Abstract
We herein report a case of allergic bronchopulmonary aspergillosis (ABPA) that occurred in a man treated with adalimumab for ankylosing spondylitis (AS). A 69‐year‐old man with a history of ankylosing spondylitis treated by adalimumab, an anti‐tumour necrosis factor‐α (TNF‐α) antibody, developed cough and wheezing. Chest computed tomography showed obstruction of dilated left upper lobe bronchus by high attenuation mucus as well as central bronchiectasis. Both Aspergillus‐specific immunoglobulin E (IgE) and Aspergillus precipitating antibody were positive and Aspergillus fumigatus was detected in a sputum culture. According to the new diagnostic criteria, the patient was diagnosed with ABPA. His condition rapidly improved after the withdrawal of adalimumab and initiation of prednisolone and itraconazole. Anti‐TNF‐α antibody might cause ABPA through both aggravation of the host's T‐helper 2 immunological response and anti‐fungal response.
Keywords: Adalimumab, allergic bronchopulmonary aspergillosis, ankylosing spondylitis, anti‐tumour necrosis factor‐α antibody
We report a case of allergic bronchopulmonary aspergillosis (ABPA) that occurred in a man receiving adalimumab for ankylosing spondylitis. This case suggests that anti‐tumour necrosis factor‐α (TNF‐α) antibody might cause ABPA in terms of disruption of the Th2/Th1 balance and the anti‐fungal response.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an eosinophilic inflammatory condition characterized by exaggerated immune responses to the fungal genus Aspergillus [1]. The development of ABPA involves the allergic host reaction to Aspergillus antigen. As the indications for treatment with anti‐tumour necrosis factor‐α (anti‐TNF‐α) antibody in patients with autoimmune diseases expand, adverse effects associated with allergic reactions have been reported [2, 3, 4, 5]. TNF‐α blockage might disrupt the T‐helper 2/T‐helper 1 (Th2/Th1) balance and decrease interferon‐γ production, which causes fungal colonization.
We herein report a case of ABPA that developed in a patient with ankylosing spondylitis treated by adalimumab, an anti‐TNF‐α antibody. Clinicians should understand the possibility of ABPA development in patients being treated with anti‐TNF‐α antibody.
Case Report
A 69‐year‐old man with a three‐year history of ankylosing spondylitis was referred to our hospital because of a one‐week history of wet cough and wheezing and the appearance of an infiltration shadow in the left upper lobe on chest X‐ray examination. He had smoked one pack of cigarettes per day until the age of 65 years. He had experienced asthma in childhood but had developed no asthma attacks even without medication since having become an adult. He had no history of atopic dermatitis. He had received therapy with adalimumab at 40 mg every two weeks for ankylosing spondylitis beginning at the age of 66 years, and his symptoms were relieved.
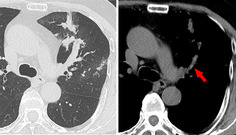
The patient's physical examination was only remarkable for wheezing on left chest auscultation. Blood tests revealed the following: white blood cell count, 6500/μL (normal range, 3300–8600/μL) with 1200/μL (18.5%) eosinophils (normal range, 100–500/μL, 1.0–5.0%); C‐reactive protein, 0.12 mg/dL (normal range, <0.14 mg/dL); β‐d glucan, <6.0 pg/mL (normal range, <11.0 pg/mL); serum immunoglobulin E (IgE), 221 IU/mL (normal range, <250 IU/mL); and Aspergillus‐specific IgE, 2.54 UA/mL (normal range, <0.35 UA/mL). Aspergillus precipitating antibody was positive. Forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), and FEV1/FVC ratio were 3.87 L (104.5% of predicted), 2.28 L (73.0% of predicted), and 59.84%, respectively. Aspergillus fumigatus was detected in a sputum culture. Chest computed tomography showed obstruction of dilated left upper lobe bronchus by high attenuation mucus and bronchiectasis (Fig. 1). Bronchoscopy revealed a mucus plug at the orifice of the left B3 bronchus. Aspergillus sp. was not cultured in the mucus plug, but eosinophilic infiltration and Charcot–Leyden crystals were present (Fig. 2). According to the new diagnostic criteria [1], the patient was diagnosed with ABPA despite not fulfilling the International Society for Human and Animal Mycology (ISHAM) criteria.
Figure 1.
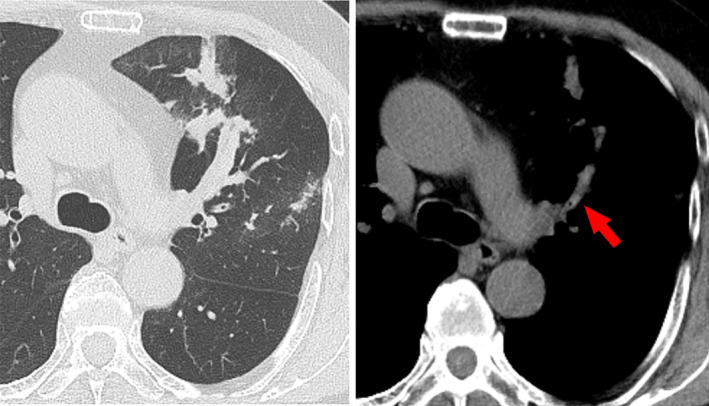
Axial plane chest computed tomography with the pulmonary window showed an obstruction of dilated left upper lobe bronchus by high attenuation mucus (arrow).
Figure 2.
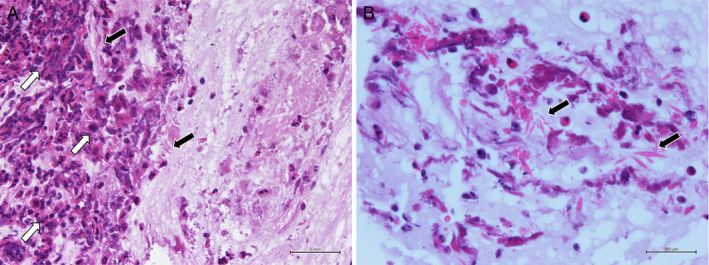
Microscopic examination. (A) Eosinophilic infiltration (white arrows) and Charcot–Leyden crystals (black arrows) are revealed (haematoxylin and eosin, 20×) and (B) Charcot–Leyden crystals (black arrows) are in the mucus plug (haematoxylin and eosin, 60×).
Adalimumab was discontinued, and systemic prednisolone (0.5 mg/kg/day = 30 mg/day) and itraconazole (200 mg/day) were initiated. The patient's symptoms and infiltration shadow on chest X‐ray examination improved. The prednisolone dosage was reduced to 15 mg/day on the 17th day after starting these therapies. Although adalimumab was resumed after two months and prednisolone was gradually reduced to 7.5 mg/day, the patient developed no recurrence of ABPA for six months.
Discussion
The development of ABPA involves an allergic host reaction to Aspergillus antigen in the bronchial tree [1]. The Th2 response towards Aspergillus can lead to allergic disease, especially in individuals with pulmonary diseases such as asthma and cystic fibrosis. Allergic forms of aspergillosis are thought to be mediated predominantly by a hyperactive Th2 response, leading to airway hypersensitivity, IgE production, and persistent airway inflammation. The hyperactive Th2 response elevates the concentrations of interleukins 5 and 13 and suppresses the interferon‐γ response, leading to further disruption of the Th2/Th1 ratio.
Treatment with anti‐TNF‐α antibody has been shown to be beneficial for Th1‐dominant diseases, including Crohn's disease, rheumatoid arthritis, and ankylosing spondylitis. Adverse effects of anti‐TNF‐α antibody include the development of Th2‐dominant diseases, such as atopic dermatitis and asthma [2, 3]. Although the detailed mechanism is still unknown, disruption of the Th1/Th2 balance by downregulation of Th1 might cause a Th2‐dominant pathological response with aggravation of airway inflammation.
Anti‐TNF‐α antibody might also aggravate intratracheal colonization or expansion with Aspergillus. Becker et al. reported that adalimumab decreased Aspergillus‐induced interferon‐γ [6], suggesting anti‐TNF‐α antibody might facilitate the development of colonization of Aspergillus, which drives Aspergillus‐specific Th2 response. ABPA patients not only show an increased Th2 response after stimulation with Aspergillus conidia, but also have an Aspergillus‐specific decreased interferon‐γ production [6].
ABPA might be caused by the synergic effect of anti‐TNF‐α antibody‐induced Th2 response in terms of disruption of the Th2/Th1 balance and Aspergillus‐specific Th2 response by fungal colony formation.
We used new diagnostic criteria consisting of 10 components for ABPA/allergic bronchopulmonary mycosis (ABPM) as proposed by Asano et al. [1] in 2020. The sensitivity of these criteria for physician‐diagnosed ABPA/ABPM is 94.4%; in contrast, the sensitivity of the Rosenberg–Patterson and ISHAM criteria is 49.2% and 82.7%, respectively [1]. The area under the curve for the receiver operating characteristic curves were 0.98, 0.85, and 0.90, respectively [1].
Many patients who develop ABPA have neither asthma nor a high total serum IgE concentration. The new criteria are useful to diagnose ABPA without needing to fulfil the old criteria. Although only four patients with ABPA after the initiation of anti‐TNF‐α therapy have been described [4, 5], clinicians might encounter patients with ABPA who do not fulfil the old criteria in terms of complicated immune modification.
In conclusion, we have herein reported a case of ABPA that occurred in a man receiving adalimumab for ankylosing spondylitis. This case suggests that anti‐TNF‐α antibody might cause ABPA in terms of disruption of the Th2/Th1 balance and the anti‐fungal response.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Author Contribution Statement
Yudai Suzuki, Sachi Matsubayashi, and Naoki Takasaka interpreted the patient's data. Ayako Kojima, Kyota Shinfuku, Tsukasa Hasegawa, Masami Yamada, Ikumi Fujisaka, Aya Seki, Yoshitaka Seki, Takeo Ishikawa, and Kazuyoshi Kuwano acquired the patient's data. All authors read and approved the final manuscript.
Acknowledgment
We thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Suzuki, Y , Takasaka, N , Matsubayashi, S , et al. (2021) Allergic bronchopulmonary aspergillosis in a patient with ankylosing spondylitis treated with adalimumab. Respirology Case Reports, 9(8), e00805. 10.1002/rcr2.805
Associate Editor: John Kolbe
References
- 1. Asano K, Hebisawa A, Ishiguro T, et al. 2021. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J. Allergy Clin. Immunol. 147:1261–8.e5. [DOI] [PubMed] [Google Scholar]
- 2. Chan JL, Davis‐Reed L, and Kimball A. 2004. Counter‐regulatory balance: atopic dermatitis in patients undergoing infliximab infusion therapy. J. Drugs Dermatol. 3:315–318. [PubMed] [Google Scholar]
- 3. Ahlmén M, Nordenskiöld U, Archenholtz B, et al. 2005. Rheumatology outcomes: the patient's perspective. A multicentre focus group interview study of Swedish rheumatoid arthritis patients. Rheumatology (Oxford) 44:105–110. [DOI] [PubMed] [Google Scholar]
- 4. Sasaki H, Miyata J, Maki Y, et al. 2019. Development of allergic bronchopulmonary aspergillosis in a patient with Crohn's disease. Intern. Med. 58:2835–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawasaki T, Kamiya M, Nakagawa A, et al. 2016. Allergic bronchopulmonary aspergillosis in a patient with rheumatoid arthritis under adalimumab therapy: a case report. Jpn. J. Clin. Immunol. 39(1):84–89. [DOI] [PubMed] [Google Scholar]
- 6. Becker K, Gresnigt M, Smeekens S, et al. 2015. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin. Exp. Allergy 45:423–437. [DOI] [PubMed] [Google Scholar]


