products that are readily converted to corresponding alcohols. 12(S)-Hydroxyeicosatetraenoyl ethanolamide (12(S)-HETE-EA) produced by the lipoxygenase ALOX12 or in low amounts by lipoxygenase ALOX15 binds to CB1R in rat brain membranes with lower efficiency (≈ 53%) than AEA and to human CB2R expressed in CHO cells also with lower efficiency than AEA (≈ 60%) (Edgemond et al. 1998). Another report indicates that 12(S)-HETE-EA binds to CB1R in rat brain membranes at 60% of the efficiency of AEA and to CB2R in rat spleen membranes 72% lower efficiency (van der Stelt et al. 2002). ALOX15 produces 15(S)-Hydroxyeicosatetraenoyl ethanolamide (15(S)-HETE-EA) from AEA that binds to rat brain CB1R with lower efficiency than AEA (14%) and does not bind to human CB2R expressed in CHO cells (Edgemond et al. 1998). A second report confirms these observations where 15(S)-HETE-EA was found to bind to CB1R in rat brain membranes but with a lower efficiency than AEA (15%) and does not bind to CB2R in rat spleen membranes (van der Stelt et al. 2002). Although the action of lipoxygenase reduces AEA signal intensity, the signal is still transmitted, and the overall effect requires knowledge of the respective metabolite’s half-life as well as the efficacy and half-lives of the metabolic products for these metabolites. Lastly, 15S-HPETE-EA produced from AEA by ALOX15 normally converted to the hydroxy product can also be converted to eoxin A4 ethanolamide (EXA4-EA) by ALOX15 itself (Feltenmark et al. 2008) which in turn can readily be converted to a series of eoxin ethanolamide products. Although eoxins are known to be potent signaling molecules in their own right, the efficacy of the ethanolamide derivative binding to CB1R or CB2R has not been reported.
Cyclooxygenase 2 (COX2) converts AEA to the unstable prostaglandin H2 ethanolamide (PGH2-EA) which is readily converted to a variety of prostanoid ethanolamides by the respective prostanoid enzymes (Biringer 2020). Although EC50 values have not been reported for ethanolamide prostamides, Berglund et al. (1999) examined the binding of methylethanolamide, ethylethanolamide, and isopropylethanolamide derivatives of PGE2 and PGF2α to both CB1R and CB2R. Here they find that both methylethanolamide and ethylethanolamide derivatives of prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) exhibit the tightest binding to both receptors with Ki values for the displacement of CP55940 range from 733 nM to 40 μM depending on the derivative and receptor.
2-AG is also metabolized to a variety of products and utilizes some of the same pathways as AEA metabolism (Baggelaar et al. 2018; Kozak et al. 2002). CYP450 enzymes, notably CYP2J2, convert AEA to both, 2-(11,12-epoxyeicosatrienoyl)glycerol (2-11,12-EG) and 2-(14,15-epoxyeicosatrienoyl)-glycerol (2-14,15-EG) which are both strong agonists for both CB1R and CB2R (Chen et al. 2008). 2-11,12-EG is the stronger binder with IC50 values that are 50–60% of those for 2-14,15-EG and both show a twofold preference for CB2R over CB1R. Lipoxygenases ALOX12 and ALOX15 in combination with GPX convert 2-AG to the corresponding Hydroxyeicosatetraenoyl glycerol (HETE-G) products. COX2 converts 2-AG to prostaglandin H2 glycerol (PGH2-G) which is then readily converted to other prostaglandin glycerols (i.e., PGE2-G, PGF2α-G, PGI2-G, and PGD2-G) by prostanoid enzymes (Kozak et al. 2002). Binding activity for any of these oxidized derivatives to either CB1R or CB2R has not been reported.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The author declares that he has no conflict of interest or competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, Ross RA. Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J Biol Chem. 2012;287(1):91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggelaar MP, Maccarrone M, van der Stelt M. 2-Arachidonoylglycerol: a signaling lipid with manifold actions in the brain. Prog Lipid Res. 2018;71:1–17. doi: 10.1016/j.plipres.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Balenga NA, Aflaki E, Kargl J, Platzer W, Schröder R, Blättermann S, Kostenis E, Brown AJ, Heinemann A, Waldhoer M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011;21(10):1452–1469. doi: 10.1038/cr.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AM, Callahan DJ, Richner JM, Choi J, DiPersio JF, Diamond MS, Bhattacharya D. GPR18 controls reconstitution of mouse small intestine intraepithelial lymphocytes following bone marrow transplantation. PLoS ONE. 2015;10(7):e0133854. doi: 10.1371/journal.pone.0133854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard A, Tremblay P, Chernomoretz A, Vallières L. Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia. 2007;55(8):777–789. doi: 10.1002/glia.20477. [DOI] [PubMed] [Google Scholar]
- Benoit ME, Hernandez MX, Dinh ML, Benavente F, Vasquez O, Tenner AJ. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-β neurotoxicity. J Biol Chem. 2013;288(1):654–665. doi: 10.1074/jbc.M112.400168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund BA, Boring DL, Berglund HAC, BA, Boring DL, Howlett AC, Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv Exp Med Biol. 1999;469:527–533. doi: 10.1007/978-1-4615-4793-8_77. [DOI] [PubMed] [Google Scholar]
- Biringer RG. The enzymology of the human prostanoid pathway. Mol Biol Rep. 2020;47(6):4569–4586. doi: 10.1007/s11033-020-05526-z. [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4(6):1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Böttcher G, Sjölund K, Ekblad E, Håkanson R, Schwartz TW, Sundler F. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul Pept. 1984;8(4):261–266. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Daniels DA, Kassim M, Brown S, Haslam CP, Terrell VR, Brown J, Nichols PL, Staton PC, Wise A, Dowell SJ. Pharmacology of GPR55 in yeast and identification of GSK494581A as a mixed-activity glycine transporter subtype 1 inhibitor and GPR55 agonist. J Pharmacol Exp Ther. 2011;337(1):236–246. doi: 10.1124/jpet.110.172650. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Laun AS, Song ZH. Cannabidiol, a novel inverse agonist for GPR12. Biochem Biophys Res Commun. 2017;493(1):451–454. doi: 10.1016/j.bbrc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SH, McQuain CA, Ross AH, Salmonsen RA, Zurier RE. Resolution of inflammation by N-arachidonoylglycine. J Cell Biochem. 2011;112(11):3227–3233. doi: 10.1002/jcb.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Leo S, Vermaercke B, Meert T, D'Hooge R. LPA5 receptor plays a role in pain sensitivity, emotional exploration and reversal learning. Genes Brain Behav. 2012;11(8):1009–1019. doi: 10.1111/j.1601-183X.2012.00840.x. [DOI] [PubMed] [Google Scholar]
- Capaldi S, Suku E, Antolini M, Di Giacobbe M, Giorgetti A, Buffelli M. Allosteric sodium binding cavity in GPR3: a novel player in modulation of Aβ production. Sci Rep. 2018;8(1):11102. doi: 10.1038/s41598-018-29475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Gutierrez T, Deng L, Lee WH, Mackie K, Hohmann AG. Inflammatory and neuropathic nociception is preserved in GPR55 knockout mice. Sci Rep. 2017;7(1):944. doi: 10.1038/s41598-017-01062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Chen JK, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, Falck JR, Capdevila JH, Harris RC. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283(36):24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212(8):1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. Novel resolvin D2 receptor axis in infectious inflammation. J Immunol. 2017;198(2):842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148(6):2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149(5):2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, Lucman A, Xing C, Sebring K, Zhou J, Wagner B, Unett D, Jones RM, Behan DP, Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol. 2010;24(1):161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuderland D, Seger R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun Integr Biol. 2008;1(1):4–5. doi: 10.4161/cib.1.1.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265(1):218–226. [PubMed] [Google Scholar]
- Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol. 2014;171(16):3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HM, Tough IR, Woolston AM, Zhang L, Nguyen AD, Sainsbury A, Herzog H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11(6):532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR, Pin JP, Spedding M, Harmar AJ. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65(3):967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E, Sperow M, Console-Bram L, Carter RL, Tilley DG, Kalamarides DJ, Kirby LG, Brailoiu GC, Brailoiu E, Benamar K, Abood ME. The lysophosphatidylinositol receptor GPR55 modulates pain perception in the periaqueductal gray. Mol Pharmacol. 2015;88(2):265–272. doi: 10.1124/mol.115.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod. 2008;78(4):667–672. doi: 10.1095/biolreprod.107.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): their affinities for cannabinoid receptors and pathways of inactivation. Mol Pharmacol. 1998;54(1):180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48(3):443–450. [PubMed] [Google Scholar]
- Feltenmark S, Gautam N, Brunnström A, Griffiths W, Backman L, Edenius C, Lindbom L, Björkholm M, Claesson HE. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci USA. 2008;105(2):680–685. doi: 10.1073/pnas.0710127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5(3):131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DB, Joseph WR, Grimsey NL. Glass M (2016) GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. PeerJ. 2016;4:e1835. doi: 10.7717/peerj.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Rempel V, Müller CE. The natural product magnolol as a lead structure for the development of potent cannabinoid receptor agonists. PLoS ONE. 2013;8(10):e77739. doi: 10.1371/journal.pone.0077739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Navarrete F, Navarro G, Reyes-Resina I, Franco R, Lanciego JL, Giner S, Manzanares J. Alterations in gene and protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics. 2018;15(3):796–806. doi: 10.1007/s13311-018-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Moreno-Delgado D, Moreno E, Pérez-Capote K, Franco R, Mallol J, Cortés A, Casadó V, Lluís C, Ortiz J, Ferré S, Canela E, McCormick PJ. Circadian-related heteromerization of adrenergic and dopamine D4 receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012;10(6):e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill M, Högenauer C, Blesl A, Haybaeck J, Golob-Schwarzl N, Ferreirós N, Thomas D, Gurke R, Trötzmüller M, Köfeler HC, Gallé B, Schicho R. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci Rep. 2019;9(1):2358. doi: 10.1038/s41598-019-38865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96(9):E1409–1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- Hassing HA, Fares S, Larsen O, Pad H, Hauge M, Jones RM, Schwartz TW, Hansen HS, Rosenkilde MM. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem Pharmacol. 2016;119:66–75. doi: 10.1016/j.bcp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23(1):183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Schröder R, Kargl JK, Platzer W, Martini L, Arthur S, Penman J, Whistler JL, Kostenis E, Waldhoer M, Irving AJ. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol. 2010;160(3):604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287(2):249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hu J, Oda SK, Shotts K, Donovan EE, Strauch P, Pujanauski LM, Victorino F, Al-Shami A, Fujiwara Y, Tigyi G, Oravecz T, Pelanda R, Torres RM. Lysophosphatidic acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. J Immunol. 2014;193(1):85–95. doi: 10.4049/jimmunol.1300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Skwarek-Maruszewska A, Horré K, Vandewyer E, Wolfs L, Snellinx A, Saito T, Radaelli E, Corthout N, Colombelli J, Lo AC, Van Aerschot L, Callaerts-Vegh Z, Trabzuni D, Bossers K, Verhaagen J, Ryten M, Munck S, D’Hooge R, Swaab DF, Hardy J, Saido TC, De Strooper B, Thathiah A. Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer's disease mouse models. Sci Transl Med. 2015;7(309):309164. doi: 10.1126/scitranslmed.aab3492. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Lintzel J, Hermans-Borgmeyer I, Kreienkamp HJ, Joost P, Thomsen S, Methner A, Schaller HC. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J Neurosci. 2003;23(3):907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov A, Lintzel J, Kreienkamp HJ, Schaller HC. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem Biophys Res Commun. 2003;311(2):329–336. doi: 10.1016/j.bbrc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Isawi IH, Morales P, Sotudeh N, Hurst DP, Lynch DL, Reggio PH. GPR6 structural insights: homology model construction and docking studies. Molecules. 2020;25(3):725. doi: 10.3390/molecules25030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96(24):14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre J, Yeh CC, Wong E, Thete M, Xu F, Zlatanova I, Lloyd E, Kobzik L, Legrand M, Hellman J. Activation of CB1R promotes lipopolysaccharide-induced IL-10 secretion by monocytic myeloid-derived suppressive cells and reduces acute inflammation and organ injury. J Immunol. 2020;204(12):3339–3350. doi: 10.4049/jimmunol.2000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152(5):825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem. 2009;284(43):29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M. The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J Biol Chem. 2012;287(53):44234–44248. doi: 10.1074/jbc.M112.364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014;39(5):233–244. doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347(3):827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318(2):619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277(47):44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44(D1):D862–868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. 2008;105(7):2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer L, Iakoubov R, Brubaker PL. GPR119: "double-dipping" for better glycemic control. Endocrinology. 2008;149(5):2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58(5):1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun AS (2018) A study of GPR3, GPR6, of GPR12 as novel molecular targets for cannabidiol. Electronic Theses and Dissertations. Paper 2945. 10.18297/etd/2945
- Laun AS, Song ZH. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun. 2017;490(1):17–21. doi: 10.1016/j.bbrc.2017.05.165. [DOI] [PubMed] [Google Scholar]
- Laun AS, Shrader SH, Song ZH. Novel inverse agonists for the orphan G protein-coupled receptor 6. Heliyon. 2018;4(11):e00933. doi: 10.1016/j.heliyon.2018.e00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun AS, Shrader SH, Brown KJ, Song ZH. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40(3):300–308. doi: 10.1038/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281(33):23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Lin ZJ, Lu XM, Zhu TJ, Fang YC, Gu QQ, Zhu W. GPR12 selections of the metabolites from an endophytic Streptomyces sp. associated with Cistanches deserticola. Arch Pharm Res. 2008;31(9):1108–1114. doi: 10.1007/s12272-001-1276-4. [DOI] [PubMed] [Google Scholar]
- Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem. 2012;287(21):17608–17617. doi: 10.1074/jbc.m111.330183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopategi A, Flores-Costa R, Rius B, López-Vicario C, Alcaraz-Quiles J, Titos E, Clària J. Frontline science: specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol. 2019;105(1):25–36. doi: 10.1002/JLB.3HI0517-206RR. [DOI] [PubMed] [Google Scholar]
- Lowther KM, Uliasz TF, Götz KR, Nikolaev VO, Mehlmann LM. Regulation of constitutive GPR3 signaling and surface localization by GRK2 and β-arrestin-2 overexpression in HEK293 cells. PLoS ONE. 2013;8(6):e65365. doi: 10.1371/journal.pone.0065365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zhang N, Meng B, Dong S, Hu Y. Involvement of GPR12 in the regulation of cell proliferation and survival. Mol Cell Biochem. 2012;366(1–2):101–110. doi: 10.1007/s11010-012-1287-x. [DOI] [PubMed] [Google Scholar]
- Lu VB, Puhl HL, 3rd, Ikeda SR. N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol Pharmacol. 2013;83(1):267–282. doi: 10.1124/mol.112.081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundequist A, Boyce JA. LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1β release. PLoS ONE. 2011;6(3):e18192. doi: 10.1371/journal.pone.0018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8(2):E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124(4):619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini M, Iaccino E, Mosca MG, Mimmi S, D’Angelo R, Quinto I, Scala G, Mariggiò S. Peptide-guided targeting of GPR55 for anti-cancer therapy. Oncotarget. 2017;8(3):5179–5195. doi: 10.18632/oncotarget.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Reyes-Resina I, Oñatibia-Astibia A, Zamarbide M, Ricobaraza A, Navarro G, Moreno E, Dopeso-Reyes IG, Sierra S, Rico AJ, Roda E, Lanciego JL, Franco R. CB1 and GPR55 receptors are co-expressed and form heteromers in rat and monkey striatum. Exp Neurol. 2014;261:44–52. doi: 10.1016/j.expneurol.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Rico AJ, Rivas-Santisteban R, Lillo J, Roda E, Navarro G, Lanciego JL, Franco R. Expression of GPR55 and either cannabinoid CB1 or CB2 heteroreceptor complexes in the caudate, putamen, and accumbens nuclei of control, parkinsonian, and dyskinetic non-human primates. Brain Struct Funct. 2020;225(7):2153–2164. doi: 10.1007/s00429-020-02116-4. [DOI] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Page J, Dunn E, Bradshaw HB. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol. 2012;165(8):2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Roskowski D, Xie S, Bradshaw HB. Δ(9)-THC and N-arachidonoyl glycine regulate BV-2 microglial morphology and cytokine release plasticity: implications for signaling at GPR18. Front Pharmacol. 2014;4:162. doi: 10.3389/fphar.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu DM, Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rümenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414(2–3):145–154. doi: 10.1016/s0014-2999(01)00789-0. [DOI] [PubMed] [Google Scholar]
- Miller S, Hu SS, Leishman E, Morgan D, Wager-Miller J, Mackie K, Bradshaw HB, Straiker A. A GPR119 signaling system in the murine eye regulates intraocular pressure in a sex-dependent manner. Investig Ophthalmol vis Sci. 2017;58(7):2930–2938. doi: 10.1167/iovs.16-21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Daily L, Leishman E, Bradshaw H, Straiker A. Δ9-tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Investig Ophthalmol vis Sci. 2018;59(15):5904–5911. doi: 10.1167/iovs.18-24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, Reggio PH. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2(1):265–273. doi: 10.1089/can.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, Hurst DP, Reggio PH. Methods for the development of in silico GPCR models. Methods Enzymol. 2017;593:405–448. doi: 10.1016/bs.mie.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Andradas C, Medrano M, Caffarel MM, Pérez-Gómez E, Blasco-Benito S, Gómez-Cañas M, Pazos MR, Irving AJ, Lluís C, Canela EI, Fernández-Ruiz J, Guzmán M, McCormick PJ, Sánchez C. Targeting CB2-GPR55 receptor heteromers modulates cancer cell signaling. J Biol Chem. 2014;289(32):21960–21972. doi: 10.1074/jbc.M114.561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda SK, Strauch P, Fujiwara Y, Al-Shami A, Oravecz T, Tigyi G, Pelanda R, Torres RM. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol Res. 2013;1(4):245–255. doi: 10.1158/2326-6066.CIR-13-0043-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odori S, Hosoda K, Tomita T, Fujikura J, Kusakabe T, Kawaguchi Y, Doi R, Takaori K, Ebihara K, Sakai Y, Uemoto S, Nakao K. GPR119 expression in normal human tissues and islet cell tumors: evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism. 2013;62(1):70–78. doi: 10.1016/j.metabol.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O'Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283(30):21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362(4):928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J Biochem. 2009;145(1):13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S, Myers AG, Prasad BM. Constitutively active GPR6 is located in the intracellular compartments. FEBS Lett. 2009;583(1):107–112. doi: 10.1016/j.febslet.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, Selley DE. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol Pharmacol. 2006;70(1):41–50. doi: 10.1124/mol.105.020552. [DOI] [PubMed] [Google Scholar]
- Penumarti A, Abdel-Rahman AA. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther. 2014;349(1):29–38. doi: 10.1124/jpet.113.209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumarti A, Abdel-Rahman AA. Neuronal nitric oxide synthase-dependent elevation in adiponectin in the rostral ventrolateral medulla underlies g protein-coupled receptor 18-mediated hypotension in conscious rats. J Pharmacol Exp Ther. 2014;351(1):44–53. doi: 10.1124/jpet.114.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastira I, Bernhart E, Goeritzer M, Reicher H, Kumble VB, Kogelnik N, Wintersperger A, Hammer A, Schlager S, Jandl K, Heinemann A, Kratky D, Malle E, Sattler W. 1-Oleyl-lysophosphatidic acid (LPA) promotes polarization of BV-2 and primary murine microglia towards an M1-like phenotype. J Neuroinflamm. 2016;13(1):205. doi: 10.1186/s12974-016-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman G, Simcocks A, Hryciw DH, Hutchinson DS, McAinch AJ. G protein coupled receptor 18: a potential role for endocannabinoid signaling in metabolic dysfunction. Mol Nutr Food Res. 2016;60(1):92–102. doi: 10.1002/mnfr.201500449. [DOI] [PubMed] [Google Scholar]
- Rapino C, Castellucci A, Lizzi AR, Sabatucci A, Angelucci CB, Tortolani D, Rossi G, D'Andrea G, Maccarrone M. Modulation of endocannabinoid-binding receptors in human neuroblastoma cells by tunicamycin. Molecules. 2019;24(7):1432. doi: 10.3390/molecules24071432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Serhan CN. Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front Immunol. 2012;3:298. doi: 10.3389/fimmu.2012.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Resina I, Navarro G, Aguinaga D, Canela EI, Schoeder CT, Załuski M, Kieć-Kononowicz K, Saura CA, Müller CE, Franco R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors Relevance in neurodegenerative diseases. Biochem Pharmacol. 2018;157:169–179. doi: 10.1016/j.bcp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology. 2011;61(1–2):43–50. doi: 10.1016/j.neuropharm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Ruz-Maldonado I, Liu B, Atanes P, Pingitore A, Huang GC, Choudhary P, Persaud SJ. The cannabinoid ligands SR141716A and AM251 enhance human and mouse islet function via GPR55-independent signalling. Cell Mol Life Sci. 2020;77(22):4709–4723. doi: 10.1007/s00018-019-03433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba SW, Jauch H, Gargouri B, Keil A, Hurrle T, Volz N, Mohr F, van der Stelt M, Bräse S, Fiebich BL. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J Neuroinflamm. 2018;15:322. doi: 10.1186/s12974-018-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Welch SP, Sim-Selley LJ. Sphingosine lysolipids in the CNS: endogenous cannabinoid antagonists or a parallel pain modulatory system? Life Sci. 2013;93(5–6):187–193. doi: 10.1016/j.lfs.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir H, Console-Bram L, Mundy C, Popoff SN, Kapur A, Abood ME. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J Neuroimmune Pharmacol. 2012;7(4):856–865. doi: 10.1007/s11481-012-9351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40(5):659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- Snider NT, Nast JA, Tesmer LA, Hollenberg PF. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol. 2009;75(4):965–972. doi: 10.1124/mol.108.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Soliman E, Van Dross R. Cannabinoids modulate neuronal activity and cancer by CB1 and CB2 receptor-independent mechanisms. Front Pharmacol. 2017;8:720. doi: 10.3389/fphar.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, Matsumoto S, Kamohara M, Hiyama H, Yoshida S, Momose K, Ueda Y, Matsushime H, Kobori M, Furuichi K. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326(4):744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, Bassoni DL, Raab WJ, Quinn E, Wehrman TS, Davenport AP, Brown AJ, Green A, Wigglesworth MJ, Rees S. Screening β-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen. 2013;18(5):599–609. doi: 10.1177/1087057113475480. [DOI] [PubMed] [Google Scholar]
- Sridar C, Snider NT, Hollenberg PF. Anandamide oxidation by wild-type and polymorphically expressed CYP2B6 and CYP2D6. Drug Metab Dispos. 2011;39(5):782–788. doi: 10.1124/dmd.110.036707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinform. 2016;54(1):1.30.1–1.30.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Succar R, Mitchell VA, Vaughan CW. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol Pain. 2007;3:24. doi: 10.1186/1744-8069-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida H, Cyster JG. G-protein coupled receptor 18 contributes to establishment of the CD8 effector T cell compartment. Front Immunol. 2018;9:660. doi: 10.3389/fimmu.2018.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am J Physiol Endocrinol Metab. 2012;303(12):E1469–E1478. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- Takenouchi R, Inoue K, Kambe Y, Miyata A. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem Biophys Res Commun. 2012;418(2):366–371. doi: 10.1016/j.bbrc.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ishii K, Kasai K, Yoon SO, Saeki Y. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J Biol Chem. 2007;282(14):10506–10515. doi: 10.1074/jbc.M700911200. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Miyagi T, Dohi E, Seki T, Hide I, Sotomaru Y, Saeki Y, Antonio Chiocca E, Matsumoto M, Sakai N. Developmental expression of GPR3 in rodent cerebellar granule neurons is associated with cell survival and protects neurons from various apoptotic stimuli. Neurobiol Dis. 2014;68:215–227. doi: 10.1016/j.nbd.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Tengholm A. Cyclic AMP dynamics in the pancreatic β-cell. Ups J Med Sci. 2012;117(4):355–369. doi: 10.3109/03009734.2012.724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A, Spittaels K, Hoffmann M, Staes M, Cohen A, Horré K, Vanbrabant M, Coun F, Baekelandt V, Delacourte A, Fischer DF, Pollet D, De Strooper B, Merchiers P. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science. 2009;323(5916):946–951. doi: 10.1126/science.1160649. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Horre K, Snellinx A, Vandewyer E, Huang Y, Ciesielska M, De Kloe G, Munck S, De Strooper B. β-arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer’s disease. Mol Neurodegener. 2013;8(Suppl 1):P41. doi: 10.1186/1750-1326-8-S1-P41. [DOI] [PubMed] [Google Scholar]
- Tudurí E, Imbernon M, Hernández-Bautista R, Tojo M, Fernø J, Diéguez C, Nogueiras R. GPR55: a new promising target for metabolism? J Mol Endocrinol. 2017;58(3):R191–R202. doi: 10.1530/JME-16-0253. [DOI] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Uhlenbrock K, Gassenhuber H, Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal. 2002;14(11):941–953. doi: 10.1016/s0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Horner K, Mehlmann LM, Conti M. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol. 2008;316(1):124–134. doi: 10.1016/j.ydbio.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Célérier E, Baranyi M, Vanderhaeghen P, Maldonado R, Sperlagh B, Vassart G, Ledent C. GPR3 receptor, a novel actor in the emotional-like responses. PLoS ONE. 2009;4(3):e4704. doi: 10.1371/journal.pone.0004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, van Kuik JA, Bari M, van Zadelhoff G, Leeflang BR, Veldink GA, Finazzi-Agrò A, Vliegenthart JF, Maccarrone M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J Med Chem. 2002;45(17):3709–3720. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science. [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121(Pt 10):1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sumida H, Cyster JG. GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J Exp Med. 2014;211(12):2351–2359. doi: 10.1084/jem.20140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284(25):17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Liu Z, Cao J, Ma Q, Gao X, Wang Q, Jin C, Zhou Y, Wen L, Ren J. GPS 2.1: enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein Eng Des Sel. 2011;24:255–260. doi: 10.1093/protein/gzq094. [DOI] [PubMed] [Google Scholar]
- Ye C, Zhang Z, Wang Z, Hua Q, Zhang R, Xie X. Identification of a novel small-molecule agonist for human G protein-coupled receptor 3. J Pharmacol Exp Ther. 2014;349(3):437–443. doi: 10.1124/jpet.114.213082. [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284(18):12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BL, Li Y, Ding JH, Dong FL, Hou YJ, Jiang BC, Shi FX, Xu YX. Sphingosine 1-phosphate acts as an activator for the porcine Gpr3 of constitutively active G protein-coupled receptors. J Zhejiang Univ Sci B. 2012;13(7):555–566. doi: 10.1631/jzus.B1100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Qiu C, Yang L, Zhang Z, Zhang Q, Wang B, Wang X. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019;217:49–56. doi: 10.1016/j.lfs.2018.11.061. [DOI] [PubMed] [Google Scholar]
- Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.