Abstract
Background
Prevalent symptoms that affect children and adolescents throughout the process of cancer diagnosis and treatment include nausea and vomiting, fatigue, pain, mucositis, and anxiety.
Aim
To examine the effect of a home‐based multimodal symptom‐management program for alleviation of nausea and vomiting, fatigue, pain, mucositis, and anxiety in children and adolescents undergoing chemotherapy for hematological malignancies or solid tumors.
Methods
In an exploratory pilot randomized study with qualitative interview, patients between 10 and 18 years of age were randomly assigned to either the symptom‐management program plus usual care (intervention group) or usual care (control group). The program consisted of multiple nonpharmacological interventional components. The targeted symptoms were measured at baseline (after diagnosis), at the first 2 weeks of each cycle of chemotherapy, and at 6 months after baseline, using the Memorial Symptom Assessment Scale 10‐18 and the State Anxiety Scale for Children.
Results
Fifty children (31 boys; mean age, 13.7 years) were randomized either to the intervention group or the control group (25 each) and underwent baseline assessment. A comparison between the groups showed that the intervention group had a significant less fatigue over time (P < .05). However, no differences were found with respect to nausea and vomiting, pain, mucositis, and anxiety between groups. Both children and parents reported a positive experience with the symptom‐management program.
Conclusion
The home‐based symptom‐management program may have helped to reduce fatigue in children and adolescents undergoing chemotherapy. In addition, qualitative data support the importance of improving children and parents' knowledge, coping skills, and psychological preparation for symptoms associated with chemotherapy.
Keywords: adolescents, anxiety, cancer, chemotherapy, fatigue, home‐based, mucositis, nausea, pain, pediatric patients, symptom cluster, symptom management, symptoms, vomiting
1. INTRODUCTION
The success achieved in recent decades in improving the survival rates of children and adolescents with cancer has been largely attributed to combined treatment approaches, the intensification of cytotoxic chemotherapy, and enhanced supportive care. 1 However, cancer‐ and treatment‐related toxicity remains a significant clinical problem in pediatric oncology. Clinically, symptoms experienced by children undergoing cancer treatment rarely occur in isolation, 2 multiple symptoms can co‐occur and be related to each other, referred to as symptom clusters. 3 , 4 Prevalent symptoms in children and adolescents throughout the process of diagnosis and cancer treatment include nausea and vomiting, fatigue, pain, mucositis, and anxiety. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Research evidence also suggests that these symptoms are inter‐correlated and can negatively influence patient outcomes. 13 , 14 , 15 , 16 , 17 Fatigue has been reported to be associated with psychological symptoms 13 and pain 14 in children with cancer. A higher level of anxiety was associated with a higher probability of mucositis, 15 while mucositis was associated with pain. 16 Previous study indicates that children with high symptom severity, including anxiety, fatigue, and pain were associated with poor functional outcomes. 17 These multiple symptoms throughout their process of cancer diagnosis and treatment continue to be a challenge in supportive care for pediatric oncology. Nevertheless, the concepts of symptom clusters and self‐care 3 , 18 , 19 offer insights into the development and application of multimodal interventions/ multiple intervention options to target these multiple symptoms simultaneously.
Nonpharmacological interventions for nausea and vomiting, fatigue, pain, and anxiety have been widely tested in cancer settings and have been found to be a promising option for patient care. For example, an increasing body of evidence suggests that the use of progressive muscle relaxation techniques can effectively reduce nausea and vomiting, pain, and anxiety in cancer settings. 7 , 20 Several studies have also reported a positive effect on fatigue after interventions with distraction and energy conservation strategies. 20 , 21 The literature also reports that systematic oral self‐care aids in mucositis prevention. 22 The current version of the MASCC/ISOO mucositis guidelines suggests that oral care protocols should be used to prevent mucositis in all age groups and across all cancer treatments. 23
However, studies of multimodal nonpharmacological symptom management approaches to address multiple symptoms in pediatric cancer populations remain limited. This pilot study evaluated multiple intervention components incorporated into a home‐based symptom‐management program to target nausea and vomiting, fatigue, pain, mucositis, and anxiety in children and adolescents who undergo chemotherapy. Intervention effects on the reported levels of the targeted symptoms were evaluated, and patients and parents' experiences and views on the symptom‐management program were explored. It was hypothesized that children and adolescents receiving home‐based symptom‐management program would have a greater decrease in symptom scores across each cycle of chemotherapy (a maximum of four cycles) and at 6 months after baseline assessment compared with control group participants.
2. METHODS
This prospective exploratory pilot randomized study with qualitative interview was conducted between December 2011 and August 2014 at a Children's Cancer Centre in Singapore after approval from the Institutional Review Board (DSRB Ref: 2011/01812). The eligible participants were children and adolescents between the ages of 10 to 18 years with a new diagnosis of a hematological malignancy or solid tumor and had planned chemotherapy. This age group was considered most likely to benefit from the symptom‐management program being evaluated in the study because of their conceptual abilities to understand the intervention components and predictable level of cooperation, alongside the consideration of study instrument of Memorial Symptom Assessment Scale (MSAS) 10‐18, which is designed for administration to children aged 10 to 18 years. 24 The patients and their parents were informed of the aims of the study and were included only after the patients gave their assent to participate and their parents provided written informed consent. After enrollment, the patients were stratified on the basis of hematologic malignancy and solid tumor and were randomly assigned by a computer‐generated system to participate in a home‐based multimodal symptom‐management program plus usual care (intervention group) or in usual care only (control group). Usual care included treatment with ancillary medications such as anti‐emetics and analgesics as prescribed by the medical doctors at the Cancer Center. Randomization was balanced for every four participants. We planned to enroll 30 participants in each arm, based on the clinical team's recommendation that approximately 60 patients could be targeted at the study site over a 2‐year period.
2.1. The symptom‐management program
The home‐based multimodal symptom‐management program was underpinned by the Social Cognitive Theory (self‐efficacy and empowering people to make positive behavior change) 25 and the Symptom Cluster Concept (most‐common symptom approach for cluster identification and interventions for multiple symptoms in cluster to improve patients' symptom experience) 3 , 4 and was based on recommendations from experts and evidence from the literature. The program was designed for children and adolescents ages 10 to 18. It was psychoeducational oriented, and aimed primarily to provide children and adolescents and their parents with the knowledge, skills, and support necessary for symptom prevention and management. The composition of the program included non‐pharmacological interventions of progressive muscle relaxation, distraction strategies, guided imagery, energy conservation, advice on meal preparation, oral care, and warm and cold packs. The program would work by taking advantage of synergistic effects of multi‐modal non‐pharmacological interventions on the targeted symptoms.
The key feature of the symptom‐management program was its inclusion of a home or clinical visit and regular phone contact. The home or clinical visit (1‐1.5 hours) took place before or during the first 2 weeks of the first chemotherapy cycle. During the visit, a trained research assistant (RA) discussed the chemotherapy and its adverse effects and symptoms, as well as coached the patients in the presence of their parent(s) for each intervention component to mastery. The coaching was aligned with the psychoeducation focus of active involvement of the RA with the patient and parents during the process. The progressive muscle relaxation involved a two‐step process of Tense and Relax through a Tense and Relax script. Distraction strategies focused on things that helped take children and adolescents' mind off their symptoms. These included playing, performing games, listening music, playing musical instruments, and/or reading. Guided imagery employed children and adolescents' own visual, auditory and/or movement imaginations, such as favorite place, TV show, song, swimming, and jogging. Energy conservation included a deliberatively planned management of children and adolescents' personal energy resources to prevent their depletion, with strategies such as set priorities, and schedule activities when children and adolescents had the most energy. Advice on meal preparation focused on things that enhanced children and adolescents' hydration and nutritional intake. These included screening of noxious stimuli and discuss ways to avoid them, assessing children and adolescents' food preference, and discussing principle in meal preparation particularly during nausea/vomiting and mucositis. Oral care involved a systematic hygienic care through tooth brushing with fluoride toothpaste and mouth rinsing with normal saline mouthwash. Warm and cold packs were used back and forth for pain relief when appropriate. In addition, patients were given a protocol‐based booklet outlining the details of each intervention component to enhance their home practice. For this study, the participants were instructed to perform each intervention component at least once a week during each cycle of chemotherapy. The participants had weekly phone contact with a RA in each cycle of chemotherapy for intervention reinforcement. The participants were asked to keep a logbook to record their self‐practice of intervention components, with assistance from their parents, during the study period.
2.2. Outcomes and assessments
The primary outcomes were the differences in the reported occurrences and levels of targeted symptoms from baseline throughout each cycle of chemotherapy to 6 months between two groups. These symptoms were measured at baseline (ie, after diagnosis and before the first cycle of chemotherapy), at the first 2 weeks in each cycle of chemotherapy (a maximum of four assessments), and at 6 months after baseline, using the relevant items from the MSAS 10‐18. 24 The MSAS 10‐18 comprises a 30‐item 4‐ or 5‐point Likert scale measuring the dimensions of frequency, severity, and distress of 30 symptoms that typically occur in cancer settings. Specifically, if a symptom is experienced, a composite of the average scores of its severity, frequency, and distress dimensions is computed to produce a symptom score between 0 and 4. 24 The MSAS 10‐18 has been validated as having good psychometric properties for children with cancer who are as young as 10 years old. 24 The MSAS 10‐18 was used rather than symptom‐specific scales to assess nausea and vomiting, fatigue, pain, and mucositis to minimize the burden on the participants.
The short‐form State Anxiety Scale for Children (CSAS) was used to measure anxiety. The CSAS is a 10‐item three‐point patient‐rated Likert scale measure and provides a valid measure of the anxiety state in children older than 6 years, with total possible scores ranging from 10 to 30 (higher scores indicate greater anxiety). 26 A standardized profile for sociodemographic data was collected by a questionnaire survey, and clinical data were gathered from the participants' medical records.
2.3. Data analysis
Data were analyzed using SPSS v26. Categorical and continuous data were presented as frequencies as a percentage and as a mean ± SD, respectively. Differences in demographic and clinical data between the groups were compared with the chi‐square test and Student's t‐test. The study outcomes were analyzed according to the intention‐to‐treat principle. Comparisons of symptom scores between groups throughout each cycle of chemotherapy were performed using repeated‐measures analysis of variance, with baseline measure values controlled for as covariates. A P value of less than 5% was considered to indicate statistical significance.
2.4. Qualitative semi‐structured interview and analysis
This study included a qualitative semi‐structured interview at 6 months after baseline to allow a deep understanding of the patients and parents' experiences with the symptom‐management program. Semi‐structured dyadic interviews were conducted by the RA either at the hospital or at the participants' home. The RA encouraged the children and parents to describe their perceptions and experiences with the symptom‐management program. The interviews were recorded on a voice recorder and transcribed verbatim by the same RA. Content analysis was conducted by the first author to analyze the qualitative data. Statements or phrases were extracted from each interview, and codes were assigned during the initial analyses of these significant statements. From the codes, categories and themes were developed. All analyses were conducted before the analysis of the quantitative data yielded from the randomized study.
3. RESULTS
Of 59 eligible pediatric and adolescent patients, 9 refused to participate because of lack of interest, 50 (84.7%) were enrolled and randomized to either the intervention group or the control group (25 each) and all completed the baseline assessment (100%) (Figure 1). As shown in Figure 1, an average of 86% in the intervention group while 91% in the control group completed the required subsequent assessments. Of note, four and six patients in the intervention group were not required to complete the cycles 3 and 4 assessments, respectively, because of no scheduled chemotherapy. Two and four patients in the control group did not have scheduled chemotherapy at cycles 3 and 4, respectively, and thus were not required to complete those assessments. For the patients who did not complete the required assessments, the reasons were due to overlooking and feeling overwhelmed with the assessments. The participants were primarily boys and their medical diagnoses were predominately leukemia. All demographic and clinical characteristics were comparable between the two groups (P > .05) (Table 1). No variabilities of supportive‐care practices between groups were observed during the study period. Per intervention logbook, an average of 83% adherence (ie, performed each intervention component at least once a week during each cycle of chemotherapy) to progressive muscle relaxation, 80% guided imagery, 88% distraction strategies and techniques, 90% advice on meal preparation, 86% energy conservation strategies, 80% warm and cold packs, and 90% oral care protocol was recorded.
FIGURE 1.
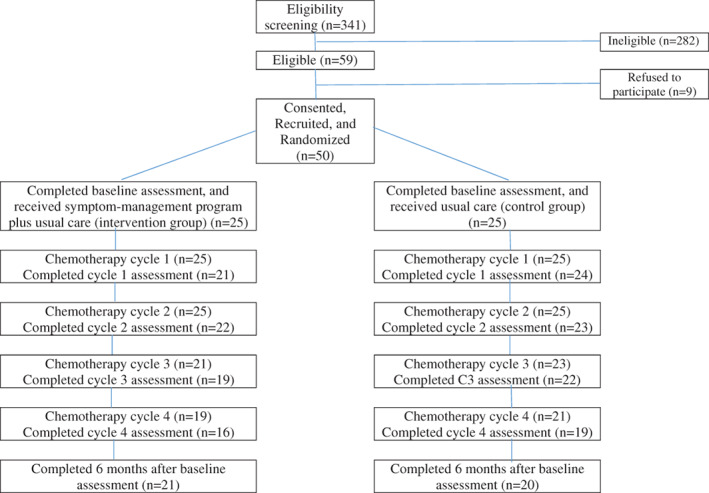
Recruitment and follow‐up assessments flow chart
TABLE 1.
Patients' characteristics and disease information
| Characteristics | Total N = 50 | Control n = 25 | Intervention n = 25 | P‐value |
|---|---|---|---|---|
| f (%) | f (%) | f (%) | ||
| Age (Mean ± SD) | 13.7 ± 2.5 | 13.4 ± 2.6 | 13.9 ± 2.4 | .540 |
| Gender | ||||
| Boy | 31 (62.0%) | 15 (60.0%) | 16 (64.0%) | .771 |
| Girl | 19 (38.0%) | 10 (40.0%) | 9 (36.0%) | |
| Education | ||||
| Primary to junior secondary | 38 (76.0%) | 21 (84.0%) | 17 (68.0%) | .185 |
| Secondary graduate | 12 (24.0%) | 4 (16.0%) | 8 (32.0%) | |
| Primary caregiver | ||||
| Mother | 41 (82.0%) | 21 (84.0%) | 20 (80.0%) | .713 |
| Father | 9 (18.0%) | 4 (16.0%) | 5 (20.0%) | |
| Caregiver education | ||||
| Primary, secondary or JC education | 19 (38.0%) | 9 (36.0%) | 10 (40.0%) | .771 |
| Secondary graduate or post‐secondary education | 31 (62.0%) | 16 (64.0%) | 15 (60.0%) | |
| Type of cancer | ||||
| Hematological malignancy | 37 (74.0%) | 19 (76.0%) | 18 (72.0%) | .747 |
| Solid tumor | 13 (26.0%) | 6 (24.0%) | 7 (28.0%) | |
| Cancer treatments | ||||
| Chemotherapy/radiotherapy | .628 | |||
| Chemotherapy alone | 31 (63.3%) | 15 (60.0%) | 16 (66.7%) | |
| Chemotherapy & radiotherapy | 18 (36.7%) | 10 (40.0%) | 8 (33.3%) | |
| Surgery | 11 (22.4%) | 6 (24.0%) | 5 (20.8%) | .791 |
| Transplantation | 8 (16.0%) | 6 (24.0%) | 2 (8.0%) | .123 |
| Targeted therapy | 7 (14.3%) | 3 (12.0%) | 4 (16.7%) | .641 |
| Chemotherapy drugs a | ||||
| Corticosteroids | 39 (79.6%) | 21 (84.0%) | 18 (75.0%) | .435 |
| Alkylating agents | 39 (79.6%) | 21 (84.0%) | 18 (75.0%) | .435 |
| Anti‐metabolites | 32 (65.3%) | 19 (76.0%) | 13 (54.2%) | .108 |
| Antimicrotubule drugs | 33 (67.3%) | 15 (60.0%) | 18 (75.0%) | .263 |
| Topoisomerase inhibitors | 27 (55.1%) | 13 (52.0%) | 14 (58.3%) | .656 |
| Cytotoxic antibiotics | 39 (79.6%) | 19 (76.0%) | 20 (83.3%) | .524 |
| L‐Asparaginas | 14 (28.6%) | 7 (28.0%) | 7 (29.2%) | .928 |
| Ancillary medications (at least in one cycle of chemotherapy throughout the study period) | ||||
| Analgesic for pain | 43 (86%) | 21 (84%) | 22 (88%) | .399 |
| Anti‐emetic for nausea/vomiting | 34 (68%) | 20 (80%) | 14 (56%) | .069 |
Chemotherapy drug and target therapy information was missing for one participant in intervention group.
3.1. Patterns of change of symptom occurrences/ symptom scores across time
In general, the patterns of the occurrence rates and the mean symptom scores were similar in the two groups; these were high at baseline and at the first cycle of chemotherapy and began to decline by the second or third cycle of chemotherapy (Figures 2A‐E and 3A‐F). Of note, the occurrence rates of nausea and vomiting and fatigue in the control group increased at 6 months after baseline, whereas fatigue and pain in the intervention group increased at 6 months after baseline. The mean symptom scores of nausea and vomiting, fatigue, pain, and mucositis reported by the control group increased at 6 months after baseline, whereas all mean symptom scores in the intervention group, except those for fatigue, continued to decline since second or third cycle of chemotherapy.
FIGURE 2.
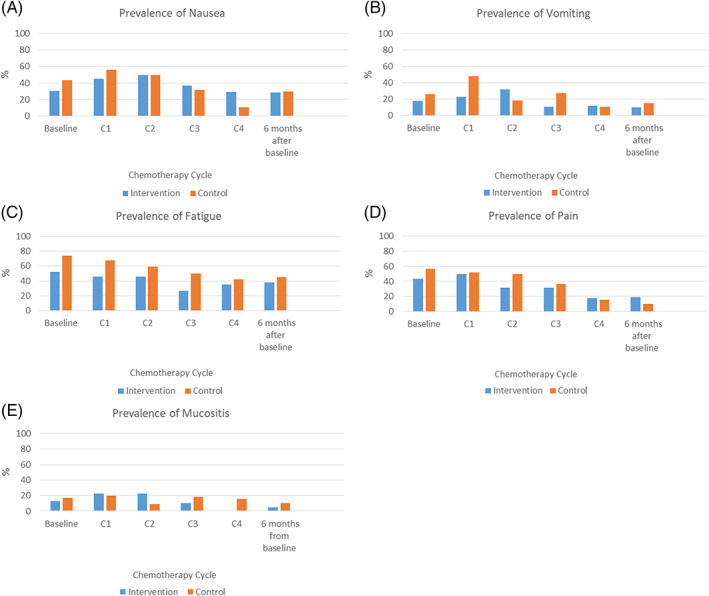
A‐E, Prevalence of nausea, vomiting, fatigue, pain and mucositis
FIGURE 3.
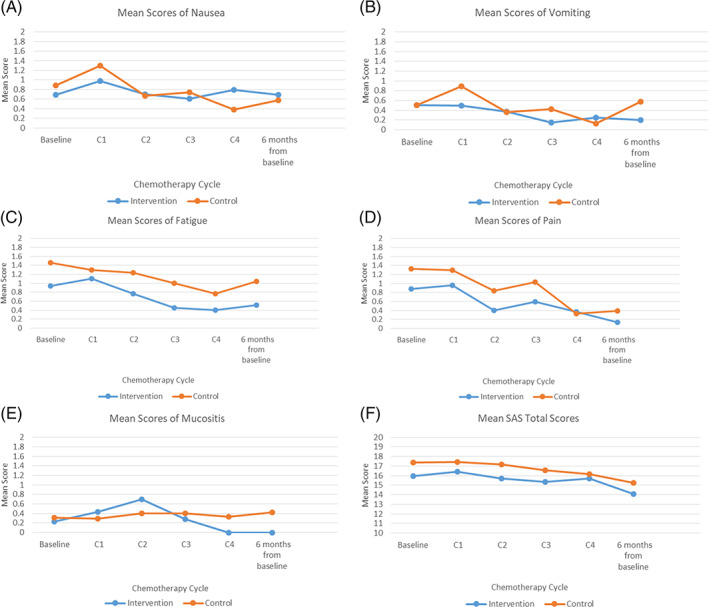
A‐F, Mean symptom scores of nausea, vomiting, fatigue, pain, mucositis and anxiety
3.2. Between‐group differences in symptom occurrences across time
In this study, 44%, 56%, 52%, 38.1%, 32%, and 30% of the participants in the intervention group reported ≥2 symptoms at baseline, cycles 1, 2, 3, and 4 assessments, and at 6 months after baseline, respectively. As for the control group, 52%, 72%, 64%, 43.5%, 38.8%, and 40% reported ≥2 symptoms, separately. As shown in Figure 2A‐E, fatigue was the predominant symptom from diagnosis and throughout chemotherapy. The children who received the symptom‐management program had a lower incidence of fatigue (26.3%‐52.2%) than those who received usual care only (42.1%‐73.9%) across all time points. Nausea (intervention group, 28.6%‐50%; control group, 10.5%‐56%) and pain (intervention group, 19%‐50%; control group, 10%‐56.5%) were the next most common symptoms. Vomiting (intervention group, 9.5%‐31.8%; control group, 15%‐48%) and mucositis (intervention group, 0%‐22.7%; control group, 9.1%‐20%) were the least frequent symptoms in both groups. However, no statistically significant differences were found with respect to the incidences of all targeted symptoms between groups (P > .05).
3.3. Between‐group differences in symptom scores across time
Of note, the symptom scores of fatigue was significantly lower across all time‐points in the intervention group compared to the control group, favored the intervention group (F = 4.95, P = .034, effect‐size = 0.32). The effect‐size of 0.32 was also clinically meaningful improved difference. According to distribution‐based methods to determining clinically meaningful change, an effect size of 0.20 has been proposed as an appropriate threshold of a minimal clinically important difference for health status measures. 27 However, for nausea and vomiting, pain, mucositis, and anxiety scores, only a trend toward lower scores in the intervention group than the control group at most time‐points (P > .05).
3.4. Patients and parents' experiences and views on the symptom‐management program
Two main themes emerged from the interview data: “Positive impact” and “Gaps,” with “Being well‐prepared” and “Get ready for symptoms” being two interrelated subcategories of “Positive impact.” Both children and parents reported a positive experience with the symptom‐management program as a whole, particularly almost all children and parents mentioned that the symptom‐management program helped increase their understanding of the symptoms associated with chemotherapy and equipped them to prepare, identify, and cope more effectively with the targeted symptoms at home. Some parents also mentioned that the acquisition of knowledge from the symptom‐management program helped them overcome their own stress, which in turn may have enhanced their ability to offer support to their children during chemotherapy. Two parents went further and suggested that the symptom‐management program should be made an adjunct of medical treatment for children. This theme is illustrated by the following quotations:
“…It helps reduce the symptoms and keeps me alert, and makes me calm down [so that] I can make it through the days easier…” (WN005LL, patient).
“…It's really good to have some coping suggestions that are noninvasive interventions to manage the symptoms…” (WN009HL, patient).
Two interrelated categories emerged from the theme of “Gaps”: “The Breadth and depth of intervention” and “Contextual consideration.” Some children and parents suggested that more information about food selection and meal preparation should be included to help support their nutritional needs with respect to the morbidity associated with nausea/vomiting and mucositis. Some children and parents also described infection/neutropenic fever and sleeping difficulties as being stressful conditions for themselves and noted that there was a need for information and strategies about infection prevention and sleep promotion during the course of chemotherapy in addition to that concerned with the targeted symptoms. Some parents reported that their children's motivation to be engaged with the intervention components was hindered by the discomfort associated with chemotherapy. At the moment when children were suffering severe symptom, they regarded ancillary medications was crucially important in alleviating their symptoms instead of nonpharmacological intervention components. Some stated:
“…After chemotherapy, his weakness, nausea and vomiting is so severe. At that time, it's impossible to urge him to practice the program. Only medications can help him…” (WN023L, parent).
“…It helps me when my symptoms are not very severe, but when my symptoms become worse, only medications can give me relief…” (WN039RS, patient).
4. DISCUSSION
Results in this study show that our multimodal symptom‐management program reduced the levels of fatigue in pediatric patients across multiple cycles of chemotherapy and at 6 months after baseline. The effect‐size of 0.32 exceeding the minimally important difference threshold of ≥0.2 was clinically meaningful improved difference. 27 This is a promising finding because cancer‐related fatigue has long been a significant concern for children and adolescents, family members, and health care professionals. 11 , 28 Recent systematic and integrative reviews of various exercise‐based interventions, complementary and alternative therapies, and health education intervention to manage fatigue among children and adolescents with cancer revealed limited research in this area and population and a dearth of strong evidence for the efficacy of any treatment approach. 28 , 29 , 30 , 31 , 32 , 33 Our study results provide evidence for the benefits of a combination of distraction strategies/techniques and energy conservation strategies to reduce the levels of fatigue in this underserved population. In a systematic review of the lived experience of fatigue in children and adolescents with cancer, Tomlinson et al (2016) also indicated that distraction is a perceived alleviator of fatigue in patients. 11 In addition, our results provide support on psychoeducation focus of active involvement of the patient and parents during the process. The present data revealed that children and adolescents adhered over 80% with the intervention components. Children and adolescents and their parents' positive experience with the symptom‐management program further supports the importance of psychoeducational‐oriented coaching to provide participants with the knowledge, skills, and support necessary for symptom prevention and management.
Although statistically significant differences could not be detected between the study groups in terms of the levels of nausea and vomiting, pain, mucositis, and anxiety, it was observed that children and adolescents who took part in the symptom‐management program tended to have reduced incidence of and less severe symptoms than those who received usual care only. The lack of statistical significance in the differences between the outcome variables of the groups might be attributable to type II error and/or the potential of suboptimal efficacious dosage of intervention components. It is the fact that only limited number of patients enrolled in this study, and the actual effect size of the symptom‐management program may be small which require a larger sample size to detect. Post hoc power analysis revealed less than 80% of power to detect a mean difference with respect to nausea and vomiting, pain, mucositis and anxiety between intervention and control groups at a 0.05 significance level. In addition, our current dosage of intervention components characterized by duration, frequency and amount might have limited the full potential of the synergistic effects of the symptom‐management program. It may be possible that more intense program could achieve greater synergistic effects. In this study, our weekly practicing intervention components during chemotherapy cycle were based on the considerations of practical feasibility. Voils et al highlighted that patient burden and adherence are needed to consider when designed behavioral interventions, as excessive burden leads to low attendance or high withdrawal. 34 Nevertheless, this lack of statistical significance should not preclude researchers and clinicians from holding positive views about the potential benefits of the intervention components. More large studies of multiple intervention components are warranted to improve the study power to detect the synergistic effects of interventions for multiple symptoms. Larger factorial research design trials are also necessary to further refine and validate the most effective dose parameters of intervention components in symptom‐management program.
This study also provided in‐depth insight into children and parents' experiences with the symptom‐management program throughout the course of chemotherapy. The qualitative data reveal the salient effect of improved knowledge and understanding of symptoms associated with chemotherapy on the children's and parents' readiness for cancer therapy. This sense of readiness is crucial to help patients and parents strengthen their perception that they have control over their care. Additional research might need to consider for inclusion infection prevention and sleep promotion interventions, and to consider whether or how nonpharmacological interventions can be combined with the use of supportive ancillary medications to create a comprehensive, integrated approach to symptom management.
This study was limited by the small sample size, and single‐clinical research site that may influence the study's power and external validity. In addition, our sample may have been biased toward inclusion of children and adolescents who were more open and had a greater interest in the program since completion of the multimodal symptom‐management program required that participants have the necessary stamina to complete the study intervention components. Nevertheless, results of this study suggest that a home‐based multiple intervention symptom‐management program may help to reduce fatigue in children and adolescents who undergo multiple cycles of chemotherapy. Moreover, the results give valuable insights into the feasibility and effects of a symptom‐cluster approach and multiple intervention components.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, K.K.F.C., L.M.L.T.; Methodology, K.K.F.C.; Investigation, K.K.F.C., L.M.L.T; Data curation, K.K.F.C.; Formal Analysis, K.K.F.C.; Project Administration, K.K.F.C., L.M.L.T.; Resources, K.K.F.C., L.M.L.T.; Software, K.K.F.C.; Writing ‐ Original Draft, K.K.F.C.; Writing ‐ Review & Editing, K.K.F.C., L.M.L.T.; Supervision, K.K.F.C., L.M.L.T.; Funding Acquisition, K.K.F.C.
ETHICS STATEMENT
The study was conducted at a Children's Cancer Centre in Singapore after approval from the Institutional Review Board (DSRB Ref: 2011/01812). The patients and their parents were informed of the aims of the study and were included only after the patients gave their assent to participate and their parents provided written informed consent.
ACKNOWLEDGEMENTS
This study was supported by a Start‐Up Grant of the National University of Singapore, Singapore. We thank clinical staff for their assistance in screening/recruitment and Ms Win Lei Phyu for her assistance in recruitment and data collection. We also wish to express our gratitude to the patients and parents for agreeing to participate in this study.
Cheng KK‐F, Tan LML. A pilot study of the effect of a home‐based multimodal symptom‐management program in children and adolescents undergoing chemotherapy. Cancer Reports. 2021;4:e1336. 10.1002/cnr2.1336
Funding information Start‐Up Grant
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Tran TH, Shah AT, Loh AT. Precision medicine in pediatric oncology: translating genomic discoveries into optimized therapies. Clin Cancer Res. 2017;23(18):5329‐5338. 10.1158/1078-0432.CCR-16-0115. [DOI] [PubMed] [Google Scholar]
- 2. Cleve LV, Muñoz CE, Savedra M, et al. Symptoms in children with advanced cancer: child and nurse reports. Cancer Nurs. 2012;35(2):115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465‐447. [PubMed] [Google Scholar]
- 4. Xiao C. The state of science in the study of cancer symptom clusters. Eur J Oncol Nurs. 2010;14:417‐434. [DOI] [PubMed] [Google Scholar]
- 5. Baggott C, Dodd M, Kennedy C, Marina N, Matthay KK, Miaskowski C. Changes in children's reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs. 2010;27(6):307‐315. 10.1177/1043454210377619. [DOI] [PubMed] [Google Scholar]
- 6. Cheng KKF. Prevention of gastrointestinal side effects in paediatric oncology: what are the guidelines? Curr Opin Support PA. 2017;11(2):120‐124. 10.1097/SPC.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 7. Dupuis LL, Lu X, Mitchell HR, et al. Anxiety, pain, and nausea during the treatment of standard‐risk childhood acute lymphoblastic leukemia: a prospective, longitudinal study from the children's oncology group. Cancer. 2016;122(7):1116‐1125. 10.1002/cncr.29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kestler SA, LoBiondo‐Wood G. Review of symptom experiences in children and adolescents with cancer. Cancer Nurs. 2012;35(2):E31‐E49. [DOI] [PubMed] [Google Scholar]
- 9. Linder LA, Al‐Qaaydeh S, Donaldson G. Symptom characteristics among hospitalised children and adolescents with cancer. Cancer Nurs. 2018;41(1):23‐32. 10.1097/NCC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 10. Rodgers CC, Hooke MC, Hockenberry MJ. Symptom clusters in children. Curr Opin Support Palliat Care. 2013;7:67‐72. 10.1097/SPC.0b013e32835ad551. [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson D, Zupanec S, Jomes H, O'Sullivan C, Hesser T, Sung L. The lived in experience of fatigue in children and adolescents with cancer: a systematic review. Support Care Cancer. 2016;24(8):3623‐3631. 10.1007/s00520-016-3253-8. [DOI] [PubMed] [Google Scholar]
- 12. Torres V, Nunes MDR, Silva‐Rodrigues FM, et al. Frequency, severity, and distress associated with physical and psychosocial symptoms at home in children and adolescents with cancer. J Pediatr Health Care. 2019;33:404‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker AJ, Johnson KP, Miaskowski C, Lee KA, Gedaly‐Duff V. Sleep quality and sleep hygiene behaviours of adolescents during chemotherapy. J Cl Sleep Med. 2010;6:439‐444. [PMC free article] [PubMed] [Google Scholar]
- 14. Gedaly‐Duff V, Lee KA, Nail L, Nicholson HS, Johnson KP. Pain, sleep disturbance, and fatigue in children with leukemia and their parents: a pilot study. Oncol Nurs Forum. 2006;33:641‐646. [DOI] [PubMed] [Google Scholar]
- 15. Schurman JV, Singh M, Singh V, Neilan N, Friesen CA. Symptoms and subtypes in pediatric functional dyspepsia: relation to mucosal inflammation and psychological functioning. J Pediatr Gastroenterol Nutr. 2010;51(3):298‐303. 10.1097/MPG.0b013e3181d1363c. [DOI] [PubMed] [Google Scholar]
- 16. Cheng KKF, Chang AM. Palliation of oral mucositis symptoms in pediatric patients treated with cancer chemotherapy. Cancer Nurs. 2003;26(6):476‐484. [DOI] [PubMed] [Google Scholar]
- 17. Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61:1282‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng KKF, Wong EMC, Ling WM, Chan CWH, Thompson DR. Measuring the symptom experience of Chinese cancer patients: a validation of the Chinese version of the memorial symptom assessment scale. J Pain Symptom Manag. 2009;37(1):44‐57. 10.1016/j.jpainsymman.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 19. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient‐reported outcomes. J Natl Cancer Inst Monogr 2007. 2007;2007(37):16‐21. [DOI] [PubMed] [Google Scholar]
- 20. Charalambous A, Giannakopoulou M, Bozas E, Marcou Y, Kitsios P, Paikousis L. Guided imagery and progressive muscle relaxation as a cluster of symptoms management intervention in patients receiving chemotherapy: a randomized control trial. PLoS One. 2016;11(6):e0156911. 10.1371/journal.pone.0156911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, Nail L. A randomized clinical trial of energy conservation for patients with cancer‐related fatigue. Cancer. 2004;100(6):1302‐1310. [DOI] [PubMed] [Google Scholar]
- 22. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev. 2009;2009(1):CD006953. 10.1002/14651858.CD006953.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ranna V, Cheng K, Castillo D, et al. Development of the MASCC/ISOO clinical practice guidelines for mucositis: an overview of the methods support care. Support Care Cancer. 2019;27(10):3933‐3948. 10.1007/s00520-019-04891-1. [DOI] [PubMed] [Google Scholar]
- 24. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice‐Hall; 1986. [Google Scholar]
- 25. Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manag. 2000;19(5):363‐377. [DOI] [PubMed] [Google Scholar]
- 26. Li HCW, Lopez V. Development and validation of a short form of the Chinese version of the state anxiety scale for children. Int J Nurs Stud. 2007;44(4):566‐573. [DOI] [PubMed] [Google Scholar]
- 27. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the health utilities index mark II. PharmacoEconomics. 1999;15:141‐155. [DOI] [PubMed] [Google Scholar]
- 28. Miyauti‐Silva MC, Lopes‐Júnior LC, Nascimento LC, Lima RAG. Fatigue in children and adolescents with cancer from the perspective of health professionals. Rev Latino‐Am Enfermagem. 2016;24:e2784. 10.1590/1518-8345.1159.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhardwaj T, Koffman J. Non‐pharmacological interventions for management of fatigue among children with cancer: systematic review of existing practices and their effectiveness. BMJ Support Palliat Care. 2017;7(4):404‐414. 10.1136/bmjspcare-2016-001132. [DOI] [PubMed] [Google Scholar]
- 30. Chang CW, Mu PF, Jou ST, Wong TT, Chen YC. Systematic review and meta‐analysis of nonpharmacological interventions for fatigue in children and adolescents with cancer. Worldviews Evidence‐Based Nurs. 2013;10(4):208‐217. 10.1111/wvn.12007. [DOI] [PubMed] [Google Scholar]
- 31. Lopes‐Júnior LC, Bomfim EO, Nascimento LC, Nunes MD, Pereira‐da‐Silva G, Lima RA. Non‐pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: an integrative review. Eur J Cancer Care. 2016;25(6):921‐935. 10.1111/ecc.12381. [DOI] [PubMed] [Google Scholar]
- 32. Nunes MDR, Bomfim E, Olson K, et al. Interventions minimizing fatigue in children/adolescents with cancer: an integrative review. J Child Health Care. 2018;22(2):186‐204. 10.1177/1367493517752498. [DOI] [PubMed] [Google Scholar]
- 33. Spathis A, Booth S, Grove S, Hatcher H, Kuhn I, Barclay S. Teenage and young adult cancer‐related fatigue. Is prevalent, distressing, and neglected: it is time to intervene. A systematic literature review and narrative synthesis. J Adolesc Young Adult Oncol. 2015;4(1):3‐17. 10.1089/jayao.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voils CI, King HA, Maciejewski ML, Allen AD, Yancy WS Jr, Shaffer JA. Approaches for informing optimal dose of behavioral interventions. Ann Behav Med. 2014;48(3):392‐401. 10.1007/s12160-014-9618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


