Abstract
BACKGROUND
Despite significant advances in our understanding of the pathophysiology of preeclampsia (PE), there are still many unknowns and controversies in the field. Women undergoing frozen-thawed embryo transfer (FET) to a hormonally prepared endometrium have been found to have an unexpected increased risk of PE compared to women who receive embryos in a natural FET cycle. The differences in risk have been hypothesized to be related to the absence or presence of a functioning corpus luteum (CL).
OBJECTIVE AND RATIONALE
To evaluate the literature on secretory products of the CL that could be essential for a healthy pregnancy and could reduce the risk of PE in the setting of FET.
SEARCH METHODS
For this review, pertinent studies were searched in PubMed/Medline (updated June 2020) using common keywords applied in the field of assisted reproductive technologies, CL physiology and preeclampsia. We also screened the complete list of references in recent publications in English (both animal and human studies) on the topics investigated. Given the design of this work as a narrative review, no formal criteria for study selection or appraisal were utilized.
OUTCOMES
The CL is a major source of multiple factors regulating reproduction. Progesterone, estradiol, relaxin and vasoactive and angiogenic substances produced by the CL have important roles in regulating its functional lifespan and are also secreted into the circulation to act remotely during early stages of pregnancy. Beyond the known actions of progesterone and estradiol on the uterus in early pregnancy, their metabolites have angiogenic properties that may optimize implantation and placentation. Serum levels of relaxin are almost undetectable in pregnant women without a CL, which precludes some maternal cardiovascular and renal adaptations to early pregnancy. We suggest that an imbalance in steroid hormones and their metabolites and polypeptides influencing early physiologic processes such as decidualization, implantation, angiogenesis and maternal haemodynamics could contribute to the increased PE risk among women undergoing programmed FET cycles.
WIDER IMPLICATIONS
A better understanding of the critical roles of the secretory products of the CL during early pregnancy holds the promise of improving the efficacy and safety of ART based on programmed FET cycles.
Keywords: corpus luteum, preeclampsia, estradiol, estradiol metabolites, progesterone, relaxin, implantation, angiogenesis, placentation, frozen-thawed embryo transfer
Introduction
In the United States, the number of assisted reproductive technologies (ART) cycles per year has nearly tripled from 2000 through 2018 (from 99,629 to 306,197), and today approximately 1.9% of all live births are the result of these techniques (Luke, 2017; Crawford and Ledger, 2019; CDC, 2020). This dramatic rise in the use of ART has allowed a continuous refinement of the techniques, but also has uncovered unanticipated outcomes. For instance, although frozen-thawed embryo-transfer (FET) has been one of the most commonly used procedures, programmed cycle FET, in which estradiol and progesterone are supplemented in the absence of a corpus luteum (CL), has recently been associated with increased rates of preeclampsia (PE) among other complications (Ginström Ernstad et al., 2019; von Versen-Hoynck et al., 2019b). Although initially this was hypothesized to result from the freeze-thaw process, a recent study found that programmed FET cycles were three times more likely to result in PE compared to modified natural FET cycles, where vitrified embryos were transferred in an unstimulated ovulatory cycle, in the presence of a single CL (von Versen-Hoynck et al., 2019b). The adverse obstetric outcome, confirmed in subsequent studies (Ginström Ernstad et al., 2019; Singh et al., 2020), supported the hypothesis presented by Conrad and Baker regarding the potential protective role of the CL and its secretory products against PE (Conrad and Baker, 2013).
PE, diagnosed as onset of hypertension after 20 weeks of gestation in association with significant proteinuria and/or evidence of organ damage, is a major cause of maternal-foetal morbidity and mortality worldwide (Ghulmiyyah and Sibai, 2012; ACOG, 2019). Although the pathophysiology of PE remains incompletely understood, a prevalent theory proposes that abnormal placentation and impaired spiral artery remodelling and angiogenesis trigger an increase in the resistance of the utero-placental circulation, resulting in hypertension, activation of an inappropriate inflammatory response and global endothelial dysfunction (Roberts and Gammill, 2005).
The CL is a transitory organ which is the major source of steroid hormones, vasoactive and angiogenic regulating substances that play critical roles in the initial stages of pregnancy (Conrad, 2011; Henríquez et al., 2016; Conrad et al., 2019a). As the increased risk of PE in programmed FET cycles occurs despite presumably adequate supplementation of estradiol (E2) and progesterone (P), other secretory products of the CL may be also essential for normal placentation and angiogenesis. Here, we review the current understanding of the role of CL as an endocrine organ in the context of normal pregnancy and PE. We identify some missing links in the chain of PE pathophysiology to guide future research on the beneficial role of the CL in FET cycles.
Evidence suggesting that the CL plays a role in reducing the risk of preeclampsia
Endocrine function and lifespan of the CL in a cycle of conception
The CL is the major source of P, the quintessential hormone of pregnancy, which transforms the uterine endometrium into a receptive state for the developing embryo. However, it also secretes steroid hormone metabolites (e.g. 5α-dihydroprogesterone, among others), estradiol (E2), oestrogen metabolites (EM; hydroxylated and methylated oestrogens), vascular endothelial growth factor (VEGF), prostaglandins and relaxin (Conrad, 2011; Henríquez et al., 2016).
The morphology, function and secretory capabilities of CL cells change throughout the luteal phase (Carrasco et al., 1996). The human CL is composed of steroidogenic (theca lutein and granulosa lutein) and non-steroidogenic cells, including endothelial and immune cells, which contribute to the synthesis and secretion of hormones (Fig.1). The secretion of P increases together with 17α-hydroxyprogesterone (17α-OHP) after the luteinizing hormone (LH) surge triggers ovulation, initiating luteinization of granulosa and theca cells (Devoto et al., 2009). Granulosa cell luteinization is associated with increased expression of LH receptors and P receptors (PR), as well as steroidogenic acute regulatory protein (STARD1), P450 cholesterol side-chain cleavage enzyme, cyclooxygenase-2 and members of the matrix metalloproteinase family, all of which are critical determinants of P synthesis, oocyte maturation and follicular rupture (Devoto et al., 2009).
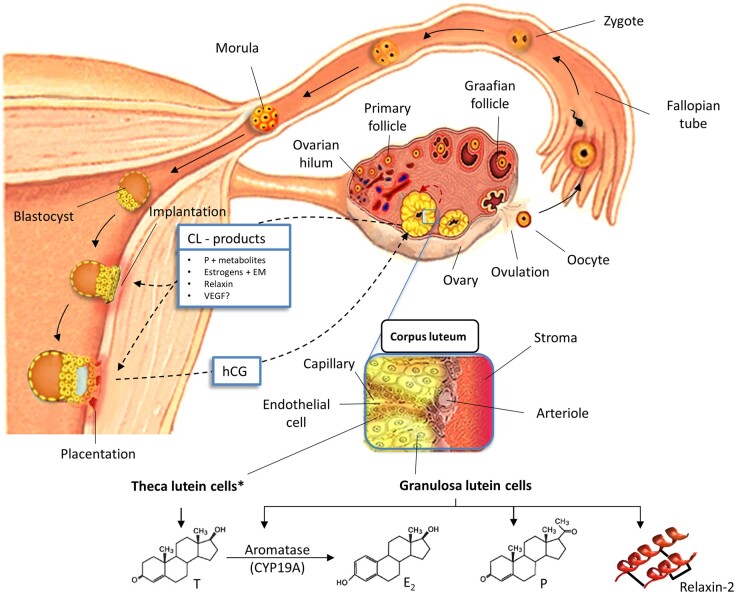
Figure 1.
Schematic representation of the systemic cross-talk (black dashed arrows) between the embryo and the CL during early pregnancy (<10 weeks). The CL produces multiple steroid and polypeptide hormones that control its own lifespan (i.e. paracrine regulation, red dashed arrow), but also that act remotely (i.e. systemic regulation) to guide embryo implantation and placentation. The elaboration of hCG by the trophoblast prevents regression of the CL (i.e. luteolysis). The latter responds to the embryo with the release of proangiogenic and vasoactive substances that further support its growth and development. The CL is mainly composed of two hormone-producing cell types, theca lutein and granulosa lutein cells, that work collaboratively in steroidogenesis. *Although most of the circulating relaxin-2 is produced by granulosa lutein cells, theca cells represent a significant local source of relaxin-2. For further information see text. CL: corpus luteum; E2: estradiol; EM: ooestrogen metabolites; hCG: human chorionic gonadotropin; P: progesterone; T: testosterone; VEGF: vascular growth factor.
E2 biosynthesis is dependent upon LH-driven androgen production by theca cells, followed by androgen aromatization by aromatase expressed in granulosa cells under the influence of FSH (Fig. 1). This two-cell, two-gonadotropin system of E2 biosynthesis in the ovarian follicle is mirrored by theca lutein and granulosa lutein cells of the CL (Henríquez et al., 2016).
LH/human chorionic gonadotropin (hCG) are of major importance in regulating CL secretory function and structure (Duncan et al., 2009). The effects of LH/hCG on CL steroidogenesis are modified by a variety of molecules encompassing growth factors, hormones, nitric oxide (NO), cytokines, insulin-like growth factor 1 and IGF-binding proteins (Devoto et al., 2000). The expression of E2 receptors (ERα and ERβ) has been consistently detected in human and monkey CL (Duffy et al., 2000; van den Driesche et al., 2008). Moreover, 17β-hydroxysteroid dehydrogenase type 1, which converts oestrone (E1) into E2 (a more potent oestrogen) in ovarian cells, reaches its maximum expression just before the late luteal phase (Vaskivuo et al., 2002). These data suggest that E2 may act as a paracrine regulator of luteal function and CL lifespan.
Oestrogen metabolites (EMs) produced by the CL may also have luteolytic (e.g. 2-methoxyestradiol [2-ME2], 2-methoxyoestrone [2-ME1]) and luteotrophic (e.g. 16-ketoestradiol [16-ketoE2], 4-hydroxyoestrone [4-OHE1]) functions in different species (Duffy et al., 2000; Henríquez et al., 2016). In an experimental study, CLs of women at varying stages of the luteal phase were collected and levels of EMs and VEGF, and their angiogenic activity, were determined (Henríquez et al., 2016). Although EMs with proangiogenic activity were higher in the early and mid-luteal phases, late luteal phase CL were characterized by significantly higher levels of EMs with antiangiogenic activity (Henríquez et al., 2016). During the early luteal phase, endothelial cells within the CL proliferate to establish a rich capillary network critical for the delivery of gonadotropins and precursors for P production synthesis (i.e. lipoprotein cholesterol) and removal of secretory products from luteal cells (Devoto et al., 2009; Lu et al., 2019). When conception occurs, hCG produced by trophoblast cells prevents regression of the CL from its programmed senescence (i.e. luteolysis), allowing for the continued secretion of substances that sustain the uterine environment until the placenta takes over its function. This physiologic milestone has been named ‘CL rescue’, and was recreated in monkeys by showing that the administration of exponentially increasing doses of hCG (mimicking conception) prolonged the lifespan of the CL (Zeleznik, 1998). However, the rescue mechanism is dependent on the age of the CL. Accordingly, althoughthe CL is relatively insensitive to exogenous hCG in the early luteal phase, the responsiveness of the CL increases from the mid-to-late luteal phase; a dramatic increase in plasma P4 and 17α-OHP and an increase in the expression of STARD1 identified by immunohistochemistry was seen following hCG treatment in the late luteal phase, compared to the early luteal phase (Kohen et al., 2003).
Although P has been proposed to be an autocrine/paracrine factor that rescues the CL in conception cycles, down-regulation of PRs in the CL during the late-luteal phase is not prevented by hCG, an observation that is not consistent with a major role for P in CL rescue (Duncan et al., 2005). However, Henríquez et al. (2016) showed that the administration of hCG also increased the production of EMs with pro-angiogenic activity and reduced the production of EMs with anti-angiogenic actions, suggesting a potential mechanism to explain, at least in part, the local role of steroid hormones in CL rescue (Henríquez et al., 2016). Conversely, in the absence of hCG the human CL undergoes functional and structural changes, including a significant reduction in P secretion and loss of the glandular vascular network (Christenson and Devoto, 2003; Devoto et al., 2001).
The human CL also represents the main source of relaxin, a 6-kDa peptide hormone with high structural similarity to insulin (Fig. 1) (Marshall et al., 2017). Relaxin production begins a few days after ovulation and reaches its peak in the latter half of the luteal phase of the ovarian cycle, after which its production is interrupted at luteolysis (Anand-Ivell and Ivell, 2014). If pregnancy occurs, relaxin continues to be produced as long as the CL functionally persists. Although the main relaxin receptor (RFXP1, also called LGR7) has been widely identified in human and non-human CLs (Maseelall et al., 2009), the local effect of relaxin as a luteotrophic/luteolytic factor is not clearly defined. Relaxin significantly increases CL production of P and E2 (and potentially VEGF) during the mid and especially late luteal phase (Beindorff and Einspanier, 2010), but also increases matrix metalloproteinases, that may mediate local connective tissue remodelling (Maseelall et al., 2009).
VEGF has been identified as a crucial substance not only in controlling CL structure but also in influencing its function. Inhibition of VEGF close to the time of ovulation and in the early luteal phase significantly impairs the development of the luteal microvasculature and also decreases P secretion (Duncan et al., 2009). Notably, VEGF expression by cultured luteinized granulosa cells and in mature CLs in vivo seems to be under hormonal control (i.e. LH/hCG) and in response to hypoxia (i.e. hypoxia-inducible factor [HIF]-1α) (Duncan et al., 2008; 2009).
Collectively, the findings reviewed above show that a number of factors influencing angiogenesis, working in concert in a time-dependent fashion, regulate the functional lifespan of the CL. By extension, the absence or imbalance of these CL factors during early stages of pregnancy may increase the risk of disorders of vascularization.
Role of secretory products of the CL in normal embryo implantation and placentation
Embryo implantation, which is dependent upon a competent blastocyst and uterine receptivity, takes place in the mid-to-late luteal phase (Zhang et al., 2013). During implantation, a subset of cytotrophoblasts adopt a vascular phenotype as they differentiate and invade the uterine spiral arteries, initiating a major remodelling of the uterine arterial wall caused by apoptosis, dedifferentiation of the muscular layer, and replacement by extravillous trophoblast cells and fibrinoid deposits (Whitley and Cartwright, 2009). This results in a loss of local vascular tone and a failure of the vessels to react to powerful vasoactive substances (Berkane et al., 2017). Moreover, as trophoblast invasion evolves, there is a temporary accumulation of trophoblast cells that occlude the spiral artery lumen, resulting in a transient hypoxic environment in the placenta, and consequently, local overexpression of hypoxia inducible factor-1α (HIF-1α) (Liu et al., 2002). The latter substance promotes VEGF mRNA expression to support angiogenesis and placental development (Wheeler et al., 1995; Liu et al., 2002). In a progressive process, the trophoblast plugs are dispersed during the first trimester of pregnancy, allowing the restoration of a normoxic environment, which is essential for foetal and placental development.
Both P and E2 secreted by the CL seem to have important roles in endometrial maturation, angiogenesis, vasodilation and placentation (Young, 2013). P acts in the process of predecidualization, in which the endometrium converts to a secretory pattern that is highly vascularized and produces secretions that encourage a favourable intrauterine milieu for embryo attachment and implantation. With pregnancy, the lining of the endometrium undergoes further morphologic and functional changes, becoming decidualized, which closes the temporal window for implantation and creates an adequate environment for placentation and embryo development. The process of decidualization, and also its maintenance, is regulated, at least in part, by P (Okada et al., 2018). In studies with embryo transfers, high levels of P led to both lower uterine contraction rates and changes in the contractility pattern, enhancing the ability of the embryo to interact with the endometrium, resulting in higher implantation rates (Fanchin et al., 2001). The expression of certain factors (e.g. insulin-growth factor binding protein-1, prolactin and interleukin-15, glycodelin [a progesterone-associated endometrial protein]) may play a key role in endometrial maturation (Founds et al., 2009). Indeed, the down-regulation of these factors in chorionic villous samples has been observed in women who subsequently developed severe PE (Founds et al., 2009). Hence, defective endometrial maturation may be implicated in the future development of PE (Conrad, 2020). Finally, P also induces uterine angiogenesis and vascular remodelling in the anti-mesometrial and mesometrial regions of the rodent uterus via VEGF-A/VEGFR-2 signalling and angiopoietin-2, respectively (Kim et al., 2013; Park et al., 2020).
Although later in pregnancy oestrogen is produced by the placental syncytiotrophoblast by conversion of androgen precursors originating mostly from the foetal adrenal glands, the main source of oestrogens in the very early stages of pregnancy is the CL. More precisely, at approximately 9 weeks of gestation, a CL-to-placenta shift occurs, resulting in a greater contribution of placental P and E2 (Berkane et al., 2017). Although endometrial proliferation is the classic and most studied role of oestrogens, their other function in implantation and placentation have been increasingly recognized. Indeed, E2 increases both NO synthesis and levels of angiogenic factors, such as VEGF and placental growth factor (PlGF) (Hervé et al., 2006; Johnson et al., 2006). Data have also suggested that EMs could enable cytotrophoblasts to differentiate into an invasive endovascular phenotype under low-oxygen conditions (Lee et al., 2010). Consistent with this observation, another study found that under oxygen levels mimicking in-vivo conditions, the decrease in 2-ME2, an EM, inhibited trophoblast cell migration (Shen et al., 2014).
Recently, the role of relaxin in implantation and placentation has regained attention. Circulating levels of this hormone peak toward the end of the first trimester and remain relatively constant at intermediate levels throughout the rest of the pregnancy (Marshall et al., 2017; Conrad et al., 2019a). As explained later in greater detail, the CL represents the primary source of circulating relaxin in pregnant women. Thus, it seems that the luteal-placental shift at the end of the first trimester occurs mainly with respect to steroidogenesis, and residual relaxin production still occurs even though the CL undergoes substantial structural regression after the first trimester (Conrad et al., 2019a). Although the most important function of relaxin seems to be related to the maternal cardiovascular adaptation to pregnancy (e.g. reduction of both arterial stiffness and peripheral vascular resistance with a consequent rise in cardiac output [CO]) (Conrad, 2011; Devarakonda and Salloum, 2018; Conrad et al., 2019b), it also regulates VEGF expression in the endometrium and supports decidualization, implantation and pregnancy (Kaczmarek et al., 2008; Anand-Ivell and Ivell, 2014). In an experimental study in the marmoset, uterine RNA and protein expression of relaxin and one of its receptors, RXFP1, were highly upregulated shortly before and during implantation (Einspanier et al., 2009). In addition, the action of relaxin on the uterus was accompanied by an increase of oestrogen-associated factors and macrophage infiltration (responsible for foetal-maternal immune adjustment), suggesting regulation of redundant systems necessary for successful implantation. Increasing angiogenesis as well as earlier and faster growth of the uterus and placenta were also seen in relaxin-treated animals (Einspanier et al., 2009). These and other observations have raised the question as to whether the introduction of relaxin in ART cycles lacking a CL would protect pregnant women from pregnancy complications associated with insufficient placentation (Conrad and Baker, 2013; Conrad, 2020).
Evidence from ART that a physiologic number of CL protects against preeclampsia
FET facilitates elective single-embryo transfer, reduces the incidence of ovarian hyperstimulation syndrome, and potentially allows time for preimplantation genetic testing, all resulting in high rates of live births (Singh et al., 2020). Furthermore, singletons born after FET seem to have a reduced risk of low birthweight, small for gestational age and preterm birth compared to singletons born after fresh embryo transfer, although outcomes are worse when compared to singletons born after unassisted conception (Sha et al., 2018; Ginström Ernstad et al., 2019). These benefits have resulted in steadily increasing use of FET, representing ∼34% of all ART procedures in the United States in 2018 (CDC, 2020). As mentioned previously, FET is commonly performed in the context of a programmed cycle in which ovulation is suppressed, resulting in the absence of a CL, and the endometrium is prepared with exogenous steroid hormone administration. In contrast, women undergoing fresh embryo transfer often have multiple CL. In a recent retrospective cohort study of 1140 live births, the placental pathology arising from autologous ART cycles with fresh or frozen programmed transfer was examined (Sacha et al., 2020). Programmed FET was more likely to be associated with more anatomic and vascular placental pathology, including marginal cord insertion, accessory lobe formation, subchorionic thrombi and features of foetal vascular malperfusion with cord anomalies (Sacha et al., 2020). Although these findings could be associated with the processes of freezing and thawing by itself, the absence or presence of a CL could also contribute to the findings (Tables I and II). Indeed, recent prospective and retrospective cohort studies showed that programmed FET cycles were associated with higher rates of PE and PE with severe features compared with stimulated or natural FET cycles (i.e. CL present) (Ginström Ernstad et al., 2019; von Versen-Hoynck et al., 2019b; Wang et al., 2020). A retrospective registry study of 9726 singleton pregnancies after autologous FET reported an incidence rate of PE of 8.2% in programmed cycles compared with 4.4% in natural ones (Ginström Ernstad et al., 2019). A larger retrospective cohort study which collected a total of 14,373 singletons born following FET (10,211 natural cycles and 4,162 programmed cycles) also found that the incidence of PE was significantly higher in programmed FET cycles (8.6 vs. 3.8%, respectively, p < 0.05) (Wang et al., 2020). These studies have also revealed other adverse obstetrical and perinatal outcomes with programmed FETs, including postpartum haemorrhage, macrosomia and post-term birth (Ginström Ernstad et al., 2019; Wang et al., 2020). Recent reports comparing three cohorts of women (conceiving naturally, by programmed FET and women undergoing ovarian stimulation) with different numbers of CL, showed that women who conceived by FET in the absence of a CL failed to show the normal cardiovascular adaptations during early pregnancy (von Versen-Hoynck et al., 2019a; Conrad et al., 2019b). Interestingly, these studies showed that pregnant women with multiple CLs had a lower rate of PE closer to that of spontaneous conceptions with similar trends for other hypertensive disorders of pregnancy (Ginström Ernstad et al., 2019; von Versen-Hoynck et al., 2019b). However, pregnancies resulting from fresh embryo transfer could be at risk of developing PE if women register an elevated peak serum E2 (>3450 pg/mL) during ovarian stimulation (Imudia et al., 2012). Currently, the ongoing, large-scale (n = 1,200) randomised open label PREECLAM-2019 (Impact of Corpus Luteum Presence or Absence in the Incidence of Preeclampsia After Frozen Embryo Transfer) trial aims to address whether the absence of a CL adversely affects pregnancy (i.e. associates with higher incidence rates of PE), and to determine if there are differences in the perinatal outcomes due to differences in the endometrial preparation protocol for a FET (NCT04092829).
Table I.
Risk of hypertensive disorders of pregnancy in different autologous ART protocols.
| First author (year) | Design of the study | Type of study (Origin) | Sample size | No oocytes transferred | Incidence of PE/PIH | Risk of PE/PIH (95%CI) |
|---|---|---|---|---|---|---|
| FET vs. fresh ET: “Is the freezing-thawed procedure associated with an increased PE risk?”* | ||||||
| Sazonova et al. (2012) | Retrospective cohort study | Multicentre (Sweden) | FET: 2,348; fresh ET: 8,944 | Single and double | PE: FET 5.3% vs. fresh ET 4.4% | PE: AOR: 1.32 (1.07-1.63) |
| Wei et al. (2019) | Randomized controlled trial | Multicentre (China) | FET: 512; fresh ET : 401 | Single | PE: FET 3.1% vs. fresh ET 1.0% | PE: RR: 3.12 (1.06-9.30) |
| Sites et al. (2017) | Retrospective cohort study | Multicentre (USA) | FET: 1,052; fresh ET: 7,453 | Single | PE: FET 7.5% vs. fresh ET 4.3% | PE: AOR: 2.17 (1.67-2.82) |
| Opdahl et al., (2015) | Retrospective cohort study | Multicentre (Nordic database) | FET : 6,444; fresh ET: 39,878 | Single | PIH: FET 7.0% vs. fresh ET 5.7% | PIH: AOR: 1.41 (1.27-1.56) |
| Ishihara et al. (2014) | Retrospective cohort study | Multicentre (Japanese database) | FET: 39,249; fresh ET: 16,909 | Single | PIH: FET 2.9% vs. fresh ET 1.9% | PIH: AOR: 1.58 (1.35-1.86) |
| Chen et al. (2016) | Randomized controlled trial | Multicentre (China) | Programmed FET: 434; fresh ET: 427 | Single and double | PE: Programmed FET 4.4% vs. fresh ET 1.4% | PE: RR: 3.12 (1.26-7.73) |
| Barsky et al. (2016) | Retrospective cohort study | Single centre (USA) | Programmed FET: 109; fresh ET 289 | Single | PE: Programmed FET 7.6% vs. fresh ET 2.6% | PE: AOR: 3.10 (1.20-8.40) |
| Belva et al. (2016) | Retrospective cohort study | Single centre (Belgium) | FET: 912; fresh ET: 1,517 | Single and double | PIH: FET 13.4% vs. fresh ET 7.2% | PIH: RR: 1.90 (1.49-2.43) |
| Ginström Ernstad et al. (2019) | Retrospective registry study | Multicentre (Swedish database) | FET: 9,726; fresh ET: 24,365 | Single | PE: FET 4.9% vs fresh ET 3.7% | PIH: AOR: 1.51 (1.35-1.68) |
| Programmed FET vs. natural FET: “Does the absence of a CL confer an increased risk of PE?” | ||||||
| Jing et al. (2019) | Retrospective cohort study | Single centre (China) | Programmed FET: 2,611; Natural FET: 8,425 | Single and multiple | PIH: Programmed FET 7.2% vs. Natural FET 4.2% | PIH: AOR: 1.78 (1.26-2.51) |
| Ginström Ernstad et al. (2019) | Retrospective cohort study | Multicentre (Swedish database) | Programmed FET: 1,446; Natural FET: 6,297 | Single and double | PE: Programmed FET 8.2% vs. Natural FET 4.4% | PIH: AOR: 1.78 (1.43-2.21) |
| Wang et al. (2020) | Retrospective cohort study | Single centre (China) | Programmed FET: 4,162; Natural FET: 10,211 | Single | PE: Programmed FET 8.6% vs. Natural FET 3.8% | PE: AOR: 2.55 (2.06-3.16) |
| von Versen-Höynck et al. (2019) | Prospective cohort study | Single centre (USA) | Programmed FET: 94; Natural FET: 127 | Single | PE: Programmed FET 12.8% vs. Natural FET 3.9% | PE: AOR : 3.55 (1.20-11.94) |
| Saito et al. (2019) | Retrospective cohort study | Multicentre (Japanese database) | Programmed FET : 24,225; Natural FET: 10,755 | Single and double* | PIH: Programmed FET 4.0% vs. Natural FET 3.0% | PIH: AOR: 1.43 (1.14-1.80) |
Pregnancy-induced hypertension (PIH) includes gestational hypertension and preeclampsia. *It is noteworthy, that the FET groups of some of these studies have included programmed FET cycles (absence of a CL), what could have driven the observed increased PE/PIH risk. **Each group >96% singleton. ART: assisted reproductive technology; FET: frozen embryo transfer; ET: embryo transfer; PE: preeclampsia; AOR: adjusted odds ratio; RR: relative risk; CI: confidence interval.
Table II.
Comparison of ART protocols.
| Protocol overview | Advantages | Disadvantages | Variants | Notes | No of CLs (ovulation status) | Hormonal profile* | Adverse obstetric and perinatal outcomes | |
|---|---|---|---|---|---|---|---|---|
| Fresh ET |
|
|
|
NA |
|
Multiple (ovulation stimulated) |
|
|
| FET |
|
|
|
Programmed FET:
|
|
No CL (ovulation suppressed) |
|
|
|
Natural FET:
|
|
Physiologic no of CL (ovulation not affected) |
|
No specific adverse outcome reported for this variant. | ||||
| Vaginal P supplementation is used routinely. |
Circulating serum levels of different hormones are compared with those measured in single spontaneous normal pregnancies (i.e. CL = 1). **An increased risk of PE has been identified in women that developed high (supraphysiologic) E2 levels during controlled ovarian hyperstimulation after fresh ET, comparing with women that register physiologic E2 levels. ART: assisted reproductive technology; CL: corpus luteum; E2: estradiol; ET: embryo transfer; FET: frozen embryo transfer; hCG: human chorionic gonadotropin; LH: luteinizing hormone; LP: luteal phase; OHSS: ovarian hyperstimulation syndrome; NA: not applicable; P: progesterone; PE: preeclampsia; PlGF: placental growth factor; sFlt-1: soluble forms of fms-like tyrosine kinase 1; SGA: small for gestational age; US: ultrasound; VEGF: vascular endothelial growth factor.
The different endocrine profiles of ART protocols
Since the birth of the first child from in-vitro fertilization >40 years ago, more than 8 million babies have been born from ART around the world, allowing us to learn more about the advantages and drawbacks of the available protocols (Tables I and II). In order to decide which protocol is best for each woman, it is necessary to consider the aetiology of infertility and maternal age, the technical requirements of each protocol and the potential complications. Each protocol has different endocrine profiles based on the presence or absence of a CL.
The most popular methods of FET are natural cycle, modified natural cycle (i.e. with ovulation triggering) and programmed cycles (Dal Prato et al., 2002; Yarali et al., 2016). All FET methods require synchronization of the endometrium with the development of the embryo (Fritz et al., 2017). Although natural FET cycles rely on the growth of a dominant follicle and formation of a functional CL for the production of endogenous products that control endometrial maturation, implantation and early placentation, programmed (artificial) FET cycles recreate this sequence with exogenous E2 and P (Pan et al., 2020). In general, the oestrogen and progestin given to program cycles, and the progestin used for LP support, are the same molecules as those produced endogenously by the CL, but along with inactive ingredients added to the formulations to improve preservation, absorption and delivery (e.g. colloidal silicon dioxide, starch, lactose monohydrate, benzyl benzoate, among others). On the other hand, in natural cycles, there is continuous secretion of CL hormones, under the control of pituitary and trophoblast-derived factors that govern hormone concentrations. These conditions are not replicated during exogenous hormone supplementation in artificial FET cycles.
The quantities and patterns of substances depend upon the route of administration, metabolism and inter-individual variations (e.g. genetic variation) (Miles et al., 1994; Ficicioglu et al., 2004; Kuhl, 2005). In addition, there are multiple protocols for hormone supplementation, where oestrogen and progestin can be administered orally, vaginally and/or transdermally (Kuhl, 2005). The transvaginal route leads to significantly higher P concentrations in the endometrial tissue, but a markedly lower serum concentration than that achieved by the intramuscular route (Miles et al., 1994; Ficicioglu et al., 2004; Shapiro et al., 2014). Thus, at some centres a common practice is to use both the intramuscular and transvaginal routes for progestin supplementation (Pabuccu et al., 2016), as it will assure optimal serum, placental and endometrial concentrations (Vaisbuch et al., 2012). AsE2 and P are subjected to significant metabolism in first-pass pre-hepatic and hepatic metabolism when given orally, several synthetic compounds have been introduced (Abu-Musa et al., 1998). Dydrogesterone is a synthetic isomer (retroprogesterone) in which the methyl group at carbon 10 is located in the α position instead of the β position of natural progesterone, and the hydrogen at carbon 9 is in the β configuration rather than the α configuaration. This structure renders the compound more stable and effective orally. It does not inhibit the formation of P in the placenta and has no androgenic effects in the foetus at recommended doses (Salehpour et al., 2013). Despite differences in chemical structure, activity and route of administration, it was concluded from meta-analyses that the form of oestrogen or progestin supplementation had no effect on pregnancy rates of FETs, as well as obstetrical outcomes (van der Linden et al., 2015; Glujovsky et al., 2020). Conrad et al. (2019a) measured the E2 and P levels in pregnant women undergoing programmed cycles with absent CL, spontaneous conception and IVF with multiple CLs formed. Although the average E2 levels were similar between the three cohorts, P was found to be elevated early in pregnancies with multiple CLs, but similar among pregnancies with no or one CL.
Although little consensus has been reached on the best protocol for endometrial preparation for FET cycles, the endometrial gene expression pattern (endometrial transcriptome) at the time of embryo implantation in natural FET cycles was more similar to the profile of fertile controls than to that of programmed FET cycles, with the latter having a stronger negative effect on expression of genes and pathways crucial for endometrial receptivity (Altmäe et al., 2010). As pointed out by Conrad, as the CL is the key regulator of endometrial function, including decidualization in the secretory phase and early pregnancy, potential explanations for the increased incidence of adverse obstetric outcomes may result from suboptimal dosage and/or inadequate timing of E2 and P administration for luteal support, and although not mutually exclusive, other CL factors(s) may be required for optimal endometrial maturation (Conrad, 2020). In theory, the transcriptome analysis could provide valuable insights into the potential biomarkers and molecular mechanisms related to endometrial receptivity and risk of PE, and offer critical information to personalize hormone supplementation and protocol selection in the subgroup of infertile patients who have experienced recurrent implantation failure, placental syndromes or PE (Messaoudi et al., 2019).
Patterns of preeclampsia biomarkers in women undergoing ART with and without a CL
There is no single biological marker to date that predicts with high confidence the occurrence of PE or its severe consequences (Brown et al., 2018). However, some reports suggest that the combination of PlGF, maternal serum pregnancy-associated plasma protein-A (PAPP-A), mean BP and uterine artery pulsatility index may have an acceptable performance for PE prediction during the first trimester of pregnancy (Akolekar et al., 2013). Similar to what is seen in PE patients, it was shown that ART pregnancies (where the type of ART was not specified) tend to have an anti-angiogenic profile characterized by significantly higher levels of the placenta-derived soluble forms of fms-like tyrosine kinase 1 (sFlt-1) and lower levels of PlGF throughout gestation (Lee et al., 2015). sFlt-1 is derived from a splice variant of the VEGF receptor Flt-1, lacking the transmembrane and cytoplasmic domains (Maynard et al., 2003). It binds to and neutralises both circulating VEGF and PlGF, playing a role in the regulation of angiogenic homeostasis during pregnancy (Maynard et al., 2003). The rising circulating levels of sFlt-1 herald the onset of PE in pregnant women (Levine et al., 2004), reflecting an imbalance between pro- and anti-angiogenic factors, resulting in a net anti-angiogenic state that favours the development of the disease (Maynard et al., 2003; Tomimatsu et al., 2019). Of note, an increased and decreased expression of sFlt1 and PlGF, respectively, has been observed among women with hydatidiform mole, a condition with an elevated risk for severe very early onset PE (<20 weeks gestation) (Koga et al., 2010).
On the other hand, only a few studies have considered the differences in biochemical marker levels between pregnancies conceived after ART with and without CL. In a study conducted by Conrad et al., sFlt-1 levels were generally higher in the two ART groups studied (programmed FET or fresh donor oocyte-generated embryos in a programmed cycle [absent CL] and fresh embryo transfer after ovarian stimulation [multiple CL]) compared to spontaneous pregnancies, throughout most of the pregnancy beginning as early as 10-12 gestational weeks, but especially at 32-35 weeks of gestation (Conrad et al., 2019a). The authors stressed that the number of participants at 32-35 gestational weeks with high sFlt-1 concentrations and a high sFlt-1/PlGF ratio was greater for subjects with no CL than in women with one CL, suggesting more severe placental pathology in the former. Conversely, in a retrospective cohort study of 152 singleton live births (103 fresh embryo transfer with multiple CL, 49 programmed FET), there were no differences in concentrations of PlGF, s-Flt1 and the sFlt-1:PlGF ratio between groups (Woo et al., 2017). No studies have addressed the levels of angiogenic biomarkers in programmed FET versus natural FET cycles. This would specifically address the effect of the presence or absence of a CL on the pattern of PE biomarkers in the same context of freezing and thawing of embryos. Further research is needed on both a molecular and clinical level, investigating prospectively clinical biomarkers and placental pathology in these two protocols for FET.
Haemodynamic and hormonal changes in early pregnancy seem to depend upon the presence of a competent CL, rather than on an intact functioning foetal-maternal-placental unit (Fig. 2) (Chapman et al., 1997). Interesting results comparing cardiovascular markers in pregnancies with different CL numbers have been reported by Conrad et al., who found that the expected gestational rise in cardiac output and other haemodynamic characteristics were attenuated in the absence of CL during the first trimester in comparison to pregnant women with one or more CL, who were similar to each other (Conrad et al., 2019b). In addition, several indices of central aortic stiffness failed to decline and left ventricle afterload remained elevated (Conrad et al., 2019b). In a similar study, von (von Versen-Hoynck et al. 2019b) found that the expected decline in carotid-femoral pulse wave velocity ( a surrogate of aortic stiffness) were attenuated in the no CL cohort compared with combined single/multiple-CL cohorts, and these observations were most striking at 10 to 12 weeks of gestation (p = 0.01 and 0.006 for one and more than one, respectively). Further, the absence of CL was also associated with impaired endothelial function (a classic hallmark of PE pathophysiology), an increase in early aortic wave reflections (i.e. indicator of an increased left ventricle afterload) and higher creatinine, sodium and total carbon dioxide levels in early pregnancy compared with natural FET cycles (Fig. 2) (von Versen-Höynck et al., 2019c). Interestingly, women with no or more than three CL lacked the drop in mean BP in the first trimester compared with women with one CL (von Versen-Hoynck et al., 2019a).
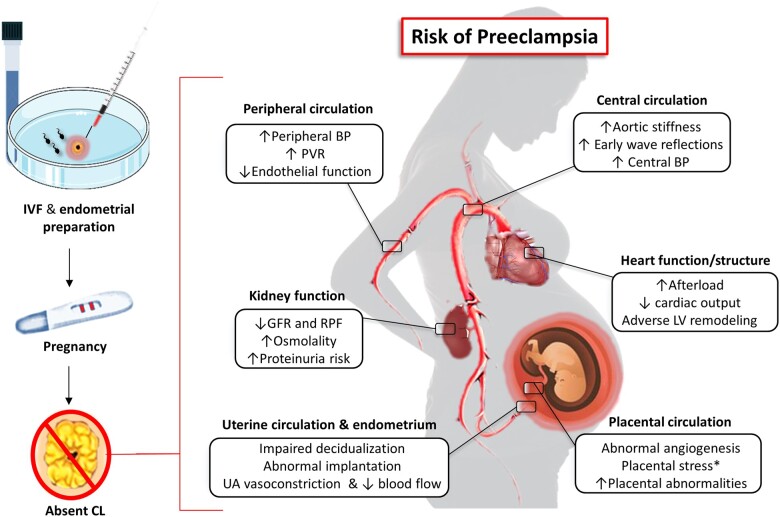
Figure 2.
Potential consequences of the absence of a CL (and its secretory products) in early pregnancy. An unbalanced early hormonal milieu would impair endometrial quality for implantation, placental angiogenesis and development, and prevent the early maternal cardiovascular adaptations required to cope with haemodynamic loads of pregnancy. All these mechanisms would play together increasing the risk of developing preeclampsia as the pregnancy progresses. *Placental hypoxia and stress trigger the release of anti-angiogenic, vasoactive and pro-inflammatory factors into the maternal systemic circulation that further impair the vascular and haemodynamic condition. BP: blood pressure; CL: corpus luteum; GFR: glomerular filtration rate; IVF in-vitro fertilization; LV: left ventricle; PVR: peripheral vascular resistance; RBF: renal plasma flow; UA: uterine artery.
Secretory products of the CL that could influence implantation, placentation and risk of preeclampsia
Progesterone and its metabolites
As mentioned previously, the CL is the primary source of P after implantation until the placenta becomes the dominant source. The effects of this hormone are primarily mediated by interaction with the two classic PR isoforms, PR-A and PR-B, both of which are highly expressed in the uterus (Devoto et al., 2009). PR-A is required for normal ovarian and uterine function, whereas PR-B is critical for mammary development. A mouse model in which both PRs were absent confirmed that these PRs are essential for the establishment and maintenance of pregnancy (Table III) (Lydon et al., 1995).
Table III.
Secretory products of the CL during normal pregnancy and complicated with PE.
| CL product | Serum levels in normal pregnancy* | Serum levels in PE** | Angiogenesis | Role in implantation/placentation | Other effects during pregnancy |
|---|---|---|---|---|---|
| P | ↑ | ↔ ↓ | Pro-angiogenic |
|
|
| 5α-DHP | ↑ | ↔ | No data | No data | No data |
| 20α-DHP | No data | ↑ | No data | No data | Partial agonistic effect on mineralocorticoid receptor. |
| 3ɑ5ɑ20ɑ-HHP | No data | ↑ | No data | No data | No data |
| 3β5α20α-HHP | No data | ↑ | No data | No data | No data |
| E2 | ↑ | ↔ ↓ | Pro-angiogenic | Proliferation, differentiation and migration of trophoblast cells. |
|
| 2-ME2 | ↑ (Plateaus in the last trimester) | ↓ | Anti-angiogenic | Differentiation of cytotrophoblast in an invasive phenotype. |
|
| 4-OHE1 | No data | ↑ in sPE | Pro-angiogenic*** | No data | No data |
| 16-KetoE2 | No data | ↑ | Pro-angiogenic*** | No data | No data |
| Relaxin | ↑ (markedly increase if multiple CLs) | ↔ ↓ | Pro-angiogenic |
|
|
| VEGF | ↑ | ↓(Free biologically active form) | Pro-angiogenic |
|
|
Serum levels compared with nonpregnant women. **Serum levels compared with normal pregnant women.
This effect has been shownlocally, in the CL. 2-ME2: 2-methoxyestradiol; 20ɑ-DHP: 20ɑ-dihydroprogesterone; 4-OHE1: 4-hydroxyoestrone; 5α-DHP: 5ɑ-dihydroprogesterone; BP: blood pressure; E2: estradiol; EF: endothelial function; GFR: glomerular filtration rate; HHP: hexahydroprogesterone; HIF: hypoxia inducible factor; HR: heart rate; NO: nitric oxide; P: progesterone; PlGF: placental growth factor; PVR: peripheral vascular resistance; sPE: severe preeclampsia; VEGF: vascular endothelial growth factor.
On the other hand, P also acts via non-genomic pathways presumably by activating two types of membrane receptors, members of the membrane progestin receptor (mPR) of the PAQR family and progesterone receptor membrane component 1 (PGRMC) that have been localized in the ovary, uterus, foetal membranes and endothelial cells of blood vessels in the uterus among other non-reproductive cells and tissues (e.g. cardiovascular system) (Gellersen et al., 2008; Garg et al., 2017). These receptors have been implicated in preparing the uterus for implantation (Gellersen et al., 2008) and placentation (Reynolds et al., 2015), as well as in regulating labour (Garg et al., 2017) and preserving foetal membrane integrity (Kowalik et al., 2018). In addition, some studies suggest that these pathways may account for P action in preserving CL cell viability in human and bovine granulosa/luteal cells before and during the first trimester of pregnancy (Engmann et al., 2006; Peluso et al., 2009; Kowalik et al., 2018). Nevertheless, the roles of these receptors and signalling pathways in pregnancy pathologies such as PE is unknown.
P can be metabolized into molecules with biological activities important for pregnancy outcomes, in addition to 17α-OH-P which is a product of theca lutein cells. Patil et al. (2015) showed that the endogenous P metabolites 16α-hydroxyprogesterone (16α-OHP) and 6β-hydroxyprogesterone (6β-OHP) have opposing effects on oxytocin-induced uterine contraction frequency. In addition, the metabolism of P into these compounds seems to be dependent on the expression of cytochrome P450 enzyme isoforms (Quinney et al., 2017). A recent publication showed a relationship between progesterone metabolism into the mineralocorticoid and glucocorticoid pathways and spontaneous preterm delivery (sPTD) (Patil et al., 2020). Briefly, the authors analysed 11-deoxycorticosterone (DOC) and 16α-OHP among other P metabolites. They found that maternal serum levels of DOC measured during the late first trimester or early second trimester correlated negatively with an increased risk for sPTD prior to 32 weeks. Moreover, they found that a decreased ratio of DOC/16α-OHP may be an early indicator of an imbalance between mineralocorticoid and glucocorticoid pathways later in pregnancy, and subsequent sPTD (Patil et al., 2020). Although a recent study of Berkane et al. (2018) reported no difference in P, 5α-dihydroprogesterone or 17α-dihydroprogesterone levels (both secreted by the CL) between PE and normal pregnancy, the concentrations of three 20α-reduced metabolites of progesterone (20α-DHP, 3α5α20α-hexahydroprogesterone [HHP], and 3β5α20α-HHP), were higher in women at 24-29 weeks of gestation who subsequently developed PE. Moreover, the activity of 20α-HSD (i.e. the enzyme that converts progesterone into 20α-DHP) reflected in the 20α-DHP/progesterone ratio, increased in the PE group compared with the control values (Berkane et al., 2018). Although these observations reveal an abnormal hormonal milieu after luteal-placental shift during pregnancy, they show that P metabolites can influence pregnancy outcomes.
In the case of programmed FET cycles, P concentrations were similar compared to natural FET cycles or spontaneous conception with one CL during pregnancy, even between 5-14 weeks of gestation (Conrad et al., 2019a; von Versen-Hoynck et al., 2019a). This is not surprising due to exogenous intramuscular or vaginal P administration during the first 10 weeks of pregnancy and placental production thereafter. Although the importance of P in early phases of pregnancy is clear, and its use for luteal phase support is mandatory for successful implantation in programmed FET cycles, very little information regarding the effects of P metabolites in the process of implantation, placentation and placental angiogenesis is available. As described above, some investigators observed differences in P metabolite levels when comparing healthy pregnant women versus pregnant women with established PE (Berkane et al., 2018), but there is no information about the association between levels of P metabolites during the first trimester of pregnancy and development of PE. We can only speculate based on the available data that an early imbalance of P metabolites could affect the process of implantation/placentation contributing to the risk of PE.
Estradiol and its metabolites
Oestrogen functions in the uterus primarily through nuclear oestrogen receptors (ER), although other receptors mediating rapid non-nuclear responses exist (see below). As a result of this interaction, changes in transcription factors occur through upregulation of gene expression resulting in the modulation of biological activities. The main ER isoforms are ERα and ERβ (Lee et al., 2012). It is believed that oestrogen signalling pathways are selectively stimulated or inhibited depending on a balance between the activities of these receptors in target organs (Lee et al., 2012). ERα is an important mediator of oestrogensignalling during early pregnancy, with roles in regulating myometrial and endometrial growth. Prior studies have shown that ERα knockout mice are unable to support implantation (Lee et al., 2012; Zhang et al., 2013). ERβ is the sole ER expressed within the endothelium of the endometrium and the fetoplacental vasculature (Su et al., 2012). Studies have shown that its activation may contribute to angiogenic and vasomotor changes that play a role in both implantation and regulation of fetoplacental blood flow (Table III) (Su et al., 2012).
Some studies have shown the existence of an oestrogen-responsive G protein coupled receptor (GPER), that could mediate the rapid actions of oestrogen (non-genomic pathways) (Eyster, 2016). Accordingly, activation of these receptors may be implicated in vasodilation by inducing NO release, as well as a rapid (5–15 minutes) prostacyclin production, as shown in human umbilical vein and ovine uterine artery endothelial cells (Berkane et al., 2017). Notably, the signals initiated by these membrane receptors do not compensate for the absence of ERα in a knock-out mouse model (Pedram et al., 2009), and both types of receptors should be considered as different components of the same functional unit with complementary mechanisms.
During normal pregnancy, maternal plasma E2 levels dramatically increase from ∼100 pg/mL (luteal phase) up to between 2,500 pg/mL during first trimester and 10,000 pg/mL at the end of pregnancy (Abbassi-Ghanavati et al., 2009). A large body of evidence suggests that women with established PE (∼34 weeks) have low E2 levels (Zeisler et al., 2002; Salas et al., 2006; Hertig et al., 2010; Bussen and Bussen, 2011; Jobe et al., 2013; Yin et al., 2013; Wan et al., 2018). Based on these studies, and with the known E2 effect on placentation and endothelial function, Berkane et al. (2017) hypothesized that early low levels of E2 may lead to insufficient trophoblast development and angiogenesis, termed the ‘oestrogen deficiency hypothesis’ of PE. If early E2 insufficiency persists after the luteal-placental shift, it may explain the reduced E2 concentrations that have been observed in women with established PE. However, other investigators have not found significant differences in E2 levels in PE versus normal pregnancy in mid- to late pregnancy (Serin et al., 2001; Miller et al., 2003; Troisi et al., 2003).
The profiling of E2 in women who subsequently develop PE has been controversial asprospective studies in early pregnancy are rare, along with significant variations in the methods employed to measure E2 levels (Berkane et al., 2017). Shao et al. (2019) found that E2 concentrations were significantly lower in PE patients compared with normal pregnant women from early pregnancy throughout gestation. On the other hand, Salas et al. (2006) found similar serum E2 concentrations in women who developed PE or IUGR versus normal healthy pregnant women from week 10 until week 34, although thereafter, E2 values were lower in PE and IUGR mothers when compared with normal pregnancies. Conversely, both Cantonwine et al. (2019) and Kale et al. (2020), found greater urine and serum levels of E2, respectively, in early to mid-pregnancy in women who later developed PE compared with normal pregnancies. Interestingly, the metabolism of E2 in those affected women was diminished (Cantonwine et al., 2019).
When administered exogenously, elevated peak serum E2 levels on the day of hCG administration during ovarian stimulation were associated with greater odds of developing PE and other complications related to abnormal placentation (e.g. IUGR) in singleton pregnancies resulting from fresh embryo transfer (Farhi et al., 2010; Imudia et al., 2012). These observations are consistent with a study using a well-established animal model (pregnant baboons), in which the investigators found that uterine artery remodelling was impaired and utero-placental blood flow dynamics were disturbed by advancing the rise in oestrogen from the second trimester to the first trimester (Aberdeen et al., 2012). The authors hypothesized that early elevation of oestrogen during pregnancy could have a detrimental effect on uterine artery remodelling, which could increase the risk of PE. Conversely, a recent study of women undergoing ART procedures over an 18-year period indicated that patients who subsequently developed PE had lower levels of E2 on the day of hCG administration (Chen et al., 2020).
In summary, estradiol is critical to endometrial and placental development. Studies in pregnant women have shown either no change or a decrease in maternal serum E2 levels at mid- to late gestation in women with PE, with an uncertain role of E2 in early human pregnancy with respect to the risk of subsequently developing PE. Nevertheless, it seems that both diminished and excess E2 in the early stages of pregnancy may have detrimental effects on placentation. Although there is consensus on the role of oestrogens in cytotrophoblast-induced spiral artery remodelling in women, the vascular effects of E2 could be complex, and any of these effects on vessel remodelling are likely to be concentration and time-dependent.
On the other hand, the role of specific EMs has been better defined. Studies have shown that PE is characterized by aberrant synthesis, metabolism and accumulation of oestrogen and EMs that are likely associated with alterations in vascular function Jobe et al. (2013). Jobe et al. (2013) showed that plasma levels of 2-hydroxyoestrone, 4-OHE1, 16-α-hydroxyoestrone and 2-hydroxyestradiol levels are altered in PE. As these metabolites have been associated with several uteroplacental vascular effects including induction of endothelial cell proliferation (Jobe et al., 2010), generation of prostacyclin (Seeger et al., 1999) and synergistic effects with NO (Dubey et al., 2004), it is reasonable to hypothesize that a tight balance of these pro- and anti-angiogenic substances is necessary to maintain an optimal environment for a healthy pregnancy (Jobe et al., 2013).
Pro-angiogenic oestrogen metabolites
Henríquez et al. (2016) studied 2-ME2, 2-methoxyoestrone (2-ME1), 4-OHE1 and 16-ketoestradiol (16-ketoE2) throughout the luteal phase. They found that the levels of 16-ketoE2 in tissue samples from CLs were greater in the early luteal phase, suggesting that 16-ketoE2 may play a role in the vascular development of the early luteal phase CL, asit increases tube formation by EA.hy 926 cells (surrogates of vascular endothelial cells). This pro-angiogenic effect of 16-ketoE2 could be explained by an increase in VEGF synthesis. In the same study, mid-luteal phase CL tissue showed a significant increase in 4-OHE1 as compared with early luteal phase CL. Interestingly, both 16-ketoE2 and 4-OHE1 have similar effects on VEGF secretion and angiogenic activity (Henríquez et al., 2016). Although these observations strictly apply to the local vascular endothelial regulation within the CL, (Jobe et al. (2013) found that plasma levels of 16-ketoE2 during late pregnancies were significantly higher in women with severe PE (n = 8) compared with normal pregnancy (n = 8), whereas2-hydroxyestradiol and 2-methoxyoestrone levels were lower in severe PE compared to women with normal pregnancy. The increasing levels of these proangiogenic metabolites in PE could reflect a compensatory mechanism to counteract placental insufficiency.
Anti-angiogenic oestrogen metabolites
2-ME2 is a natural metabolite of 2-hydroxyestradiol synthesised by the enzyme catechol-O-methyltransferase (COMT). It has been one of the most studied CL-derived compounds with anti-angiogenic properties. It is characterized by a low oestrogenic activity compared to E2 due to its low affinity for ERα and ERβ (LaVallee et al., 2002). Its mechanism of action seems to be through the impairment of HIF nuclear translocation by binding to a buried pocket in the HIF-1α (PAS)-B domain (Poch et al., 2019). Binding of 2-ME2 to HIF-1α protein alters the HIF-1α-HIFβ interaction (preventing heterodimerization), a key step in HIF nuclear translocation, thus preventing transcriptional actions of HIF proteins, including VEGF gene activation (Poch et al., 2019).
During uncomplicated pregnancies, 2-ME2 levels rise steadily and reach a plateau in the last trimester. Notably, low levels of 2-ME2 have been implicated in the process of abnormal placentation (Zhang et al., 2014; Wantania et al., 2017). Although this could seem paradoxical, Lee et al. (2010) showed that 2-ME2 enables cytotrophoblasts to differentiate into a more invasive endovascular phenotype under low-oxygen conditions in mice. Similarly, using HTR-8/SVneo cells (human trophoblast cell line), 2-ME2 prompted cell migration under oxygen levels mimicking in-vivo conditions suggesting that a decrease in 2-ME levels could affect the migration of trophoblast cells (Shen et al., 2014).
COMT-deficient mice have been shown to show placental hypoxia, high levels of HIF-1α and significantly higher plasma concentration of sFlt-1, with the development of a PE-like phenotype (Kanasaki et al., 2008). The observation of increased foetal wastage and reduced placental weight in the same study supports the notion that reduced placental COMT activity could alter the placentation process (Kanasaki et al., 2008). Accordingly, Lee et al. (2010) showed that overnight treatment of hypoxic placental explants with 2-ME, rescued altered levels of VEGFR-2, sFlt-1 and HIF1α, suggesting a protective role against the development of PE. Furthermore, in COMT knockout mice, the administration of 2-ME2 was capable of preventing development of a PE phenotype and also suppressed placental hypoxia, HIF-1α expression and sFLT-1 elevation (Kanasaki et al., 2008). 2-ME2 levels have also been significantly correlated (positively and negatively, respectively) with PlGF and sFlt-1 molecules suggesting its participation in the classic antiangiogenic profile of PE (Pertegal et al. (2015).
Studies have also shown a role of E2 and 2-ME2 in the control of vascular tone and endothelial function which allow an optimal haemodynamic adaptation to pregnancy (Bonacasa et al., 2008; Lin et al., 2020). 2-ME2 increases endothelial NO bioavailability, by increasing its release and decreasing its oxidation (Dubey et al., 2004; Hernandez et al., 2013). Interestingly, the pharmacological inhibition of COMT activity (e.g. with entacapone) in pregnant rats has been shown to provoke hypertension and endothelial dysfunction (Hernandez et al., 2013).
Not only have low levels of 2-ME2 have been detected in the setting of early onset PE (Zhang et al., 2014; Wantania et al., 2017), but also a relationship between 2-ME2 plasma levels and clinical severity of the syndrome has been observed (Zhang et al., 2014). Pertegal et al. (2015) found that PE women with lower levels of 2-ME2 had higher values of systolic BP and proteinuria and suffered a more serious clinical condition requiring more aggressive treatment to control their BP. Indeed, 2-ME2 plasma levels significantly and negatively correlated with SBP (r = -0.62, p = 0.0001) and DBP (r = -0.63, p = 0.0001) after comparing normotensive, non-severe and severe PE patients in a recent case-control study (Tripathi et al., 2019).
Relaxin
In humans, there are three relaxin peptides, relaxin-1, relaxin-2 and relaxin-3, with relaxin-2 being the major circulating form and the most important in the female reproductive tract (Marshall et al., 2017). As mentioned previously, circulating relaxin is thought to be produced predominantly (if not solely) by the CL in human pregnancy. However, as initially found by others (Quagliarello et al., 1979), Conrad et al. (2019a) observed that at 23-25 and 32-35 gestational weeks, relaxin concentrations were maintained at ∼50% of the peak levels found during first trimester in unassisted pregnancies with a CL, despite the belief that the CL of pregnancy involutes by the end of the first trimester (Schindler, 2004). One potential explanation is that the placenta could represent a mid-to-late pregnancy source of circulating relaxin in human pregnancies, as occurs in other mammals, and also for other hormones (Sherwood, 2004). Although relaxin levels are significantly elevated in most (but not all) women following IVF with a fresh embryo transfer, with multiple CL, compared with singleton pregnancies (Haning et al., 1996; Mushayandebvu et al., 1998; Conrad et al., 2019a; von Versen-Hoynck et al., 2019b), its concentration was markedly lower and at the lower range of detectability in pregnant women with no CL (programmed FET) versus one CL (spontaneous conception and natural cycle FET). Relaxin levels were also lower in the one CL group compared to more than three CL group (von Versen-Hoynck et al., 2019a). Relaxin levels are also elevated in twin pregnancies whether spontaneously conceived or conceived by ART, compared with singleton pregnancies (Haning et al., 1996). Finally, relaxin levels were not different between natural FET cycle and spontaneous conception (von Versen-Hoynck et al., 2019a). Asthe association of relaxin and CL is evident, it has been hypothesized that relaxin levels present in the third trimester in human pregnancies could be explained by a persistent functional CL (Conrad et al., 2019a). Regardless, the persistence of relaxin may have a direct influence on the regulation of maternal haemodynamics throughout most of the pregnancy, even after the luteal-placental shift takes place at the end of the first trimester.
Relaxin administration in non-pregnant animals promotes many of the classic cardiovascular changes that occur in normal pregnancy, such as the increase in cardiac output, heart rate, global arterial compliance while decreasing peripheral vascular resistance and blood pressure (Conrad, 2011; Devarakonda and Salloum, 2018). The potent vasodilatory properties of this hormone also seem to mediate other circulatory changes, such as increasing glomerular filtration rate and effective renal plasma flow (Novak et al., 2001). In an ART pregnant group with no CL and undetectable relaxin, creatinine, sodium, and total CO2 levels were significantly higher compared with other ART and non-ART pregnancies where relaxin was present (one or more CL) (von Versen-Höynck et al., 2019c). Relaxin peptides act on a group of four G protein-coupled receptors known as relaxin family peptide receptors 1-4 (RXFP1-4). Relaxin-2 mediates its physiological actions through the activation of RXFP1 (Fig. 3) (Marshall et al., 2017). One of the proposed molecular mechanisms underlying the vasodilatory action of relaxin is via an increase of cyclic adenosine monophosphate (cAMP) through stimulation of adenylate cyclase. Binding of relaxin-2 to RXFP1 may also activate a tyrosine kinase pathway that inhibits the activity of a phosphodiesterase that degrades cAMP (Marshall et al., 2017; Devarakonda and Salloum, 2018). It also activates endothelial NO synthase, the latter mediated by VEGF and PlGF, and increases in arterial gelatinase(s) activity (Conrad, 2011; Devarakonda and Salloum, 2018). The gelatinases, in turn, hydrolyse big endothelin (ET) to form ET (1-32), which activates the endothelial ET(B) receptor/NO vasodilatory pathway (Conrad, 2011). A number of uterotropic effects, including stimulating uterine growth and vascularization, remodelling extracellular matrix components and regulating VEGF in preparation for implantation, have been attributed to this hormone (Table III) (Einspanier et al., 2009; Marshall et al., 2017; Jelinicet al., 2018). This also includes an enhancement of endothelial vasodilator function and reduced smooth muscle reactivity, which are critical events for uterine artery adaptation and optimal placental perfusion (Marshall et al., 2017). Compared to wild-type mice, relaxin knockout animals (Rln−/−) showed an increased myogenic tone and impaired endothelial vasodilator function of the uterine arteries in late pregnancy, in association with an increased risk of lower foetal weights (Marshall et al., 2018). Relaxin deficiency also caused altered uterine artery remodelling (e.g. increased elastin) especially in older pregnant mice, resulting in significantly stiffer uterine arteries (Gooi et al., 2013). Most strikingly, elevated arterial stiffness and reduced foetal weight were reversed after relaxin treatment (Gooi et al., 2013). Notably, low relaxin levels in the first trimester of human pregnancies were associated with late-onset PE (>34 weeks) (Post Uiterweer et al., 2020), pointing towards a possible pathophysiologic role for early low relaxin levels and impaired placental dysfunction. Further, although programmed FET cycles that lack relaxin were more likely associated with anatomic and vascular abnormalities of the placenta compared to fresh ET (Sacha et al., 2020), natural FET cycles and fresh embryo transfer (both CL present) were not associated with notable differences in placental histopathology (Mizrachi et al., 2020). Although these studies do not show causality, and the reason for these findings could be multifactorial, the lack of relaxin in early pregnancies without a CL might contribute to placental pathology, which could be translated into the PE risk observed in programmed FET cycle.
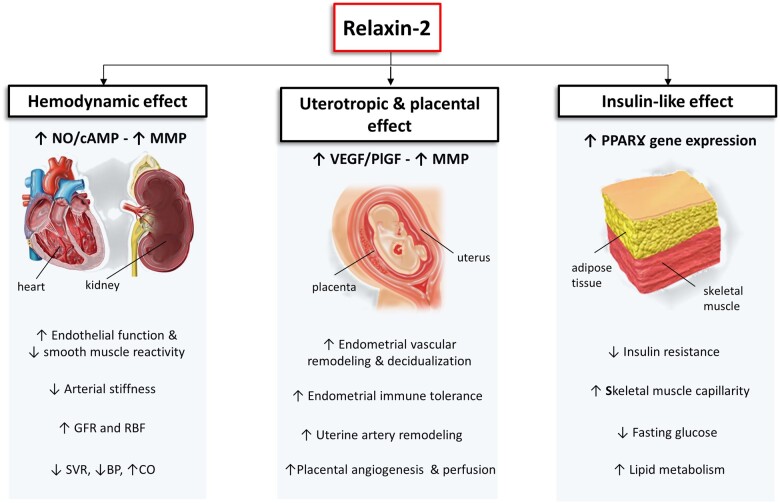
Figure 3.
Proposed physiological roles of relaxin-2 during pregnancy. Relaxin-2 produces its major effects through the activation of its membrane receptor, RFXP1, which in turns leads to an up-regulation of cAMP, NO and gene transcription (e.g. VEGF, MMP and PPARṾ), promoting cardiovascular and renal adaptation, endometrial remodelling, vascularization and placental development. BP: blood pressure; cAMP: cyclic adenosine monophosphate; CO: cardiac output; GFR: glomerular filtration rate; MMP: matrix metalloproteinases; NO: nitric oxide; PlGF: placental growth factor; PPARṾ: peroxisome proliferator-activated receptor gamma; RBF: renal blood flow; SVR: systemic vascular resistance; VEGF: vascular endothelial growth factor.
All these observations have led the investigators to propose that in the setting of programmed FET cycles in which the CL is absent, relaxin should be supplemented to contribute to the implantation and placentation process, as well as to optimize maternal cardiovascular adaptation mechanisms during early pregnancy with potential effects on reducing PE risk (Jelinicet al., 2018).
Other angiogenic factors: intra-luteal or secreted?
An increasing number of ‘omic’ technologies have become available to better profile biological systems and identify novel compounds in different disease processes (Hasin et al., 2017) Recently, ‘multi-omics’ and ‘untargeted metabolomics’, have been used to identify novel biomarkers, quantify metabolites and generate a detailed characterization of biological pathways. These tools could fully characterize the presence of all circulating metabolites (including those that are CL-derived) and their potential implications for PE (Benny et al., 2020). Although promising, complexity in interpreting the results, together with the lack of standardization and consistency in sample processing among different studies, have limited the discrimination ability of untargeted metabolomic dataset (i.e. artefacts, contaminants, redundant biological compounds versus novel unknown or unique novel compounds), and thus its clinical application (Benny et al., 2020; Sindelar and Patti, 2020).
By using different techniques, a large list of angiogenic factors produced by the CL has been identified including VEGF, fibroblast growth factor (FGF), transforming growth factor (TGF) family, angiopoietins, epidermal growth factor and insulin growth factors (IGFs) (Galvão et al., 2018; Lu et al., 2019; Benny et al., 2020). Yet, it has been debated whether these regulatory factors other than the major steroid hormones and peptide hormones locally produced in the CL also act remotely, making significant contributions to processes such as implantation and placentation. Here, we will focus on VEGF as it is the main regulator of angiogenesis and has been extensively studied. VEGF is a specific mitogen and survival factor for endothelial cells and a key regulator of vasculogenesis and angiogenesis in both physiological and patho-physiological conditions which mediates its biological effects through two specific cell surface receptors (VEGFR1 [Flt-1] and VEGFR2) (Lu et al., 2019).
Although Zamudio et al. (2013) showed that free VEGF concentrations in mother and fetus can be profoundly altered by blood collection techniques and could contribute to the variation in VEGF concentrations reported in the literature, longitudinal studies have shownthat there is a progressive increase in total serum VEGF concentrations (i.e. free plus bound forms) during unassisted pregnancies (Evans et al., 1998). The main source of this substance during pregnancy is thought to be decidualized endometrial cells and trophoblasts (Hannan et al., 2011), yet high VEGF expression within the CL has been consistently detected during early pregnancy, and occurs under hCG and estradiol control (Lee et al., 1997; Kashida et al., 2001). In a study conducted to determine the relative contributions of extra-ovarian versus ovarian sources of circulating VEGF, a group of investigators suggested that circulating VEGF levels during early gestation largely originated from the CL (Lee et al., 1997). Similarly, serum VEGF concentrations in 141 unassisted pregnancies (with CLs) were significantly higher compared with VEGF concentrations in 18 singleton pregnancies from programmed FETs (without CLs) at early stages of pregnancy, although these differences became less marked with advancing gestation (Evans et al., 1998). Further, some authors went on to propose that multiple CLs developed after ovarian stimulation in ART may result in early overproduction of VEGF, being strongly implicated in the development of ovarian hyperstimulation syndrome (Duncan et al., 2009; Kwik and Maxwell, 2016). Taken together, these data suggest that the CL could be a significant source of circulating VEGF over the first 10 weeks of pregnancy, although the lower VEGF concentrations associated with FET may also reflect the slower embryonic growth in FET cycles (Evans et al., 1998).
Total serum VEGF concentrations are elevated in PE pregnancies (∼35 weeks) compared to normal pregnancy (Lee et al., 2007) (Table III). However, the free biologically active form is significantly reduced, which is explained by an excessive production of sFlt-1 that binds and inactivates circulating VEGF (Maynard et al., 2003; Levine et al., 2004; Lee et al., 2007; Tomimatsu et al., 2019). Interestingly, cancer patients treated with bevacizumab, a recombinant humanized monoclonal antibody that binds and blocks VEGF, have an increased risk of developing a ‘pre-eclampsia-like syndrome’ in a dose-dependent manner (Vigneau et al., 2014).
Increased preeclampsia risk with other disorders of ovarian steroidogenesis
Polycystic ovary syndrome (PCOS) is the classic paradigm of abnormal ovarian steroidogenesis in women of reproductive age, being one of the most common causes of infertility in women (Sawant and Bhide, 2019). Affected women characteristically develop follicular arrest leading to anovulation or oligoovulatory cycles and polycystic ovarian morphology, in the setting of clinical and/or biochemical features of hyperandrogenism (Costello et al., 2019; Henríquez et al., 2020). One large meta-analysis that included >4000 PCOS women showed a 3-fold increased risk of developing PE, among other pregnancy complications (Qin et al., 2013). Moreover, the increased risk of developing PE in PCOS women seems to remain even after controlling for confounding factors such as obesity, ART and chronic hypertension (Mills et al., 2020).
It has been suggested that the characteristic follicular arrest could be explained by increased expression of anti-Müllerianhormone by granulosa cells that reduces the sensitivity to FSH. FSH positively regulates angiogenesis by stimulating HIF-1α expression and VEGF secretion (Kuo et al., 2011). A recent study found that PCOS women with anovulation had an anti-angiogenic environment caused by reduced levels of pro-angiogenic EMs (2-OHE2, 4-OHE1 and 16-kE2) in the follicular fluid with associated low levels of VEGF (Henríquez et al., 2020). Notably, treatment with exogenous hCG during ART improved the production of pro-angiogenic EMs and VEGF in PCOS women (Henríquez et al., 2020).
Hyperandrogenism is a hallmark of PCOS and occurs due to a combination of thecal hyperplasia and impaired aromatase activity (Henríquez et al., 2020). Aromatase (CYP19A) is a rate-limiting enzyme for oestrogen biosynthesis, which converts testosterone and androstenedione to E2 and E1, respectively. Different studies have also found evidence of aromatase dysfunction in women with PE (Perez-Sepulveda et al., 2015; Berkane et al., 2018). Moreover, Berkane et al. (2018) detected impaired aromatase activity (low E1/androstenedione ratio) long before the clinical signs of PE, which was consistent with a decrease in placental aromatase expression (RNA and protein levels) at delivery in a different small set of women with PE. The independent association between PE and PCOS in women who conceive naturally (not corrected by exogenous gonadotropins) could be explained at least in part by persistent abnormalities in the structure (i.e. thecal hyperplasia) and function (i.e. abnormal androgen, EMs, VEGF levels) of the developing CL during early pregnancy. A study that analysed placental histology from women with PCOS found decreased endovascular trophoblast invasion independent from pregnancy complications (Koster et al., 2015). The percentage of the implantation site vessels with endovascular trophoblast invasion and its extension measured by computerized analysis of biochemical and histological data were both lower in PCOS women compared with women without PCOS (Palomba et al., 2012).
PCOS is also associated with insulin resistance and risk of type 2 diabetes mellitus, a condition that increases independently the risk of PE (Wei et al., 2019; Sanchez-Garrido and Tena-Sempere, 2020). Although the decreased ovarian and peripheral insulin sensitivity in PCOS women is thought to be multifactorial (Sanchez-Garrido and Tena-Sempere, 2020), abnormal gene expression of peroxisome proliferator-activated receptor gamma (PPARṾ) may play a critical role (Wang et al., 2014; Cao et al., 2019). PPARṾ modulates glucose and lipid metabolism, as well as insulin sensitivity, inflammation, adipogenesis, vasculature function and tissue remodelling (Singh et al., 2015). Inhibition of the expression of PPARγ mRNA in ovarian granulosa cells may be related not only to the characteristic insulin resistance but also directly to the mechanism of follicular growth arrest and absence of CL-derived products (Wang et al., 2014; Cao et al., 2019). As mentioned, relaxin is structurally similar to insulin. This structural similarity is explained by differentiation of duplicated genes originated from a common ancestral gene (Hoffmann and Opazo, 2011). It seems that the close structural resemblance may lead to some functional similarities. In a study of non-pregnant women with type 2 diabetes mellitus, relaxin was positively related to insulin sensitivity and negatively to beta-cell function (Szepietowska et al., 2008). Further, serum relaxin levels were found to be elevated in pregnant women with early gestational diabetes mellitus (Alonso Lopez et al., 2017). Yet, whether relaxin may also synergise with insulin to optimise blood glucose homeostasis and ameliorate the PE risk in PCOS women remains to be determined. In studies performed in a type 2 diabetic mouse model, the chronic infusion of relaxin attenuated skeletal muscle insulin resistance and lowered fasting blood glucose levels (Fig. 3) (Bitto et al., 2013; Bonner et al., 2013), by increasing the endothelial-dependent vascular reactivity and proliferation in skeletal muscle capillarity (Bonner et al., 2013). Others found that the activation of RXFP1 by relaxin induces an increase in PPARY gene expression (Singh et al., 2015). Thus, the ‘metabolic arm of relaxin’ could also be critical in PCOS women who undergo ART by counteracting the insulin resistance, with a potential role in reducing the PE risk.
Characteristically, women with PCOS who do not respond to ovulation induction agents (e.g. clomiphene citrate) have higher body mass index, anti-Müllerian hormone values, more severe hyperandrogenism and metabolic derangement, among other factors (Ellakwa et al., 2016; Sachdeva et al., 2019). Strikingly, most of these coexisting hormonal and metabolic disturbances have been associated with an anti-angiogenic profile, placental pathology and risk of PE (Koster et al., 2015; Bartsch et al., 2016; Spradley, 2017; Mills et al., 2020). More frequently, this subset of women with PCOS end up undergoing programmed FET cycles, especially to avoid the risk of ovarian hyperstimulation syndrome which is particularly elevated in patients (Chen et al., 2016). However, the presence of a competent CL seems to be required for PCOS women who undergo successful ART. For instance, women with PCOS randomized to programmed FET experienced an increased risk of PE compared with fresh embryo transfer (4.4% vs. 1.4%, p = 0.009) (Chen et al., 2016). Interestingly, a subsequent sub-analysis of the previous study revealed that most of the increased risk of PE was confined to twin pregnancies after programmed FET (Zhang et al., 2018). Although the underlying mechanisms for this association remain to be elucidated, a ‘CL insufficiency’ in the setting of greater needs for its secretory products (i.e. no CLs, but multiple placentas-foetuses) may be a plausible explanation.
Conclusions
Although most pregnant women who develop PE have a physiologic number of CL, there is increasing evidence suggesting that the absence of the CL (or its insufficiency) could lead to alterations in endometrial preparation impairing implantation, placentation and maternal vascular health. The disparity in gestational ages between the lifespan of the CL and the onset of clinical features of PE can be explained by the fact that those abnormalities in early physiological events regulated by the CL contribute to the subsequent mid- to late pregnancy maternal and foetal outcomes. This hypothesis might explain the increased risk of PE associated with ART pregnancies, particularly those established from programmed FET cycles.
The function of the CL is controlled, in part, by angiogenic factors that participate in luteotrophic and luteolytic processes. Some of these factors, such as P, E2, EMs, relaxin and possibly VEGF, are secreted into the circulation and act remotely, with important implications for implantation and placentation. Beyond these roles, the CL appears to be critical for initiating the early maternal systemic haemodynamic adaptations to pregnancy. However, the persistence of circulating relaxin throughout the pregnancy challenges the classic luteal-placental shift suggesting that the CL also contribute to mid-late pregnancy health.
The development of PE in programmed FET cycles could be explained by the sum of multiple factors. Although E2 and P are supposed to be adequately supplemented, this might not be true for all women. Further, each woman could have different combinations of risk factors, cardiovascular profiles, endothelial function and genetic characteristics, resulting in different ‘backgrounds’ that would determine, at least in part, the outcomes for each woman. Following this line of thinking, women without a CL who do not develop PE may not have sufficient predisposing factors to develop the disease. Although many CL-derived products have redundant effects on pregnancy, we cannot disregard the possibility of ‘placental compensation’ for many of those products. In other words, those women who lack a CL (undergoing programmed FET cycles), but are adequately supplemented with E2 and P, have a healthy cardiovascular system able to adapt to the haemodynamic challenges of pregnancy, may be able to have pregnancies unaffected by PE. Further research is needed to advance our understanding of PE and to optimize lART protocols to improve the safety and outcomes.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. No original data are presented in this review. The authors will make available figures and tables if requested.
Authors' roles
J.S. and M.P. designed the review and performed the literature research and wrote the manuscript along with M.M. M.P. designed the figures and tables. All authors approved the final version to be published.
Funding
This work was funded in part by National Institutes of Health grant R01 HD083323.
Conflict of interest
The authors have declared that no competing interests exist.
References
- Abbassi-Ghanavati M, Greer LG, Cunningham FG.. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009;114:1326–1331. [DOI] [PubMed] [Google Scholar]
- Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED.. Suppression of trophoblast uterine spiral artery remodelling by oestrogen during baboon pregnancy: impact on uterine and foetal blood flow dynamics. Am J Physiol Heart Circ Physiol 2012;302: H1936–H1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Musa A, Hannoun A, Khalil A, Masaad Z, Karam K.. Artificial endometrial preparation for oocyte donation using synthetic oestrogen and progestogen. Clin Exp Obstet Gynecol 1998;3:83–85. [PubMed] [Google Scholar]
- ACOG. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019;1:e1–e25. [DOI] [PubMed] [Google Scholar]
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH.. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Foetal Diagn Ther 2013;33:8–15. [DOI] [PubMed] [Google Scholar]
- Alonso Lopez Y, Dereke J, Landin-Olsson M, Strevens H, Nilsson C, Hillman M.. Plasma levels of relaxin-2 are higher and correlated to C-peptide levels in early gestational diabetes mellitus. Endocrine 2017;57:545–547. [DOI] [PubMed] [Google Scholar]
- Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A.. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 2010;16:178–187. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Ivell R.. Regulation of the reproductive cycle and early pregnancy by relaxin family peptides. Mol Cell Endocrinol 2014;382:472–479. [DOI] [PubMed] [Google Scholar]
- Barsky M, , St. Marie P, , Rahil T, , Markenson GR, , Sites CK. Are perinatal outcomes affected by blastocyst vitrification and warming? Am J Obstet Gynecol 2016;215:603.e1–e5. [DOI] [PubMed] [Google Scholar]
- Bartsch E, Medcalf KE, Park AL, Ray JG.. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016;i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beindorff N, Einspanier A.. Luteotrophic effects of relaxin, chorionic gonadotrophin and FSH in common marmoset monkeys (Callithrix jacchus. ). Reproduction 2010;139:923–930. [DOI] [PubMed] [Google Scholar]
- Belva F, , Bonduelle M, , Roelants M, , Verheyen G, , Van Landuyt L. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod 2016;31:1610–1620. [DOI] [PubMed] [Google Scholar]
- Benny PA, Alakwaa FM, Schlueter RJ, Lassiter CB, Garmire LX.. A review of omics approaches to study preeclampsia. Placenta 2020;92:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkane N, Liere P, Oudinet JP, Hertig A, Lefèvre G, Pluchino N, Schumacher M, Chabbert-Buffet N.. From pregnancy to preeclampsia: a key role for oestrogens. Endocr Rev 2017;38:123–144. [DOI] [PubMed] [Google Scholar]
- Berkane N, Liere P, Lefevre G, Alfaidy N, Nahed RA, Vincent J, Oudinet JP, Pianos A, Cambourg A, Rozenberg P. et al. Abnormal steroidogenesis and aromatase activity in preeclampsia. Placenta 2018;69:40–49. [DOI] [PubMed] [Google Scholar]
- Bitto A, Irrera N, Minutoli L, Calò M, Lo Cascio P, Caccia P, Pizzino G, Pallio G, Micali A, Vaccaro M. et al. Relaxin improves multiple markers of wound healing and ameliorates the disturbed healing pattern of genetically diabetic mice. Clin Sci (Lond) 2013;125:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bonacasa B, Sanchez ML, Rodriguez F, Lopez B, Quesada T, Fenoy FJ, Hernandez I.. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodelling in spontaneously hypertensive rats. Maturitas 2008;61:310–316. [DOI] [PubMed] [Google Scholar]
- Bonner JS, Lantier L, Hocking KM, Kang L, Owolabi M, James FD, Bracy DP, Brophy CM, Wasserman DH.. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes 2013;62:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S.. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- Bussen S, Bussen D.. Influence of the vascular endothelial growth factor on the development of severe pre-eclampsia or HELLP syndrome. Arch Gynecol Obstet 2011;284:551–557. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, McElrath TF, Trabert B, Xu X, Sampson J, Roberts JM, Hoover RN, Troisi R.. Oestrogen metabolism pathways in preeclampsia and normal pregnancy. Steroids 2019;144:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Maowulieti G, Yu T.. Effect of testosterone on the expression of PPARγ mRNA in PCOS patients. Exp Ther Med 2019;3:1761–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco I, Troncoso JL, Devoto L, Vega M.. Differential steroidogenic response of human luteal cell subpopulations. Hum Reprod 1996;11:1609–1614. [DOI] [PubMed] [Google Scholar]
- CDC. Centre for Disease Control and Prevention. American Society for Reproductive Medicine, and Society for Assisted Reproductive Technology. 2018 Assisted reproductive technology success rates: national summary and fertility clinic reports. US Department of Health and Human Services, Washington (DC)2020. US Department of Health and Human Services, Washington (DC)20202020.
- Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT. et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol 1997;273:F777–F782. [DOI] [PubMed] [Google Scholar]
- Chen YC, Lai YJ, Su YT, Tsai NC, Lan KC.. Higher gestational weight gain and lower serum estradiol levels are associated with increased risk of preeclampsia after in vitro fertilization. Pregnancy Hypertens 2020;22:126–131. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N. et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–533. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Devoto L.. Cholesterol transportsteroidogenesis by the corpus luteum . Reprod Biol Endocrinol 2003;90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol 2011;301:R267–R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Evidence for corpus luteal and endometrial origins of adverse pregnancy outcomes in women conceiving with or without assisted reproduction. Obstet Gynecol Clin North Am 2020;47:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Baker VL.. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 2013;304:R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Graham GM, Chi YY, Zhai X, Li M, Williams RS, Rhoton-Vlasak A, Segal MS, Wood CE, Keller-Wood M.. Potential influence of the corpus luteum on circulating reproductive and volume regulatory hormones, angiogenic and immunoregulatory factors in pregnant women. Am J Physiol Endocrinol Metab 2019a;317:E677–E685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Petersen JW, Chi YY, Zhai X, Li M, Chiu KH, Liu J, Lingis MD, Williams RS, Rhoton-Vlasak A. et al. Maternal cardiovascular dysregulation during early pregnancy after in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019b;74:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello M, Garad R, Hart R, Homer H, Johnson L, Jordan C, Mocanu E, Qiao J, Rombauts L, Teede HJ. et al. A review of first line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med Sci (Basel) 2019;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GE, Ledger WL.. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG: Int J Obstet Gy 2019;126:237–243. [DOI] [PubMed] [Google Scholar]
- Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C.. Endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with gonadotropin-releasing hormone agonist. Fertil Steril 2002;77:956–960. [DOI] [PubMed] [Google Scholar]
- Devarakonda T, Salloum FN.. Heart disease and relaxin: new actions for an old hormone. Trends Endocrinol Metab 2018;29:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto L, Vega M, Kohen P, Castro A, Castro O, Christenson LK, Carvallo P, Strauss JF. 3rd. Endocrine and paracrine-autocrine regulation of the human corpus luteum during the mid-luteal phase. J Reprod Fertil Suppl 2000;13–20. [PubMed] [Google Scholar]
- Devoto L, Fuentes A, Kohen P, Céspedes P, Palomino A, Pommer R, Muñoz A, Strauss JF.. The human corpus luteum: life cycle and function in natural cycles. Fertil Steril 2009;92:1067–1079. [DOI] [PubMed] [Google Scholar]
- Devoto L, Kohen P, Gonzalez RR, Castro O, Retamales I, Vega M, Carvallo P, Christenson LK, Strauss JF. III. Expression of steroidogenic acute regulatory protein in the human corpus luteum throughout the luteal phase. J Clin Endocrinol Metab 2001;86:5633–5639. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Tofovic SP, Jackson EK.. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther 2004;308:403–409. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Chaffin CL, Stouffer RL.. Expression of oestrogen receptor alpha and beta in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology 2000;141:1711–1717. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Gay E, Maybin JA.. The effect of human chorionic gonadotrophin on the expression of progesterone receptors in human luteal cells in vivo and in vitro. Reproduction 2005;130:83–93. [DOI] [PubMed] [Google Scholar]
- Duncan WC, van den Driesche S, Fraser HM.. Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1alpha in the follicle and corpus luteum. Endocrinology 2008;149:3313–3320. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Myers M, Dickisnon R.. Paracrine regulation of luteal development and luteolysis in the primate. Anim Reprod 2009;1:34–46. [Google Scholar]
- Einspanier A, Lieder K, Husen B, Ebert K, Lier S, Einspanier R, Unemori E, Kemper M.. Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann N Y Acad Sci 2009;1160:140–146. [DOI] [PubMed] [Google Scholar]
- Ellakwa HE, Sanad ZF, Hamza HA, Emara MA, Elsayed MA.. Predictors of patient responses to ovulation induction with clomiphene citrate in patients with polycystic ovary syndrome experiencing infertility. Int J Gynaecol Obstet 2016;133:59–63. [DOI] [PubMed] [Google Scholar]
- Engmann L, Losel R, Wehling M, Peluso JJ.. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab 2006;12:4962–4968. [DOI] [PubMed] [Google Scholar]
- Evans PW, Wheeler T, Anthony FW, Osmond C.. A longitudinal study of maternal serum vascular endothelial growth factor in early pregnancy. Hum Reprod 1998;13:1057–1062. [DOI] [PubMed] [Google Scholar]
- Eyster KM. The oestrogen receptors: an overview from different perspectives. Methods Mol Biol 2016;1–10. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledée N, Frydman R.. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril 2001;75:1136–1140. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J.. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online 2010;21:331–337. [DOI] [PubMed] [Google Scholar]
- Ficicioglu C, Gurbuz B, Tasdemir S, Yalti S, Canova H.. High local endometrial effect of vaginal progesterone gel. Gynecol Endocrinol 2004;18:240–243. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP.. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 2009;30:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz R, Jindal S, Feil H, Buyuk E.. Elevated serum estradiol levels in artificial autologous frozen embryo transfer cycles negatively impact ongoing pregnancy and live birth rates. J Assist Reprod Genet 2017;34:1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão AM, Skarzynski D, Ferreira-Dias G.. luteolysis and the auto-, paracrine role of cytokines from tumor necrosis factor α and transforming growth factor β superfamilies. Vitam Horm 2018;287–315. [DOI] [PubMed] [Google Scholar]
- Garg D, Ng SSM, Baig KM, Driggers P, Segars J.. Progesterone-mediated non-classical signalling. Trends Endocrinol Metab 2017;28:656–668. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Fernandes MS, Brosens JJ.. Non-genomic progesterone actions in female reproduction. Hum Reprod Update 2008;15:119–138. [DOI] [PubMed] [Google Scholar]
- Ghulmiyyah L, Sibai B.. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 2012;36:56–59. [DOI] [PubMed] [Google Scholar]
- Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C.. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126.e1–e18. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Pesce R, Sueldo CQ, Retamar AM, Hart RJ, Ciapponi A.. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2020;Cd006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi JH, RichardsonMl, Jelinic M, Girling JE, Wlodek ME, Tare M, Parry LJ.. Enhanced uterine artery stiffness in aged pregnant relaxin mutant mice is reversed with exogenous relaxin treatment. Biol Reprod 2013;1:18. [DOI] [PubMed] [Google Scholar]
- Haning RV Jr, Goldsmith LT, Seifer DB, Wheeler C, Frishman G, Sarmento J, Weiss G.. Relaxin secretion in in vitro fertilization pregnancies. Am J Obstet Gynecol 1996;174:233–240. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Paiva P, Meehan KL, Rombauts LJ, Gardner DK, Salamonsen LA.. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology 2011;152:4948–4956. [DOI] [PubMed] [Google Scholar]
- Hasin Y, Seldin M, Lusis A.. Multi-omics approaches to disease. Genome Biol 2017;1:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henríquez S, Kohen P, Xu X, Veenstra TD, Muñoz A, Palomino WA, Strauss JF, Devoto L.. Oestrogen metabolites in human corpus luteum physiology: differential effects on angiogenic activity. Fertil Steril 2016;106:230–237.e1. [DOI] [PubMed] [Google Scholar]
- Henríquez S, Kohen P, Xu X, Villarroel C, Muñoz A, Godoy A, Strauss JF, Devoto L.. Significance of pro-angiogenic oestrogen metabolites in normal follicular development and follicular growth arrest in polycystic ovary syndrome. Hum Reprod 2020;35:1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Hernandez I, Rodriguez F, Pertegal M, Bonacasa B, Salom MG, Quesada T, Fenoy FJ.. Endothelial dysfunction in gestational hypertension induced by catechol-O-methyltransferase inhibition. Exp Physiol 2013;98:856–866. [DOI] [PubMed] [Google Scholar]
- Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G. et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol 2010;203:477.e1––e9.. [DOI] [PubMed] [Google Scholar]
- Hervé MA, Meduri G, Petit FG, Domet TS, Lazennec G, Mourah S, Perrot-Applanat M.. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol 2006;188:91–99. [DOI] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC.. Evolution of the relaxin/insulin-like gene family in placental mammals: implications for its early evolution. J Mol Evol 2011;72:72–79. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK.. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 2012;97:1374–1379. [DOI] [PubMed] [Google Scholar]
- Ishihara O, , Araki R, , Kuwahara A, , Itakura A, , Saito H, , Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–133. [DOI] [PubMed] [Google Scholar]
- Jelinic M, Marshall SA, Stewart D, Unemori E, Parry LJ, Leo CH.. Peptide hormone relaxin: from bench to bedside. R753-r60 2018;314:R753–R760. [DOI] [PubMed] [Google Scholar]
- Jing S, , Li X F, , Zhang S, , Gong F, , Lu G, , Lin G. Increased pregnancy complications following frozen-thawed embryo transfer during an artificial cycle. J Assist Reprod Genet 2019;36:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Tyler CT, Magness RR.. Aberrant synthesis, metabolism, and plasma accumulation of circulating oestrogens and oestrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013;61:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR.. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of oestrogen receptor-alpha versus oestrogen receptor-beta. Hypertension 2010;55:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP.. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes.Endocrinology 2006;30:333–342. [DOI] [PubMed] [Google Scholar]
- Kaczmarek MM, Blitek A, Kaminska K, Bodek G, Zygmunt M, Schams D, Ziecik AJ.. Assessment of VEGF-receptor system expression in the porcine endometrial stromal cells in response to insulin-like growth factor-I, relaxin, oxytocin and prostaglandin E2. Mol Cell Endocrinol 2008;1-2:33–41. [DOI] [PubMed] [Google Scholar]
- Kale K, Vishwekar P, Balsarkar G, JassawallaMJSawant G, Madan T.. Differential levels of surfactant protein A, surfactant protein D, and progesterone to estradiol ratio in maternal serum before and after the onset of severe early-onset preeclampsia. 2020;2:e13208. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J. et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008;453:1117–1121. [DOI] [PubMed] [Google Scholar]
- Kashida S, Sugino N, Takiguchi S, Karube A, Takayama H, Yamagata Y, Nakamura Y, Kato H.. Regulation and role of vascular endothelial growth factor in the corpus luteum during mid-pregnancy in rats. Biol Reprod 2001;64:317–323. [DOI] [PubMed] [Google Scholar]
- Kim M, Park HJ, Seol JW, Jang JY, Cho Y‐S, Kim KR, Choi Y, Lydon JP, DeMayo FJ, Shibuya M. et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med 2013;5:1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Osuga Y, Tajima T, Hirota Y, Igarashi T, Fujii T, Yano T, Taketani Y.. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril 2010;94:305–308. [DOI] [PubMed] [Google Scholar]
- Kohen P, Castro O, Palomino A, Muñoz A, Christenson LK, Sierralta W, Carvallo P, Strauss JF, Devoto L.. The steroidogenic response and corpus luteum expression of the steroidogenic acute regulatory protein after human chorionic gonadotropin administration at different times in the human luteal phase. J Clin Endocrinol Metab 2003;88:3421–3430. [DOI] [PubMed] [Google Scholar]
- Koster MP, de Wilde MA, Veltman-Verhulst SM, Houben ML, Nikkels PG, van Rijn BB, Fauser BC.. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod 2015;12:2829–2837. [DOI] [PubMed] [Google Scholar]
- Kowalik MK, Rekawiecki R, Kotwica J.. Expression of membrane progestin receptors (mPRs) in the bovine corpus luteum during the estrous cycle and first trimester of pregnancy. Domest Anim Endocrinol 2018;63:69–76. [DOI] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of oestrogens and progestogens: influence of different routes of administration. Climacteric 2005;8:3–63. [DOI] [PubMed] [Google Scholar]
- Kuo SW, Ke FC, Chang GD, Lee MT, Hwang JJ.. Potential role of follicle-stimulating hormone (FSH) and transforming growth factor (TGFβ1) in the regulation of ovarian angiogenesis. J Cell Physiol 2011;226:1608–1619. [DOI] [PubMed] [Google Scholar]
- Kwik M, Maxwell E.. Pathophysiology, treatment and prevention of ovarian hyperstimulation syndrome. Curr Opin Obstet Gynecol 2016;28:236–241. [DOI] [PubMed] [Google Scholar]
- LaVallee TM, ZhanXHHerbstrittCJKough EC, Green SJ, Pribluda VS.. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of oestrogen receptors alpha and beta. Cancer Res 2002;13:3691–3697. [PubMed] [Google Scholar]
- Lee A, Christenson LK, Patton PE, Burry KA, Stouffer RL.. Vascular endothelial growth factor production by human luteinized granulosa cells in vitro. Hum Reprod 1997;12:2756–2761. [DOI] [PubMed] [Google Scholar]
- Lee ES, Oh MJ, Jung JW, Lim JE, Seol HJ, Lee KJ, Kim HJ.. The levels of circulating vascular endothelial growth factor and soluble Flt-1 in pregnancies complicated by preeclampsia. J Korean Med Sci 2007;22:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Kim TH, Choi KC.. Functions and physiological roles of two types of oestrogen receptors, ERα and ERβ, identified by oestrogen receptor knockout mouse. Lab Anim Res 2012;28:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Cantonwine D, Little SE, McElrath TF, Parry SI, Lim KH, Wilkins-Haug LE.. Angiogenic markers in pregnancies conceived through in vitro fertilization. Am J Obstet Gynecol 2015;213:212.e1–e8. [DOI] [PubMed] [Google Scholar]
- Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, Whitesides GM, Kalluri R.. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol 2010;176:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- Lin ZH, Jin J, Shan XY.. The effects of estradiol on inflammatory and endothelial dysfunction in rats with preeclampsia. Int J Mol Med 2020;3:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LX, Lu H, Luo Y, Date T, Belanger AJ, Vincent KA, Akita GY, Goldberg M, Cheng SH, Gregory RJ. et al. Stabilization of vascular endothelial growth factor mRNA by hypoxia-inducible factor 1. Biochem Biophys Res Commun 2002;291:908–914. [DOI] [PubMed] [Google Scholar]
- Lu E, Li C, Wang J, Zhang C.. Inflammation and angiogenesis in the corpus luteum. J Obstet Gynaecol Res 2019;45:1967–1974. [DOI] [PubMed] [Google Scholar]
- Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol 2017;217:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shyamala G, Conneely OM, O'Malley BW.. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995;9:2266–2278. [DOI] [PubMed] [Google Scholar]
- Marshall SA, Senadheera SN, Parry LJ, Girling JE.. The role of relaxin in normal and abnormal uterine function during the menstrual cycle and early pregnancy. Reprod Sci 2017;24:342–354. [DOI] [PubMed] [Google Scholar]
- Marshall SA, SenadheeraSnJelinic M, O'Sullivan K, Parry LJ, Tare M.. Relaxin deficiency leads to uterine artery dysfunction during pregnancy in mice. Front Physiol 2018;255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseelall PB, Seungdamrong A, Weiss G, Wojtczuk AS, Donnelly R, Stouffer RL, Goldsmith LT.. Expression of LGR7 in the primate corpus luteum implicates the corpus luteum as a relaxin target organ. Ann N Y Acad Sci 2009;1:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi SE, Kasmi I, Bourdiec A, Crespo K, Bissonnette L, Le Saint C, Bissonnette F, Kadoch I-J.. 15 years of transcriptomic analysis on endometrial receptivity: what have we learnt? Fertil Res Pract 2019;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV.. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril 1994;62:485–490. [DOI] [PubMed] [Google Scholar]
- Miller NR, Garry D, Cohen HW, Figueroa R.. Serum androgen markers in preeclampsia. J Reprod Med 2003;4:225–229. [PubMed] [Google Scholar]
- Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH.. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum Reprod 2020;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- Mizrachi Y, Weissman A, Buchnik Fater G, Torem M, Horowitz E, Schreiber L, Raziel A, Bar J, Kovo M.. Placental histopathology in IVF pregnancies resulting from the transfer of frozen-thawed embryos compared with fresh embryos. J Assist Reprod Genet 2020;37:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushayandebvu TI, Goldsmith LT, Von HS, Santoro N, Thurston D, Weiss G.. Elevated maternal serum relaxin concentrations throughout pregnancy in singleton gestations after superovulation. Obstet Gynecol 1998;92:17–20. [DOI] [PubMed] [Google Scholar]
- Novak J, Danielson LA, KerchnerLJSherwood OD, Ramirez RJ, Moalli PA, Conrad KP.. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest 2001;11:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Tsuzuki T, Murata H.. Decidualization of the human endometrium. Reprod Med Biol 2018;17:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdahl S, , Henningsen AA, , Tiitinen A, , Bergh C, , Pinborg A, , Romundstad PR, , Wennerholm UB, , Gissler M, , Skjærven R, , Romundstad LB. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod 2015;30:1724–1731. [DOI] [PubMed] [Google Scholar]
- Pabuccu EG, Pabuccu R, Evliyaoglu Ozdegirmenci O, Bostancı Durmus A, Keskin M.. Combined progesterone (IM + V) versus vaginal progesterone for luteal support in cleavage-stage embryo transfer cycles of good prognosis patients. Gynecol Endocrinol 2016;32:366–369. [DOI] [PubMed] [Google Scholar]
- Palomba S, Russo T, Falbo A, Di Cello A, Amendola G, Mazza R, Tolino A, Zullo F, Tucci L, La Sala GB.. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab 2012;97:2441–2449. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li B, Wang Z, Wang Y, Gong X, Zhou W, Shi Y.. Hormone replacement versus natural cycle protocols of endometrial preparation for frozen embryo transfer. Front Endocrinol (Lausanne) 2020;11:546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YG, Choi J, Seol JW.. Angiopoietin-2 regulated by progesterone induces uterine vascular remodelling during pregnancy. Mol Med Rep 2020;22:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AS, GaikwadNWGrotegutCADowden SD, Haas DM.. Alterations in endogenous progesterone metabolism associated with spontaneous very preterm delivery. Hum Reprod Open 2020;2:hoaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AS, Swamy GK, Murtha AP, Heine RP, Zheng X, Grotegut CA.. Progesterone metabolites produced by cytochrome P450 3A modulate uterine contractility in a murine model. Reprod Sci 2015;22:1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER.. Developmental phenotype of a membrane only oestrogen receptor alpha (MOER) mouse. J Biol Chem 2009;284:3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E.. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab 2009;94:2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, España-Perrot PP, Venegas-Araneda P, Guzmán-Rojas AM, González MI, Palominos-Rivera M, Irarrazabal CE, Figueroa-Diesel H. et al. Placental aromatase is deficient in placental ischemia and preeclampsia. PLoS One 2015;10:e0139682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertegal M, Fenoy FJ, Bonacasa B, Mendiola J, Delgado JL, Hernandez M, Salom MG, Bosch V, Hernandez I.. 2-methoxyestradiol plasma levels are associated with clinical severity indices and biomarkers of preeclampsia. Reprod Sci 2015;22:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch A, Villanelo F, Henriquez S, Kohen P, Muñoz A, Strauss JF, Devoto L.. Molecular modelling predicts that 2-methoxyestradiol disrupts HIF function by binding to the PAS-B domain. Steroids 2019;144:21–29. [DOI] [PubMed] [Google Scholar]
- Post Uiterweer ED, Koster MPH, Jeyabalan A, Kuc S, Siljee JE, Stewart DR, Conrad KP, Franx A.. Circulating pregnancy hormone relaxin as a first trimester biomarker for preeclampsia. Pregnancy Hypertension 2020;22:47–53. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY.. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello J, Szlachter N, Steinetz BG, Goldsmit LT, Weiss LT.. Serial relaxin concentrations in human pregnancy. Am J Obstet Gynecol 1979;135:43–44. [PubMed] [Google Scholar]
- Quinney SK, Benjamin T, Zheng X, Patil AS.. Characterization of maternal and foetalCYP3A-mediated progesterone metabolism. Foetal Pediatr Pathol 2017;36:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Haring JS, Johnson ML, Ashley RL, Redmer DA, Borowicz PP, Grazul-Bilska AT.. Placental development during early pregnancy in sheep: oestrogen and progesterone receptor messenger RNA expression in pregnancies derived from in vivo-produced and in vitro-produced embryos. Domest Anim Endocrinol 2015;53:60–69. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Gammill HS.. Preeclampsia: recent insights. Hypertension 2005;46:1243–1249. [DOI] [PubMed] [Google Scholar]
- Sacha CR, Harris AL, James K, Basnet K, Freret TS, Yeh J, Kaimal A, Souter I, Roberts DJ.. Placental pathology in live births conceived with in vitro fertilization after fresh and frozen embryo transfer. Am J Obstet Gynecol 2020;222:360.e1–e16. [DOI] [PubMed] [Google Scholar]
- Sachdeva G, Gainder S, Suri V, Sachdeva N, Chopra S.. Comparison of clinical, metabolic, hormonal, and ultrasound parameters among the clomiphene citrate-resistant and clomiphene citrate-sensitive polycystic ovary syndrome women. J Hum Reprod Sci 2019;12:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, , Kuwahara A, , Ishikawa T, , Morisaki N, , Miyado M, , Miyado K, , Fukami M, , Miyasaka N, , Ishihara O, , Irahara M et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod 2019;34:1567–1575. [DOI] [PubMed] [Google Scholar]
- Salas SP, Marshall G, Gutiérrez BL, Rosso P.. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or foetal growth restriction. Hypertension 2006;47:203–208. [DOI] [PubMed] [Google Scholar]
- Salehpour S, Tamimi M, Saharkhiz N.. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal-phase support in in vitro fertilization (IVF): a randomized clinical trial. Iran J Reprod Med 2013;11:913–918. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Garrido MA, Tena-Sempere M.. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant S, Bhide P.. Fertility treatment options for women with polycystic ovary syndrome. Clin MedInsightsReprodHealth 2019;13:651–672.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazonova A, , Kallen K, , Thurin-Kjellberg A, , Wennerholm U-B, , Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod 2012;27:1343–1350. [DOI] [PubMed] [Google Scholar]
- Schindler AE. First trimester endocrinology: consequences for diagnosis and treatment of pregnancy failure. Gynecol Endocrinol 2004;18:51–57. [DOI] [PubMed] [Google Scholar]
- Seeger H, Mueck AO, Lippert TH.. Effect of estradiol metabolites on prostacyclin synthesis in human endothelial cell cultures. Life Sci 1999;65:PL167–PL170. [DOI] [PubMed] [Google Scholar]
- Serin IS, Kula M, Başbuğ M, Unlühizarci K, Güçer S, Tayyar M.. Androgen levels of preeclamptic patients in the third trimester of pregnancy and six weeks after delivery. Acta Obstet Gynecol Scand 2001;80:1009–1013. [DOI] [PubMed] [Google Scholar]
- Sha T, Yin X, Cheng W, Massey IY.. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril 2018;109:330–342.e9. [DOI] [PubMed] [Google Scholar]
- Shao X, Wang Y, Liu Y, Guo X, Li D, Huo R, Jia W, Cao G, Li YX, Liu M.. Association of imbalanced sex hormone production with excessive procoagulation factor SerpinF2 in preeclampsia. J Hypertens 2019;1:197–205. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Boostanfar R, Silverberg K, Yanushpolsky EH.. Examining the evidence: progesterone supplementation during fresh and frozen embryo transfer. Reprod Biomed Online 2014;29:S1–S14; quiz S15-S16. [DOI] [PubMed] [Google Scholar]
- Shen Z, Wu Y, Chen X, Chang X, Zhou Q, Zhou J, Ying H, Zheng J, Duan T, Wang K.. Decreased maternal serum 2-methoxyestradiol levels are associated with the development of preeclampsia. Cell Physiol Biochem 2014;34:2189–2199. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev 2004;25:205–234. [DOI] [PubMed] [Google Scholar]
- Sindelar M, Patti GJ.. Chemical discovery in the era of metabolomics. J Am Chem Soc 2020;142:9097–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Reschke L, Segars J, Baker VL.. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril 2020;113:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Simpson RL, Bennett RG.. Relaxin activates peroxisome proliferator-activated receptor γ (PPARγ) through a pathway involving PPARγ coactivator 1α (PGC1α). J Biol Chem 2015;290:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sites CK, , Wilson D, , Barsky M, , Bernson D, , Bernstein IM, , Boulet S, , Zhang Y. Embryo cryopreservation and preeclampsia risk. Fertil Steril 2017;108:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradley FT. Metabolic abnormalities and obesity's impact on the risk for developing preeclampsia. Am J Physiol Regul Integr Comp Physiol 2017;312:R5–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su EJ, Xin H, Monsivais D.. The emerging role of oestrogen receptor-β in human reproduction. Semin Reprod Med 2012;30:62–70. [DOI] [PubMed] [Google Scholar]
- Szepietowska B, Gorska M, Szelachowska M.. Plasma relaxin concentration is related to beta-cell function and insulin sensitivity in women with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008;79:e1–e3. [DOI] [PubMed] [Google Scholar]
- Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T.. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci 2019;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Jaiswar SP, Deo S, Shankhwar P.. Association of 2-methoxyestradiol (2ME) plasma levels with clinical severity indices and biomarkers of preeclampsia. J Obstet Gynecol India 2019;69:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN.. Maternal serum ooestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol 2003;32:455–460. [DOI] [PubMed] [Google Scholar]
- Vaisbuch E, Leong M, Shoham Z.. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online 2012;25:139–145. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Smith VM, Myers M, Duncan WC.. Expression and regulation of ooestrogen receptors in the human corpus luteum. Reproduction 2008;135:509–517. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M.. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7:Cd009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Ottander U, Oduwole O, Isomaa V, Vihko P, Olofsson JI, Tapanainen JS.. Role of apoptosis, apoptosis-related factors and 17beta-hydroxysteroid dehydrogenases in human corpus luteum regression. Mol Cell Endocrinol 2002;194:191–200. [DOI] [PubMed] [Google Scholar]
- Vigneau C, Lorcy N, Dolley-Hitze T, Jouan F, Arlot-Bonnemains Y, Laguerre B, Verhoest G, Goujon JM, Belaud-Rotureau MA, Rioux-Leclercq N.. All anti-vascular endothelial growth factor drugs can induce ‘pre-eclampsia-like syndrome’: a RARe study. Nephrol Dial Transplant 2014;29:325–332. [DOI] [PubMed] [Google Scholar]
- von Versen-Hoynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, Winn VD.. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension 2019a;73:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen-Höynck F, Schaub AM, Chi Y-Y, Chiu K-H, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR. et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019b;73:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen-Höynck F, Strauch NK, Liu J, Chi YY, Keller-Woods M, Conrad KP, Baker VL.. Effect of mode of conception on maternal serum relaxin, creatinine, and sodium concentrations in an infertile population. Reprod Sci 2019c;26:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M, Chen Q.. The reduction in circulating levels of oestrogen and progesterone in women with preeclampsia. Pregnancy Hypertens 2018;11:18–25. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhu WJ, Xie BG.. Expression of PPAR-γ in adipose tissue of rats with polycystic ovary syndrome induced by DHEA. Mol Med Rep 2014;9:889–893. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu H, Song H, Li X, Jiang J, Sheng Y, Shi Y.. Increased risk of pre-eclampsia after frozen-thawed embryo transfer in programming cycles. Front Med (Lausanne) 2020;104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantania J, Attamimi A, Siswishanto R.. A comparison of 2-methoxyestradiol value in women with severe preeclampsia versus normotensive pregnancy. J Clin Diagn Res 2017;3:Qc35–Qc38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Xu Q, Yang H, Yang Y, Wang L, Chen H, Anderson C, Liu X, Song G, Li Q. et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med 2019;16:e1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T, Elcock CL, Anthony FW.. Angiogenesis and the placental environment. Placenta 1995;16:289–296. [DOI] [PubMed] [Google Scholar]
- Whitley GS, Cartwright JE.. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat 2009;215:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo I, Chan Y, Sriprasert I, Louie K, Ingles S, Stanczyk F, McGinnis LK, Chung K.. The role of angiogenic markers in adverse perinatal outcomes: fresh versus frozen embryo transfers. J Assist Reprod Genet 2017;34:1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G.. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet 2016;33:1287–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Zhu X, Guo C, Yang Y, Han T, Chen L, Yin W, Gao P, Zhang H, Geng J. et al. Differential expression of estradiol and oestrogen receptor α in severe preeclamptic pregnancies compared with normal pregnancies. Mol Med Rep 2013;7:981–985. [DOI] [PubMed] [Google Scholar]
- Young SL. Ooestrogen and progesterone action on endometrium: a translational approach to understanding endometrial receptivity. Reprod Biomed Online 2013;27:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Kovalenko O, Echalar L, Torricos T, Al-Khan A, Alvarez M, Illsley NP.. Evidence for extraplacental sources of circulating angiogenic growth effectors in human pregnancy. Placenta 2013;34:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisler H, Jirecek S, Hohlagschwandtner M, Knöfler M, Tempfer C, Livingston JC.. Concentrations of oestrogens in patients with preeclampsia. Wien Klin Wochenschr 2002;12:458–461. [PubMed] [Google Scholar]
- Zeleznik AJ. In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci U S A 1998;95:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, Hong Y, Sun G, Zhang T, Li W. et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary syndrome: results from a randomized trial. Fertil Steril 2018;109:324–329. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR.. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013;34:939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang T, Shen Y, Wang X, Baker PN, Zhao A.. 2-Methoxyestradiol deficiency is strongly related to hypertension in early onset severe pre-eclampsia. Pregnancy Hypertens 2014;4:215–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. No original data are presented in this review. The authors will make available figures and tables if requested.