Abstract
Doege‐Potter syndrome is a rare hypoglycemic paraneoplastic disorder. This case describes that severe and symptomatic hypoglycemia can occasionally be due to a rare malignant neoplasm, and the differential diagnosis of malignancy should not be overlooked in this setting.
Keywords: big‐IGF2, Doege‐Potter syndrome, hypoglycemia, paraneoplastic syndrome, solitary fibrous tumor, STAT6
Doege‐Potter syndrome is a rare hypoglycemic paraneoplastic disorder. This case describes that severe and symptomatic hypoglycemia can occasionally be due to a rare malignant neoplasm, and the differential diagnosis of malignancy should not be overlooked in this setting.

1. INTRODUCTION
In 1930, Karl Doege and Roy Potter described an infrequent paraneoplastic syndrome characterized by nonislet cell tumor hypoglycemia (NICTH) mostly associated with solitary fibrous tumors (SFT), currently known as Doege‐Potter syndrome (DPS). 1
Solitary fibrous tumors is an uncommon tumor with a mesenchymal origin; it has 12%‐13% rate of malignancy, mostly located in the pleura. 1 Doege‐Potter syndrome is defined as NICTH associated with a SFT and has only been reported in <5% of SFT cases. 1
We present a case of diagnosis and management of DPS in a patient with an extrapleural malignant SFT.
2. CASE PRESENTATION
A 31‐year‐old Latin American woman with no previous pathological medical history was admitted to the emergency room with episodes of sweating, tremors, and decreased level of consciousness with documented hypoglycemia. A right indurated malar mass, measuring 10 cm, was observed.
She explained a 3‐year evolution right malar tumor; its appearance coincided with an exodontia. A surgical resection was proposed in her country, but was not performed.
Blood tests showed a noninsulin‐mediated hypoglycemia (24 mg/dL [70‐110 mg/dL]) with low levels of insulin (0.26 μIU/mL [2.00‐25.00 μIU/mL]), C‐peptide (0.10 ng/mL [1.10‐4.40 ng/mL]), and proinsulin (0.68 pmol/L [0.5‐3.5 pmol/L]).
Hypoglycemia with hypoinsulinemia secondary to severe hepatic insufficiency and cortisol deficiency was ruled out. The sulfonylureas test was negative. We also measured the levels of IGFII (1040 ng/mL [442‐1049 ng/mL]), IGFI (46.1 ng/mL [109‐284 ng/mL]), and IGFBP3 (3.8 mcg/mL [3.0‐7.8 mcg/mL]; Table 1).
TABLE 1.
Differential diagnosis of hypoglycemia
| Causes of hypoglycemia | |
|---|---|
| Endogenous hyperinsulinism |
Insulinoma Post‐bariatric surgery hypoglycemia Insulin autoimmune hypoglycemia |
| Factitious or accidental hypoglycemia |
Insulin Sulfonylureas Glinides |
| Hereditary diseases |
Hereditary hypoglycemia‐hyperammonemia syndrome Exercise‐induced hypoglycemia Ackee fruit toxicity |
| Drugs | Insulin, alcohol, pentamidine, quinine, ciprofloxacin, cybenzoline, indomethacin |
| Critical illness |
Sepsis Lactic acidosis Hepatic, renal or cardiac failure |
| Endocrine pathology |
Cortisol, growth hormone or glucagon deficiency Pheochromocytoma surgery |
| Paraneoplastic syndrome (IGF‐2) | Doege‐Potter syndrome |
| Malnutrition | Inanition |
| Nervous anorexia | Inanition |
A CT scan was performed and showed a solid tumor extending from the right temporal region to the nasopharynx, contacting the parotid gland, affecting the neck structures, and causing destruction of the zygomatic arch. Liver metastases were also present.
The MRI revealed a mass of 95 × 69 × 61 mm, invading the right intratemporal fossa, the right pterygopalatine fossa, and the right parotid space.
Octreotide scan demonstrated a significant uptake in the tumor indicating the presence of somatostatin receptors in the malar lesion, in contrast to a low uptake in the liver lesions.
The facial biopsy showed a spindle cell neoplasm having uniform fibroblastic cytomorphology, a patternless architecture of thin‐walled branching vessels with focally prominent hyaline collagenous stroma. The biopsy from the liver showed similar features but was more cellular, with mildly increased nuclear atypia and mitotic figures were identified, numbering up to 12 × 10 high‐power fields. An extensive immunohistochemistry study was performed on both biopsies, which showed negativity for CD34 and strong and diffuse nuclear positive staining for STAT6 in all tumor cells (Figure 1). Cell proliferation marker Ki67 resulted positive in 5% of the cells in the facial biopsy and in 20% in the hepatic biopsy. The case was finally diagnosed as a malignant SFT.
FIGURE 1.
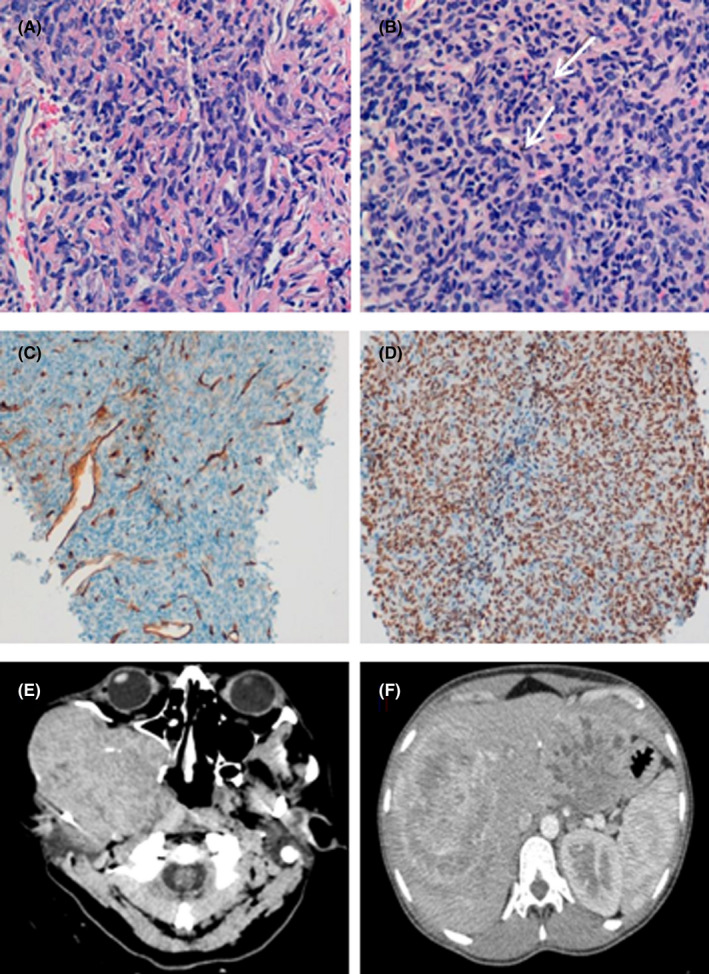
Histology and immunohistochemistry of SFT. A, Facial biopsy: spindle tumor cells with fibroblastic appearance and uniform extension with a patternless architecture and branched vessels with thin walls. B, Liver biopsy: tumor of similar characteristics, but more cellular, with greater nuclear atypia and with evidence of mitotic figures (arrows). C, Internal control: The CD34 marker is negative in tumor cells and positive in vascular walls. D, STAT6 staining shows intense, diffuse, and a uniform nuclear expression in the spindle tumor cells. E‐F: CT images. E, Large expansive lesion in the right chewing area. F, Liver with multiple metastatic lesions. The two largest lesions, in segment III and segment VIII of 110 and 150 mm, respectively
Initially, the patient required a continuous supply of glucose 10% solution concomitant with corticosteroid therapy and a high intake of carbohydrates to solve and avoid episodes of hypoglycemia.
Long‐acting subcutaneous octreotide was administered to the patient. This allowed for the cessation of the infusion of glucose 10% solution and a reduction in the corticosteroid therapy.
Chemotherapy with adriamycin and ifosfamide was initiated; the patient received a total of four cycles. The follow‐up with a CT scan showed a reduction in the size of the primary tumor (50 × 70 × 55 mm) and a liver progression as shown by an increase in the number and the size of the lesions.
Eventually, the patient decided to return to her home country, so further treatment and subsequent follow‐up could not be continued.
3. DISCUSSION
Doege‐Potter syndrome is a rare clinical entity and manifests itself as hypoglycemia associated with a SFT.
Hypoglycemia occurs due to the overproduction of the incomplete form of the insulin growth factor, known as big‐IGF2, by the tumor. 1 , 2 Increased glucose intake has also been reported in tumors greater than 10 cm, a measurement similar to the reported case.
Given the impossibility to directly measure big‐IGF2 levels, the ratio of IGF2:IGF1, an indirect marker used to quantify the mentioned levels was determined. A 3:1 ratio is considered normal while clinically significant levels are above 10. In our case, the levels were up to 22.
The most characteristic (albeit nonspecific) immunohistochemical finding is the CD34 expression; however, 5% of the STF may be negative. The NAB2‐STAT6 gene fusion, resulting in a chimeric protein (STAT6), was identified as a consistent finding in SFT. Nuclear expression of the carboxy‐terminal part of STAT6 is a highly sensitive and almost perfectly specific immunohistochemical marker for SFT and can be helpful to distinguish this tumor type from histologic mimics. 3
In our case, the immunohistochemistry for CD34 was negative. However, all tumor cells showed strong and diffuse nuclear positivity for STAT6, being consistent with the diagnosis of SFT.
Functionality tests showed an increased octreotide uptake in the primary tumor contrasting with fewer uptake in the liver metastases. It is not uncommon to observe a different behavior in growth, as well as response to treatment, between the primary tumor and the metastases.
The increased expression of somatostatin receptors in the malar tumor is consistent with a greater response to the treatment, but is in contrast to the progression of the liver metastasis. The discordance in the Ki67 index (lower in the primary tumor) is also remarkable.
The treatment of choice of SFT is surgical resection and usually resolves hypoglycemia definitively. 2 The clinical management of hypoglycemia includes oral or intravenous glucose therapy, corticosteroids, and somatostatin analogs.
Tumor responses of up to 50‐60% of patients have been reported for various regimens of anthracycline plus ifosfamide in soft tissue sarcomas (STS). 2 We chose the combination of adriamycin and ifosfamide as first‐line chemotherapy based on the experience in other STS subtypes as well as SFT. 4 , 5
We have analyzed the crude incidence rate of the STF in our geographical area (728.734 inhabitants according to the census, www.idescat.cat) since 1999 to 2016, using data from the Girona Cancer Registry. Crude incidence rate was 0.262 cases per 100 000 inhabitants year, supporting the data that SFT cases are rare. 6
In our cohort, 35 SFT were diagnosed, 15 were malignant (43%), mainly located in the thorax (59%). Twelve were localized and could be removed surgically. Three also received radiotherapy. Of the three cases diagnosed in the advanced stage, two received only chemotherapy and one best supportive care.
4. CONCLUSION
Doege‐Potter syndrome is a rare paraneoplastic syndrome associated with SFT, with physiopathological mechanism that is difficult to demonstrate. However, medical management can control symptoms regardless of the results of the chemotherapy treatment.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors: helped to draft the manuscript, read, and approved the final manuscript. RF‐C and RBSM are co‐authors and involved in collecting the data, performed the analysis, and wrote the paper. IG‐F: performed analysis of the data. AQG: performed the pathological diagnosis. AGM: performed the pathological and initial treatment of the patient. MPG: collected the data. JR‐C, MRS, and RPB: coordinated the drafting of the manuscript and its preparation for publication.
ETHICAL APPROVAL
This manuscript was originally written by the authors and has not been published elsewhere. Patient´s identity has been protected and treated confidentially.
CONSENT STATEMENT
Published with written consent of the patient.
ACKNOWLEDGMENTS
We thank Katie Linder and Marianela Pérez for reviewing the use of English in the manuscript.
Fort‐Culillas R, Barahona San Millán R, Garcia‐Fructuoso I, et al. Doege‐Potter syndrome in a facial solitary fibrous tumor: Diagnose and clinical management discussion. Clin Case Rep. 2021;9:e04291. 10.1002/ccr3.4291
Funding information
This publication has been supported by the Biomedical Research Institute of Girona (IDIBGI). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors have approved the final manuscript and had access to raw data in the study. The corresponding author had full access to all the data and the final responsibility to submit the manuscript for publication
DATA AVAILABILITY STATEMENT
All relevant data are included in the manuscript and can be obtained from the corresponding author upon reasonable request.
REFERENCES
- 1. Zafar H, Takimoto CH, Weiss G. Doege‐Potter syndrome: hypoglycemia associated with malignant solitary fibrous tumor. Med Oncol. 2003;20(4):403‐408. 10.1385/MO:20:4:403 [DOI] [PubMed] [Google Scholar]
- 2. Khowaja A, Johnson‐Rabbett B, Bantle J, Moheet A. Hypoglycemia mediated by paraneoplastic production of insulin like growth factor‐2 from a malignant renal solitary fibrous tumor ‐ clinical case and literature review. BMC Endocr Disord. 2014;14:49. 10.1186/1472-6823-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390‐395. 10.1038/modpathol.2013.164 [DOI] [PubMed] [Google Scholar]
- 4. Schöffski P, Timmermans I, Hompes D, et al. Clinical presentation, natural history, and therapeutic approach in patients with solitary fibrous tumor: a retrospective analysis. Sarcoma. 2020;2020:1385978. 10.1155/2020/1385978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leyvraz S, Zweifel M, Jundt G, et al. Long‐term results of a multicenter SAKK trial on high‐dose ifosfamide and doxorubicin in advanced or metastatic gynecologic sarcomas. Ann Oncol. 2006;17(4):646‐651. 10.1093/annonc/mdl020 [DOI] [PubMed] [Google Scholar]
- 6. Thorgeirsson T, Isaksson HJ, Hardardottir H, Alfredsson H, Gudbjartsson T. Solitary fibrous tumors of the pleura: an estimation of population incidence. Chest. 2010;137(4):1005‐1006. 10.1378/chest.09-2748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the manuscript and can be obtained from the corresponding author upon reasonable request.


