Abstract
Hypopituitarism secondary to a pituitary metastasis is rare and difficult to diagnose since its symptoms are nonspecific. The presence of visual deficits and nerve palsies should suggest the presence of a pituitary metastasis in a cancer patient.
Keywords: breast carcinoma, hemianopsia, hypopituitarism, ophthalmoplegia, pituitary metastasis, ptosis
Hypopituitarism secondary to a pituitary metastasis is rare and difficult to diagnose since its symptoms are nonspecific. The presence of visual deficits and nerve palsies should suggest the presence of a pituitary metastasis in a cancer patient.
1. INTRODUCTION
Tumors of the pituitary gland and the sellar region represent approximately 15% of all brain tumors. 1 Adenomas are the most frequent tumors of the pituitary gland. However, other tumors have been described such as carcinoma, blastoma, craniopharyngioma, germ cell tumors, mesenchymal tumors, and secondary tumors. The sellar region is known as a low‐risk brain metastasis area. 2 The prevalence of pituitary metastasis represents 1% of all surgical tumors of the pituitary gland. 3 Their most common primary sources are breast and lung cancer. 4 They tend to affect elderly patients in the sixth or seventh decade of life, and they are more frequent in patients with disseminated systemic metastases. 5 , 6 The diagnosis and the treatment of pituitary metastases are challenging.
Herein, we report a case of hypopituitarism secondary to a pituitary metastatic breast cancer in a 32‐year‐old woman.
2. OBSERVATION
A 32‐year‐old woman was referred to our department of endocrinology for hypopituitarism. Her past personal medical history included an invasive ductal breast carcinoma diagnosed 2 years earlier, treated with a left mastectomy, axillary lymph node dissection, and chemotherapy. During the last year, multiple bone metastases were diagnosed and she was treated with palliative radiotherapy.
As symptoms, she presented with a deterioration of her general condition with weakness and weight loss, nausea and vomiting, headache, blurred vision, and decreased visual acuity in the 2 weeks prior to referral. She had a secondary amenorrhea for 6 months. No polyuria was reported.
On physical examination, she had a supine blood pressure of 100/70 mm Hg, a standing blood pressure of 90/70 mm Hg, a regular pulse of 86 beats/min, a unilateral ptosis, and an ophthalmoplegia. Thyroid, abdominal, and neurological examinations were normal.
Laboratory investigations showed a fasting blood glucose of 5.5 mmol/L, a serum sodium level of 131 mmol/L, a serum potassium level of 4.2 mmol/L, a serum urea level of 0.35 g/L, a serum creatinine level of 13 mg/L, a serum calcium level of 86 mg/L, a morning cortisol level of 1.45 µg/dL (normal ranges: 4‐20), an ACTH level of 5.9 pg/mL, a FT4 level of 0.2 ng/dL (normal ranges: 0.7‐1.5), a TSH level of 0.3 mIU/L (normal ranges: 0.35‐4.95), a prolactin level of 5 µg/L (normal ranges <25), a FSH level <1 IU/L (normal ranges: 3‐8), and a LH level <1 IU/L (normal ranges: 0.6‐12).
The ophthalmologic examination revealed a bilateral papilledema and a bitemporal hemianopsia. The magnetic resonance imaging (MRI) showed a large heterogeneous mass involving the pituitary anterior lobe measuring 33 × 23 × 19 mm with an isointense signal on both T1‐ and T2‐weighted imaging and an intensive homogeneous enhancement after gadolinium administration. The lesion extended into the optic chiasm, the left cavernous sinus and the sphenoid sinus (Figure 1). There were no signs of apoplexy, and the posterior lobe bright spot was normal.
FIGURE 1.
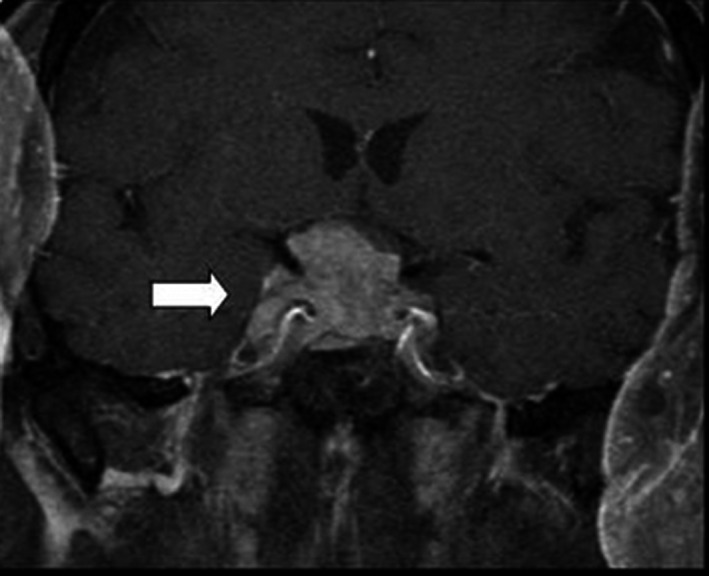
Magnetic resonance imaging of the pituitary gland showing a large heterogeneous mass involving the pituitary anterior lobe with extension into the optic chiasm, and the cavernous and the sphenoid sinus
The patient was treated with hydrocortisone and lévothyroxine, and underwent an endoscopic transsphenoidal surgery with a subtotal resection of the tumor. The histopathological findings identified the mass as a pituitary metastasis. After surgery, the patient was lost to follow‐up.
3. DISCUSSION
Pituitary metastasis is a rare condition, which was described for the first time by L. Benjamin in 1857. 7 It can arise from many tumors, but it is more often observed in lung and breast cancers, which represent over 60% of reported pituitary metastasis cases. 8 , 9
Pituitary metastases occur in 6%‐8% of breast cancer cases and usually affect elderly patients in the sixth or seventh decade of life. 6 The mean age at presentation is about 60 years. 10 Young cases as in our patient are very rare. 11
Pituitary metastases may involve both the anterior and the posterior lobe. The latter is more susceptible to metastatic lesions by a hematogenous spread due to its direct systemic arterial blood supply. 12 For this reason, the most common presentations include diabetes insipidus. 9 Metastatic colonization may also reach the anterior lobe through the hypothalamus‐hypophyseal portal circulation system. 12 The anterior lobe is affected alone as in our patient in 15.4% of cases. 3 A further involvement of the posterior lobe remains possible.
Pituitary metastases are usually asymptomatic and incidentally discovered. 3 However, a variety of symptoms are present in 2.5%‐18.2% of cases. 3 Diabetes insipidus is the most frequent symptom. 9 Other reported symptoms are headache and hypopituitarism and visual field deficits. Patients may present symptoms of gonadotropin, thyrotropin, and corticotropin deficiencies. 13 The latter may mask the ADH deficiency until corticosteroid treatment is initiated. 12 This was not the case of our patient since her posterior pituitary lobe was not involved. Symptoms such as weakness, vomiting, and weight loss are nonspecific and may mask anterior pituitary deficiency in cancer patients. 14 Visual deficits secondary to suprasellar extension were reported in 50% of cases. 14 Diplopia, ptosis, and painful ophthalmoplegia were frequent in patients with cavernous sinus involvement. 15
Differential diagnoses of pituitary metastases include adenomas, craniopharyngiomas, Rathke's cleft cysts, and aneurysms. 16 The imaging techniques are not very helpful for the diagnosis of pituitary metastasis because of the lack of specific radiological signs. Magnetic resonance imaging (MRI) usually reveals a non‐homogenous invasive mass with a suprasellar extension and infundibulum thickening. 3 , 8 , 17 Pituitary metastases are hypointense or isointense to the brain on T1‐weighted images and hyperintense on T2‐weighted images with homogeneous enhancement after gadolinium. 3 The infundibulum thickening, the invasion of the cavernous sinus, and the sclerosis of the surrounding sella turcica are helpful signs for the diagnosis of metastatic masses. 17 Other suggestive images of pituitary metastases also include a dumbbell‐shaped intra‐ and suprasellar tumor with a clear indentation at the level of the diaphragma sellae. 3 , 5 Furthermore, the rapid growth of the lesion on sequential MRI suggests a malignant disease. 18 The diagnosis of pituitary metastasis is confirmed by histopathological investigation as in our case.
The treatment options of pituitary metastases include surgical resection, radiosurgery, radiotherapy, chemotherapy, and hormone therapy. Radiotherapy and chemotherapy are indicated in patients with multifocal metastases. Surgical resection is often difficult because of the invasion of the cavernous sinus and nearby structures and the hypervascularization of the tumor. 3 It requires highly trained neurosurgeons. Surgical resection is indicated to decompress the optic nerves and chiasm and enables histological diagnosis. Transsphenoidal surgery is the preferred approach. Radiosurgery is limited in the delivery of high doses because of the adjacent anatomic structures; nevertheless, it has an effective local control and helps reduce neurological deficits and symptoms of diabetes insipidus. 19 Surgical and postoperative radiations are combined to reduce the risk of tumor progression and to treat persistent tumor. Those therapies may cause panhypopituitarism. Therefore, hormonal assessment and replacement therapy are required.
Poor prognosis is reported in patients with pituitary metastases, and surgery, radiotherapy, and chemotherapy are unable to increase survival. The mean survival is about 6‐7 months after the diagnosis, and the one‐year survival rate is 10%‐36%. 3 , 14 A shorter survival is expected in younger patients <65 years with a shorter time frame between the initial cancer diagnosis and the pituitary metastasis detection. 3
4. CONCLUSION
Hypopituitarism secondary to a pituitary metastasis is a rare condition and is difficult to diagnose since its symptoms are nonspecific. However, the presence of visual deficits and cranial nerve palsies should suggest the presence of a pituitary metastasis in a cancer patient, particularly in a context of a disseminated disease. Hormone replacement therapy is indicated if necessary. The management of the pituitary metastasis depends on the extent of the disease and the severity of symptoms.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
IO: conceived and designed the study, performed acquisition and interpretation of data, and contributed to manuscript creation and drafting. SA performed acquisition of data and manuscript drafting. MC critically revised the article for important intellectual content. All authors were involved in the management of this patient and the revision of the manuscript, and approved the final version.
ETHICAL APPROVAL
Ethical approval for this case report was not required. A written informed consent was obtained from the patient for the publication of this report.
ACKNOWLEDGMENT
Nil.
Oueslati I, Ayari S, Yazidi M, Bouali S, Khessairi N, Chihaoui M. Hypopituitarism secondary to a pituitary metastasis in a young woman with an invasive breast carcinoma. Clin Case Rep. 2021;9:e04175. 10.1002/ccr3.4175
DATA AVAILABILITY STATEMENT
No data available.
REFERENCES
- 1. Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28(3):228‐243. [DOI] [PubMed] [Google Scholar]
- 2. Lin B, Huang D, Yang X, et al. Distribution of brain metastases: low‐risk metastasis areas may be avoided when treating with whole‐brain radiotherapy. Cancer Imaging. 2020;20(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89(2):574‐580. [DOI] [PubMed] [Google Scholar]
- 4. Ravnik J, Smigoc T, Bunc G, et al. Hypophyseal metastases: A report of three cases and literature review. Neurol Neurochir Pol. 2016;50(6):511‐516. [DOI] [PubMed] [Google Scholar]
- 5. Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am. 1999;28:81‐117. [DOI] [PubMed] [Google Scholar]
- 6. Fortunati N, Felicetti F, Donadio M, et al. Pituitary lesions in breast cancer patients: a report of three cases. Oncol Lett. 2015;9(6):2762‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiang MFBM, Patt S. Pituitary metastases. Neurochirurgia (Stuttg). 1990;33:127‐131. [DOI] [PubMed] [Google Scholar]
- 8. He W, Chen F, Dalm B, et al. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary. 2015;18:159‐168. [DOI] [PubMed] [Google Scholar]
- 9. Javanbakht A, D'Apuzzo M, Badie B, et al. Pituitary metastasis: a rare condition. Endocr Connect. 2018;7(10):1049‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng S, Fomekong F, Delabar V, et al. Current status and treatment modalities in metastases to the pituitary: a systematic review. J Neurooncol. 2020;146(2):219‐227. [DOI] [PubMed] [Google Scholar]
- 11. Ito I, Ishida T, Hashimoto T, et al. Hypopituitarism due to pituitary metastasis of lung cancer: case of a 21‐year old man. Intern Med. 2001;40(5):414‐417. [DOI] [PubMed] [Google Scholar]
- 12. Shimon I. Metastatic spread to the pituitary [published online ahead of print, 2020 Feb 27]. Neuroendocrinology. 2020;110(9‐10):805‐808. 10.1159/000506810 [DOI] [PubMed] [Google Scholar]
- 13. Marsh JC, Garg S, Wendt JA, et al. Intracranial metastatic disease rarely involves the pituitary: retrospective analysis of 935 metastases in 155 patients and review of the literature. Pituitary. 2010;13(3):260‐265. [DOI] [PubMed] [Google Scholar]
- 14. Morita A, Meyer FB, Laws ER Jr. Symptomatic pituitary metastases. J Neurosurg. 1998;89(1):69‐73. [DOI] [PubMed] [Google Scholar]
- 15. Altay T, Krisht KM, Couldwell WT. Sellar and parasellar metastatic tumors. Int J Surg Oncol. 2012;2012:647256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuang Y‐C, Tsai C‐C. Pituitary metastasis of breast cancer: a case report. Inter J Gerontol. 2018;12(3):267‐270. [Google Scholar]
- 17. Post KD. Pituitary metastases: what is the role of surgery? World Neurosurg. 2013;79:251‐252. [DOI] [PubMed] [Google Scholar]
- 18. Bonneville JF. Pituitary metastases. In MRI of the Pituitary Gland. Cham: Springer; 2016:229‐234. 10.1007/978-3-319-29043-0_33 [DOI] [Google Scholar]
- 19. Kano H, Niranjan A, Kondziolka D, et al. Stereotactic radiosurgery for pituitary metastases. Surg Neurol. 2009;72:248‐255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data available.


