Abstract
Background
The relation between immunity, inflammation, and tumor development and progression has been emphasized in colorectal cancer widely and the prognosis is linked to the inflammatory reaction of the host as well as the biological behavior of the tumor.
Aim
In this study, we aimed to find out the predictive power of C‐reactive protein‐ lymphocyte ratio (CLR) for in‐hospital mortality after colorectal surgery.
Methods and Results
A series of 388 CRC patients were enrolled in the present retrospective study which was conducted in a tertiary state Hospital in Ankara, Turkey. In‐hospital mortality was the main outcome to evaluate the predictive power of inflammatory markers, while the other outcomes that would be evaluated as separate variables were LOS in hospital and LOS in ICU.
In this study, there were 260 males and 128 females, and the mean age was 60.9. The in‐hospital mortality rate was 3.4% (n = 13) and age, APACHE II score and Charlson comorbidity index score were related to in‐hospital mortality statistically. The mean LOS in the hospital was 13.9 days and LOS in ICU was 4.5 days. The CRP levels and the CLR levels were higher both in the preoperative and postoperative periods in the mortality (+) group and the difference was significant statistically (P = .008/ .002 and .004/ <.001, respectively). CLR in the postoperative period had the best predictive power with AUC: 0.876.
Conclusion
In conclusion, within the context of our study there appears to be a relationship between CLR, as measured on day 2 postoperatively, and in‐hospital mortality. It is observed to be more effective than NLR, ALC, and CRP.
Keywords: colorectal cancer, inflammatory marker, in‐hospital mortality, predictive power, surgery
1. INTRODUCTION
Colorectal cancer (CRC) aligns third in terms of incidence ‐comprising 11% of all cancer diagnoses‐ and second in terms of mortality according to GLOBOCAN 2018. 1 After the identification of the association between tumor growth and the inflammation reaction by Rudolf Virchow, 2 many studies have investigated the extent of the inflammatory reactions and the prognostic effect in oncological cases. 3 The relation between innate immunity, inflammation, and tumor development and progression have been specifically emphasized in cases of CRC arising from inflammatory bowel disease (IBD) and it is concluded that the prognosis of CRC is associated with the inflammatory reaction of the host as well as the biological behavior of the tumor. 4
The inflammatory response‐tumor interaction is extending out of the local tumor environment and provoking a systemic response. It is regulated by proinflammatory cells like natural killer cells and M1 macrophages, anti‐inflammatory cells like type 2 helper T cells. While the other components of immunity, including B lymphocytes, neutrophils, eosinophils, mast cells, and plasma cells collaborate with immunosuppressing and immunoenhancing cells. 5 , 6 Cancer progression and the clinical outcomes are linked to this interaction and systemic response and searched via a variety of biomarkers. 7
These inflammation‐related markers are either derived from complete blood count like neutrophil‐lymphocyte ratio (NLR) and platelet related inflammatory markers or derived from routine laboratory tests like C‐reactive protein (CRP). The most concerning inflammatory markers are NLR, platelet lymphocyte ratio (PLR), CRP, and CRP‐albumin ratio (CAR), and they have been studied in a wide range of cancer, including breast cancer, CRC, lung cancer, gastric cancer, and ovarian cancer. 5 , 8 The prognostic factors for colorectal cancer are well‐defined as tumor stage, histological grade, lymph node status, and intravascular invasion, whereas due to the influence of systemic inflammation in CRC progression, biomarkers offer potential supplemental predictive parameters. 9 , 10
In a recent study, it was stressed that neutrophil, platelet, and CRP levels were related to up‐regulation in disease progression while lymphocyte and albumin values were related to down‐regulation in disease progression for CRC. 11 Approximately 20% to 40% increment in the serum levels of acute‐phase proteins like CRP in the case of resectable CRC was reported, while the amount of preoperative or postoperative level was correlated with poor prognosis. 7 The lymphocyte‐to‐monocyte ratio (LMR), the NLR, the platelet ‐lymphocyte ratio (PLR), and prognostic scores like modified Glasgow Prognostic Score (mGPS) and systemic inflammation score (SIS) which comprise the serum albumin levels, serum C‐reactive protein (CRP) or LMR are the other investigated predictive biomarkers for prognosis in CRC cases. 9 , 12 , 13
In this study, we aimed to find out the predictive power of C‐reactive protein‐ lymphocyte ratio (CLR) for in‐hospital mortality after colorectal surgery and to compare it with the other well‐known inflammatory markers like CRP and NLR. As far as we know, C‐reactive protein‐ lymphocyte ratio (CLR) has not been investigated as a prognostic or predictive factor in the CRC cases in the literature.
2. METHODS
2.1. Design and data acquisition
A series of 388 CRC patients were enrolled in the present retrospective study, which was conducted in the Department of Intensive Care of a tertiary state Hospital in Ankara, Turkey. The analysis was done through the institutional database regarding data of patients who underwent curative surgery with the diagnosis of CRC between June 30, 2015 and July 30, 2018. The inclusion criteria for the study were defined as histopathology‐proven colorectal adenocarcinoma; underwent curative surgery with tumor‐free resection margins; older than 18 years old; and elective surgery. The patients who underwent palliative or emergent surgery were excluded from the study. The other criteria for exclusion were benign pathologies like IBD or ischemia, history of other malignancies, the requirement for a second operation in the first month after CRC surgery, and clinical evidence of autoimmune disorder, systemic inflammation, or infection.
All demographic and clinical data, including gender, age, Acute Physiologic Assessment, and Chronic Health Evaluation II (APACHE II) score, Charlson Comorbidity Index score, the surgical procedure, and the outcomes in the postoperative period were derived from the institutional database. Our study was designed in a retrospective manner and did not require any specific laboratory test or clinical data other than obtained data from patients' files or nurse sheets. The clinicopathological data like tumor location, the histological grade, the clinical tumor‐node‐metastasis (TNM) stage [in accordance with the TNM staging system of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC eighth edition, 2017)], 14 and the laboratory values like NLR, CRP, were also provided from the institutional database.
In‐hospital mortality was the main outcome to evaluate the predictive power of inflammatory markers while the other outcomes that would be evaluated as separate variables were length of stay (LOS) in hospital and intensive care unit (ICU). Preoperative and postoperative values of absolute neutrophil count (ANC), absolute lymphocyte count (ALC), CRP, NLR, and CLR were the assessed inflammatory markers in this study. All of these biochemical markers were analyzed daily as a routine blood test in the preoperative and postoperative periods on a daily basis during their management in our ICU. The day before surgery and 2 days after the surgery were determined as time points to evaluate the predictive power. We chose postoperative day 2 as the time point because in the previous studies the peak value of CRP was observed 48 hours after major surgery. 15 , 16
2.2. Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD), whereas categorical variables were described as total number and percentage. The normality of the variables was assessed by Kolmogorov Smirnov test and according to the result of this test‐ variables did not show normal distribution‐ nonparametric tests were utilized. The Spearman rho test and the Mann‐Whitney U test were used to clarify the relation between inflammatory markers and variables. To compare the predictive power of CLR in 30‐day mortality, the receiver operating characteristic (ROC) curve analysis, and the area under curves (AUC) were utilized. The optimal cut‐off value for CLR was determined by the Youden index (YI). The IBM SPSS software version 25 (IBM SPSS, Armonk, NY) was used for statistical analyses. Each test was two‐tailed and the statistical significance was stated with a P‐value less than .05. This study was designed in a retrospective and observational manner and because of that it was exempted from the ethics committee approval and signed informed consent.
3. RESULTS
Between June 2015 and July 2018, 388 patients who underwent CRC surgery were included in this study after the exclusion of the 190 cases which was described in Figure 1. There were 260 males and 128 females, and the mean age was 60.9. The demographic and descriptive variables were listed in Table 1. The in‐hospital mortality rate was 3.4% (n = 13) and age, APACHE II score and Charlson comorbidity index score were related to in‐hospital mortality statistically. The location of the tumor, the histological grade, and the TNM stage‐ even the metastasis‐ did not differ between mortality (+) and mortality (−) groups statistically (P > .05). Among the 13 who died, the most common reasons for in‐hospital mortality were cardiovascular disease (acute myocardial infarction and arrhythmia; n = 4), respiratory disease (pneumonia and acute exacerbation of chronic obstructive pulmonary disease; n = 6), and sepsis (n = 2). In one patient, the cause of death was acute respiratory failure after stroke. As it was described in detail in Table 2, the operative variables like surgical procedure type and the duration of surgery, and the postoperative outcomes like the length of stay (LOS) in hospital and intensive care unit (ICU) did not differ between groups as well. The mean LOS in hospital was 13.9 days and LOS in ICU was 4.5 days. The anastomoses leak rate was 3.6% (n = 14) and did not correlate with mortality statistically (P = .423). In Table 3, the levels of the inflammatory markers in the preoperative and postoperative periods were given and compared according to the in‐hospital mortality. The CRP levels and the CLR levels were higher both in the preoperative and postoperative periods in the mortality (+) group and the difference was significant statistically (P = .008/.002 and .004/ <.001, respectively). Postoperative the ALC and the NLR levels differed statistically (P < .001) while the difference was not significant in the preoperative period (P = .131 and .088, respectively). The statistical difference was not observed in the ANC levels both in the preoperative and postoperative periods as well (P = .212 and .367, respectively).
FIGURE 1.
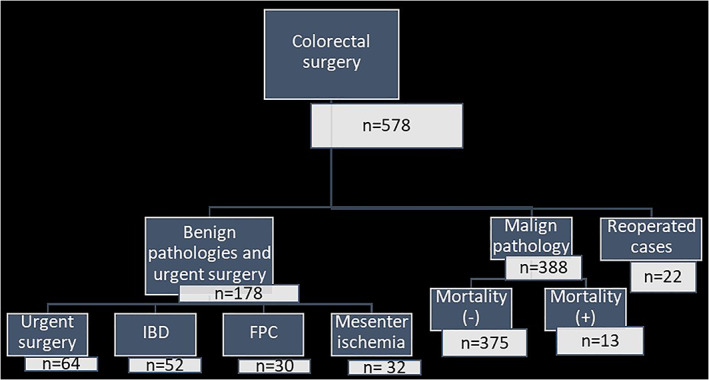
The flowchart of the study population of colorectal cancer surgery patients after exclusion of the benign pathologies, urgent surgeries and reoperated cases in the postoperative period. Abbreviations: FPC; familial polyposis coli, IBD; inflammatory bowel disease
TABLE 1.
The demographic and descriptive variables of the patients (n = 388) and the statistical assessment according to mortality
| Variables | Total patients (n = 388) | Mortality (+) (n = 13) | Mortality (−) (n = 375) | P |
|---|---|---|---|---|
| Age (years, mean ± SD) | 60.9 ± 12.9 | 74.8 ± 6.7 | 60.4 ± 12.8 | <.001 |
| Sex (Male) [n (%)] | 260 (67) | 8 (61.5) | 252 (67.2) | .341 |
| APACHE II (mean ± SD) | 7.9 ± 3.5 | 14.3 ± 6.5 | 7.7 ± 3.1 | <.001 |
| Charlson comorbidity index(mean ± SD) | 5.7 ± 2.9 | 8.5 ± 3.9 | 5.6 ± 2.8 | .005 |
| Location of the tumor | .950 | |||
| Right Colon [n (%)] | 205 (52.8) | 6 (46.2) | 199 (53.1) | |
| Left colon [n (%)] | 89 (22.9) | 5 (38.5) | 84 (22.4) | |
| Rectum [n (%)] | 94 (24.2) | 2 (15.4) | 92 (24.5) | |
| Histological grade | .454 | |||
| Well‐differentiated [n (%)] | 98(25.3) | 2(15.5) | 96(25.6) | |
| Moderate differentiated [n (%)] | 198(51) | 7 (53.8) | 191(50.9) | |
| Poorly differentiated [n (%)] | 92(23.7) | 4(30.7) | 88(23.5) | |
| TNM stage | .267 | |||
| Stage I [n (%)] | 64(16.5) | 3(23) | 61(16.3) | |
| Stage II [n (%)] | 124 (32) | 2(15.4) | 122(32.5) | |
| Stage III [n (%)] | 136(35) | 6(46.2) | 130(34.7) | |
| Stage IV [n (%)] | 64(16.5) | 2(15.4) | 62(16.5) | |
| Metastasis [n (%)] | 68 (17.5) | 3 (23.1) | 65 (17.3) | .593 |
Note: P‐value was determined with either Spearman Rho or Mann‐Whitney U test.
Abbreviations: APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; SD, standard deviation; TNM stage, clinical tumor‐node‐metastasis stage.
TABLE 2.
The operative and postoperative variables of the patients (n = 388) including the outcomes in the postoperative period and the statistical assessment according to mortality
| Variables | Total patients (n = 388) | Mortality (+) (n = 13) | Mortality (−) (n = 375) | P |
|---|---|---|---|---|
| Surgical procedure type [n (%)] | .201 | |||
| Right hemicolectomy [n (%)] | 195 (50.3) | 8 (61.5) | 187 (49.9) | |
| Left hemicolectomy [n (%)] | 74 (19.1) | 4 (30.8) | 70 (18.7) | |
| Total colectomy [n (%)] | 31(8) | 0 | 31 (8.3) | |
| Low anterior resection [n (%)] | 88 (22.7) | 1 (7.7) | 87 (23.2) | |
| Colostomy rate [n (%)] | 119(30.7) | 7 (53.8) | 112 (29.9) | .066 |
| Duration of surgery (hours) (mean ± SD) | 3.2 ± 1.4 | 3.4 ± 1.4 | 3.2 ± 1.4 | .25 |
| LOS in hospital(days) (mean ± SD) | 13.9 ± 11.5 | 23.3 ± 32.5 | 13.6 ± 9.9 | .590 |
| LOS ICU (days) (mean ± SD) | 4.5 ± 8.8 | 20.7 ± 33.5 | 3.9 ± 5.9 | <.001 |
| Anastamoses leak rate [n (%)] | 14 (3.6) | 1 (7.7) | 13 (3.5) | .423 |
Note: P‐value was determined with either Spearman Rho or Mann‐Whitney U test.
Abbreviations: ICU, intensive care unit; LOS, length of stay; SD, standard deviation.
TABLE 3.
The values of the chosen inflammatory markers in the preoperative period (Day 0), and the postoperative period (Day 2), the difference between postoperative and preoperative values were listed and compared according to mortality
| Variables | Total patients (n = 388) | Mortality (+) (n = 13) | Mortality (−) (n = 375) | P |
|---|---|---|---|---|
| Preoperative CRP (mg/L) (mean ± SD) | 27.8 ± 36.3 | 66.3 ± 68 | 26.4 ± 34.1 | .008 |
| Postoperative CRP (mg/L) (mean ± SD) | 114.6 ± 78.7 | 183.3 ± 95.2 | 112.2 ± 77.2 | .002 |
| CRP difference (mg/L) (mean ± SD) | 88.8 ± 77.5 | 117 ± 109.6 | 85.8 ± 76.1 | .125 |
| Preoperative ANC (×109/l) (mean ± SD) | 6.5 ± 4 | 8.1 ± 5.5 | 6.4 ± 3.9 | .212 |
| Postoperative ANC (×109/l) (mean ± SD) | 7.6 ± 3.9 | 8.9 ± 5.8 | 7.6 ± 3.8 | .367 |
| ANC difference (×109/l) (mean ± SD) | 1.1 ± 4.5 | 0.9 ± 4.9 | 1.1 ± .4.5 | .798 |
| Preoperative ALC (×109/l) (mean ± SD) | 1.7 ± 0.8 | 1.4 ± 0.5 | 1.7 ± 0.8 | .131 |
| Postoperative ALC (×109/l) (mean ± SD) | 1.3 ± 1.4 | 0.7 ± 0.5 | 1.3 ± .1.4 | <.001 |
| ALC difference (×109/l) (mean ± SD) | −0.5 ± 1.5 | −0.8 ± 0.8 | −0.5 ± 1.5 | .128 |
| Preoperative NLR (mean ± SD) | 4.9 ± 5.3 | 7 ± 5.8 | 4.9 ± 5.3 | .088 |
| Postoperative NLR (mean ± SD) | 7.9 ± 7 | 21 ± 16.7 | 7.5 ± 5.9 | <.001 |
| NLR difference (mean ± SD) | 2.9 ± 7.8 | 14 ± 15.4 | 2.6 ± 7.1 | .001 |
| Preoperative CLR (mg/dL)/(×109/l) (mean ± SD) | 21.1 ± 33.1 | 57.1 ± 64.8 | 19.8 ± 30.9 | .004 |
| Postoperative CLR (mg/dL)/(×109/l) (mean ± SD) | 147.5 ± 363.8 | 978.9 ± 1727 | 118.7 ± 127.7 | <.001 |
Note: P‐value was determined with either Spearman Rho or Mann‐Whitney U test.
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CLR, the ratio of C‐reactive protein to lymphocyte count; CRP, C‐reactive protein; NLR, the ratio of neutrophil count to lymphocyte count; SD, standard deviation.
The predictive power of the inflammatory markers for in‐hospital mortality was compared by AUC and ROC curves in Table 4 and Figure 2. It was clearly shown that the CLR had the best predictive power and especially CLR in the postoperative period was the best with AUC: 0.876 (95% CI, 0.778‐0.974; P < .001). The second best predictor for in‐hospital mortality was postoperative NLR (AUC: 0.829, 95% CI, 0.684‐0.973; P < .001). The ROC curve was detailed for postoperative CLR value in Figure 3 and the cut‐off value was determined by the Youden index (256.84). The sensitivity and specificity were high enough to rely on postoperative CLR as a prognostic indicator (76.9 and 90.7, respectively). Yet, the sensitivity and specificity of preoperative CLR were lower likewise AUC (69.2 and 73.6, respectively, AUC: 0.733, P = .002).
TABLE 4.
The AUC values of the CRP, ANC, ALC, NLR, and CLR as the inflammatory markers were compared for the predictive power of in‐hospital mortality
| Variable | AUC | SE | 95% CI | P |
|---|---|---|---|---|
| preopCRP | 0.717 | 0.070 | 0.580‐0.853 | .008 |
| postopCRP | 0.747 | 0.048 | 0.653‐0.842 | .002 |
| preopANC | 0.602 | 0.083 | 0.440‐0.764 | .212 |
| postopANC | 0.574 | 0.108 | 0.362‐0.785 | .367 |
| preopALC | 0.377 | 0.075 | 0.229‐0.525 | .131 |
| postopALC | 0.185 | 0.077 | 0.034‐0.337 | <.001 |
| NLRpreop | 0.639 | 0.087 | 0.469‐0.809 | .088 |
| NLRpostop | 0.829 | 0.074 | 0.684‐0.973 | <.001 |
| CLR_pre | 0.733 | 0.071 | 0.594‐0.873 | .004 |
| CLR_post | 0.876 | 0.050 | 0.778‐0.974 | <.001 |
Abbreviations: AUC, Area under the curve; CI, Confidence interval; CLR_post, the ratio of C‐reactive protein to lymphocyte count value in the postoperative period; CLR_pre, the ratio of C‐reactive protein to lymphocyte count value in the preoperative period; NLR postop, the ratio of neutrophil count to lymphocyte count value in the postoperative period; NLR preop, the ratio of neutrophil count to lymphocyte count value in the preoperative period; postop ALC, absolute lymphocyte count value in the postoperative period; postop ANC, absolute neutrophil count value in the postoperative period; postopCRP, C‐reactive protein value in the postoperative period; preop ALC, absolute lymphocyte count value in the preoperative period; preop ANC, absolute neutrophil count value in the preoperative period; preopCRP, C‐reactive protein value in the preoperative period; SE, Standard error.
FIGURE 2.
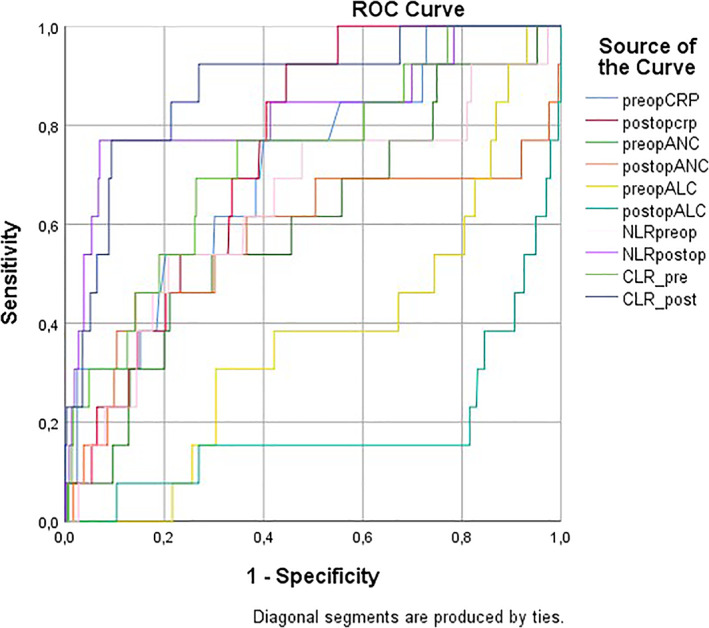
The ROC curves of the CRP, ANC, ALC, NLR, and CLR as the inflammatory markers were compared for the predictive power of in‐hospital mortality. Abbreviations: CLR_post, the ratio of C‐reactive protein to lymphocyte count value in the postoperative period; CLR_pre, the ratio of C‐reactive protein to lymphocyte count value in the preoperative period; NLR postop, the ratio of neutrophil count to lymphocyte count value in the postoperative period; NLR preop, the ratio of neutrophil count to lymphocyte count value in the preoperative period; postop ALC, absolute lymphocyte count value in the postoperative period; postop ANC, absolute neutrophil count value in the postoperative period; postopCRP, C‐reactive protein value in the postoperative period; preop ALC, absolute lymphocyte count value in the preoperative period; preop ANC, absolute neutrophil count value in the preoperative period; preopCRP, C‐reactive protein value in the preoperative period
FIGURE 3.
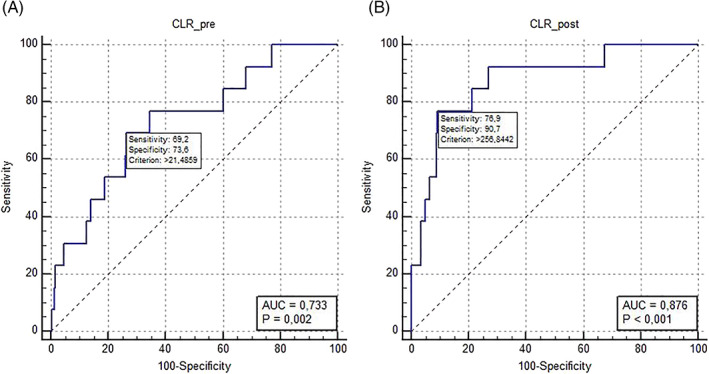
The ROC curves of the CLR in the preoperative and postoperative periods were compared for the predictive power of in‐hospital mortality with A and B. Abbreviations: AUC, area under the curve; CLR_post, the ratio of C‐reactive protein to lymphocyte count value in the postoperative period; CLR_pre, the ratio of C‐reactive protein to lymphocyte count value in the preoperative period; ROC curve, receiver operating characteristic curve
4. DISCUSSION
Colorectal cancer (CRC) continues to be a global and considerable health problem despite advancements in early diagnosis and proper management. In Turkey, 20 031 new CRC cases were reported by GLOBOCAN 2018 and it is the third most common cancer both in males and females. 1 Surgery is still the main treatment strategy while postoperative mortality and morbidity along with recurrence or distant metastasis are still bothering the surgeons and intensivists. The 5‐year survival was estimated as 50% for CRC and this poor overall prognosis leads to studies for the identification of ideal biomarkers for morbidity and mortality. 17 However, there is not any proven ideal biomarkers for CRC yet new inflammation‐related laboratory parameters or indices are investigated. We hypothesized that the ratio of CRP to ALC (CLR) – which were related to up‐regulation and down‐regulation in cancer progression, respectively – was comparable and even superior to other well‐known and investigated traditional inflammatory markers like CRP and NLR. To compare the CLR with other markers after CRC surgery, we analyzed the predictive power of CLR for in‐hospital mortality and the association with surgical outcomes which were easy to obtain via the institutional database. To avoid bias about surgical technique complications and infectious complications we excluded the reoperated cases from the study population.
CRP, NLR, and PLR were associated with poor survival in patients with CRC, gastric cancer, ovarian cancer, breast cancer, and nonsmall lung cell cancer yet the association between postoperative outcomes and CLR levels in the patients with CRC was not studied as far as we know. 4 , 8 Zou et al 4 concluded that the preoperative NLR was a significant independent prognostic factor in patients with CRC similar to other studies. 18 Some researchers investigated the combination of CRP and albumin (CAR) and some researchers combined CRP and lymphocyte value. 11 , 17 In a recent meta‐analysis, Lagunas‐Rangel et al 19 emphasized that decreased lymphocyte‐ CRP ratio (LCR) levels reflect an enhanced inflammatory process and poor prognosis in severe coronavirus disease 2019. Okugawa et al emphasized the usefulness of LCR in the appropriate management of patients with gastric cancer, 20 and in CRC. 11 In the second study, he deduced that the combination of lymphocyte count along with C‐reactive protein levels was more correlated with recurrence, overall survival (OS), and disease‐free survival (DFS) than other biomarkers in CRC patients. 11 Also, Daldal et al 21 concluded that low LCR values could be used as poor prognosis predictors in malignant bowel obstruction cases. The only CLR‐ cancer relation investigated study was about the prognosis of hepatocellular carcinoma after surgical resection. 22 Although our study did not evaluate these factors ‐OS or DFS‐ and assessed short term prognosis, the relatively high correlation with CLR and clinical outcomes gave a clue about the clinical feasibility of CLR in CRC patients. The other parameters like the gender of the patient, location, histological grade, and TNM stage of the tumor, duration of the surgery, and the surgical procedure type did not affect the in‐hospital mortality.
5. CONCLUSION
In conclusion, within the context of our study there appears to be a relationship between CLR, as measured on day 2 postoperatively, and in‐hospital mortality. It was observed to be more effective than NLR, ALC, and CRP. Hence, the predictive power should be assessed with prospective controlled studies, but we believed that CLR has the potential to complete the missing piece of the puzzle as a prognostic biomarker in CRC patients.
6. LIMITATIONS
Besides the findings of our study, there were some limitations to conclude more specific determinations. Being a single‐center, small‐scale, and short‐termed follow‐up study were the main limitations. The retrospective and cohort nature of this study were the other limitations. Prospective multi‐center studies would be more accurate to confirm these initial results.
CONFLICT OF INTEREST
The authors declare that they have no competing interests or no conflict of interest.
ETHICAL STATEMENT
Because our study was in the category of noninterventional clinical research with its retrospective structure, we did not apply to ethics committee approval and an extra formal consent other than the patients had given prior to hospitalization was waived. This situation complies with the principles outlined in the Helsinki Declaration of 1975, as revised in 2008.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, İ.M., E.B.B.; Methodology, İ.M., M.C., S.T.; Investigation, İ.M., E.T., B.T., S.S., M.C.; Formal Analysis, İ.M., B.T., D.K., S.T.; Resources, E.T., M.N.A., M.C.; Writing ‐ Original Draft, İ.M., S.S., E.B.B., S.T.; Writing ‐ Review & Editing, İ.M., E.B.B., D.K., S.T., B.T.; Visualization, M.N.A., S.S.; Supervision, E.B.B., D.K., S.T.; Funding Acquisition, E.T.; Data Curation, M.N.A.
ACKNOWLEDGMENTS
The authors of this original article certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Mungan İ, Bostancı EB, Türksal E, et al. The predictive power of C‐reactive protein‐ lymphocyte ratio for in‐hospital mortality after colorectal cancer surgery. Cancer Reports. 2021;4:e1330. 10.1002/cnr2.1330
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Virchov R. Cellular Pathology as Based upon Physiological and Pathological Histology. Philadelphia, PA: J. B. Lippincott; 1863. [Google Scholar]
- 3. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539‐545. 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4. Zou ZY, Liu HL, Ning N, et al. Clinical significance of pre‐operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241‐2248. 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Späth SS, Weissman SM, Katz SG. An overview of advances in cell‐based cancer immunotherapies based on the multiple immune‐cancer cell interactions. Methods Mol Biol. 2020;2097:139‐171. 10.1007/978-1-0716-0203-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383‐4404. 10.3748/wjg.v25.i31.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solak Mekić M, Pedišić I, Šobat H, et al. The role of complete blood count parameters in Patıents Wıth colorectal cancer. Acta Clin Croat. 2018;57(4):624‐629. 10.20471/acc.2018.57.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40‐51. 10.1038/s41416-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89‐103. 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte‐C‐reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2019;272:342‐351. 10.1097/sla.0000000000003239. [DOI] [PubMed] [Google Scholar]
- 12. Wang F, He W, Jiang C, et al. Prognostic value of inflammation‐based scores in patients receiving radical resection for colorectal cancer. BMC Cancer. 2018;18(1):1102. 10.1186/s12885-018-4842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki Y, Okabayashi K, Hasegawa H, et al. Comparison of preoperative inflammation‐based prognostic scores in patients with colorectal cancer. Ann Surg. 2018;267:527‐531. 10.1097/SLA.0000000000002115. [DOI] [PubMed] [Google Scholar]
- 14. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25(6):1454‐1455. PubMed PMID: 29616422. Epub April 5, 2018. Eng. [DOI] [PubMed] [Google Scholar]
- 15. Santonocito C, De Loecker I, Donadello K, et al. C‐reactive protein kinetics after major surgery. Anesth Analg. 2014;119(3):624‐629. 10.1213/ANE.0000000000000263 PMID: 24878684. [DOI] [PubMed] [Google Scholar]
- 16. Straatman J, Cuesta MA, Gisbertz SS, Van der Peet DL. Value of a step‐up diagnosis plan: CRP and CT‐scan to diagnose and manage postoperative complications after major abdominal surgery. Rev Esp Enferm Dig. 2014;106(8):515‐521. PMID: 25544408. [PubMed] [Google Scholar]
- 17. Zhou QP, Li XJ. C‐reactive protein to albumin ratio in colorectal cancer: a meta‐analysis of prognostic value. Dose‐Response. 2019;17(4):1559325819889814. 10.1177/1559325819889814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang SF, Hung HY, Tang R, et al. Can neutrophil‐to‐lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Color Dis. 2012;27:1347‐1357. 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- 19. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020;92(10):1733‐1734. 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte‐to‐C‐reactive protein ratio and score are clinically feasible nutrition‐inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2019;39(4):1209‐1217. [DOI] [PubMed] [Google Scholar]
- 21. Daldal E, Akbas A, Dasiran MF, et al. Prognostic importance of neutrophil/lymphocyte and lymphocyte/crp ratio in cases with malignant bowel obstruction. Medicine. 2019;8(4):927‐930. [Google Scholar]
- 22. Liao M, Chen P, Liao Y, et al. Preoperative high‐sensitivity C‐reactive protein to lymphocyte ratio index plays a vital role in the prognosis of hepatocellular carcinoma after surgical resection. Onco Targets Ther. 2018;11:5591‐5600. 10.2147/OTT.S167857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


