Abstract
BACKGROUND
The function of the gestational sac (GS) and the placenta in the closely related processes of embryogenesis and teratogenicity in the first trimester has been minimally described. The prevailing assumption is that direct teratogenic effects are mediated by the critical extraembryonic organ, the placenta, which either blocks or transfers exposures to the foetus. Placental transfer is a dominant mechanism, but there are other paradigms by which the placenta can mediate teratogenic effects. Knowledge of these paradigms and first trimester human developmental biology can be useful to the epidemiologist in the conduct of biomarker-based studies of both maternal and child health.
OBJECTIVE AND RATIONALE
Our aim is to provide a causal framework for modelling the teratogenic effects of first trimester exposures on child health outcomes mediated by the GS and placenta using biomarker data collected in the first trimester. We initially present first trimester human developmental biology for the sake of informing and strengthening epidemiologic approaches. We then propose analytic approaches of modelling placental mechanisms by way of causal diagrams using classical non-embryolethal teratogens (diethylstilboestrol [DES], folic acid deficiency and cytomegalovirus [CMV]) as illustrative examples. We extend this framework to two chronic exposures of particular current interest, phthalates and maternal adiposity.
SEARCH METHODS
Information on teratogens was identified by a non-systematic, narrative review. For each teratogen, we included papers that answered the five following questions: (i) why were these exposures declared teratogens? (ii) is there a consensus on biologic mechanism? (iii) is there reported evidence of a placental mechanism? (iv) can we construct a theoretical model of a placental mechanism? and (v) can this knowledge inform future work on measurement and modelling of placental-foetal teratogenesis? We prioritized literature specific to human development, the organogenesis window in the first trimester and non-embryolethal mechanisms.
OUTCOMES
As a result of our review of the literature on five exposures considered harmful in the first trimester, we developed four analytic strategies to address first trimester placental mechanisms in birth cohort studies: placental transfer and direct effects on the foetus (DES and maternal adiposity), indirect effects through targeted placental molecular pathways (DES and phthalates), pre-placental effects through disruptions in embryonic and extraembryonic tissue layer differentiation (folic acid deficiency), and multi-step mechanisms that involve maternal, placental and foetal immune function and inflammation (DES and CMV).
WIDER IMPLICATIONS
The significance of this review is to offer a causal approach to classify the large number of potentially harmful exposures in pregnancy when the exposure occurs in the first trimester. Our review will facilitate future research by advancing knowledge of the first trimester mechanisms necessary for researchers to effectively associate environmental exposures with child health outcomes.
Keywords: placenta, gestational sac, teratogen, biomarkers, diethylstilboestrol (DES), phthalates, folic acid, cytomegalovirus (CMV), epidemiology, first trimester
Introduction
The gestational sac (GS) and placenta have been minimally described as key components in the two closely related fields of embryology and teratology. A predominant premise in the teratology literature is that the placenta acts as a barrier or a transporter of teratogens to foetal tissues (Walker et al., 2017; Koren and Ornoy, 2018). As a result, there is a limited scope of published data and theory on movement of molecules and molecular mechanisms associated with teratogenic effects within the GS, which includes both the placenta and the embryo, during the early first trimester.
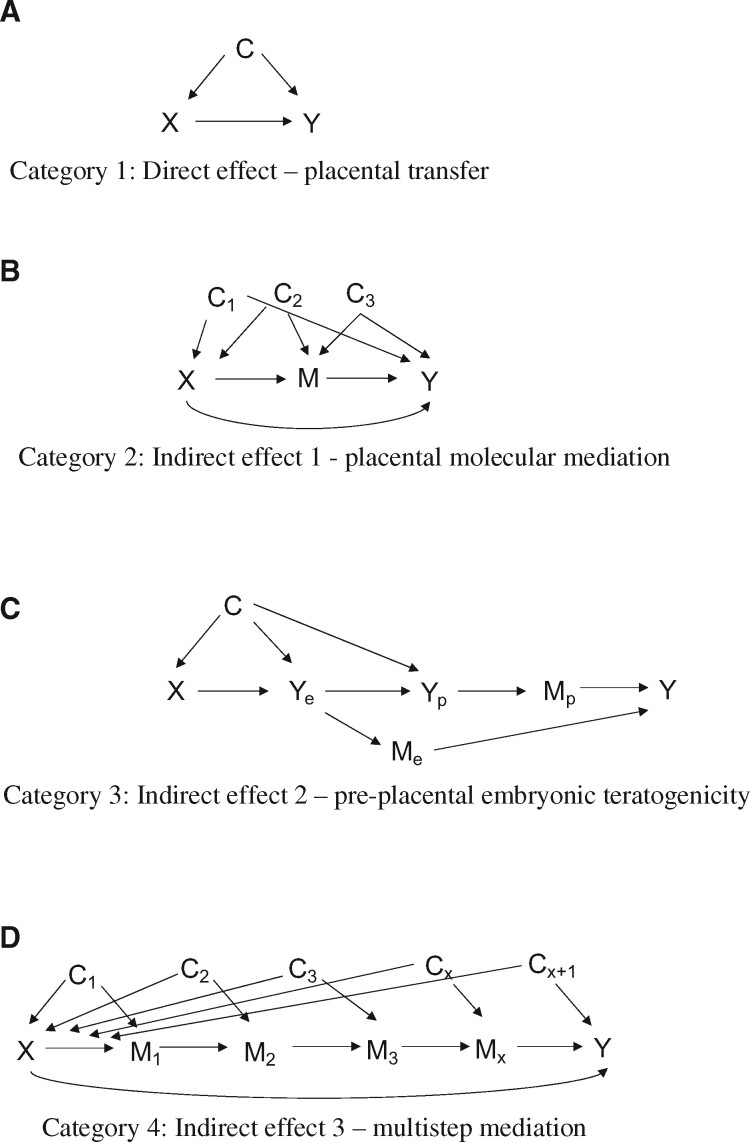
The aim of this review is to expand the scope of research by which the placenta is both measured and modelled as a critical component of historically established teratogenic mechanisms in the first trimester. We propose four mechanisms to model teratogens in observational studies that consider relevant developmental biology and apply the use of directed acyclic graphs (DAGs), an epidemiologic tool for causal inference. The four mechanisms include (i) direct teratogenic effects, (ii) placental molecular mediation, (iii) pre-placental embryonic teratogenicity and (iv) multi-step mediation. We use diethylstilboestrol (DES) as the primary example to illustrate how these four mechanisms of teratogenicity can be applied to a classic teratogen with complex pathophysiology. In addition to DES, we conducted a semi-structured literature review of folic acid, cytomegalovirus (CMV), phthalates and maternal adiposity for two examples of well-established teratogens and two non-traditional examples of teratogens, respectively.
In the remainder of this introduction, we discuss pertinent developmental biology and epidemiologic methodology that support the basis for the proposed teratogenic mechanisms. To consider how placental mechanisms in the first trimester might influence measurement and analytical strategy, an overview is presented of first trimester human GS and placental biology. Subsequently, we provide an explanation of DAGs to familiarize the reader with how biological processes are translated into causal models for observational studies. Finally, we summarize the history of DES as context for the public health significance and rationale for modelling a classic teratogen with respect to first trimester GS biology. DES provides an illustrative example of how the proposed framework applies to a teratogen.
Primer on first trimester GS and placental biology
The gestational sac
The GS is the term used to describe the placenta-embryo during the period of organogenesis (4–12 weeks of gestation) discussed here and includes multiple structures (Carlson, 2014; Fig. 1). The embryo gives rise to the tissues within the GS, some of which are embryonic and others which are extraembryonic. The GS and placenta are genetically the same as the foetus (i.e. foetal sex and karyotype). Correct form and function of the GS ensure success of the pregnancy. Key structures are the placental villi, which are the largest of gestational tissue structures in volume and surface area. In this early period, the villi cover the full surface of the chorion and are bathed in intervillous fluid (clear fluid made up of uterine gland secretions; Benirschke and Kaufmann, 1995). Uterine glands which form in the decidua after conception are the source of nutrients and immune factors circulating in the intervillous space to support these early stages of development (Burton et al., 2002). In this early period before 10-week gestation, the chorion is 20% thicker than at the end of pregnancy and has embryonic mesodermal and extraembryonic epithelial layers which contain stem cell and progenitor cell populations (Benirschke and Kaufmann, 1995; Genbacev et al., 2011). The chorion houses a network of foetal vessels that travel through the umbilical cord. Foetal circulation through these vessels is established at 10-week pregnancy (Jauniaux et al., 2003). Contained within the chorion is the fluid-filled exocoelomic cavity (ECC). The ECC forms at 4 weeks of gestation between the amnion and the chorion. It contains coelomic fluid (CF) which is yellow in colour with high concentrations of nutrients and proteins produced by the villi (Jauniaux and Gulbis, 2000).
Figure 1.
A timeline of human development, with an emphasis on gestational sac and placental development, fluid cavities and changes in these structures over time relative to milestones in foetal development.
Within the ECC lies the yolk sac, a distinct structure with its own membrane and unique fluid composition. The yolk sac forms from the embryonic endoderm and the extraembryonic mesoderm by 5-weeks of gestation and is the primary source of nutrients and essential molecules for the embryo during the first stages of development (Benirschke and Kaufmann, 1995; Shahbazi et al., 2016; Cindrova-Davies et al., 2017). Teratogen transport mechanisms within the yolk sac before 10 weeks overlap with nutrient transport mechanisms (Cindrova-Davies et al., 2017). In the 10th week, the yolk sac starts to degenerate and ceases to function as nutrients in maternal blood replace it (Jones and Jauniaux, 1995).
Contained within the ECC is the amnion. The amnion is a thinner and more translucent membrane than the chorion and encapsulates the embryo in clear amniotic fluid (AF). The GS can be thought of as a highly engineered system of fluid compartments and channels to move molecules and control pressures in a co-ordinated fashion. It is the combination of these molecules and biomechanical pressures that results in a highly reproducible process of embryogenesis (Davidson et al., 2009). The details in Figs 1 and 2 are helpful to understand how teratogens can move through this complex structure to disrupt embryo development. The definition of teratogens could be extended to those that are toxic to foetal tissues at a molecular level and which can result in malformations and/or disruptions in the engineering of the GS.
Figure 2.
An illustration of the four different ways in which teratogens (grey dots) can disrupt development in the first trimester. (A) Category 1, direct effects of teratogens on embryo development. Representation of structures during 10–12-week gestation. (B) Category 2, placental molecular mediation. Representation of teratogen movement and structures during 10–12-week gestation. (C) Category 3, embryonic teratogenicity. Representation of teratogen access to structures in the first month after conception and before formation of the placental villi. (D) Category 4, multi-step mediation. Representation of teratogen access to cells and structures over the course of the first trimester. (E) Enlargement from A of the process by which the trophoblast plugs dissolve and maternal blood flow into the intervillous space begins. (F) Enlargement from D of the different tissue and cell types where teratogens can induce molecular and/or morphologic effects.
The entire GS is maintained in a low oxygen environment by virtue of ‘trophoblastic plugs’ that block maternal blood flow at the border between the GS and the myometrium (uterine wall) during the first 10 weeks of pregnancy (Hustin and Schaaps, 1987; Jaffe et al., 1997). Trophoblastic plugs are aggregates of endovascular trophoblasts that effectively block the flow of blood out of the maternal arteries and into the intervillous space (Burton et al., 1999). Maternal-foetal transport into the GS occurs by way of secretions from uterine glands into the intervillous space and also a plasma ultrafiltrate that seeps through the plugs closer to 10 weeks. These secretions might represent but do not mimic in concentration and composition the components of maternal blood (Burton et al., 1999). Once inside the intervillous space, placental transport occurs by way of receptor molecules on the villi, or else by diffusion through extracellular space.
While molecules are not readily moving from maternal blood into the intervillous space, placental transport might not be a primary route of teratogenic actions before 10 weeks. At 10 weeks, these trophoblastic plugs dissipate and maternal blood flows into the intervillous space (Weiss et al., 2016; Roberts et al., 2017; James et al., 2018) (Fig. 1). This changes the distribution of molecules in and out of the GS (Jauniaux et al., 2006). There are multiple scenarios for passive and/or active transport systems of substances into and through the GS which might be the basis for teratogenic mechanisms (Walker et al., 2017; Koren and Ornoy, 2018). The GS itself could be a target of teratogens and be subject to morphologic defects.
GS transport of exogenous non-teratogenic compounds
In situations of presumed direct teratogenic effects within foetal cells, it is helpful to conceive of a pathway by which teratogens can either travel through the GS or access the embryo as distinct from situations where the teratogen might be prevented from accessing the embryo. In a small cross-sectional investigation of first trimester pregnancies that measured levels of the same five exogenous non-teratogenic compounds in all of the GS and maternal compartments, there was considerable variation in whether and how the exogenous compounds reached the early embryo (Jauniaux and Gulbis, 2000). Three compounds given as discrete doses over a 30-min period had full entry and were detectable in all compartments: (i) diazepam, an anti-anxiety medication; (ii) inulin, a soluble fibre used here as an inert substance and delivered by injection; and (iii) cotinine, a metabolite of nicotine. The diazepam concentration was an order of magnitude higher in maternal serum, suggesting restricted transport. Inulin was detected according to a high to low gradient from maternal serum to CF (fluid in the ECC) to AF, suggesting movement across membranes. Interestingly, cotinine was higher in the CF and AF compared with maternal serum in 40 active and 5 passive smokers (Jauniaux and Gulbis, 2000). In this scenario, pre-conception exposures may have accumulated in tissues before the formation of the placenta. Cotinine has a long half-life in the foetal compartment which may be due to lack of active transport out of the GS by the P-glycoprotein transporter (Wang et al., 2005). Another explanation is that nicotine (acquired through active smoking in the first trimester) was transported through the plasma ultrafiltrate and uterine gland secretions to the placental villi and metabolized by the placenta, then accumulated within the GS (Pastrakuljic et al., 1998).
Fentanyl, a synthetic opioid, was detected in maternal serum and in AF at about equal concentrations. The fifth compound, propofol (an anaesthetic), was only detected in maternal serum and, therefore, presumed unable to cross the membranes into the GS. This may be a rare example of the placenta acting as a true barrier. This type of investigation is valuable in understanding whether teratogenesis occurs as direct exposure or whether there is effective restriction. However, the technique used to harvest these fluids (coleocentesis) carries risk and is not feasible to apply in the setting of large-scale cohort studies (Jauniaux and Gulbis, 2000).
Stage-related changes in the function of the GS
Key aspects of GS morphogenesis likely play a role in the linkage between organogenesis and teratogenesis (Fig. 1). At 10 weeks, there is a transition when the GS turns into the placenta as it is more commonly described as a maternal-foetal transport organ. This is the period when the trophoblastic plugs dissolve and maternal blood flow begins into the space between placenta villi. This period presents changes in placental physiologic function, morphology and molecular machinery (Genbacev et al., 1997). There is a switch from histiotrophic nutrition (uterine glands-placental villi-chorion-ECC-yolk sac-amnion-embryo) to haemotrophic nutrition (maternal blood-placental villi-foetal circulation; Burton et al., 2001).
The ECC is gone by 14 weeks (Benirschke and Kaufmann, 1995). The amnion and the chorion fuse, leaving no fluid cavity inbetween. Levels of human chorionic gonadotropin (hCG), a human and placenta-specific hormone, in maternal serum begin rising within 7 days post-conception and peak at 10 weeks of gestation, then subsequently decrease (Nagy et al., 1994a,b). After the ECC disappears, the placental villi along the smooth chorion gradually become avascular and decrease in total surface area. The smooth chorion (chorion laeve) in the final form makes up two-thirds of the placenta surface and has no villi. The chorion frondosum (chorion with villi attached or rough chorion) becomes one-third of the total surface area. This shrinkage in villous surface area might result in net lower transfer of some types of teratogens. It may enhance the transfer of other types of teratogens given the close proximity of uterine wall, chorion smooth/amnion and foetus (Benirschke and Kaufmann, 1995; Genbacev et al., 2015). Transport of teratogens across the smooth chorion in the first trimester has not been studied. All of these details hold importance in terms of the ‘engineering’ of the foetal environment and may offer salient insights to link placental development to changing levels of placental hormones in maternal circulation.
Directed acyclic graphs (DAGs): translation of biology to statistical models
DAGs visually represent the background knowledge and assumptions applied to the statistical estimation of a causal relationship (Greenland et al., 1999; Pearl, 2000; Rothman et al., 2008). They are useful in the analysis of observational data, such as those collected in a birth cohort study. An investigator can use a DAG to hypothesize which variables represent exposures, outcomes, intermediate variables between exposure and outcome, confounders and sources of selection bias (also referred to as collider bias). In the setting outlined here, a collider is a variable that represents a process that occurred after the exposure in time and in causal sequence. A collider might be correlated with the pathophysiology that led to the outcome. For example, the common practice of adjusting for gestational age at birth when estimating effects of maternal exposures on birth and child health outcomes is a violation of the rule that all confounders have to precede the exposure in time (Wilcox et al., 2011). Another common source of collider bias is the practice of restricting analysis to those pregnancies which had a pathology examination at birth. Adjustment for variables that increase precision in the outcome but which are not causes of the exposure can produce collider or selection bias. Specific rules guide the use of arrows and nodes in a DAG (not reviewed here) Shrier and Platt, 2008). A ‘backdoor pathway’ refers to a line that connects the exposure and the outcome in unintended ways. It can falsely create an association that is otherwise not there, and attenuate or block an existing association. Backdoor pathways due to measured or unmeasured confounders, intermediate variables or colliders can be identified with the use of a DAG. Appropriate statistical methods can be selected to effectively remove arrows and/or block pathways, facilitating causal inference assuming an unbiased association of an accurately measured exposure and outcome.
Summary of introduction
The aim of this narrative review and commentary is to combine description of the unique biology of the first trimester and a causal modelling approach using DAGs. The first trimester is of particular importance because of GS and placental development, as well as the definitive formation of foetal organs including the neocortex, the brain, the gonad and the genitalia. This is covered in detail elsewhere (Moore et al., 2008; Carlson, 2014). We acknowledge that there are two general categories driving this process: (i) genetically controlled developmental processes essential to survival of the embryo and the species (show little variation across individuals), and (ii) those processes which show greater variation among individuals and reflect differences in gene expression, epigenetics, exposures, etc. This review focuses on four processes (direct effects, placental molecular mediation, pre-placental embryonic teratogenicity and multi-step mediation) which fall into the second category. Changes in these processes are not uniformly embryo-lethal. Child health outcomes associated with these mechanisms might fit into the category of ‘adaptive foetal programming’ (Myatt, 2006; Jansson and Powell, 2007; O'Donnell et al., 2009; Sferruzzi-Perri and Camm, 2016). Teratogen exposures reviewed here (DES, folic acid, CVM, phthalates and obesity) and which are associated with placental biomarkers through a reproducible mechanism can lead to meaningful differences in chronic health risk of the offspring and future generations.
Methods
The teratogens were selected to cover the following categories: endocrine disruptors (DES, phthalates), nutritional deficiency (folic acid deficiency) and viral teratogens (CMV). Maternal adiposity, measured as body mass index (BMI), was selected as a highly prevalent, modifiable and well-studied foetal exposure. The literature review for the selected teratogens’ roles was carried out as a non-systematic, narrative review between February and August 2019, with updates in 2020. PubMed and Google search engines were used to conduct searches with key terms including name of teratogen or maternal exposure, embryo, GS, placenta, placental mechanism, placental transfer, trophoblast, first trimester, epidemiology, toxicology and foetal origins.
Five criteria determined by expert consensus were applied for each teratogen for review: (i) insight into how these exposures were declared teratogens, (ii) basic biological mechanisms of these teratogens, (iii) evidence of placental mechanisms, (iv) construction of testable theoretical models of placental mechanisms and (v) practical approaches (i.e. candidate biomarkers, causal diagrams) on how best to measure and model placental-foetal teratogenesis.
Efforts were taken to include mechanisms specific to the organogenesis window, and which did not result in foetal death and/or spontaneous abortion. Animal studies were minimally included given the inability to translate across species with regard to placental transport mechanisms and placental endocrinology (Walker et al., 2017). We drew causal diagrams that correspond to the four teratogenic mechanisms, measurement and analysis strategies (Shrier and Platt, 2008).
Proposed conceptual framework
Placental transfer and direct effects
The first category of teratogens includes those which pass through the placenta to directly affect the developing foetus (Fig. 2A). Direct effects are those attributed to exposure to the original teratogenic molecule. Placental transfer may occur actively through specific transporters or protein receptors, passively through diffusion processes or through transformation of a parent compound into its more toxic metabolite (Walker et al., 2017; Koren and Ornoy, 2018).
In the placenta, active transport is carried out by proteins that fall into two classes: ATP-binding cassettes (ABC) and solute carriers (SLC). From a comprehensive review of these two transporter classes, it is clear that transporters likely play an important and understudied role in teratogen exposure (Walker et al., 2017). Some are expressed according to specific temporal patterns, either increasing or decreasing with gestational age. Transporter proteins are differentially expressed on one side (apical) or the other (basal) of the syncytiotrophoblast membrane (multinucleated trophoblast layer that lines the outer edge of the placental villi) so as to move molecules towards or away from foetal circulation. The under- and over-expression of transporters is likely influenced by teratogens and other maternal exposures.
It is important to distinguish teratogenic effects of a parent compound versus its metabolites and conjugates. Some teratogens may initially enter the body in a non-toxic form, termed a pro-teratogen, that is transformed into a toxic form (Wells and Winn, 1996). Upon entry into the cell, molecules can be transformed into reactive or potent intermediates that cause teratogenic effects by different enzymatic systems, such as cytochrome P450, prostaglandin H synthase and lipoxygenase (van Gelder et al., 2010). Examples of teratogens subject to such transformations are thalidomide, Benzo(a)pyrene, Aflatoxin B1 and DES (Wells and Winn, 1996). In the Wells and Winn’s authoritative review on this topic, it is assumed that these transformations either occur within maternal or embryonic/foetal tissue (Wells and Winn, 1996). Notably, xenobiotic metabolism and other similar pathways are also active within the placenta and important for its function (Hakkola et al., 1998; Myllynen et al., 2007). Some teratogens can preferentially accumulate in the foetus rather than in the maternal compartment, which reflects both transport and metabolism. Foetal detoxification mechanisms are not as well developed as in the adult. For the sake of truly understanding direct teratogenicity, the presence/absence of transport and detoxification mechanisms in foetal placental cells and in foetal somatic cells needs to be established.
Biomarkers, direct effects
The gold standard for this type of mechanism would be a real-time imaging biomarker that could visualize and quantify the movement of the teratogen from the maternal tissues, through the placenta and report final dose to foetus. The next best biomarker would be the foetal tissue concentrations of the teratogen that could be correlated with maternal levels. Foetal tissue is not available in a first trimester viable pregnancy, and even the next best option of a measure of teratogen concentration in placental tissue itself is not available until the placenta is recovered during delivery. With 26–36 weeks elapsing between first trimester teratogenesis and a full-term delivery, temporality is lost. Hence, maternal circulating levels of the teratogen in the first trimester are generally the most commonly used biomarker to estimate direct effects of a teratogen (i.e. phthalate levels in urine, folic acid levels in blood, CMV antibodies in maternal blood). Human placental perfusion studies are one approach to the validation of assumptions of placental transport of maternal exposures (Mose et al., 2007; Mathiesen et al., 2014; Koren and Ornoy, 2018).
DAG, direct effects
This DAG for estimating a causal effect of this type of teratogen requires individual-level measures of the first trimester foetal exposure and the child health outcome (Fig. 3A). It is important to emphasize that X in the figure represents foetal teratogen exposure, even though it is generally measured as maternal exposure. Confounding bias can occur in the case of measured and unmeasured factors that (i) affect the placental transfer of the specific teratogen of interest from the mother to the foetus; and (ii) affect the foetus in the absence of the teratogen. For example, expression of a transporter protein in the first trimester would be a source of confounding in this model. Placental receptors can transport the specific teratogen and also are designed to transport a variety of molecules including nutrients. Transporters are not secreted into maternal blood and therefore there is no way to measure and adjust for this type of confounding in a birth cohort study. Information on confounding by transporter expression can only be obtained through validation studies, such as placental perfusion studies or direct measures of teratogen using coleocentesis (see GS transport of exogenous non-teratogenic compounds), and addressed in the study design.
Figure 3.
Four directed acyclic graphs to guide the analysis of first trimester teratogens, biomarkers and child outcomes. (A) The directed acyclic graph for direct effects. (X: prenatal exposure, Y: foetal outcome, C: set of confounders). (B) The directed acyclic graph for placental molecular mediation (indirect effects). (X: prenatal exposure, M: placental mediator/biomarker, Y: foetal outcome, C1,2,3: set of confounders). (C) The directed acyclic graph for pre-placental embryonic teratogenicity. (X: pre-conception/prenatal exposure, Ye: embryo outcome, Yp: extraembryonic/placental outcome, C: set of confounders, Mp: extraembryonic/placental secreted biomarker, Me: embryonic secreted biomarker). (D) The directed acyclic graph for multi-step mediation. (X: prenatal exposure, M1…x: mediator/biomarker, Y: foetal outcome, C1…X+1: set of confounders).
If validation studies demonstrate that the exposure measure correlates with the foetal tissue concentration, there is no need to measure and account for the placenta in this setting. However, the delivery of the teratogen from the mother to the foetus may differ by factors, such as maternal metabolism (liver and kidney function) of the parent compound or the presence of other exposures, such as smoking, morbidities or medications. Foetal sex could be a confounder in this situation given that expression of some of the placental transporter genes and enzymes differ by XX and XY karyotype, which is also a determinant of foetal development (Walker et al., 2017). Maternal psychosocial and physiological stress (or sources of) are potential confounders here given the overlap in enzymes that metabolise glucocorticoids and xenobiotics. All of the above are likely to also have causal effects on foetal development regardless of the teratogen exposure, qualifying them as confounders. Gestational age at the time of the blood or urine sample would also be a confounder and/or an effect modifier of a teratogen. Gestational age is a cause of changes in placental transporter expression and maternal blood volume and kidney function, which are both determinants of measured biomarker levels. Given the profound changes in placental morphology and function at 10-week gestation (see Stage-related changes in the function of the GS), a dummy variable can be created to compare exposure and biomarker levels before and after this timepoint. This can improve interpretation when analysed as an effect modifier or confounder of the teratogen effect. A DAG is useful in this setting as it allows the investigator to call upon previous knowledge and make their assumptions explicit regarding how and whether the maternal exposure reaches the foetus.
Examples, direct effects
Literature on the effects of maternal adiposity in pregnancy was reviewed, considering adiposity as a ‘teratogen’ with direct effects on the foetus in Table I. This is an example where the teratogen occurs as a ‘mixture’ given maternal obesity/elevated adiposity includes abnormal levels of lipids, sugars, insulin and other molecules. There are likely multiple mechanisms involving the placenta. For the sake of sharpening the causal question, we organized the literature review based on the direct effects of glucose and fatty acids.
Table I.
Exposures illustrative of four gestational sac/placental mechanisms of teratogenicity in the first trimester.
| Title | Direct effect: placental transfer | Indirect effects: placental molecular mediation | Indirect effects: pre-placental embryonic teratogenicity | Indirect effects: multi-step mediation | |
|---|---|---|---|---|---|
| Teratogen | Exposure | Obesity | Phthalates | FA deficiency | CMV |
| Timing | Chronic | Chronic | Chronic | Acute | |
| Definition | Maternal BMI ≥30 kg/m2 (Leddy et al., 2008) | Chemicals used in plastics (plasticisers), personal care products, furniture, medical supplies, etc. | Water-soluble B vitamin and essential coenzyme for biochemical reactions (e.g. DNA synthesis). (Beaudin and Stover, 2009; Meethal et al., 2013) | DNA virus of the Herpesviridae family (Cannon et al., 2010) | |
| History |
•2013 ACOG guidelines for BMI of 30 or greater; •BMI not reviewed as foetal teratogen (ACOG, 2013) |
•Production began in the 1930s. In 2000, CDC scientists published first human population exposure report (Blount et al., 2000); •Phthalates labelled as teratogenic in rodent studies (Aldyreva et al., 1975; Ema et al., 1995; Sharpe et al., 1995; Foster et al., 2000) | •FA supplementation showed to reduce risk of neural tube defects in 1990s. (MRC Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992; Crider et al., 2011); •In 1998, the US government instituted guidelines for supplementation in flour | •Despite teratogenic effects reported beginning in the 1950s, there is currently no newborn or prenatal screening for CMV due to the unpredictability of vertical infection and the variability in developmental outcomes | |
| Exposure | •40% of the US population is obese. •Characterized by high circulating glucose, hormones, fatty acids and inflammatory markers (Schmatz et al., 2010); •For the sake of illustration of direct effects, this review only addresses exposure to glucose and fatty acids | •>95% of pregnant women in the USA are exposed (Woodruff et al., 2011); •Exposure measured as urinary metabolites (phthalate monoester) of the parent compound (phthalate diester); •Parent compound is rapidly hydrolyzed to phthalate monoesters by lipases (Frederiksen et al., 2007); •Half-life in the body is 12–48 h | • Low maternal serum or plasma levels of folate; • FA deficiency results in high levels of homocysteine which is thought to be neurotoxic (Molloy et al., 2017); •Folate acid receptor antagonism activates the same mechanism (van Gelder et al., 2010) | •Pervasive in women of reproductive age with prevalence rates ranging from 45% to 100% (Cannon et al., 2010); •Vertical transmission is 40% for women with active CMV infections during the first trimester of pregnancy (Weisblum et al., 2014) and 0.2–2% for women with latent, pre-conception CMV infections (Boppana et al., 1999) | |
| Sources of exposure | •High-fat/sugar diet, •Physical inactivity (Racette et al., 2003); •Genetic predisposition (Racette et al., 2003); •Corticosteroids, anti-psychotics, anti-depressants. (Ness-Abramof and Apovian, 2005) | • Oral (i.e. diet); dermal (i.e. personal care product use); and inhalation (i.e. off-gassing of furniture); •Exposure is involuntary and universal | •Poor dietary intake of folate-rich foods or supplements (Beaudin and Stover, 2009); •Use of anti-folate drugs (e.g. valproic acid, methotrexate) (Hernández-Díaz et al., 2001) | •Contact with saliva or urine from an infected person; •Contact with infected children | |
| Fate and transport |
•Increased expression of the placenta glucose transporter GLUT1 due to insulin resistance and hyperlipidaemia (Hauguel-de Mouzon et al., 1994; Jones et al., 2009); •Increased expression of placenta fatty acid transporters FATP6 and FATB3 (Diaz et al., 2015) |
•Phthalate metabolites detected in human placental tissue (Poole and Wibberley, 1977; Mose et al., 2007) and amniotic fluid in late pregnancy (Jensen et al., 2012); •Not known if active or passive transport; •Variation in placental transfer and metabolism of phthalates has not been assessed |
•FA is actively transported across the placental trophoblast layer after 10 weeks of gestation.(Zhao et al., 2011) | •Fc receptor-mediated transcytosis by STBs to villous cytotrophoblasts, where the virus replicates. (Arora et al., 2017) | |
| Gestational sac | Molecular level |
•Increased syncytiotrophoblast basal membrane GLUT1 transporter protein expression (Acosta et al., 2015); •Increased STB basal membrane FATP2 protein expression (Lager et al., 2016) |
•Activation of PPARγ receptor and pathway by phthalates; (Maloney and Waxman, 1999; Hurst and Waxman, 2003; Schlezinger et al., 2004; Kaya et al., 2006); •Changes in placental human chorionic gonadotropin in vitro and in vivo, and anti-androgenic actions in foetus (Swan et al., 2005; Adibi et al., 2017b; Shoaito et al., 2019); •Oxidative stress in placental cells (Meruvu et al., 2016; Pérez-Albaladejo et al., 2017; van et al., 2019); •DNA methylation in term placenta (LaRocca et al., 2014); •Non-coding RNA expression in term placenta (LaRocca et al., 2016; Machtinger et al., 2018) |
•Hypomethylation of genes required for embryonic development and organogenesis (e.g. GR and PPARα) (Kim et al., 2009; Koukoura et al., 2012); •Impaired DNA synthesis for cell proliferation and differentiation (e.g. neural crest cells and trophoblasts) (Sato et al., 2006; Williams et al., 2011; Chen et al., 2012) |
•Elevated cytokines and chemokines, including tumour necrosis factor alpha and monocyte chemoattractant protein-1 expression in CMV-infected placentas (Hamilton et al., 2012) and amniotic fluid (Scott et al., 2012) |
| Physiologic | •Increased placental transfer/diffusion of glucose and free fatty acids to the foetus (Jones et al., 2007; Jones et al., 2009; Brett et al., 2014) |
•Increased rat placental short chain fatty acid transport (Xu et al., 2008; Xu et al., 2005; Xu et al., 2006); •Thyroid hormone transport and metabolism (Huang et al., 2007, 2016; Wadzinski et al., 2014; Johns et al., 2015, 2016; Gao et al., 2017) |
•Abnormal trafficking of proteins and/or fluids (e.g. amniotic staining, high alpha fetoprotein) |
CMV infection perturbs: •Proliferation and differentiation of trophoblast progenitor cells (Tabata et al., 2015); •STB proliferation, resulting in leakiness of the STB layer |
|
| Morphologic | •Increased STB surface and a larger fetal-placental endothelial surface, theorized to increase transfer of glucose an fatty acids (Castillo-Castrejon and Powell, 2017) |
•Normal in terms of placental pathology; •Variation in placental weight (Zhu et al., 2018; Mustieles et al., 2019; Philippat et al., 2019) |
•Abnormal stem cell differentiation (Beaudin and Stover, 2009); •Defects in formation of embryonic and extraembryonic structures (Kim et al., 2009); •ADAM syndrome (amniotic deformity, adhesion and mutilation); theorized (Cignini et al., 2012) |
•Changes to the decidua, including plasma cell deciduitis and necrosis (Uenaka et al., 2019) | |
| Birth outcomea | Placental outcome | •Abnormal transfer of glucose and fatty acids, theorized to occur in presence of chronic villitis (Brouwers et al., 2019) | •None established (Marie et al., 2015). |
•Amniotic banding or adhesions of placental tissue to foetal organs; theorized (Cignini et al., 2012); •Chorionic aberrations (Wegrzyn et al., 2013); •Placental abruption (Ray and Laskin, 1999); •Pre-eclampsia (Wen et al., 2008; Liu et al., 2018) |
•Chronic villitis (Uenaka et al., 2019) |
| Short-term |
•Macrosomia (Sebire et al., 2001); •Congenital heart disease (Leddy et al., 2008); •Neural tube defects (Werler et al., 1996) |
•Disruption of the timing of labor (Adibi et al., 2009; Latini et al., 2003; Meeker et al., 2009; Chin et al., 2019) |
•Neural tube defects: failure of neural tube closure resulting in spinal or cranial defects (MRC Vitamin Study Research Group, 1991; Safi et al., 2012; Greene and Copp, 2014); •Cleft palate (Wehby and Murray, 2010); •Congenital heart defects (De-Regil et al., 2015); •Syndactyly/limb amputation secondary to amniotic bands; theorized based on (Cignini et al., 2012) |
•Perturbed STB layer: impaired nutrition of the foetus, yielding lower birth weights | |
| Long term, speculated to involve placental mediation |
•Later-life obesity and diabetes (Gillman et al., 2003; Tenenbaum-Gavish and Hod, 2013); •Cardiovascular disease (Drake and Reynolds, 2010); •Cognitive impairment (Krakowiak et al., 2012) |
• Future obesity and diabetes risk (Grün and Blumberg, 2007; Bolling et al., 2012; Valvi et al., 2015; Shoaito et al., 2019; Venturelli et al., 2019); • Reproductive system development in F1 offspring (Gray et al., 2000; Albert and Jegou, 2014; Adibi et al., 2015); •Germ cell development in F2/F3 offspring (Doyle et al., 2013; Zhou et al., 2017; Rattan et al., 2019); • Neurodevelopmental differences (Andrade et al., 2006; Huang et al., 2007; Engel et al., 2009) |
•Organ dysfunction secondary to congenital defects; •Cognitive and psychomotor impairment (Tamura and Picciano, 2006) |
•CMV placental infection is associated with neurocognitive defects in children, including hearing loss, microcephaly and motor defects (Boppana et al., 2013); •Foetal thrombotic vasculopathy in placental tissue is associated with CMV infection in stillborn infants (Iwasenko et al., 2011) |
CMV, cytomegalovirus; FA, folic acid; FATB3, fatty acid binding protein 3; FATP6, fatty acid transport protein 6; GLUT1, glucose transporter 1, GR, glucocorticoid receptor gene; PPARα, peroxisome proliferator-activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor gamma; STB, syncytiotrophoblast.
Outcomes highlighted in bold are the classic presentation of the teratogen with strongest supporting evidence.
Italicized text in the morphology and birth outcome rows indicate theorized supporting evidence.
Placental molecular mediation
The second category addresses those teratogens which can exert effects on the foetus even in the absence of the direct transfer of the teratogen by the placenta (Fig. 2B). This corresponds to the unique aspects of GS biology reviewed above (see Introduction). This is called an indirect effect which is estimated alongside the direct effect in the DAG (Fig. 3B). The assumption is that some of the teratogen effect will be direct (not involving the placenta) and some of it will be a placentally mediated effect.
In this case, direct transfer of the teratogen to the embryo during the period of foetal development is minimal or absent. In the above-described examples (see GS transport of exogenous compounds), diazepam and propofol would be candidates for this model given evidence of restricted transport across the GS. Therefore, the teratogen interacts directly with the outer layer of the placental villi and affects trophoblast gene and protein expression. This, in turn, changes the secreted products of the placenta and their availability to the early embryo (Fig. 2B). Indirect effects could include disruptions in the timing, the availability or the dose of key hormones, growth factors, morphogens, etc., and could potentially have adverse effects on organ structure, organ function or on the programming of future organ function.
Biomarkers, placental molecular mediation
The gold standard measure for this type of mechanism would be a real-time imaging biomarker that could tag in vivo the relevant placental hormone and make its expression and movement within the GS visible and quantifiable to the investigator. Working backwards from here, placenta-specific molecules (RNAs and proteins) reflective of the specific hormonal pathway can be measured in placental tissue at birth. As with the direct effect scenario, temporality is lost as these biomarkers are only available 26–36 weeks after the teratogenic effects occurred. Alternatively, circulating or excreted placental and foetal hormones and other molecules (cytokines, growth factors, metabolites, nucleic acids and extracellular vesicles) can be measured in maternal circulation and urine respectively in the first trimester. This is the most realistic and widely available approach.
DAG, placental molecular mediation
The DAG includes a mediator between X and Y (Fig. 3B). In this case, X is measured as the maternal or placental teratogen exposure. M represents the placental measure of the specific hormonal pathway that is disrupted by X, and which is causally related to foetal development. The pathway from X to Y is the direct pathway, and the pathway through M is the indirect pathway. The mediator is a placenta-specific molecule that is changed by the exposure and which is causally related to foetal development. If a circulating blood biomarker is used, then validation work must be done to understand its correlation to its first trimester placental tissue expression and secretion. Otherwise, it is difficult to exclude the possibility that it is a biomarker of expression levels in maternal tissues.
There are three distinct sets of confounders to enumerate in the DAG for causal mediation. C1 are the confounders that are common causes of the teratogen exposure and the child health outcome. This could include factors that precede pregnancy and will also be the same after pregnancy with impacts on childrearing, such as maternal race, neighbourhood and dietary habits. C2 represent those confounders which are causes of the teratogen exposure and the placental hormone level. C2 can include pre-pregnancy and pregnancy specific factors that affect teratogen exposure and placental development and function, such as maternal age, maternal race, chronic disease status, reproductive history or neighbourhood. C3 includes those confounders which are causes of the mediator and foetal development. The C2 and C3 sets can overlap as they both include pregnancy-specific sources of confounding. However, C3 will include only those factors that are contemporaneous to the current pregnancy and occur after the baby is conceived and before the baby is born. This would include prenatal vitamins, pregnancy-specific social stressors, weight gain and nausea. The only variables in C3 that would predate pregnancy are paternal epigenetics (i.e. paternal age as a proxy variable) at the time of conception since the placental genotype and epigenome are half-paternal, and in principle could contribute to levels of placental hormones (Sharma et al., 2015).
Sex-specific mechanisms in the placenta have been offered to explain why associations between prenatal exposures and child outcomes are stronger or weaker, or differ in direction, between males and females (Adibi et al., 2017a). In one case, an association between maternal asthma and foetal growth restriction was stronger in female than in male offspring (Clifton, 2005). This was explained by sex differences in placental glucocorticoid metabolism. In another example, the effects of maternal obesity on placental mitochondrial function differed by sex. This is offered as a basis for the sex difference in the risk of obesity in the offspring of obese mothers (Muralimanoharan et al., 2016). Foetal sex could be a C1, C2 or C3 confounder or effect modifier. C1 confounding by foetal sex can occur if foetal sex is a cause of variability in placental metabolism and transport of the teratogen'cause' of the internal dose or X (Figure 3B).
It is possible to analyse and report these associations or causal effects (x > y, x > m, m > y) separately in three tables or figures, and offer a speculative interpretation on the overall model. Alternatively, statistical techniques to address causal mediation and interaction can be employed to offer an interpretation of the teratogen and the outcome assuming that the role of the placenta was causal (Fig. 3B). Causal mediation approaches, nuanceas and limitations are described elsewhere (VanderWeele and Knol, 2014; VanderWeele, 2015, 2016).
In the analysis of the data, the mediator (M) cannot simply be included in the model as a covariate as that would potentially attenuate or block the pathway between X and Y. In the decomposition approach, the placental hormone is evaluated as a mediator and as a modifier of the teratogen exposure (VanderWeele, 2015, 2016; Discacciati et al., 2019). This is particularly powerful as it allows for simultaneous estimation and comparison of the direct and indirect effects.
Examples, placental molecular mediation
One example of this type of co-ordinated mechanism by which an abnormally functioning placenta can alter the normal development of the foetus is the well-studied association between pre-eclampsia/hypertension in pregnancy and hypospadias in the male neonates. Based on a meta-analysis with data from 15 studies, women with pre-eclampsia had 2-fold higher odds of giving birth to male babies with hypospadias (Sheriff et al., 2019). Pre-eclampsia is a structural and molecular disorder of the placenta that occurs in 3–4% of livebirths (NICHD, 2017). Women at the 85th percentile of first trimester hCG had 1.5 times higher odds (95% CI 1.00–2.23) of developing pre-eclampsia later in pregnancy (Barjaktarovic et al., 2019). Hypospadias is a common birth defect that occurs in 0.5% of male livebirths (USCDC, 2020). Hypospadias reflects improper genital development in the foetus arising from molecular defects in cellular differentiation during the period from 8 to 12 weeks of gestation (Scott et al., 2009). This is the same critical window in which hCG plays a causal role in the formation of the genitalia. The binding of placental hCG to LHCGR (luteinising hormone chorionic gonadotropin receptor) expressed by cells within the nascent male foetal gonad initiates testosterone production by Leydig cells from ∼8 weeks of gestation (Huhtaniemi et al., 1977a,b; Teerds and Huhtaniemi, 2015). Higher first trimester hCG was associated with shorter anogenital distance in male babies in a birth cohort study (Adibi et al., 2015). This supports the general finding of a stable inverse association between first trimester placental hCG (due to multiple causes) and reduced masculinization of the genitalia (Adibi et al., 2015). The mechanistic details of how this occurs are unknown.
hCG is an example of a placental hormone that is associated with a long list of environmental chemicals, including DES (Bechi et al., 2013), phthalates (Table I; Adibi et al., 2017b), chlorpyrifos (Ridano et al., 2012), triclosan (Honkisz et al., 2012) and others reviewed elsewhere (Adibi et al., 2020). There may be similar examples in the literature or yet unknown examples involving other placental hormones, placental growth factors, placental cytokines that could be causally associated with teratogen exposure and with foetal developmental pathways.
Phthalates, as putative endocrine disrupting teratogens (like DES), may also operate according to this paradigm of placental molecular mediation in the first trimester. A summary of the relevant phthalate literature is presented in Table I and the DES evidence is outlined in Figure 4 .
Pre-placental, embryonic teratogenicity
The third category includes those teratogens that were present before the formation of the GS or the placenta. Nonetheless, placental biomarkers offer a way to measure this type of time-dependent, direct teratogenic effect. A chronic exposure at the time of conception would be in direct contact with target cells (or their parent stem cells) at the earliest stages of formation of the embryo, the amniotic cavity and the yolk sac. This is a situation where teratogenicity can occur without placental transfer. The compound would have the ability to affect cell lineage determination after gastrulation and during formation of the GS (Fig. 2C). The prediction of this type of teratogenicity assumes sequential effects on embryonic structures and the extraembryonic structures that arise from them.
Teratogenic effects that occur through this mechanism might include babies born with limb wall birth defects, such as neural tube defects, gastroschisis and cleft palate. An example of a GS pathology (later becoming placental pathology) in this category is the ADAM syndrome (amniotic deformity, adhesion and mutilation). This has been proposed as a group of placental birth defects that have been associated with specific types of chemical and mechanical exposures (Keller et al., 1978; Opitz et al., 2015). In a 1984 paper on ADAM syndrome, the authors speculated that the cause may be more environmental than hereditary, and that it originates from a defect in the ‘germinal disk’ (Herva and Karkinen-Jääskeläinen, 1984). In a 1988 report, occurrence of the early amnion rupture syndrome (TEARS) was reported to differ by age and race in Atlanta, Georgia over a 15-year period and was associated with a wide range of structural birth defects including limb wall defects. These infants were all alive at 1 year and the cause of the defects were likewise attributed to maternal exposures versus genetics (Garza et al., 1988).
Biomarkers, embryonic teratogenicity
The gold standard for a biomarker in this category remains theoretical. It would require detection of abnormal cellular differentiation occurring deep within the GS that is a few millimetres in diameter and embedded within the uterine wall. At birth, these types of defects might be identified as malformations of the amnion (described above). In the first trimester, the only hope of detection is in the form of a circulating biomarker that is expressed in high enough abundance to be detected. The embryo or the placenta are the likely sources of such circulating biomarkers. Precise temporality may be lost in this case, but detection of a biomarker within the first trimester (within 10 weeks of the original defect) is still more useful in a causal model than something measured at birth (Fig. 3C).
One example of such a biomarker is alpha fetoprotein (AFP) which is measured in maternal blood, but originates in the first trimester yolk sac and has been measured in amniotic and coelomic fluids (Gitlin et al., 1972; Jauniaux et al., 1995). High levels of AFP early in pregnancy are predictive of spina bifida (Brock and Sutcliffe, 1972). Inappropriate trafficking of AFP follows from malformation of early embryonic and extraembryonic structures. Other circulating molecules may be elevated for similar reasons and could be useful in identifying this type of teratogenicity.
DAG, embryonic teratogenicity
In the DAG, we assume two processes that are relevant to the causal model (Fig. 3C). X represents the pre-conception maternal exposure. There is an embryo endpoint (Ye) or cellular differentiation defect that occurs first and that gives rise to a placental differentiation defect or endpoint (Yp). Ultimately, Y is the child health outcome. The affected embryo may secrete a biomarker (Me) as is the case with foetal AFP that is leaked and detected in maternal blood when the neural tube fails to close (described above). The placenta may also secrete a biomarker (Mp) at abnormal levels or at a faster rate of increase/decrease by gestational age than a normal placenta.
This DAG would lead to an analytical strategy more similar to direct effects (Fig. 3A) because the intermediate variables Ye and Yp are not measurable in the first trimester when they occur. M in this DAG is not a causal mediator but instead acting as a reporter or a proxy for the outcome. C would be the group of confounding variables that were causes of both the pre-conception teratogen exposure and the health of the mother that could cause the embryo defect. This could include most factors related to where the mother lives, what she eats, chronic health conditions, reproductive history and genetic and non-genetic factors that influence how the mother’s body metabolizes the teratogen.
Examples, embryonic teratogenicity
This mechanism is also distinctive in that it can be the absence or subtraction of a compound from the system, as opposed to the presence or addition of an unwanted compound into a system, that is causally related to the birth defect. One example is folic acid deficiency and the general absence of critical substances or molecules at the time of conception (Table I). Exposures which antagonize the folic acid receptor, such as antiepileptic drugs, can cause teratogenic effects through the same mechanism as folic acid deficiency (van Gelder et al., 2010).
Other teratogens may disrupt formation of the placental and/or foetal vasculature resulting in placental and/or foetal birth defects (van Gelder et al., 2010). This might also be considered a pre-placental teratogenic mechanism if the disruption is occurring in a parallel fashion between the embryo and GS structures. This type of teratogenesis is predicted to occur in women exposed to vasoconstrictive or vasodilating substances, such as misoprostol, aspirin, ergotamine or pseudoephedrine (van Gelder et al., 2010). The types of effects could include anything that alters the flow of blood and other substances necessary for embryogenesis (hyperperfusion, hypoperfusion, hypoxia, obstruction and placental insufficiency). In order to be classified as a teratogen in this case, the exposure–outcome relationship would need to occur predictably and reproducibly.
Multi-step mediation
This is the most loosely defined of those proposed in this review, and the most open to methodologic validation and innovation. It includes teratogens that are suspected to cause a cascade of effects that include, but are not specific to, the placenta. For example, the maternal-placental immune response represents a complex system of signalling and multiple cell types: maternal immune cells, the decidua, placental trophoblasts, placental macrophages (Hofabauer) and foetal endothelium (Erlebacher, 2013; Fig. 2D). There is a growing list of viral teratogens (CMV, rubella and most recently Zika) which likely exert their toxic actions by way of the maternal-placental immune mechanism (Pereira, 2018; Table I). At the time of writing, SARS-CoV-2 infection in pregnancy has not been associated with clear teratogenic effects in offspring. SARS-CoV-2 has been associated with effects in multiple cell types within the placental-foetal unit and not excluding direct transfer and toxicity (Vivanti et al., 2020). Placental inflammation and maternal-placental immune toxicity are being loosely grouped together in this category. Placental inflammation may mediate teratogenic effects of diverse exposures including maternal obesity (Muralimanoharan et al., 2016) and maternal stress (Bronson and Bale, 2014).
Key distinctions between placental molecular mediation and multi-step mediation are that: (i) teratogenic effects in multi-step mediation could be occurring both at the microscopic molecular level and at the visible morphologic level, whereas we hypothesise that placental molecular mediation effects occur primarily at the molecular level; (ii) placental molecular mediation effects are more specific than multi-step mediation and might involve a transcription factor or receptor-mediated pathway rather than a broad system-level effect on immune function or inflammation; and (iii) we are defining multi-step mediation molecular effects to be more easily measured in placental tissue than placental molecular mediation effects which might be more easily measured in circulation at time points relevant to placental-foetal development.
Multi-step mediation exposures are acute or chronic and have effects on the earliest stages of placental formation and function. This results in abnormal production and secretion of inflammatory markers and cytokines. The inflammatory and immune cascades produce cytotoxic defects independent of the actual teratogenic agent. The direct transfer and direct effect of a teratogen would partially be a function of ‘leakiness’ of the trophoblast layer after destruction of trophoblast cells by these immune or inflammatory cytotoxic cascades (Kim et al., 2015).
Biomarkers, multi-step mediation
The gold standard would be a panel of biomarkers that are representative of the teratogen effects at (i) the maternal–placental interface, (ii) the outer layer of the placenta and (iii) the foetal endothelium (the cell type that lines the vessels within the placenta and mediates placental-foetal effects). If additional tissue or cell layers are identified, the list can be expanded (Fig. 3D). The emphasis is still the first trimester and therefore the biomarkers are most useful if they can be measured in maternal circulation. However, placental inflammation is well-measured as lesions in the villous tissue, chorion and basal plate at birth (Redline, 2015; Romero et al., 2018). Measures can be highly quantitative even when abstracted from a pathology report (Catov et al., 2015). Validation work can link these types of inflammatory markers measured at birth with specific types of insults in the first trimester (Salafia et al., 2016).
DAG, multi-step mediation
The DAG can be organized according to logical and temporal sequence (Fig. 3D). For example, M1 might be a biomarker of the immune response within the maternal decidua. M2 might be a biomarker of an immune response within the trophoblast layer that then results in a set of effector genes that results in a cascade of secreted biomarkers from both the trophoblast (M3) and the foetal endothelium (M4). A distinction between M1 and Mx could be that M1 is most proximal to the maternal tissue and Mx is most proximal to the foetal tissue in space and in temporal sequence. There may be unique sets of confounders to each exposure–mediator relationship that may be important to account for. For example, a decidual biomarker might be confounded by factors that are unique to pre-pregnancy maternal health (i.e. cellular memory of previous infections), whereas a placental biomarker could be confounded by aspects of paternal pre-conception health (i.e. exposures, genetics and epigenetics of sperm cells) and maternal prenatal health (current infections). The number and types of biomarkers selected might depend on their specificity to the placenta and temporal expression during organogenesis. This is described in DAG theory as context-dependent mediation (Matthay and Glymour, 2020). The statistical techniques to address multi-step mediation are less straightforward. Recently, proposed methods may address multiple mediators (Naimi et al., 2017; Bellavia and Valeri, 2018) or use non-parametric approaches to model complex longitudinal associations (Naimi et al., 2017).
Examples, multi-step mediation
An example of a teratogen that may produce teratogenic effects for the foetus by multi-step mediation is CMV infection. CMV is a viral teratogen that produces a systemic maternal-placental immune response harmful to the foetus. Multi-step mediation as a teratogenic mechanism is described for CMV in Table I.
Diethlystilbesterol (DES) an illustrative example
History of DES
DES was widely prescribed by physicians for pregnancies with bleeding or broadly at risk for miscarriage from 1938 to 1971 (Bamigboye and Morris, 2003). In 1971, a paper was published reporting eight cases of a rare vaginal adenocarcinoma in girls and young women in Boston exposed to DES in utero (Folkman, 1971). The Food and Drug Administration (FDA) acted quickly after publication of these findings to ban the practice of prescribing DES to pregnant women (Vessey, 1989). The ban came long after completion of a randomized trial in the early 1950s that demonstrated no benefit of DES for reducing miscarriage. In a 2003 Cochrane review of the evidence of DES as an intervention, authors wrote, ‘Had the principle of “best evidence” been followed, the embarrassment of diethylstilboestrol as a medical intervention, and the effects on offspring who were exposed to it before birth, would have been avoided’ (Bamigboye and Morris, 2003).
The placenta figures into DES history in multiple ways, which is why it was chosen as an illustrative example for this review. First, the term ‘transplacental carcinogenesis’ was used to describe the phenomenon of vaginal adenocarcinoma in girls exposed to DES in utero (Folkman, 1971). Carcinogenesis in this context implied cellular changes that began in utero but were not readily apparent at birth. The terminology marked a departure from the concept of teratogenesis as defects which are visible at birth. Secondly, DES was designed to improve placental function by augmenting placental hCG and oestrogen (produced by the corpus luteum and the placenta) in first trimester (Smith et al., 1941; Smith and Smith, 1944). That marked an important clinical strategy, but one that was based on a faulty biological premise as DES did not augment hCG.
Newer studies showed DES administration lowered hCG secretion by trophoblasts (Bechi et al., 2013) Moreover, there is evidence that endocrine disrupting compounds (EDCs) can impact hCG production differently depending on the sex of the embryo-placenta (Adibi et al., 2017b). These nuances of placental hormone biology were not factored into the science and evaluation of DES therapy or the epidemiologic studies to assess effects, but played a major role in how the DES story unfolded.
The DES tragedy may have played out differently if the framework presented here on first trimester mechanisms of teratogenicity would have been proposed and implemented 70 years ago. The medical and public health communities may have employed a biomarker-based approach in pre-clinical studies. This would have identified placental effects in the first trimester. Even if unable to predict the vaginal adenocarcinoma risk in childhood, a strong finding on DES and placental biomarkers in the first trimester may have raised flags in terms of short-term toxicity. If DES still made it to the clinical trial phase, these types of biomarkers could have been instrumental in monitoring toxicity and could have informed earlier decisions to monitor different types of outcomes or to stop the use of DES without waiting the 40 years required for a cluster of childhood cancer cases to be identified. There would have been translation of the DES teratogenic model to subsequent endocrine disrupting chemicals used in commercial products and pharmaceuticals, to make swifter and evidence-based determinations regarding allowable risks associated with prenatal exposures. For all of the above to occur, a paradigm shift would have occurred whereby strong evidence of placental toxicity in the first trimester would be considered tantamount to evidence of foetal developmental toxicity.
DES direct effects
DES as the parent compound and as conjugates (metabolites) can cross the placenta from the mother to the foetus (Fig. 4). Through labelling of the DES molecule in pregnant mice, investigators observed a phenomenon whereby the placental levels increased and then decreased in a time-dependent fashion (Shah and McLachlan, 1976). Foetal levels increased in a steady time-dependent fashion. The dosing in this study began at the equivalent of 10-weeks human gestation, the time point when blood flow to the placenta begins (Fig. 1). In a dosing study, DES conjugate levels were reported to be 20–25 times higher in rat foetal plasma than in maternal plasma (Miller et al., 1982). Thereby is evidence that there is a mammalian placental transport and/or metabolism mechanism whereby DES preferentially accumulates in foetal tissues. Hence, DES and its metabolites are both in contact with the embryo/foetus (Metzler, 1981). This process of placental transfer varied over gestational time in the rodent. However, literature that established these relationships in humans was not identified.
Figure 4.
Schematic of gestational sac pathways in diethylstilbesterol-induced toxicity in utero and long-term health outcomes. F0 outcomes (exposed mothers): (Bamigboye and Morris, 2003). F1 outcomes (exposed children): (Folkman, 1971; Gill et al., 1979; Beral and Colwell, 1981; Vessey, 1989; Mittendorf, 1995; Mittendorf and Williams, 1995; Wilcox et al., 1995; Salle et al., 1996; Perez et al., 2005; Troisi et al., 2007; Titus-Ernstoff et al., 2006; Hatch et al., 2011; Hoover et al., 2011; Troisi et al., 2013; Jensen and Longnecker, 2014). F2 outcomes (offspring of F1 and their placentas, exposed as precursor germ cells): (Troisi et al., 2007; Titus-Ernstoff et al., 2008, 2010; Kalfa et al., 2011; Jukic et al., 2011; Kioumourtzoglou et al., 2018; Titus et al., 2019).
Of the foetal tissues assayed, the reproductive tract had the highest concentration of DES. This tendency of a compound to preferentially be trafficked to a specific tissue is called organotropism (Shah and McLachlan, 1976; Metzler, 1981). These findings were confirmed in Wistar rats (Miller et al., 1982). In this study, DES levels in the placenta and yolk sac were 1.5–2 times higher than in foetal plasma, which could also suggest that the structures in the human GS may have had higher concentrations as compared to the maternal compartment.
A gold standard observational study to estimate the direct effect of DES on reproductive tract development would include a measure of the individual-level DES concentration within the foetal compartment (X) during the period between 8- and 12-week gestation when masculinization of the reproductive tract occurs, and an anatomic measure within the reproductive tract of the foetus (Y). In the absence of these measures, the option is to use her medical record of DES dose and anogenital distance at birth (for example). Gestational age at the time of DES dose in the first trimester would be treated as an effect modifier of this association. If there is prior knowledge of factors that strongly determine placental metabolism of DES (i.e. smoking, foetal sex, diet, genetics, other medications) and contribute to overall foetal development, they can be included in the analysis as potential sources of confounding and/or effect modification.
DES indirect effects: placental molecular mediation
Hypothesised indirect effects of DES on the developing foetus by way of the placenta are depicted in Fig. 4. The outcomes are categorized according to which generation they occurred in. All of the outcomes reported in Fig. 4 are supported by epidemiologic findings. F0 are the women who were treated with DES; F1 are the children of the exposed pregnancy; and F2 are the grandchildren of the exposed pregnancy, who were also directly exposed to DES as arrested germ cells.
In the F1 generation of women who were exposed to DES in utero and newly pregnant with F2 offspring, a delay was observed in their rise of urinary hCG levels in the 7 days post-implantation compared with women who were not exposed in utero to DES. However, after Day 5, their hCG levels increased more rapidly as compared to their unexposed counterparts. This is based on only seven exposed F1 women with F2 placentas but offers a hypothesis regarding a potential mechanism by which DES effects on the female germline (F1 generation) can also influence placental function in F2 progeny (Jukic et al., 2011). The risk of pre-eclampsia was also higher suggesting that there was some memory in the germ cell that gave rise to the future placenta (Mittendorf and Williams, 1995; Troisi et al., 2007; Fig. 4).
A gold standard study of DES teratogenicity, assuming this model, would include the same X and Y as described above (see Direct effects), but would additionally include a placental biomarker, such as hCG. This hormone could be measured in maternal circulation and not confused with maternal expression, as the placenta would be the dominant source of the hormone. Foetal sex and gestational age at the time of the DES dose would be C1 and C2 confounders. Gestational age at the time of the blood draw would not be a confounder as it is not a cause of the outcome; yet it would be important to normalise the placental hormone for the day of gestation. Placental hormones in the first trimester are generally either steeply increasing or decreasing (Nagy et al., 1994b).
DES indirect effects: pre-placental, embryonic teratogenicity
In the case of DES, there is no example that falls under this category as women were prescribed DES no earlier than 6 weeks after conception.
DES indirect effects: multi-step mediation
DES effects in the current pregnancy (F0 generation) likely occur by the dysregulation of multiple pathways in the placenta and which determine the overall health and function of the placenta. However, these do not qualify as teratogenic effects. For example, mouse trophoblast stem cells treated with DES showed an abnormally large number of trophoblast giant cells at the expense of diploid trophoblast cells (Tremblay et al., 2001). This was consistent with an earlier study in mice that showed thinning of the labyrinthe zone, a layer analogous to villi in the human placenta (Scott and Adejokun, 1980). This produced a disorganized and poorly functioning placenta.
This subset of DES effects results in adverse pregnancy outcomes (higher incidence of miscarriage, preterm labour, pre-eclampsia and growth restriction) which ironically are the types of outcomes DES use was intended to prevent (Bamigboye and Morris, 2003; Fig. 4). This could occur through effects on the early stages of trophoblast differentiation that might lead to an incorrectly organized or formed placenta. The incorrect organization of the outer layers of the placenta would be the starting point for abnormal immune and inflammatory responses. This could set off a cascade of reactions in multiple cell layers and cell types that would be damaging to the current pregnancy, but maybe not teratogenic.
An ideal study of DES multi-step mediation effects would include the above-described X, M and Y and additionally include a biomarker indicative of the number of trophoblasts and the integrity of the trophoblast layer. A set of circulating biomarkers that are elevated in cases where the syncytiotrophoblast layer (outer layer of trophoblasts that line the placental villi) of the placenta is deficient could be used. The end result of this cascade might be placental inflammation or defective immune responses at the placental–maternal interface that could be causally related to higher risk of preterm birth and miscarriage (Bamigboye and Morris, 2003).
Discussion
We present four mechanisms by which first trimester exposures to teratogens can be causally and non-causally associated with placental biomarkers and with child outcomes. These mechanisms are synthesized from a comprehensive but non-systematic review of the historic and current literature on three known teratogens (DES, folic acid deficiency and CMV infection) and two chronic exposures (phthalates and maternal obesity). The goal is to unify and organize methodologies and knowledge conceptually and practically on teratogens and child health outcomes. For each of the four mechanisms, biologic rationale, specific types of biomarkers and a proposal of how to structure an analysis of causal effects are presented.
This approach is based on the convenience of detecting placental (extraembryonic) biomarkers that are more abundant and accessible than foetal (embryonic) biomarkers. This is responsive to current endeavours to address causality in child health disorders as well as population-level interventions (Buckley et al., 2020).
In terms of a paradigm shift, these mechanisms argue for more data collection in the lengthy period immediately after a woman misses her menstrual period (∼4 weeks of gestation) up until when standard prenatal screening begins (∼11 weeks of gestation). This is the period when the GS and the placenta first form and embryogenesis and organogenesis take place. The period from 4 to 11 weeks of gestation could be a period of central importance to population health and could be tackled more specifically and proactively to increase understanding of environmental influences during foetal organ-specific sensitive periods. Even stronger would be an approach that includes the pre-conception period with high-frequency sampling from 0- to 14-week gestation.
At a population level, this approach could solve a missing data problem in that the majority of cohort studies begin data collection at the first prenatal visit, or often sometime after. Earlier recruitment strategies around the experience of a missed period could also address the livebirth bias which occurs when we limit our data collection to pregnancies that end in livebirth (Raz et al., 2018). The reasons why these data are challenging to collect is because women may not know they are pregnant yet; there is a chance they will still lose their pregnancy, and we lack well-established methods to recruit and collect biospecimens from women outside of the prenatal care framework.
The mechanisms might help narrow and map first trimester exposures and placental-foetal biomarkers to specific types of child health outcomes, such as brain development, asthma, diabetes, obesity and ultimately fertility. Mechanistic and biological knowledge can be useful for within and between species translation. The four mechanisms might also help point to exposure–outcome relationships where the murine model is not useful given the presence/absence of morphologic structures, developmental and molecular pathways (i.e. aromatase and hCG are not expressed by the rodent placenta), differences in the design of the maternal–foetal interface and differences in maternal–foetal transport mechanisms (Walker et al., 2017). Others have provided analyses of species differences in placental mechanisms that can serve as a reference for when animal models can be used to understand human mechanisms and when they cannot (Malassineet al., 2003; Mendelson and Kamat, 2007; Rawn and Cross, 2008; Maltepe et al., 2010; Schmidt et al., 2015; Walker et al., 2017).
Lastly, this effort is important to public health practice where observational research is conducted with the goal of identifying the best possible intervention to protect foetal health. In this case, intervention is the removal of an exposure when deemed harmful. Once clearly and convincingly identified as teratogens, swift actions are generally taken to remove the exposure and protect the foetus. This review lays out a set of steps that if taken can help to answer the question of teratogenicity of individual agents.
Compared to the embryo, the placenta may be more directly manipulated through its compensatory and highly evolved mechanisms to protect and to ensure the survival and future reproductive success of the foetus. ‘Manipulation’ refers here to intervention by removing the exposure and improving nutrition and general health. Attempts to directly alter hormone production by the placenta are at risk of going down a similar path as the DES experiment with potentially tragic consequences.
Examples of biomarkers and application of the four DAGs are provided in greater detail for DES and in less detail for other teratogens and chronic exposures. Another innovation here is to redraw the classical teratogen figure to emphasize GS morphology in the first trimester (Fig. 1). This will help correct the current practice of considering teratogens and the embryo and foetus in isolation of the GS and placenta (Moore et al., 2008). Foetal endocrine and organ development can be overlaid on the GS timeline to give greater understanding of sensitive timepoints and options for informative biomarkers, and ultimately for the interpretation of statistical relationships.
More work is needed to address rigour and reproducibility in specific statistical approaches applied to the mechanisms described here and these types of data. The application of these approaches to modelling placental mechanisms in the first trimester can grant insight into how the maternal environment contributes to child health and can support a framework for designing and evaluating interventions in the first trimester. Using these models, it will be possible to evaluate how useful a particular biomarker is, and most importantly, how strong, weak or incomplete the evidence is to declare an exposure as truly teratogenic.
Data availability
No new data were generated or analysed in support of this research.
Acknowledgements
We acknowledge the support of all members of the Adibi Research and R01 group who participated in group discussions on this project. We thank Dr. Diana Laird for her critical comments on DES and germ cell effects. We thank Sadeq Saleh for the creation of Figs 1 and 2.
Authors’ roles
J.J.A. led the project, mentored students in the review and interpretation of literature on specific teratogens, conducted the review of the DES literature and drafted the manuscript. J.J.A. presented this article at the 2019 Society of Teratology meeting. A.J.L. participated in the formulation of the theory, conducted the review of folic acid deficiency, assisted with review of the maternal adiposity literature and created Table I. R.L.B. reviewed the adiposity and CMV literature, created Table I and gave critical feedback on the manuscript; A.M. participated in the organization of the project and the review of the CMV literature; X.X. and M.E.M. reviewed the literature on maternal adiposity; M.C.S. reviewed the phthalate literature; Q.Y. contributed to the discussion of statistical methods; T.O.C. inserted the text on foetal programming; S.P. contributed to the discussion of the biostatistical methods; R.T.M. gave critical feedback and assisted with the publication strategy. E.S.B. and N.S. gave critical feedback. All authors contributed to the editing of the manuscript.
Funding
Funding was provided by the National Institute of Environmental Health Sciences (NIEHS): 5R01ES029336-03 (J.J.A., R.L.B., M.C.S.,X.X., Q.Y., E.S.B., N.S., T.O.C. and R.T.M.), 4R00ES017780-0 (J.J.A.), 5K99ES017780-02 (J.J.A.), National Center for Advancing Translational Sciences (NRSA): 5TL1TR001858-04 (A.J.L.), and Department of Epidemiology, University of Pittsburgh Graduate School of Public Health.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- ACOG. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol 2013;121:210–212. [DOI] [PubMed] [Google Scholar]
- Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T.. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol 2015;212:227.e221–e227. [DOI] [PubMed] [Google Scholar]
- Adibi J, Burton GJ, Clifton V, Collins S, Frias AE, Gierman L, Grigsby P, Jones H, Lee C, Maloyan A. et al. IFPA meeting 2016 workshop report II: placental imaging, placenta and development of other organs, sexual dimorphism in placental function and trophoblast cell lines. Placenta 2017a;60:S10–S15. [DOI] [PubMed] [Google Scholar]
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH.. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol 2009;169:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Layden AJ, Yin Q, Xun X, Peddada S, Birru R.. A toolkit for the application of placental-fetal molecular biomarkers in epidemiologic studies of the fetal origins of chronic disease. Curr Epidemiol Rep 2020. 10.1007/s40471-020-00258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, Zhao Y, Thiet MP, Redmon JB, Swan SH.. Human chorionic gonadotropin partially mediates phthalate association with male and female anogenital distance. J Clin Endocrinol Metab 2015;100:E1216–E1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Zhao Y, Zhan LV, Kapidzic M, Larocque N, Koistinen H, Huhtaniemi IT, Stenman UH.. An investigation of the single and combined phthalate metabolite effects on human chorionic gonadotropin expression in placental cells. Environ Health Perspect 2017b;125:107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert O, Jegou B.. A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum Reprod Update 2014;20:231–249. [DOI] [PubMed] [Google Scholar]
- Aldyreva MV, Klimova TS, Iziumova AS, Timofeevskaia LA.. The effect of phthalate plasticizers on the generative function. Gig Tr Prof Zabol 1975;25–29. [PubMed] [Google Scholar]
- Andrade AJM, Grande SW, Talsness CE, Grote K, Chahoud I.. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology 2006;227:185–192. [DOI] [PubMed] [Google Scholar]
- Arora N, Sadovsky Y, Dermody TS, Coyne CB.. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017;21:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamigboye AA, Morris J.. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database Syst Rev 2003;60:CD004353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjaktarovic M, Korevaar TIM, Jaddoe VWV, Rijke YB, Peeters RP, Steegers EAP.. Human chorionic gonadotropin and risk of pre-eclampsia: prospective population-based cohort study. Ultrasound Obstet Gynecol 2019;54:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE, Stover PJ.. Insights into metabolic mechanisms underlying folate-responsive neural tube defects: a minireview. Birth Defect Res A 2009;85:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechi N, Sorda G, Spagnoletti A, Bhattacharjee J, Vieira Ferro EA, de Freitas Barbosa B, Frosini M, Valoti M, Sgaragli G, Paulesu L. et al. Toxicity assessment on trophoblast cells for some environment polluting chemicals and 17beta-estradiol. Toxicol In Vitro 2013;27:995–1000. [DOI] [PubMed] [Google Scholar]
- Bellavia A, Valeri L.. Decomposition of the total effect in the presence of multiple mediators and interactions. Am J Epidemiol 2018;187:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P.. Pathology of the Human Placenta, 3rd edn. New York: Springer, 1995. [Google Scholar]
- Beral V, Colwell L.. Randomised trial of high doses of stilboestrol and ethisterone therapy in pregnancy: long-term follow-up of the children. J Epidemiol Commun Health 1981;35:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW.. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 2000;108:979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling AK, Ovrevik J, Samuelsen JT, Holme JA, Rakkestad KE, Mathisen GH, Paulsen RE, Korsnes MS, Becher R.. Mono-2-ethylhexylphthalate (MEHP) induces TNF-alpha release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicol Lett 2012;209:43–50. [DOI] [PubMed] [Google Scholar]
- Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF.. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 1999;104:55–60. [DOI] [PubMed] [Google Scholar]
- Boppana SB, Ross SA, Fowler KB.. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 2013;57:S178–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB.. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 2014;15:16153–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock DJ, Sutcliffe RG.. Alpha-fetoprotein in the antenatal diagnosis of anencephaly and spina bifida. Lancet 1972;300:197–199. [DOI] [PubMed] [Google Scholar]
- Bronson SL, , Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 2014;155:2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers L, Franx A, Vogelvang TE, Houben ML, van Rijn BB, Nikkels PG.. Association of maternal prepregnancy body mass index with placental histopathological characteristics in uncomplicated term pregnancies. Pediatr Dev Pathol 2019;22:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, Fry RC, Funk WE, Hamra GB, Hecht SS. et al. ; on behalf of program orators for ECHO. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) Program. J Expo Sci Environ Epidemiol 2020;30:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E.. Nutrition of the human fetus during the first trimester—a review. Placenta 2001;22:S70–S77. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL.. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999;181:718–724. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E.. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002;87:2954–2959. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB.. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010;20:202–213. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Human Embryology and Developmental Biology, 5th edn. Philadelphia, PA: Saunders/Elsevier, 2014. [Google Scholar]
- Castillo-Castrejon M, Powell TL.. Placental nutrient transport in gestational diabetic pregnancies. Front Endocrinol (Lausanne) 2017;8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Peng Y, Scifres CM, Parks WT.. Placental pathology measures: can they be rapidly and reliably integrated into large-scale perinatal studies? Placenta 2015;36:687–692. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Z, Xie Y, Guo X, Tang X, Wang S, Yang S, Chen K, Niu Y, Ji W.. Folic acid deficiency inhibits neural rosette formation and neuronal differentiation from rhesus monkey embryonic stem cells. J Neurosci Res 2012;90:1382–1391. [DOI] [PubMed] [Google Scholar]
- Chin HB, Jukic AM, Wilcox AJ, Weinberg CR, Ferguson KK, Calafat AM, McConnaughey DR, Baird DD.. Association of urinary concentrations of early pregnancy phthalate metabolites and bisphenol A with length of gestation. Environ Health 2019;18:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignini P, Giorlandino C, Padula F, Dugo N, Cafà EV, Spata A.. Epidemiology and risk factors of amniotic band syndrome, or ADAM sequence. J Prenat Med 2012;6:59–63. [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T, Jauniaux E, Elliot MG, Gong S, Burton GJ, Charnock-Jones DS.. RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken. Proc Natl Acad Sci U S A 2017;114:E4753–E4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res 2005;322:63–71. [DOI] [PubMed] [Google Scholar]
- Crider KS, Bailey LB, Berry RJ.. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3:370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I.. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–1835. [DOI] [PubMed] [Google Scholar]
- Davidson L, von Dassow M, Zhou J.. Multi-scale mechanics from molecules to morphogenesis. Int J Biochem Cell Biol 2009;41:2147–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P.. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev 2015;CD007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P, Harris J, Rosario FJ, Powell TL, Jansson T.. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am J Physiol Regul Integr Comp Physiol 2015;309:R1569–R1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discacciati A, Bellavia A, Lee JJ, Mazumdar M, Valeri L.. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int J Epidemiol 2019;48:15–20. [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH.. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod 2013;88:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Reynolds RM.. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140:387–398. [DOI] [PubMed] [Google Scholar]
- Ema M, Kurosaka R, Amano H, Ogawa Y.. Developmental toxicity evaluation of mono-n-butyl phthalate in rats. Toxicol Lett 1995;78:101–106. [DOI] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS.. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology 2009;30:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol 2013;31:387–411. [DOI] [PubMed] [Google Scholar]
- Folkman J. Transplacental carcinogenesis by stilbestrol. N Engl J Med 1971;285:404–405. [DOI] [PubMed] [Google Scholar]
- Foster PM, Cattley RC, Mylchreest E.. Effects of di-n-butyl phthalate (DBP) on male reproductive development in the rat: implications for human risk assessment. Food Chem Toxicol 2000;38:S97–99. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM.. Metabolism of phthalates in humans. Mol Nutr Food Res 2007;51:899–911. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu W, Xu Y, Jin Z, Bao H, Zhu P, Su P, Sheng J, Hao J, Tao F.. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep 2017;7:13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza A, Cordero JF, Mulinare J.. Epidemiology of the early amnion rupture spectrum of defects. Arch Pediatr Adolesc Med 1988;142:541–544. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, Larocque N, Goldfien G, Zdravkovic T, McMaster MT. et al. Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells (Dayton, Ohio) 2011;29:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Vicovac L, Larocque N.. The role of chorionic cytotrophoblasts in the smooth chorion fusion with parietal decidua. Placenta 2015;36:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ.. Regulation of human placental development by oxygen tension. Science 1997;277:1669–1672. [DOI] [PubMed] [Google Scholar]
- Gill WB, Schumacher GF, Bibbo M, Straus FH, Schoenberg HW.. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. JURO 1979;122:36–39. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA.. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–e226. [DOI] [PubMed] [Google Scholar]
- Gitlin D, Perricelli A, Gitlin GM.. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res 1972;32:979–982. [PubMed] [Google Scholar]
- Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L.. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 2000;58:350–365. [DOI] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ.. Neural tube defects. Annu Rev Neurosci 2014;37:221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM.. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- Grün F, Blumberg B.. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord 2007;8:161–171. [DOI] [PubMed] [Google Scholar]
- Hakkola J, Pelkonen O, Pasanen M, Raunio H.. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol 1998;28:35–72. [DOI] [PubMed] [Google Scholar]
- Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD.. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 2012;7:e52899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Troisi R, Wise LA, Titus-Ernstoff L, Hyer M, Palmer JR, Strohsnitter WC, Robboy SJ, Anderson D, Kaufman R.. Preterm birth, fetal growth, and age at menarche among women exposed prenatally to diethylstilbestrol (DES). Reprod Toxicol 2011;31:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Leturque A, Alsat E, Loizeau M, Evain-Brion D, Girard J.. Developmental expression of Glut1 glucose transporter and c-fos genes in human placental cells. Placenta 1994;15:35–46. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA.. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol 2001;153:961–968. [DOI] [PubMed] [Google Scholar]
- Herva R, Karkinen-Jääskeläinen M.. Amniotic adhesion malformation syndrome: fetal and placental pathology. Teratology 1984;29:11–19. [DOI] [PubMed] [Google Scholar]
- Honkisz E, Zieba-Przybylska D, Wojtowicz AK.. The effect of triclosan on hormone secretion and viability of human choriocarcinoma JEG-3 cells. Reprod Toxicol 2012;34:385–392. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL. et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 2011;365:1304–1314. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC.. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod 2007;22:2715–2722. [DOI] [PubMed] [Google Scholar]
- Huang PC, Tsai C-H, Liang W-Y, Li S-S, Huang H-B, Kuo P-L.. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS One 2016;11:e0159398-0159318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi IT, Korenbrot CC, Jaffe RB.. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab 1977a;44:963–967. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Korenbrot CC, Serón-Ferré M, Foster DB, Parer JT, Jaffe RB.. Stimulation of testosterone production in vivo and in vitro in the male rhesus monkey fetus in late gestation. Endocrinology 1977b;100:839–844. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ.. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 2003;74:297–308. [DOI] [PubMed] [Google Scholar]
- Hustin J, Schaaps JP.. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987;157:162–168. [DOI] [PubMed] [Google Scholar]
- Iwasenko JM, Howard J, Arbuckle S, Graf N, Hall B, Craig ME, Rawlinson WD.. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis 2011;203:1526–1533. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Jauniaux E, Hustin J.. Maternal circulation in the first-trimester human placenta–myth or reality? YMOB 1997;176:695–705. [DOI] [PubMed] [Google Scholar]
- James JL, Saghian R, Perwick R, Clark AR.. Trophoblast plugs: impact on utero-placental haemodynamics and spiral artery remodelling. Hum Reprod 2018;33:1430–1441. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL.. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci 2007;113:1–13. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B.. Fluid compartments of the embryonic environment. Hum Reprod Update 2000;6:268–278. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B.. In vivo investigation of placental transfer early in human pregnancy. Eur J Obstet Gynecol Reprod Biol 2000;92:45–49. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Burton GJ.. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus—a review. Placenta 2003;24:S86–S93. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Jurkovic D, Gavriil P, Campbell S.. The origin of alpha-fetoprotein in first-trimester anembryonic pregnancies. YMOB 1995;173:1749–1753. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ.. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006;12:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ET, Longnecker MP.. Pharmacologic sex hormones in pregnancy in relation to offspring obesity. Obesity (Silver Spring) 2014;22:2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Nørgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, Thulstrup AM, Ivell R, Anand-Ivell R, Lindh CH. et al. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect 2012;120:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD.. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect 2016;124:1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF. et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 2015;13:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Jauniaux E.. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron 1995;26:145–173. [DOI] [PubMed] [Google Scholar]
- Jones HN, Powell TL, Jansson T.. Regulation of placental nutrient transport—a review. Placenta 2007;28:763–774. [DOI] [PubMed] [Google Scholar]
- Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T.. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 2009;23:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AM, Weinberg CR, Baird DD, Wilcox AJ.. The association of maternal factors with delayed implantation and the initial rise of urinary human chorionic gonadotrophin. Hum Reprod 2011;26:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfa N, Paris F, Soyer-Gobillard M-O, Daures J-P, Sultan C.. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril 2011;95:2574–2577. [DOI] [PubMed] [Google Scholar]
- Kaya T, Mohr SC, Waxman DJ, Vajda S.. Computational screening of phthalate monoesters for binding to PPARgamma. Chem Res Toxicol 2006;19:999–1009. [DOI] [PubMed] [Google Scholar]
- Keller H, Neuhauser G, Durkin-Stamm MV, Kaveggia EG, Schaaff A, Sitzmann F, Holmes LB.. "ADAM complex" (amniotic deformity, adhesions, mutilations)—a pattern of craniofacial and limb defects. Am J Med Genet 1978;2:81–98. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Kim J-S.. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213:S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-C, Friso S, Choi S-W.. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem 2009;20:917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou M-A, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG.. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatr 2018;172:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren G, Ornoy A.. The role of the placenta in drug transport and fetal drug exposure. Expert Rev Clin Pharmacol 2018;11:373–385. [DOI] [PubMed] [Google Scholar]
- Koukoura O, Sifakis S, Spandidos DA.. DNA methylation in the human placenta and fetal growth (review). Mol Med Rep 2012;5:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I.. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129:e1121–e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL.. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta 2016;40:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca J, Binder AM, McElrath TF, Michels KB.. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res 2014;133:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca J, Binder AM, McElrath TF, Michels KB.. First-trimester urine concentrations of phthalate metabolites and phenols and placenta miRNA expression in a cohort of U.S. women . Environ Health Perspect 2016;124:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P.. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. Biol Neonate 2003;83:22–24. [DOI] [PubMed] [Google Scholar]
- Leddy MA, Power ML, Schulkin J.. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- Liu C, Liu C, Wang Q, Zhang Z.. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: a meta-analysis. Arch Gynecol Obstet 2018;298:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Zhong J, Mansur A, Adir M, Racowsky C, Hauser R, Brennan K, Karlsson O, Baccarelli AA.. Placental lncRNA expression is associated with prenatal phthalate exposure. Toxicol Sci 2018;163:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malassine A, Frendo JL, Evain-Brion D.. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update 2003;9:531–539. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ.. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 1999;161:209–218. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Bakardjiev AI, Fisher SJ.. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest 2010;120:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat M-P.. Obstetrical outcomes and biomarkers to assess exposure to phthalates: a review. Environ Int 2015;83:116–136. [DOI] [PubMed] [Google Scholar]
- Mathiesen L, Mørck TA, Zuri G, Andersen MH, Pehrson C, Frederiksen M, Mose T, Rytting E, Poulsen MS, Nielsen JK. et al. Modelling of human transplacental transport as performed in Copenhagen. Basic Clin Pharmacol Toxicol 2014;115:93–100. [DOI] [PubMed] [Google Scholar]
- Matthay EC, Glymour MM.. A graphical catalog of threats to validity. Epidemiology (Cambridge, Mass) 2020;31:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Téllez-Rojo MM.. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect 2009;117:1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meethal SV, Hogan KJ, Mayanil CS, Iskandar BJ.. Folate and epigenetic mechanisms in neural tube development and defects. Childs Nerv Syst 2013;29:1427–1433. [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Kamat A.. Mechanisms in the regulation of aromatase in developing ovary and placenta. J Steroid Biochem Mol Biol 2007;106:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Choudhury M.. Mono-(2-ethylhexyl) phthalate increases oxidative stress responsive miRNAs in first trimester placental cell line HTR8/SVneo. Chem Res Toxicol 2016;29:430–435. [DOI] [PubMed] [Google Scholar]
- Metzler M. The metabolism of diethylstilbestrol. CRC Crit Rev Biochem 1981;10:171–212. [DOI] [PubMed] [Google Scholar]
- Miller RK, Heckmann ME, McKenzie RC.. Diethylstilbestrol: placental transfer, metabolism, covalent binding and fetal distribution in the Wistar rat. J Pharmacol Exp Ther 1982;220:358–365. [PubMed] [Google Scholar]
- Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology 1995;51:435–445. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Williams MA.. Stilboestrol exposure in utero and risk of pre-eclampsia. Lancet 1995;345:265–266. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Pangilinan F, Brody LC.. Genetic risk factors for folate-responsive neural tube defects. Annu Rev Nutr 2017;37:269–291. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN,, Torchia MG.. The Developing Human: Clinically Oriented Embryology, 8th edn.Philadelphia, PA: Saunders/Elsevier, 2008. [Google Scholar]
- Mose T, Knudsen LE, Hedegaard M, Mortensen GK.. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int J Toxicol 2007;26:221–229. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338:131–137. [PubMed] [Google Scholar]
- Muralimanoharan S, Gao X, Weintraub S, Myatt L, Maloyan A.. Sexual dimorphism in activation of placental autophagy in obese women with evidence for fetal programming from a placenta-specific mouse model. Autophagy 2016;12:752–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V, Minguez-Alarcon L, Christou G, Ford JB, Dimitriadis I, Hauser R, Souter I, Messerlian C, Environment R, Health Study T.. Placental weight in relation to maternal and paternal preconception and prenatal urinary phthalate metabolite concentrations among subfertile couples. Environ Res 2019;169:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L. Placental adaptive responses and fetal programming. J Physiol 2006;572:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllynen P, Pasanen M, Vähäkangas K.. The fate and effects of xenobiotics in human placenta. Expert Opin Drug Metabol Toxicol 2007;3:331–346. [DOI] [PubMed] [Google Scholar]
- Nagy AM, Glinoer D, Picelli G, Delogne-Desnoeck J, Fleury B, Courte C, Kaufman JM, Robyn C, Meuris S.. Total amounts of circulating human chorionic gonadotrophin alpha and beta subunits can be assessed throughout human pregnancy using immunoradiometric assays calibrated with the unaltered and thermally dissociated heterodimer. J Endocrinol 1994a;140:513–520. [DOI] [PubMed] [Google Scholar]
- Nagy AM, Jauniaux E, Jurkovic D, Meuris S.. Placental production of human chorionic gonadotrophin alpha and beta subunits in early pregnancy as evidenced in fluid from the exocoelomic cavity. J Endocrinol 1994b;142:511–516. [DOI] [PubMed] [Google Scholar]
- Naimi AI, Cole SR, Eh K.. An introduction to g methods. Int J Epidemiol 2017;46:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness-Abramof R, Apovian CM.. Drug-induced weight gain. Drugs Today 2005;41:547–555. [DOI] [PubMed] [Google Scholar]
- NICHD. Preeclampsia and Eclampsia. Bethesda, MA: NIH, 2017. [Google Scholar]
- O’Donnell K, O’Connor TG, Glover V.. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci 2009;31:285–292. [DOI] [PubMed] [Google Scholar]
- Opitz JM, Johnson DR, Gilbert-Barness EF.. ADAM "sequence" part II: hypothesis and speculation. Am J Med Genet A 2015;167:478–503. [DOI] [PubMed] [Google Scholar]
- Pastrakuljic A, Schwartz R, Simone C, Derewlany LO, Knie B, Koren G.. Transplacental transfer and biotransformation studies of nicotine in the human placental cotyledon perfused in vitro. Life Sci 1998;63:2333–2342. [DOI] [PubMed] [Google Scholar]
- Pearl J. Causality: models, Reasoning, and Inference. Cambridge, U.K.; New York: Cambridge University Press, 2000. [Google Scholar]
- Pereira L. Congenital viral infection: traversing the uterine-placental interface. Annu Rev Virol 2018;5:273–299. [DOI] [PubMed] [Google Scholar]
- Perez KM, Titus-Ernstoff L, Hatch EE, Troisi R, Wactawski-Wende J, Palmer JR, Noller K, Hoover RN.. Reproductive outcomes in men with prenatal exposure to diethylstilbestrol. Fertil Steril 2005;84:1649–1656. [DOI] [PubMed] [Google Scholar]
- Pérez-Albaladejo E, Fernandes D, Lacorte S, Porte C.. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol In Vitro 2017;38:41–48. [DOI] [PubMed] [Google Scholar]
- Philippat C, Heude B, Botton J, Alfaidy N, Calafat AM, Slama R; the EDEN Mother–Child Cohort Study Group. Prenatal exposure to select phthalates and phenols and associations with fetal and placental weight among male births in the EDEN Cohort (France). Environ Health Perspect 2019;127:017002–017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CF, Wibberley DG.. Determination of di-(2-ethylhexyl)phthalate in human placenta. J Chromatogr 1977;132:511–518. [DOI] [PubMed] [Google Scholar]
- Racette SB, Deusinger SS, Deusinger RH.. Obesity: overview of prevalence, etiology, and treatment. Phys Ther 2003;83:276–288. [PubMed] [Google Scholar]
- Rattan S, Beers HK, Kannan A, Ramakrishnan A, Brehm E, Bagchi I, Irudayaraj JMK, Flaws JA.. Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol Appl Pharmacol 2019;379:114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn SM, Cross JC.. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol 2008;24:159–181. [DOI] [PubMed] [Google Scholar]
- Ray JG, Laskin CA.. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta 1999;20:519–529. [DOI] [PubMed] [Google Scholar]
- Raz R, Kioumourtzoglou MA, Weisskopf MG.. Live-birth bias and observed associations between air pollution and autism. Am J Epidemiol 2018;187:2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015;213:S21–S28. [DOI] [PubMed] [Google Scholar]
- Ridano ME, Racca AC, Flores-Martin J, Camolotto SA, de Potas GM, Genti-Raimondi S, Panzetta-Dutari GM.. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod Toxicol 2012;33:331–338. [DOI] [PubMed] [Google Scholar]
- Roberts VHJ, Morgan TK, Bednarek P, Morita M, Burton GJ, Lo JO, Frias AE.. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: new insights from contrast-enhanced ultrasound and tissue histopathology. Hum Reprod 2017;32:2382–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, Bhatti G, Kim J-S, Qureshi F, Jacques SM. et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinatal Med 2018;46:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL.. Modern Epidemiology, 3rd edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. [Google Scholar]
- Safi J, Joyeux L, Chalouhi GE.. Periconceptional folate deficiency and implications in neural tube defects. J Pregn 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafia CM, Thomas DM, Roberts DJ, Straughen JK, Catalano PM, Perez-Avilan G.. First trimester detection of placental disease: challenges and opportunities. Am J Perinatol 2016;33:1306–1312. [DOI] [PubMed] [Google Scholar]
- Salle B, Sergeant P, Awada A, Bied-Damon V, Gaucherand P, Boisson C, Guibaud S, Benchaib M, Rudigoz RC.. Transvaginal ultrasound studies of vascular and morphological changes in uteri exposed to diethylstilbestrol in utero. Hum Reprod 1996;11:2531–2536. [DOI] [PubMed] [Google Scholar]
- Sato K, Kanno J, Tominaga T, Matsubara Y, Kure S.. De novo and salvage pathways of DNA synthesis in primary cultured neurall stem cells. Brain Res 2006;1071:24–33. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Howard GJ, Hurst CH, Emberley JK, Waxman DJ, Webster T, Sherr DH.. Environmental and endogenous peroxisome proliferator-activated receptor gamma agonists induce bone marrow B cell growth arrest and apoptosis: interactions between mono(2-ethylhexyl)phthalate, 9-cis-retinoic acid, and 15-deoxy-Delta12,14-prostaglandin J2. J Immunol 2004;173:3165–3177. [DOI] [PubMed] [Google Scholar]
- Schmatz M, Madan J, Marino T, Davis J.. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol 2010;30:441–446. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Morales-Prieto DM, Pastuschek J, Frohlich K, Markert UR.. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol 2015;108:65–71. [DOI] [PubMed] [Google Scholar]
- Scott GM, Chow SS, Craig ME, Pang CN, Hall B, Wilkins MR, Jones CA, Lloyd AR, Rawlinson WD.. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J Infect Dis 2012;205:1305–1310. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM.. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 2009;30:883–925. [DOI] [PubMed] [Google Scholar]
- Scott JN, Adejokun F.. Placental changes due to administration of diethylstilbestrol (DES). Virchows Archiv B Cell Pathol 1980;34:261–267. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S.. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes 2001;25:1175–1182. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Camm EJ.. The programming power of the placenta. Front Physiol 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HC, McLachlan JA.. The fate of diethylstilbestrol in the pregnant mouse. J Pharmacol Exp Ther 1976;197:687–696. [PubMed] [Google Scholar]
- Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NME, Campbell A, Devito LG, Ilic D. et al. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 2016;18:700–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF.. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol 2015;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP.. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect 1995;103:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff FR, Lopez A, Lupo PJ, Seth A, Jorgez C, Agopian AJ.. Maternal hypertension and hypospadias in offspring: a systematic review and meta-analysis. Birth Defects Res 2019;111:9–15. [DOI] [PubMed] [Google Scholar]
- Shoaito H, Petit J, Chissey A, Auzeil N, Guibourdenche J, Gil S, Laprevote O, Fournier T, Degrelle SA.. The role of peroxisome proliferator-activated receptor gamma (PPARgamma) in Mono(2-ethylhexyl) Phthalate (MEHP)-mediated cytotrophoblast differentiation. Environ Health Perspect 2019;127:027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier I, Platt, RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8. https://doi.org/10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith O, Smith G.. Pituitary stimulating property of stilbesterol as compared with that of estrone. Proc Soc Exper Med Biol 1944;57:198–200. [Google Scholar]
- Smith O, Smith G, Schiller S.. Estrogen and progestin metabolism in pregnancy: spontaneous and induced labor. J Clin Endocrinol 1941;1:461–469. [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S. et al. ; the Study for Future Families Research Team. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005;113:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Zydek M, Fang-Hoover J, Larocque N, Tsuge M, Gormley M, Kauvar LM, Pereira L.. Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J Virol 2015;89:5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Picciano MF.. Folate and human reproduction. Am J Clin Nutr 2006;83:993–1016. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Huhtaniemi IT.. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update 2015;21:310–328. [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Gavish K, Hod M.. Impact of maternal obesity on fetal health. Fetal Diagn Ther 2013;34:1–7. [DOI] [PubMed] [Google Scholar]
- Titus L, Hatch EE, Drake KM, Parker SE, Hyer M, Palmer JR, Strohsnitter WC, Adam E, Herbst AL, Huo D. et al. Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): a report from the US National Cancer Institute DES Third Generation Study. Reprod Toxicol 2019;84:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Hyer M, Wise LA, Palmer JR, Kaufman R, Adam E, Noller K, Herbst AL. et al. Offspring of women exposed in utero to diethylstilbestrol (DES). Epidemiology 2008;19:251–257. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN.. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). Int J Androl 2010;33:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K. et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int J Epidemiol 2006;35:862–868. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V.. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev 2001;15:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Hyer M, Hatch EE, Titus-Ernstoff L, Palmer JR, Strohsnitter WC, Herbst AL, Adam E, Hoover RN.. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiology 2013;24:430–438. [DOI] [PubMed] [Google Scholar]
- Troisi R, Titus-Ernstoff L, Hyer M, Hatch EE, Robboy SJ, Strohsnitter W, Palmer JR, Oglaend B, Adam E, Kaufman R. et al. Preeclampsia risk in women exposed in utero to diethylstilbestrol. Obstet Gynecol 2007;110:113–120. [DOI] [PubMed] [Google Scholar]
- Uenaka M, Morizane M, Tanimura K, Deguchi M, Kanzawa M, Itoh T, Yamada H.. Histopathological analysis of placentas with congenital cytomegalovirus infection. Placenta 2019;75:62–67. [DOI] [PubMed] [Google Scholar]
- USCDC. Facts about Hypospadias. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention: Atlanta, GA: 2020. https://www.cdc.gov/ncbddd/birthdefects/hypospadias.html. [Google Scholar]
- Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M.. Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect 2015;123:1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MM, van Rooij IA, Miller RK, Zielhuis GA, de Jong-van den Berg LT, Roeleveld N.. Teratogenic mechanisms of medical drugs. Hum Reprod Update 2010;16:378–394. [DOI] [PubMed] [Google Scholar]
- van’t Erve TJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, Calafat AM, Swan SH, Ferguson KK.. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol 2019;53:3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;25:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford, UK: Oxford University Press, 2015a. [Google Scholar]
- VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Knol MJ.. A tutorial on interaction. Epidemiol Meth 2014;3:1–40. [Google Scholar]
- Venturelli AC, Meyer KB, Fischer SV, Kita DH, Philipsen RA, Morais RN, Martino Andrade AJ.. Effects of in utero and lactational exposure to phthalates on reproductive development and glycemic homeostasis in rats. Toxicology 2019;421:30–40. [DOI] [PubMed] [Google Scholar]
- Vessey MP. Epidemiological studies of the effects of diethylstilboestrol. IARC Sci Publ 1989;335–348. [PubMed] [Google Scholar]
- Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D.. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski TL, Geromini K, McKinley Brewer J, Bansal R, Abdelouahab N, Langlois M-F, Takser L, Zoeller RT.. Endocrine disruption in human placenta: expression of the dioxin-inducible enzyme, Cyp1a1, is correlated with that of thyroid hormone-regulated genes. J Clin Endocrinol Metabol 2014;99:E2735–E2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA.. Placental transporter localization and expression in the human: the importance of species, sex, and gestational age differencesdagger. Biol Reprod 2017;96:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Markowitz JS, Donovan JL, Devane CL.. P-glycoprotein does not actively transport nicotine and cotinine. Addict Biol 2005;10:127–129. [DOI] [PubMed] [Google Scholar]
- Wegrzyn P, Brawura-Biskupski-Samaha R, Borowski D, Górski A, Wielgoś M.. The chorionic bump associated with acrani-case report. Ginekol Pol 2013;84:1055–1058. [DOI] [PubMed] [Google Scholar]
- Wehby GL, Murray JC.. Folic acid and orofacial clefts: a review of the evidence. Oral Dis 2010;16:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG.. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 2014;36:615–625. [DOI] [PubMed] [Google Scholar]
- Weiss G, Sundl M, Glasner A, Huppertz B, Moser G.. The trophoblast plug during early pregnancy: a deeper insight. Histochem Cell Biol 2016;146:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, Winn LM.. Biochemical toxicology of chemical teratogenesis. Crit Rev Biochem Mol Biol 1996;31:1–40. [DOI] [PubMed] [Google Scholar]
- Wen SW, Zhou J, Yang Q, Fraser W, Olatunbosun O, Walker M.. Maternal exposure to folic acid antagonists and placenta-mediated adverse pregnancy outcomes. CMAJ 2008;179:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werler MM, Louik C, Shapiro S, Mitchell AA.. Prepregnant weight in relation to risk of neural tube defects. JAMA 1996;275:1089–1092. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL.. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med 1995;332:1411–1416. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Basso O.. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol 2011;174:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Bulmer JN, Innes BA, Broughton Pipkin F.. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol Reprod 2011;84:1148–1153. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM.. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 2011;119:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT.. Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta 2008;29:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Cook TJ, Knipp GT.. Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol Sci 2005;84:287–300. [DOI] [PubMed] [Google Scholar]
- Xu Y, Knipp GT, Cook TJ.. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch Toxicol 2006;80:293–298. [DOI] [PubMed] [Google Scholar]
- Zhao R, Diop-Bove N, Visentin M, Goldman ID.. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 2011;31:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, Flaws JA.. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 2017;158:1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y-D, Gao H, Huang K, Zhang Y-W, Cai X-X, Yao H-y, Mao L-J, Ge X, Zhou S-S, Xu Y-Y. et al. Prenatal phthalate exposure and placental size and shape at birth—A birth cohort study. Environ Res 2018;160:239–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.