Abstract
Phagocytic cells, such as macrophages, neutrophils, and dendritic cells, ingest particles larger than about 0.5 μM and thereby clear microbial pathogens and malignant cells from the body. These phagocytic cargoes are proteolytically degraded within the lumen of phagosomes, and peptides derived from them are presented on Major Histocompatibility Complexes (MHC) for the activation of T cells. Mammalian PLA2 isozymes belong to a large family of enzymes that cleave phospholipids at the second position of the glycerol backbone, releasing a free fatty acid and a lysolipid moiety. In human macrophages, at least 15 different PLA2 forms are expressed, and expression of many of these is dependent on pathogenic stimulation. Intriguing questions are why so many PLA2 forms are expressed in macrophages, and what are the functional consequences of their altered gene expression after encountering pathogenic stimuli. In this review, we discuss the evidence of the differential roles of different forms of PLA2 in phagocytic immune cells. These roles include: lipid signaling for immune cell activation, initial phagocytic particle uptake, microbial action for the killing and degradation of ingested microbes, and the repair of membranes induced by oxygen radicals. We also discuss the roles of PLA2 in the subsequent digestion of ingested phagocytic cargoes for antigen presentation to T cells.
Keywords: phagocytosis, PLA2, pathogens, macrophages, trafficking
Introduction
A role for phospholipase A2 (PLA2) in phagocytic immune cells has been suggested since the 1980s (Waite et al., 1979; Scott et al., 1980). These phagocytic cells, such as macrophages, neutrophils, and dendritic cells, ingest particles larger than about 0.5 μM and thereby clear microbial pathogens and malignant cells from the body (Uribe-Querol and Rosales, 2020). Particularly macrophages and dendritic cells also present peptides derived from these phagocytosed antigens on major histocompatibility complex (MHC) types I and II to activate cytolytic and helper T cells, respectively, while lipids can be presented on CD1 (Burgdorf and Kurts, 2008). The process of phagocytosis and the subsequent processing of the ingested antigen are essential processes that contribute to both innate and adaptive immunity. As we will discuss in this review, evidence suggests that PLA2 is involved in all stages of pathogen encounter, from the initial signaling, to the uptake, degradation and presentation of the antigen.
The nomenclature of PLA2 is very complex and disordered as it follows a chronology reflecting their time-line of discovery (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). Mammalian PLA2 isozymes belong to a large family, which have been assigned into groups I to XVI based on their primary amino acid sequence (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). Based on their subcellular localization and Ca2+ requirement, these groups can be further categorized into six types called sPLA2, iPLA2, cPLA2, LPLA2, aiPLA2, and PAF-AH, and all these types are further categorized into subtypes (Table 1) (i) sPLA2 are Ca2+-dependent secretory PLA2 forms found in secretions, such as a tears, plasma and pancreatic juice (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). These enzymes belong to groups I, II, III, V, IX, X, XI, XII, XIII, and XIV (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). Within the sPLA2 subgroup, a capital English letter indicates the subtype of enzymes. For example, sPLA2-IIA is present in synovial fluid, while sPLA2-IID is present in pancreas and spleen (Dennis et al., 2011). (ii) cPLA2 are Ca2+-dependent cytosolic enzymes which belong to group IV, and its subtypes are cPLA2α, -β, -γ, -δ, -ε, and -ζ (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). (iii) iPLA2 are Ca2+-independent cytosolic forms which belong to group VI, and its subtypes are iPLA2-β, -γ, -δ, -ε, -ζ, and -η (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). Within the cPLA2 and iPLA2 types, the different subtypes are mostly indicated by Greek letters (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). However, this nomenclature is not always consistently used and for example cPLA2-IVα and iPLA2-VIβ are also known as cPLA2-IVA and iPLA2-VIA. (iv) LPLA2 is a lysosomal PLA2 which belongs to group XV (Shayman et al., 2011; Shayman and Tesmer, 2019). (v) aiPLA2 are acidic Ca2+-independent PLA2 forms which belong to group XVI, and are better known as peroxisomal PLA2 (Sorokina et al., 2009; Fisher, 2018). (vi) platelet-activating factor hydrolases (PAFAH) belong to groups VIIA (also known as lipoprotein-associated PLA2; Lp-PLA2), VIIB and VIII (Stafforini, 2009; Dennis et al., 2011).
TABLE 1.
Phospholipase A2 (PLA2) family and role of specific forms discussed in this review.
| Type | Group | Sub types | Other name | Molecular weight (kDa) | Catalytic residue | References | PLA2 forms discussed |
| cPLA2 | IV | α (A) β (B) γ (C) δ (D) ε (E) ζ (F) | 60–114 | Ser/asp | Dennis et al., 2011; Leslie, 2015 | cPLA2-IVα in lipid signaling | |
| iPLA2 | VI | β (A) γ (B) δ (C) ε (D) ζ (E) η (F) | PNPLA9 PNPLA8 PNPLA6 PNPLA3 PNPLA2 PNPLA4 | 27–146 | Ser/Asp | Dennis et al., 2011; Ramanadham et al., 2015 | iPLA2-VIβ in supporting focal exocytosis at the phagocytic cup |
| sPLA2 | I II III V IX X XI XII XIII XIV | A, B A, B, C, D, E, F A, B A, B | 10–19 | His/Asp | Dennis et al., 2011; Murakami et al., 2015; Murakami, 2017 | sPLA2-IIA, V, X, XII as antimicrobial forms at the phagocytic cup and within closed phagosomes | |
| LPLA2 | XV | 45 | Ser/His/Asp | Dennis et al., 2011; Fisher, 2018 | LPLA2 having bacteriocidal activity at the phagocytic cup and within phagolysosomes Also, its membrane repair mechanism | ||
| aiPLA2 | XVI | Peroxiredoxin 6 | 25 | Ser/His/Asp | Fisher, 2018 | aiPLA2regulating NOX2 assembly, and its membrane repair mechanism | |
| PAF-AH | VIIA VIIB VIII | Lp-PLA2, PLA2VII PAF-AH II PAF-AH I (PAFAH1B1 PAFAH1B2 PAFAH1B3) | 26–45 | Ser/His/Asp | Dennis et al., 2011; Karasawa and Inoue, 2015 | Not discussed as relevant information was not found |
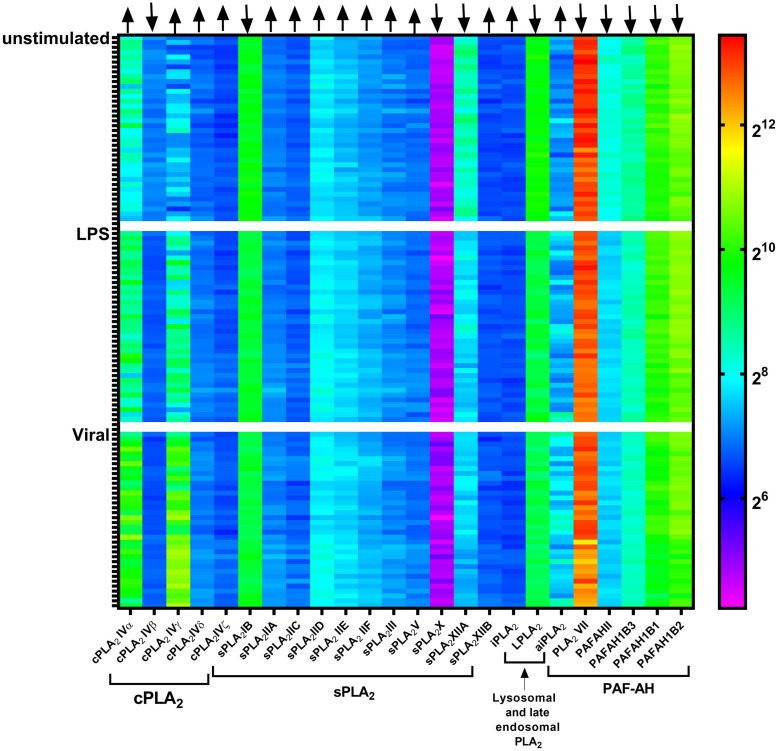
In human macrophages, 15 PLA2 forms are found at the transcript level, including sPLA2 (sPLA2-IID, -V, and -XIIA), cPLA2 (cPLA2-IV α, β, and γ), iPLA2 (iPLA2-VI β, γ, δ, ε, ζ, and η), LPLA2, LpPLA2, and aiPLA2 (Elstad et al., 1989; Rubio et al., 2015). Of these forms, LPLA2 and LpPLA2 carry a signal peptide required for cotranslational insertion into the ER and subsequent transport through the Golgi where they undergo N-glycosylation, while others are either cytosolic or membrane bound proteins (Dennis et al., 2011; Murakami et al., 2015; Ramanadham et al., 2015). Analysis of published gene expression data of blood monocyte-derived dendritic cells from 38 healthy individuals (Lee et al., 2014) revealed that the expression of some of these PLA2 forms is dependent on pathogenic stimulation (Figure 1). For example, the expression of cPLA2-IVα and -IVγ are upregulated, while sPLA2-XIIA and PAFAHII are downregulated upon stimulation of dendritic cells with lipopolysaccharide (LPS) or influenza virus. This information is important, because it shows that many forms of PLA2 undergo substantial up- or down-regulation upon pathogenic stimulation, suggesting that they have roles in the immune function of the phagocytes.
FIGURE 1.
Gene microarray heatmap of phospholipase A2 (PLA2) gene expression in monocyte-derived dendritic cells from a published microarray study. Each column shows a PLA2 gene. Each row shows dendritic cells derived from an individual donor, either unstimulated or stimulated with LPS or the influenza virus (Viral). Microarray data is from the reference (Lee et al., 2014).
Indeed, although the roles of most PLA2 forms in macrophages and dendritic cells are largely unknown, literature shows that various PLA2 forms function during all the events that occur following the encounter of a pathogen: (i) the immune signaling by lipids carried out by cPLA2α, (ii) the focal exocytosis to support phagosome formation by iPLA2β, (iii) the killing of the microbe by sPLA2-V, and LPLA2 forms, and (iv) the repair of damaged phagosomal membranes inflicted by reactive oxygen species by LPLA2 and aiPLA2. In this review, we aim is to provide an overview of these functions of PLA2 forms.
Lipid Signaling by cPLA2α
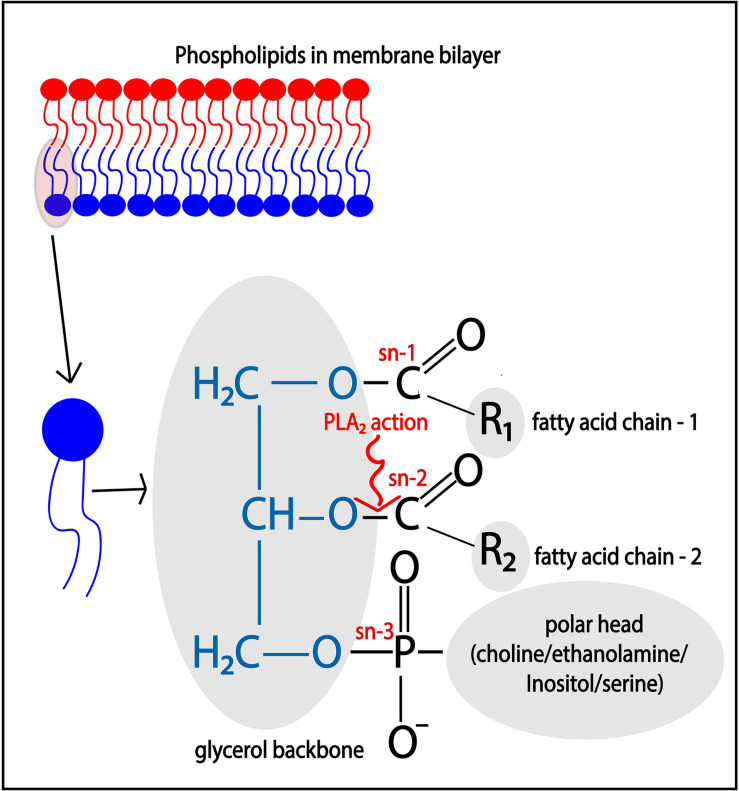
Phospholipase A2 enzymes hydrolyze membrane phospholipids that are composed of a glycerol backbone esterified to two hydrophobic fatty acids tails at the sn- (stereospecifically numbered) 1 and 2 positions, and a hydrophilic head group at the sn-3 position (Figure 2). Phospholipids with the head groups choline, ethanolamine, serine, and inositol form the main classes of phospholipids and are called phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI), respectively. The catalytic action of PLA2 releases the free fatty acid from the sn-2 position, such as arachidonic acid (ARA; 20:4), docosahexaenoic acid (C22:6), oleic acid (C18:1), while lysophospholipids, such as lyso-phosphatidyl-choline/-ethanolamine/-inositol (LPC/LPE/LPI), remain esterified in the membrane.
FIGURE 2.
Catalytic action of PLA2. Membrane phospholipids such as phosphatidylcholine (PC)/phosphatidylethanolamine (PE)/phosphatidylinositol (PI), and phosphatidylserine (PS), are cleaved at the sn-2 position of the glycerol backbone by the action of PLA2, thereby releasing free fatty acid such as arachidonic acid.
Both the lyso-phospholipids and the free fatty acids produced by PLA2 can be bioactive molecules and/or form precursors for the generation of bioactive lipid hormones (Brash, 2001; Carneiro et al., 2013). PLA2 activity is especially important for the generation of a class of lipid hormones called eicosanoids. Eicosanoids are a broad family of oxygenated lipid compounds that include prostaglandins, thromboxanes, and leukotrienes. Eicosanoids are generated via non-enzymatic oxidation of ARA or by the action of enzymes, such as cyclooxygenase (COX), lipooxygenase (LOX), and cytochrome P450 (CYP) (Dennis and Norris, 2015; Gil-de-Gómez et al., 2020). Eicosanoids exert immunomodulatory functions and, depending on the species of eicosanoids, can have pro- or anti-inflammatory effects (Dennis and Norris, 2015). Upon encountering a pathogen, immune phagocytes produce elevated levels of eicosanoids and an increased amount of eicosanoids in circulation is considered a hallmark of inflammation (Dennis and Norris, 2015). Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, indomethacin, and asprin, target COX and PLA2 to alleviate pain, redness, and swelling associated with inflammation (Singh et al., 2005; Dennis and Norris, 2015).
Mammalian cells are rich in ARA, but this is mostly incorporated in phospholipids by esterification to the glycerol backbone at the sn-2 position. For example, human cultured platelets contain ∼30 μg esterified arachidonate per 109 cells, which approximately corresponds to 5 mM, while free ARA is only present at ∼3 pmol per 106 cells, corresponding to about 0.5–1 μM (Brash, 2001). However, the concentration of free ARA significantly increases upon pathogenic stimulation. For example, plasma concentrations in mice increase from ∼1 to ∼1.5 mM upon infection with Salmonella pneumonia (Eijkelkamp et al., 2018). Similarly, concentrations of ARA in the whole blood of sepsis patients increase significantly from ∼200 to ∼250 ng/ml (Bruegel et al., 2012). Phagocytosis also triggers ARA release in vitro, as shown for macrophages labeled with radioisotope labeled ARA (Waite et al., 1979; Gil-de-Gómez et al., 2020). This has been observed for a wide range of phagocytic cargoes, such as serum-opsonized zymosan, native zymosan, and live pathogenic bacteria (Adolph et al., 2012; Gil-de-Gómez et al., 2020). These studies indicate that pathogenic encounter results in an increased PLA2 activity resulting in the production of free ARA. The elevated ARA levels, in turn, promote the production of eicosanoids for inflammatory signaling. As will be discussed in more detail below, the elevated ARA levels might also facilitate the phagocytic process because supplementation of ARA to macrophage-like cell lines RAW264.7 and THP-1 accelerated phagocytosis, and potentiated their anti-microbial ability against intracellular microbes (Adolph et al., 2012; Eijkelkamp et al., 2018).
The most important PLA2 form involved in eicosanoid signaling is cPLA2α. cPLA2 isozymes are characterized by an N-terminal CalB domain for Ca2+ binding, an active site Ser-Asp dyad for lipid hydrolysis, and a C-terminal phospholipid-binding domain for interacting with membranes (Leslie, 2015). cPLA2 forms require mM concentrations of free Ca2+ for calcium-dependent membrane binding and activation (Dennis et al., 2011; Leslie, 2015). Members of this family include cPLA2α, -β, -γ, -δ, -ε, and -ζ, which vary in their molecular weight, tissue expression, and subcellular localization (Dennis et al., 2011; Leslie, 2015). These members have about 30% sequence homology and although they have overlapping activities, their functions are largely non-redundant. cPLA2α is the only PLA2 form that contains mitogen-activated protein kinase (MAPK) phosphorylation sites (S505 and S727) (Murakami, 2017). Thereby, infection-induced activation of the Ras and MAPK pathways results in the phosphorylation of cPLA2α (Zhou et al., 2003; Su et al., 2004). This phosphorylation results in increased activation of cPLA2α, which in turn leads to increased production of ARA (Dieter et al., 2002). Infection also induces cPLA2α transcription via transcriptional factors, such as nuclear factor κB (NF-κB), Krüppel-like factor, hypoxia-inducible factor (Hif), specificity protein 1 (Sp1), and c-Jun, that are well known to regulate immune cell activation (Su et al., 2004; Dennis and Norris, 2015; Lane et al., 2019). In line with this, transcriptomics analysis of human blood monocyte-derived dendritic cells revealed that both LPS and viral stimulation increase cPLA2α expression (Figure 1).
cPLA2α localizes in the cytosol in unstimulated RAW264.7 macrophage-like cells (Casas et al., 2006) and in mouse peritoneal primary macrophages (Gijón and Leslie, 1999). It translocates to a perinuclear membrane-rich area (likely the Golgi network) in response to chemically induced increases in PI (4,5)-bisphosphate [PI(4,5)P2] (Bechler et al., 2012) and Ca2+ concentrations at this location (Gijón and Leslie, 1999). A similar translocation has been observed in LPS-stimulated P388D1 macrophage-like cells (Balboa et al., 2003b; Shirai et al., 2005). In mouse peritoneal macrophages, cPLA2α and COX2 are both located close to the perinuclear Golgi network, which likely facilitates their functional coupling (Gijón and Leslie, 1999; Su et al., 2004). Thus, the increased expression and MAPK-mediated phosphorylation of cPLA2α upon encountering a pathogen, lead to its translocation to the Golgi, resulting in increased production of ARA (Gijón and Leslie, 1999). This mechanism underlies the increased production of eicosanoids upon infection.
However, in human monocyte-derived macrophages, immunofluorescence microscopy experiments revealed that GFP–cPLA2α also translocates to the phagocytic cup (Casas et al., 2010) and to zymosan containing phagosomes (Casas et al., 2009). This suggests that, next to lipid signaling, cPLA2α has additional roles in the phagocytic process. These additional roles seem to be independent of its catalytic activity, because mutated cPLA2α versions with an inactive catalytic domain showed no phagocytic defect in RAW264.7 macrophage-like cells, whereas mutation of the C2 domain, which is needed for membrane attachment, resulted in significantly less phagocytic ability (Zizza et al., 2012).
iPLA2β Supports Focal Exocytosis for Pathogen Uptake
Unlike cPLA2 forms, group-VI iPLA2s do not require Ca2+ for their activity and membrane association (Dennis et al., 2011; Ramanadham et al., 2015). These are intracellular membrane-associated and cytoplasmic isozymes with a molecular weight ranging from 27 to 146 kDa (Dennis et al., 2011; Ramanadham et al., 2015). These isozymes are characterized by lipase (GXSXG) and nucleotide-binding (GXGXXG) consensus sequences (Dennis et al., 2011; Ramanadham et al., 2015). They also carry ankyrin repeats that mediate protein-protein interactions and this enables them to form homo-oligomers which is essential for their activity (Dennis et al., 2011; Ramanadham et al., 2015). Members of this family include iPLA2-β, -γ, -δ, -ε, -ζ, and -η. Notably, transcripts of iPLA2-β, -γ, -δ, -ε, -ζ, and -η have been detected in human monocyte derived macrophages (Rubio et al., 2015), iPLA2-β in human primary blood monocytes (Mishra et al., 2008) and the human monocyte cell line U937 (Tay and Melendez, 2004). Of these, only iPLA2-β (a cytoplasmic form), and -γ (membrane-associated form) isozymes are widely characterized (Dennis et al., 2011; Ramanadham et al., 2015).
iPLA2-β localizes to the ER–Golgi intermediate compartment (ERGIC), likely by associating to the cytosolic leaflet of the ERGIC membrane (Ben-Tekaya et al., 2010; Bechler et al., 2012). Studies in mammalian cell lines, such as HeLa, showed that iPLA2-β mediates the formation of membrane tubules that bridge between separate ERGIC clusters and thereby regulate intra-ERGIC trafficking (Ben-Tekaya et al., 2010; Bechler et al., 2012). However, the widely described role of iPLA2-β is a housekeeping function of remodeling phospholipids (Winstead et al., 2000; Ramanadham et al., 2015) that occurs in nearly all cellular membranes via two pathways: the Lands and the Kennedy (or de novo) pathways (Pasternak and Bergeron, 1970; Dabral and Coorssen, 2017). The Lands pathway maintains cellular homeostasis and operates in the presence of low concentrations of free ARA, which typically is the case in a resting cell, and iPLA2-β is a critical enzyme for this pathway (Kennedy and Weiss, 1956; Lands, 1958). In the Lands pathway, iPLA2-β generates free ARA, but most of it rapidly gets re-incorporated into phospholipids by first linking to Co-Enzyme A (CoA) by long-chain fatty acyl CoA synthetases. CoA-linked ARA then gets incorporated into PC by a CoA-dependent acyltransferase (Balsinde et al., 1997). Thus, iPLA2β-produced ARA is an intermediate of connected de-acylation and re-acylation reactions of membrane phospholipids. In unstimulated P388D1 macrophages, re-acylation dominates over de-acylation, which limits the presence of free ARA (Chilton et al., 1996; Balsinde et al., 1997), thereby also limiting eicosanoid production. The Kennedy pathway is a low-affinity mechanism that incorporates free ARA in membranes as triacylglycerol (TAG) and diacylglycerol (DAG) species (Kennedy and Weiss, 1956; Astudillo et al., 2012; Gil-de-Gómez et al., 2020). Thus, the incorporation of free ARA into membranes depends upon the concentration of free ARA: at low concentrations most ARA will be incorporated in phospholipids via the Lands pathway, while at higher concentrations it will be incorporated in TAG and DAG species via the Kennedy pathway. Since pathogenic stimulation results in increased activities of cPLA2 as described above, the rate of ARA hydrolysis exceeds that of its reincorporation into membrane phospholipids, leading to activation of the Kennedy pathway to esterify bulk ARA back in the membrane.
However, evidence suggests that iPLA2-β has a second role in supporting the phagocytic uptake. During phagocytosis, iPLA2-β translocates to the plasma membrane within 5 mins after particle engagement (Tay and Melendez, 2004). The catalytic activity of iPLA2-β is essential for particle uptake, as its inhibition using bromoenolactone (BEL)–a selective iPLA2-β and -γ inhibitor (Ramanadham et al., 2015)–leads to the polarized accumulation of electronlucent structures that appear as intracellular vesicles in the cytosol polarized toward the target attachment site in primary human monocytes (Lennartz et al., 1997). Moreover, enrichment of BEL-sensitive iPLA2 to F-actin rich pseudopods occurs in murine monocytes that are stimulated with monocyte chemoattractant protein-1 (MCP-1) (Mishra et al., 2008), a chemokine known to induce increased ARA release in human monocytes (Gschwandtner et al., 2019). Together, these findings indicate that vesicles carrying BEL-sensitive iPLA2 translocate to the plasma membrane at the site of the nascent phagosome, presumably to support membrane extension by promoting the local fusion of these vesicles with the plasma membrane (Bajno et al., 2000). These iPLA2 carrying vesicles might be of endosomal nature, as iPLA2 has been reported on endosomes (Karli et al., 1990; Mayorga et al., 1993, 1994). Alternatively or additionally, these might be secretory lysosomes, as BEL-sensitive iPLA2 is required for lysozyme secretion from these compartments (Balboa et al., 2003a).
Anti-Microbial Roles of sPLA2
Bactericidal Roles of sPLA2 Forms
Members of the sPLA2 group were first discovered in snake and bee venom, and in bovine pancreatic juice (Dennis et al., 2011). Humans express 10 catalytically active sPLA2 forms (IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA), and one inactive form (XIIB) (Murakami et al., 2015). Several of these forms are expressed in phagocytic immune cells. For instance, human primary macrophages express sPLA2-IIA, -IID, -V, -XIIA, and -XIIB (Anthonsen et al., 2000; Rubio et al., 2015), the macrophage-like cell line U937 expresses sPLA2-IID, -V, and -XIIA (Dennis et al., 2011), human monocyte-derived dendritic cells express sPLA2-IIA, -IIC, -IID, -IIE, -IIF, -III, -V, -X, -XIIA, and -XIIB (Anthonsen et al., 2000; Lee et al., 2014), and human monocytes express sPLA2-IIA, and -V (Anthonsen et al., 2000). sPLA2 forms are Ca2+-dependent and low molecular weight proteins (<10–19 kDa) which are secreted into the extracellular environment. They carry a His-Asp catalytic dyad, have a 6–8 intramolecular disulfide bonds, and contain a highly conserved Ca2+ binding loop (Dennis et al., 2011; Murakami et al., 2015). Intracellular sPLA2 is believed to be inactive and reside within the lumen of secretory vesicles and it becomes active after its secretion (Murakami et al., 2015).
Because its activity is largely confined to the extracellular environment, sPLA2 in principle can only cleave phospholipids that are exposed to the outside of the cell. Thus, sPLA2 enzymes can mainly hydrolyze lipids in the exoplasmic leaflet of the plasma membrane, and might hence act on the same cell that produces it in an autocrine manner, or paracrine on other cells. In addition, sPLA2 might act on other membranous extracellular structures such as extracellular vesicles, mitochondria, lipoproteins, and microbes (Dennis et al., 2011; Murakami et al., 2015). Both sPLA2-IIA and -V have specificity for phospholipids head-groups and cleave phosphatidylglycerol (PG) and phosphatidylethanolamine (PE) more efficiently than phosphatidylcholine (PC) (Dennis et al., 2011; Murakami et al., 2015). Because the outer monolayer of the mammalian plasma membrane is rich in PC, whereas bacterial membranes are mainly composed of PG and PE, sPLA2-IIA and -V are considered so-called bactericidal or inflammatory sPLA2s that primarily hydrolyze phospholipids of invading bacteria (Murakami et al., 2015).
Pathogenic stimulation promotes the secretion and expression of sPLA2-IIA and -V (Lee et al., 2014). In healthy human individuals, circulating sPLA2-IIA levels are low (∼3 ng/ml), and likely insufficient to kill bacteria (Grönroos et al., 2002; Movert et al., 2013). However, upon microbial infection, sPLA2-IIA production increases resulting in increased levels (250–500 ng/ml) in human and mouse serum, which are sufficient to kill microbe as shown by in vitro dose-response curves (Grönroos et al., 2002; Bruegel et al., 2012; Movert et al., 2013; Murakami et al., 2015). Unstimulated mouse peritoneal macrophages express sPLA2-V and an intracellular pool is present at the Golgi network and recycling endosomes (Balestrieri et al., 2006). Activation of macrophages results in the release of this sPLA2-V pool, as stimulating P388D1 macrophage-like cells with LPS for 6 h resulted in an increased localization of sPLA2-V in caveolin-rich vesicles near the perinuclear area (Balboa et al., 2003b). The pathogenic stimulation also increases the expression of sPLA2-V, as has been reported for LPS-stimulated primary human monocyte-derived macrophages (Rubio et al., 2015) and rat liver macrophages (Dieter et al., 2002). In line with this, transcriptomics analysis (Lee et al., 2014) also showed an upregulation in the expression of sPLA2-V in LPS and viral stimulated human monocyte-derived dendritic cells (Figure 1). However, sPLA2-XIIA seems down regulated upon pathogenic stimulation of monocyte-derived dendritic cells (Figure 1), but the functional significance of this is unknown.
The release of sPLA2-V might occur in a polarized fashion at the phagocytic cup, as it translocates from the Golgi and recycling endosomes to the forming phagosome in zymosan-stimulated mouse peritoneal macrophages (Balestrieri et al., 2006, 2009). This translocation of sPLA2-V from its juxtanuclear resting position to the forming phagosome was also observed within 5 mins after phagocytic cup formation in zymosan-stimulated peritoneal mouse macrophages (Balestrieri et al., 2006). Therefore, sPLA2-V at the phagocytic cup could potentially damage the attached microbe even before its internalization, especially since its activity will be promoted by the high extracellular calcium concentration (mM) (Mitsuishi et al., 2006; Balestrieri et al., 2009). Evidence supporting such a role in the killing of pathogenic microbes at the phagocytoc cup and/or later in closed phagosomes, comes from the finding that mouse peritoneal macrophages lacking sPLA2-V show delayed phagocytosis, delayed phagosomal maturation, and impaired Candida albicans killing (Lennartz et al., 1997). Similarly, human recombinant sPLA2-IIA, V, X, and XII substantially inhibited growth of Staphylococcus aureus, and Listeria monocytogenes in colony forming unit (CFU) assays (Koduri et al., 2002). However, the contribution of these sPLA2 forms in the killing of infectious pathogens in vivo is yet unknown.
Bacterial Killing and Immune Modulation by LPLA2
Lysosomal PLA2 (LPLA2; group XV) is a glycoprotein and is highly mannosylated. The glycosylated form has a molecular weight of about 45 kDa (Shayman et al., 2011; Shayman and Tesmer, 2019). It carries a signal peptide for targeting to the ER, and is characterized by the conserved lipase motif AXSXG, containing an active site serine which is essential for its hydrolytic action (Shayman et al., 2011; Shayman and Tesmer, 2019). Its catalytic activity is calcium-independent, and it primarily localizes to the lumen of acidic lysosomes and late endosomes (Shayman et al., 2011; Shayman and Tesmer, 2019). Besides the canonical PLA2-mediated hydrolysis which releases a free sn-2 fatty acid from a phospholipid, LPLA2 is capable of transferring the fatty acid to the OH group at the C1 position of a short-chain ceramide in the so-called transacylase reaction. LPLA2 has an acidic pH optimum and is expressed in murine alveolar macrophages, RAW264.7 macrophage-like cells, and peritoneal mouse macrophages (Shayman et al., 2011; Shayman and Tesmer, 2019). The physiological roles of LPLA2 are still incompletely understood, and several functions have been proposed.
First, LPLA2 might regulate phospholipid metabolism, because phospholipids (i.e., PC, PE, and plasminogen-PE) were found to be accumulated in the alveolar lavage fluid of LPLA2 knock-out mice (Shayman and Tesmer, 2019).
Second, LPLA2 might mediate pathogen killing similar to sPLA2 described above, because LPLA2 is also released in the extracellular space of zymosan-stimulated mouse peritoneal and alveolar macrophages (Wightman et al., 1981; Abe et al., 2008). Also similar to sPLA2, LPLA2 translocates to forming phagosomes in RAW264.7 macrophage-like cells within 4 mins after particle engagement, and the fusion of lysosomes with the plasma membrane results in the exocytosis of LPLA2 (Sun et al., 2020). Because the V-ATPase at the plasma membrane pumps H+ ions across the membrane to the extracellular side, potentially lowering the local extracellular pH at the phagocytic cup, this might provide the required acidic microenvironment for secreted LPLA2 to attack the microbial cargo.
Third, LPLA2 might play a role in antigen presentation of lipids to T cells in CD1, because LPLA2 knock-out mice displayed lower T cell activation and recruitment to infected lungs (Shayman and Tesmer, 2019). Furthermore, Mycobacterium infected LPLA2 knock-out mice show an enhanced bacterial burden and a low recruitment of CD1-expressing cells to the infection site (Schneider et al., 2014). This role in lipid presentation is supported by the finding that LPLA2 can cleave bacterial cardiolipin, and the resulting lipid species are incorporated into the membranes of phagosomes and other organelles in Mycobacterium bovis infected murine macrophages (Fischer et al., 2001). Similarly, LPLA2 can cleave mycobacterial tetra-acylated glycolipid antigens (phosphatidyl-myo-inositol mannosides) into diacylated forms in THP-1 monocyte-like cells (Balboa et al., 2003a). Because cardiolipin carries four fatty acid chains, just as mycobacterial tetra-acylated glycolipids, LPLA2 appears to preferentially cleave tetra-acylated lipids (Fischer et al., 2001; Gilleron et al., 2016). Thus, current evidence suggests that LPLA2 processes microbial tetra-acylated lipids within phagolysosomes, and the resulting diacylated forms might be presented on CD1 molecules to stimulate T cells.
Fourth, LPLA2 could play a role in membrane repair, as explained in the next section.
Membrane Repair by LPLA2 and aiPLA2
Transacylase Activity of LPLA2 for Repair of Membrane Damage
In addition to its antimicrobial roles described in the previous section, LPLA2 also has a potential role in membrane repair. It can remove oxidized fatty acids at the sn-2 position of phospholipids and transfer these to ceramides in the transacylase reaction (Shayman and Tesmer, 2019). This ability of LPLA2 might be important, because in neutrophils, macrophages, and dendritic cells, the killing of ingested pathogens is a radical-mediated mechanism where the NADPH oxidase NOX2 generates large amounts of reactive oxygen species (ROS) in the lumen of phagolysosomes (Vulcano et al., 2004). These radicals not only damage the ingested pathogen, but also oxidize membranes of the host cell (Dingjan et al., 2016). Therefore, the host cell could need a mechanism to repair damage to the phagosomal membrane, particularly because polyunsaturated fatty acids, such as ARA, are highly susceptible to lipid peroxidation (Yin et al., 2011). LPLA2 might thus potentially limit or repair the damaging effects of ROS, by removing oxidized ARA from phospholipids and transfer it to ceramides. Supporting this transfer, ceramides containing long acyl chains such as ARA are high on mature phagosomes while ceramide synthase 2 expression and activity are high on early phagosomes in RAW264.7 macrophage-like cells (Pathak et al., 2018).
Regulation of Oxidative Damage by aiPLA2
Acidic Ca2+-independent PLA2 (aiPLA2) has a molecular weight of ∼25 kDa and is better known as peroxiredoxin 6 (Fisher, 2011, 2018). It is expressed in mouse alveolar macrophages (Chatterjee et al., 2011) and mainly localizes to the cytosol where it has only low PLA2 activity due to its acidic optimum of pH 4.0 (Fisher, 2018). However, a fraction of aiPLA2 is also targeted to the lumen of lysosomes and late endosomes, in a manner depending on direct interactions with the chaperone 14-3-3ε (Sorokina et al., 2009; Fisher, 2018). Besides possessing PLA2 activity, aiPLA2 is a multifunctional enzyme that also possesses lyso-phosphatidyl acyltransferase and glutathione peroxidase activities (Fisher, 2011).
Because aiPLA2 binds to liposomes carrying oxidized lipids, and translocates to the plasma membrane in A549 cells following treatment with an oxidization agent (Manevich et al., 2009), membrane oxidation might promote association of aiPLA2 with the membrane. Hence, oxidized sn-2 fatty acids might be replaced by the lyso-phosphatidyl acyltransferase activity of aiPLA2 (Fisher, 2018; Fisher et al., 2018). Additionally, aiPLA2 can reduce peroxidized phospholipids to their corresponding alcohol due to its glutathione peroxidase activity (Chatterjee et al., 2011; Fisher, 2018; Fisher et al., 2018). Therefore, similar to LPLA2, aiPLA2 can protect against oxidative damage due to its ability to (i) hydrolyze the sn-2 position peroxidized fatty acids to generate lyso-phospholipids, (ii) reacylate these lyso-phospholipids to form a new phospholipid, and (iii) convert oxidized phospholipids to their corresponding alcohol (Fisher et al., 2018). The physiological roles of aiPLA2 therefore include the repair of oxidized membranes (Chatterjee et al., 2011).
However, aiPLA2 also promotes ROS production by promoting the assembly of the NOX2 complex in alveolar macrophages (Chatterjee et al., 2011). As mentioned above, NOX2 generates ROS within the lumen of phagolysosomes, and also at the plasma membrane, in order to kill and degrade microbial pathogens (Burgdorf and Kurts, 2008; Uribe-Querol and Rosales, 2020). The assembly and activation of NOX2 occurs when MAPK phosphorylates cytosolic aiPLA2 at T177 which results in the translocation of aiPLA2 to the plasma membrane (Fisher et al., 2018). At the plasma membrane, aiPLA2 generates lyso-PC which in turn converts to lyso-phospatidic acid (LPA) by lysophospholipase D (Vázquez-Medina et al., 2016; Fisher, 2018). Binding of LPA to the LPA receptor-1 activates the small-GTPase Rac, which is a component required for the activation of the NOX2 complex (Fisher, 2018). In stark contrast, another component of the NOX2, p67phox, can bind to phosphorylated form of aiPLA2, and this inhibits its PLA2 activity (Fisher, 2018).
Thus, aiPLA2 has a dual function: it both repairs oxidation-induced membrane damage and promotes NOX2-mediated ROS formation. Perhaps at initial stages of pathogen recognition, the membrane damage by NOX2-produced ROS is still low. At this stage, unassembled p67phox (i.e., not in the NOX2 complex) might negatively regulate aiPLA2 activity, as the need to repair damaged membranes is low. However, as more NOX2 is assembled and ROS production is increased, most p67phox might be assembled in the NOX2 complex and no longer be available to inhibit aiPLA2. At this stage, aiPLA2 would be free to repair peroxidized phospholipids in the membrane.
aiPLA2 might also mediate the repair of membranes damaged by intracellular pathogens. The expression of aiPLA2 shows a biphasic response in Brucella suis infected RAW267.4 macrophages: aiPLA2 expression initially decreases until 10 h post-infection, after which it increases until 50 h post-infection (Wang et al., 2019). During the first phase, B. suis is non-replicative within phagosomes (Celli, 2019), whereas it becomes replicative in the second phase and this eventually leads to rupture of the phagosomal membrane (Celli, 2019; Wang et al., 2019). Therefore, the expression of aiPLA2 might initially be low because there is limited need for repair of membrane damage, whereas later its expression increases to perhaps repair the damaged phagosomal membrane. This mechanism is speculative and needs experimental support.
Discussion and Concluding Remarks
As discussed above, PLA2 forms have different effects on phagocytosis: aiPLA2, LPLA2, and sPLA2 forms mainly act on the luminal and/or extracellular leaflet of the (nascent) phagosomal membrane and play roles in the killing of the pathogen and the repair of the membrane from oxidative damage. In contrast, iPLA2 and cPLA2 forms mainly act on the cytosolic leaflets of the plasma membrane, phagosomes and other organelles, and play roles in eicosanoid signaling and regulation of organellar trafficking. These roles are summarized in Figure 3. In addition, since the products from PLA2 hydrolysis directly alter the physicochemical properties of the membrane, PLA2s might also directly affect the phagocytic process. ARA and LPC have negative and positive spontaneous curvatures, respectively, and thereby can directly stabilize or destabilize membrane assemblies (Chernomordik et al., 1995). Increased levels of ARA in the cis leaflets of merging bilayers (i.e., the cytoplasmic leaflet of the plasma membrane and the outer leaflet of the organellar membrane) promote fusion, while more LPC blocks fusion (Chernomordik et al., 1995). In contrast, more ARA in the trans leaflets (i.e., outer leaflet of the plasma membrane and luminal leaflet of the organellar membrane) of merging bilayers blocks fusion, while more LPC promotes fusion (Chernomordik et al., 1995). Therefore, membrane fusion might be both promoted and inhibited by these PLA2 metabolites depending on the site of PLA2 action, the PLA2 substrates, and downstream metabolism of the PLA2 products. Moreover, the fusion of intracellular membrane compartments at the phagocytic cup results in the delivery of more PLA2s to the nascent phagosome, including cPLA2, sPLA2, LPLA2, aiPLA2, and iPLA2 forms. This local delivery of more PLA2 at the nascent phagosome might thus modulate the phagocytic process by either facilitating or inhibiting the membrane reshaping required for the membrane wrapping and internalization of the phagocytic cargo.
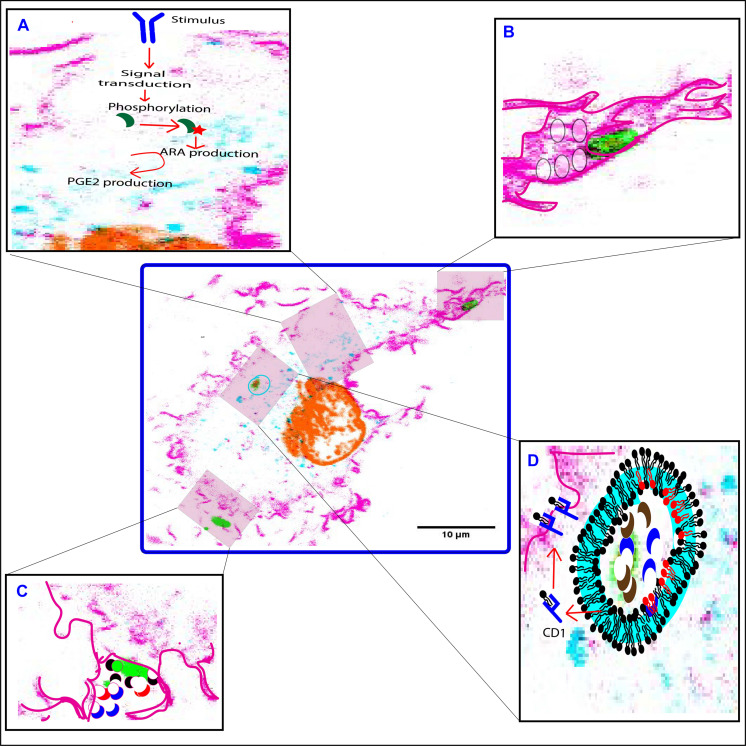
FIGURE 3.
Functions of PLA2 forms at different stages of phagocytosis. (A) cPLA2α (green crescent) is phosphorylated in response to an extracellular pathogenic stimulus. This leads to translocation of cPLA2α to the perinuclear area, where it hydrolyzes membrane phospholipids to generate arachidonic acid (ARA) which in-turn gets metabolized to eicosanoids by the action of the COX-2 enzyme. (B) iPLA2 (not shown) translocates to the phagocytic cup, and facilitates fusion of secretory vesicles (translucent circles) to provide additional membrane required for the extension of the pseudopodia. (C) sPLA2-II/V (red and black crescent) and LPLA2 (blue crescent) are released at the phagocytic cup to degrade invading microbes (green). (D) LPLA2 (blue crescent) and aiPLA2 (brown crescent) work together to degrade and process microbes within the phagolysosome. The processed lipids are then loaded onto a CD1 molecule (blue structure) to be presented on the cell surface to activate T cells. Both LPLA2 and aiPLA2 repair peroxidized lipids of the phagosomal membrane that might occur due to increased NOX2 activity. The repaired phospholipids are shown in red. F-actin is pink, DNA is orange, LAMP1 is turquoise and LPS is green. Highlighted pink boundary in insets (B–D) shows pseudopodia, phagocytic cup, and plasma membrane, respectively. Highlighted turquoise boundary at the phagosome membrane is to show recruited LAMP1.
In addition to these spontaneous effects on membrane fusion by the physicochemical properties of PLA2 products, PLA2 might potentially also affect phagocytosis by affecting soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE)-mediated membrane fusion. In animal cells, all organellar membrane fusion (except mitochondrial fusion) is mediated by members of the SNARE protein family (Hong, 2005). Cognate SNARE proteins in both the vesicular and target membranes, called v- and t-SNAREs, engage and “zipper” from their N-terminal toward their C-terminal termini, thereby forming a tight alpha-helical coiled-coil bundle that overcomes the energy barrier of membrane fusion (Dingjan et al., 2018). In mouse chromaffin cells, the transmembrane domain of the v-SNAREs VAMP2 is affected by the PLA2 metabolites LPC and oleic acid in a leaflet specific manner that either promotes or inhibits fusion pore formation and expansion, hence affecting neurotransmitter release (Dhara et al., 2020). In line with this, the administration of LPC and oleic acid to the cell culture medium, which increases their concentrations in the outer leaflet of the plasma membrane, accelerated and deaccelerated nurotransmitter release, respectively (Dhara et al., 2020). In contrast, the intracellular administration of LPC and oleic acid by microinjection, which increased their concentration in the inner leaflet of the plasma membrane, blocked and promoted membrane fusion, respectively (Dhara et al., 2020). Similar results were also obtained in homotypic fusion of cortical vesicles isolated from eggs of the sea urchin, in which increasing the LPC concentrations in the outer leaflets of these vesicles by external LPC blocked fusion (Dabral and Coorssen, 2019). However, the PLA2 products might not only affect the membrane fusion, but also upstream events as the external application of ARA also affected the SNARE dependent docking or priming in homotypic fusion of cortical vesicles (Dabral and Coorssen, 2019). Because many SNARE proteins are both functionally and structurally homologous, and since many SNARE proteins are involved in endosomal trafficking and phagocytosis (Dingjan et al., 2018), similar interactions with PLA2 metabolites could potentially inhibit or promote membrane fusion in phagocytosis as well.
Whereas the roles of the different PLA2 types in phagocytes are fairly well established, as discussed in this review, a main open question is why immune cells express so many different members of each PLA2 group. For example, human monocyte derived macrophages express three forms of sPLA2 (sPLA2-IID, -V, and -XIIA), three forms of cPLA2 (cPLA2IV-α, -β, and -γ) and six forms of iPLA2 (iPLA2VI-β, -γ, -δ, -ε, -ζ, and -η) (Rubio et al., 2015). While these different types have non-overlapping functions in eicosanoid signaling, membrane repair, organellar trafficking and pathogen killing, it is largely unknown why multiple members of each different type are expressed. PLA2 members from each type perhaps are essential for carrying out the same critical processes. Hence, multiple genes coding for these essential enzymes might ensure the functional redundency in the absence of one of the forms, thereby increasing the fidelity of the immune response. It might also be that these different members have different specificities for headgroups and acyl chains, and/or they might have different subcellular localizations. Thereby, the various PLA2 subgroup members might have non-overlapping roles, and could for instance act during different stages of the phagocytic process: antigen recognition, formation of the phagocytic cup, particle internalization, and maturation of the phagosome into a phagolysosome. Supporting such non-overlapping roles, is the finding that expression of different subtype members of cPLA2 is differently regulated upon pathogenic stimulation: transcript levels of cPLA2-IVα and -IVγ are upregulated, whereas cPLA2-IVβ is downregulated (Figure 1) (Lee et al., 2014).
Another open question is whether microbial PLA2 forms also modulate phagocytic process. Several bacterial pathogens express PLA2 enzymes as virulence factors. For example, PLA2 activity of Helicobacter pylori is responsible for the degradation of the gut mucosal barrier (Baj et al., 2020). The putative PLA2 protein RV3091 of Mycobacterium tuberculosis is involved in the phagosomal escape of this pathogen (Cui et al., 2020) and a secreted form of PLA2 by Toxoplama gondii contributes to its replicative cycle (Cassaing et al., 2000). These bacterial PLA2s are structurally similar to mammalian sPLA2 forms and thus are evolutionary conserved (Murakami et al., 2015). Hence, we presume that within the lumen of a phagosome or at the nascent phagocytic cup, both host and microbial PLA2 likely engage in a contest to cleave the lipids of the bacterial and host membranes, respectively, but the functional impact of such engagement in phagocytic cells is yet unexplored.
Experimentally, it is difficult to discriminate the actions of the different PLA2 forms, both from host and microbial origin, because many PLA2 inhibitors are not entirely specific for one specific species of PLA2. Moreover, all PLA2 forms act on membrane phospholipids, and lipidomics approaches therefore do not readily allow the assignment of the results to a single species of PLA2. The potential functional redundancy of PLA2s also make them difficult to study, as knockout or knockdown might not always result in a clear phenotype. Nevertheless, we expect that the side-by-side comparison of mammalian cell lines with specific knockouts of one or more PLA2 forms, for instance using CRISPR technology, coupled with MS based organellar lipidomics and high resolution microscopy, will enable to address this functional redundancy and better delineate the unique and overlapping roles of the different PLA2 forms.
As summarized in Figure 3, PLA2 is important for the clearance of pathogens, because it (i) triggers eicosanoid signaling shaping the downstream immune response, (ii) stimulates or inhibits the phagocytic process, (iii) directly supports the killing of pathogenic microbes, and (iv) repairs oxidation induced phagosomal membrane damage. Therefore, the understanding of the expression, membrane association, substrate specificity and the associated immunomodulatory actions of PLA2 forms in regulating the immune response in phagocytic cells is critical, as this might also lead to new therapeutic approaches to combat microbial infections.
Author Contributions
DD conceptualize and drafted the original draft. GB reviewed and provided critical feedback. Both authors equally contributed to the final preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. GB is funded by a Young Investigator Grant from the Human Frontier Science Program (HFSP; RGY0080/2018). GB has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (Grant Agreement No. 862137).
References
- Abe A., Kelly R., Kollmeyer J., Hiraoka M., Lu Y., Shayman J. A. (2008). The secretion and uptake of lysosomal phospholipase A2 by alveolar macrophages. J. Immunol. 181 7873–7881. 10.4049/jimmunol.181.11.7873 [DOI] [PubMed] [Google Scholar]
- Adolph S., Fuhrmann H., Schumann J. (2012). Unsaturated fatty acids promote the phagocytosis of P. aeruginosa and R. equi by RAW264.7 macrophages. Curr. Microbiol. 65 649–655. 10.1007/s00284-012-0207-3 [DOI] [PubMed] [Google Scholar]
- Anthonsen M. W., Stengel D., Hourton D., Ninio E., Johansen B. (2000). Mildly oxidized LDL induces expression of group IIa secretory phospholipase A2 in human monocyte derived macrophages. Arterioscler. Thromb. Vasc. Biol. 20 1276–1282. 10.1161/01.atv.20.5.1276 [DOI] [PubMed] [Google Scholar]
- Astudillo A. M., Balgoma D., Balboa M. A., Balsinde J. (2012). Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim. Biophys. Acta. 1821 249–256. 10.1016/j.bbalip.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Baj J., Forma A., Sitarz M., Portincasa P., Garruti G., Krasowska D., et al. (2020). Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 10:27. 10.3390/cells10010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno L., Peng X. R., Schreiber A. D., Moore H. P., Trimble W. S., Grinstein S. (2000). Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149 697–706. 10.1083/jcb.149.3.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa M. A., Sáez Y., Balsinde J. (2003a). Calcium-independent phospholipase A2 is required for lysozyme secretion in U937 promonocytes. J. Immunol. 170 5276–5280. 10.4049/jimmunol.170.10.5276 [DOI] [PubMed] [Google Scholar]
- Balboa M. A., Shirai Y., Gaietta G., Ellisman M. H., Balsinde J., Dennis E. A. (2003b). Localization of group V phospholipase A2 in caveolin-enriched granules in activated P388D1 macrophage-like cells. J. Biol. Chem. 278 48059–48065. 10.1074/jbc.m305904200 [DOI] [PubMed] [Google Scholar]
- Balestrieri B., Hsu V. W., Gilbert H., Leslie C. C., Han W. K., Bonventre J. V., et al. (2006). Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J. Biol. Chem. 281 6691–6698. 10.1074/jbc.m508314200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri B., Maekawa A., Xing W., Gelb M. H., Katz H. R., Arm J. P. (2009). Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. immunol. 182 4891–4898. 10.4049/jimmunol.0803776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J., Balboa M. A., Dennis E. A. (1997). Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 272 29317–29321. 10.1074/jbc.272.46.29317 [DOI] [PubMed] [Google Scholar]
- Bechler M. E., de Figueiredo P., Brown W. J. (2012). A PLA1-2 punch regulates the Golgi complex. Trends Cell Biol. 22 116–124. 10.1016/j.tcb.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tekaya H., Kahn R. A., Hauri H.-P. (2010). ADP ribosylation factors 1 and 4 and group VIA phospholipase A2 regulate morphology and intraorganellar traffic in the endoplasmic reticulum-Golgi intermediate compartment. Mol. Biol. Cell 21 4130–4140. 10.1091/mbc.e10-01-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash A. R. (2001). Arachidonic acid as a bioactive molecule. J. Clin. Investig. 107 1339–1345. 10.1172/jci13210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruegel M., Ludwig U., Kleinhempel A., Petros S., Kortz L., Ceglarek U., et al. (2012). Sepsis-associated changes of the arachidonic acid metabolism and their diagnostic potential in septic patients. Crit. Care. Med. 40 1478–1486. 10.1097/ccm.0b013e3182416f05 [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Kurts C. (2008). Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20 89–95. 10.1016/j.coi.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Carneiro A. B., Iaciura B. M. F., Nohara L. L., Lopes C. D., Veas E. M. C., Mariano V. S., et al. (2013). Lysophosphatidylcholine triggers TLR2- and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation. PLoS One 8:e76233. 10.1371/journal.pone.0076233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas J., Gijón M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006). Phosphatidylinositol 4,5-bisphosphate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Mol. Biol. Cell 17 155–162. 10.1091/mbc.e05-06-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas J., Meana C., Esquinas E., Valdearcos M., Pindado J., Balsinde J., et al. (2009). Requirement of JNK-mediated phosphorylation for translocation of group IVA phospholipase A2 to phagosomes in human macrophages. J. Immunol. 183 2767–2774. 10.4049/jimmunol.0901530 [DOI] [PubMed] [Google Scholar]
- Casas J., Valdearcos M., Pindado J., Balsinde J., Balboa M. A. (2010). The cationic cluster of group IVA phospholipase A2 (Lys488/Lys541/Lys543/Lys544) is involved in translocation of the enzyme to phagosomes in human macrophages. J. Lipid Res. 51 388–399. 10.1194/jlr.m001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaing S., Fauvel J., Bessières M.-H., Guy S., Séguéla J.-P., Chap H. (2000). Toxoplasma gondii secretes a calcium-independent phospholipase A2. Int. J. Parasitol. 30 1137–1142. 10.1016/s0020-7519(00)00101-6 [DOI] [PubMed] [Google Scholar]
- Celli J. (2019). The intracellular life cycle of Brucella spp. Microbiol. Spectr. 7:10.1128/microbiolsec.BAI-0006-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Feinstein S. I., Dodia C., Sorokina E., Lien Y. C., Nguyen S., et al. (2011). Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 286 11696–11706. 10.1074/jbc.m110.206623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L., Chanturiya A., Green J., Zimmerberg J. (1995). The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys. J. 69 922–929. 10.1016/s0006-3495(95)79966-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton F. H., Fonteh A. N., Surette M. E., Triggiani M., Winkler J. D. (1996). Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta. 1299 1–15. 10.1016/0005-2760(95)00169-7 [DOI] [PubMed] [Google Scholar]
- Cui Z., Dang G., Song N., Cui Y., Li Z., Zang X., et al. (2020). Rv3091, an extracellular patatin-like phospholipase in Mycobacterium tuberculosis, prolongs intracellular survival of recombinant Mycolicibacterium smegmatis by mediating phagosomal escape. Front. Microbiol. 11:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabral D., Coorssen J. R. (2017). Phospholipase A2: potential roles in native membrane fusion. Int. J. Biochem. Cell Biol. 85 1–5. 10.1016/j.biocel.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Dabral D., Coorssen J. R. (2019). Arachidonic acid and lysophosphatidylcholine inhibit multiple late steps of regulated exocytosis. Biochem. Biophys. Res. Commun. 515 261–267. 10.1016/j.bbrc.2019.05.106 [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Norris P. C. (2015). Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15 511–523. 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. A., Cao J., Hsu Y.-H., Magrioti V., Kokotos G. (2011). Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111 6130–6185. 10.1021/cr200085w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhara M., Mantero Martinez M., Makke M., Schwarz Y., Mohrmann R., Bruns D. (2020). Synergistic actions of v-SNARE transmembrane domains and membrane-curvature modifying lipids in neurotransmitter release. eLife 9:e55152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P., Kolada A., Kamionka S., Schadow A., Kaszkin M. (2002). Lipopolysaccharide-induced release of arachidonic acid and prostaglandins in liver macrophages: regulation by Group IV cytosolic phospholipase A2, but not by Group V and Group IIA secretory phospholipase A2. Cell Signal 14 199–204. 10.1016/s0898-6568(01)00243-1 [DOI] [PubMed] [Google Scholar]
- Dingjan I., Linders P. T. A., Verboogen D. R. J., Revelo N. H., Beest M. Ter, van den Bogaart G. (2018). Endosomal and phagosomal SNAREs. Physiol. Rev. 98 1465–1492. 10.1152/physrev.00037.2017 [DOI] [PubMed] [Google Scholar]
- Dingjan I., Verboogen D. R., Paardekooper L. M., Revelo N. H., Sittig S. P., Visser L. J., et al. (2016). Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 6:22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp B. A., Begg S. L., Pederick V. G., Trapetti C., Gregory M. K., Whittall J. J., et al. (2018). Arachidonic acid stress impacts pneumococcal fatty acid homeostasis. Front. Microbiol 9:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstad M. R., Stafforini D. M., McIntyre T. M., Prescott S. M., Zimmerman G. A. (1989). Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J. Biol. Chem. 264 8467–8470. 10.1016/s0021-9258(18)81811-7 [DOI] [PubMed] [Google Scholar]
- Fischer K., Chatterjee D., Torrelles J., Brennan P. J., Kaufmann S. H. E., Schaible U. E. (2001). Mycobacterial lysocardiolipin is exported from phagosomes upon cleavage of cardiolipin by a macrophage-derived lysosomal phospholipase A2. J. Immunol. 167 2187–2192. 10.4049/jimmunol.167.4.2187 [DOI] [PubMed] [Google Scholar]
- Fisher A. B. (2011). Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal 15 831–844. 10.1089/ars.2010.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B. (2018). The phospholipase A(2) activity of peroxiredoxin 6. J. Lipid Res. 59 1132–1147. 10.1194/jlr.r082578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B., Vasquez-Medina J. P., Dodia C., Sorokina E. M., Tao J. Q., Feinstein S. I. (2018). Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 14 41–46. 10.1016/j.redox.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijón M. A., Leslie C. C. (1999). Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 65 330–336. 10.1002/jlb.65.3.330 [DOI] [PubMed] [Google Scholar]
- Gil-de-Gómez L., Monge P., Rodríguez J. P., Astudillo A. M., Balboa M. A., Balsinde J. (2020). Phospholipid arachidonic acid remodeling during phagocytosis in mouse peritoneal macrophages. Biomedicines 8:274. 10.3390/biomedicines8080274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron M., Lepore M., Layre E., Cala-De Paepe D., Mebarek N., Shayman J. A., et al. (2016). Lysosomal lipases PLRP2 and LPLA2 process mycobacterial multi-acylated lipids and generate T cell stimulatory antigens. Cell Chem. Biol. 23 1147–1156. 10.1016/j.chembiol.2016.07.021 [DOI] [PubMed] [Google Scholar]
- Grönroos J. O., Laine V. J. O., Nevalainen T. J. (2002). Bactericidal group IIA phospholipase A2 in serum of patients with bacterial infections. J. Infect. Dis. 185 1767–1772. 10.1086/340821 [DOI] [PubMed] [Google Scholar]
- Gschwandtner M., Derler R., Midwood K. S. (2019). More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immun 10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. (2005). SNAREs and traffic. Biochim. Biophys. Acta, Mol. Cell Res. 1744 120–144. [DOI] [PubMed] [Google Scholar]
- Karasawa K., Inoue K. (2015). Overview of PAF-degrading enzymes. Enzymes 38 1–22. 10.1016/bs.enz.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Karli U. O., Schäfer T., Burger M. M. (1990). Fusion of neurotransmitter vesicles with target membrane is calcium independent in a cell-free system. Proc. Natl. Acad. Sci. U.S.A. 87 5912–5915. 10.1073/pnas.87.15.5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Weiss S. B. (1956). The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 222 193–214. 10.1016/s0021-9258(19)50785-2 [DOI] [PubMed] [Google Scholar]
- Koduri R. S., Grönroos J. O., Laine V. J., Calvez C. Le, Lambeau G., Nevalainen T. J., et al. (2002). Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A(2). J. Biol. Chem. 277 5849–5857. 10.1074/jbc.m109699200 [DOI] [PubMed] [Google Scholar]
- Lands W. E. (1958). Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231 883–888. 10.1016/s0021-9258(18)70453-5 [DOI] [PubMed] [Google Scholar]
- Lane K., Andres-Terre M., Kudo T., Monack D. M., Covert M. W. (2019). Escalating threat levels of bacterial infection can be discriminated by distinct MAPK and NFkB signaling dynamics in single host cells. Cell Syst. 8 183–196.e4. [DOI] [PubMed] [Google Scholar]
- Lee M. N., Ye C., Villani A. C., Raj T., Li W., Eisenhaure T. M., et al. (2014). Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science 343:1246980. 10.1126/science.1246980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz M. R., Yuen A. F., Masi S. M., Russell D. G., Buttle K. F., Smith J. J. (1997). Phospholipase A2 inhibition results in sequestration of plasma membrane into electronlucent vesicles during IgG-mediated phagocytosis. J. Cell Sci. 110(Pt 17) 2041–2052. 10.1242/jcs.110.17.2041 [DOI] [PubMed] [Google Scholar]
- Leslie C. C. (2015). Cytosolic phospholipase A2: physiological function and role in disease. J. Lipid Res. 56 1386–1402. 10.1194/jlr.r057588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y., Shuvaeva T., Dodia C., Kazi A., Feinstein S. I., Fisher A. B. (2009). Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities. Arch. Biochem. Biophys. 485 139–149. 10.1016/j.abb.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Berón W., Sarrouf M. N., Colombo M. I., Creutz C., Stahl P. D. (1994). Calcium-dependent fusion among endosomes. J. Biol. Chem. 269 30927–30934. 10.1016/s0021-9258(18)47370-x [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Colombo M. I., Lennartz M., Brown E. J., Rahman K. H., Weiss R., et al. (1993). Inhibition of endosome fusion by phospholipase A2 (PLA2) inhibitors points to a role for PLA2 in endocytosis. Proc. Natl. Acad. Sci. U.S.A. 90 10255–10259. 10.1073/pnas.90.21.10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. S., Carnevale K. A., Cathcart M. K. (2008). iPLA2β: front and center in human monocyte chemotaxis to MCP-1. J. Exp. Med 205 347–359. 10.1084/jem.20071243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi M., Masuda S., Kudo I., Murakami M. (2006). Group V and X secretory phospholipase A2 prevents adenoviral infection in mammalian cells. Biochem. J. 393(Pt 1), 97–106. 10.1042/bj20050781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movert E., Wu Y., Lambeau G., Kahn F., Touqui L., Areschoug T. (2013). Secreted group IIA phospholipase A2 protects humans against the group b Streptococcus: experimental and clinical evidence. J. Infect. Dis. 208 2025–2035. 10.1093/infdis/jit359 [DOI] [PubMed] [Google Scholar]
- Murakami M. (2017). Lipoquality control by phospholipase A2 enzymes. Proc. Jpn. Acad., Ser. B Phys. Biol. Sci. 93 677–702. 10.2183/pjab.93.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Sato H., Miki Y., Yamamoto K., Taketomi Y. (2015). A new era of secreted phospholipase A2. J. Lipid Res. 56 1248–1261. 10.1194/jlr.r058123.1015.1.test [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Bergeron J. J. (1970). Turnover of mammalian phospholipids. Stable and unstable components in neoplastic mast cells. Biochem. J. 119 473–480. 10.1042/bj1190473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D., Mehendale N., Singh S., Mallik R., Kamat S. S. (2018). Lipidomics suggests a new role for ceramide synthase in phagocytosis. ACS Chem. Biol. 13 2280–2287. 10.1021/acschembio.8b00438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanadham S., Ali T., Ashley J. W., Bone R. N., Hancock W. D., Lei X. (2015). Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J. Lipid Res. 56 1643–1668. 10.1194/jlr.r058701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio J. M., Rodríguez J. P., Gil-de-Gómez L., Guijas C., Balboa M. A., Balsinde J. (2015). Group V secreted phospholipase A2 Is upregulated by IL-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids. J. Immunol. 194 3327–3339. 10.4049/jimmunol.1401026 [DOI] [PubMed] [Google Scholar]
- Schneider B. E., Behrends J., Hagens K., Harmel N., Shayman J. A., Schaible U. E. (2014). Lysosomal phospholipase A2: a novel player in host immunity to Mycobacterium tuberculosis. Eur. J. Immunol. 44 2394–2404. 10.1002/eji.201344383 [DOI] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. (1980). Regulation of arachidonic acid metabolites in macrophages. J. Exp. Med. 152 324–335. 10.1084/jem.152.2.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman J. A., Tesmer J. J. G. (2019). Lysosomal phospholipase A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman J. A., Kelly R., Kollmeyer J., He Y., Abe A. (2011). Group XV phospholipase A2, a lysosomal phospholipase A2. Prog. Lipid Res. 50 1–13. 10.1016/j.plipres.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y., Balsinde J., Dennis E. A. (2005). Localization and functional interrelationships among cytosolic Group IV, secreted Group V, and Ca2+-independent Group VI phospholipase A2s in P388D1 macrophages using GFP/RFP constructs. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1735 119–129. 10.1016/j.bbalip.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Singh R. K., Ethayathulla A. S., Jabeen T., Sharma S., Kaur P., Singh T. P. (2005). Aspirin induces its anti-inflammatory effects through its specific binding to phospholipase A2: crystal structure of the complex formed between phospholipase A2 and aspirin at 1.9 Å resolution. J. Drug Target. 13 113–119. 10.1080/10611860400024078 [DOI] [PubMed] [Google Scholar]
- Sorokina E. M., Feinstein S. I., Milovanova T. N., Fisher A. B. (2009). Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 297 L871–L880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini D. M. (2009). Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc. Drugs Ther. 23 73–83. 10.1007/s10557-008-6133-8 [DOI] [PubMed] [Google Scholar]
- Su H., McClarty G., Dong F., Hatch G. M., Pan Z. K., Zhong G. (2004). Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for chlamydial acquisition of host glycerophospholipids. J. Biol. Chem. 279 9409–9416. 10.1074/jbc.m312008200 [DOI] [PubMed] [Google Scholar]
- Sun X., Xu M., Cao Q., Huang P., Zhu X., Dong X.-P. (2020). A lysosomal K+ channel regulates large particle phagocytosis by facilitating lysosome Ca2+ release. Sci. Rep. 10:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay H. K., Melendez A. J. (2004). FcγRI-triggered generation of arachidonic acid and eicosanoids requires iPLA2 but Not cPLA2 in human monocytic cells. J. Biol. Chem. 279 22505–22513. 10.1074/jbc.m308788200 [DOI] [PubMed] [Google Scholar]
- Uribe-Querol E., Rosales C. (2020). Phagocytosis: our current understanding of a universal biological process. Front. Immun 11:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Dodia C., Weng L., Mesaros C., Blair I. A., Feinstein S. I., et al. (2016). The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. Faseb. J. 30 2885–2898. 10.1096/fj.201500146r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcano M., Dusi S., Lissandrini D., Badolato R., Mazzi P., Riboldi E., et al. (2004). Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J. Immunol. 173 5749–5756. 10.4049/jimmunol.173.9.5749 [DOI] [PubMed] [Google Scholar]
- Waite M., DeChatelet L. R., King L., Shirley P. S. (1979). Phagocytosis-induced release of arachidonic acid from human neutrophils. Biochem. Biophys. Res. Commun. 90 984–992. 10.1016/0006-291x(79)91924-7 [DOI] [PubMed] [Google Scholar]
- Wang L.-L., Chen X.-F., Hu P., Lu S.-Y., Fu B.-Q., Li Y.-S., et al. (2019). Host Prdx6 contributing to the intracellular survival of Brucella suis S2 strain. BMC Vet. Res. 15:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. (1981). The selective release of phospholipase A2 by resident mouse peritoneal macrophages. Biochem. J. 200 441–444. 10.1042/bj2000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead M. V., Balsinde J., Dennis E. A. (2000). Calcium-independent phospholipase A(2): structure and function. Biochim. Biophys. Acta. 1488 28–39. [DOI] [PubMed] [Google Scholar]
- Yin H., Xu L., Porter N. A. (2011). Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111 5944–5972. 10.1021/cr200084z [DOI] [PubMed] [Google Scholar]
- Zhou H., Das S., Murthy K. S. (2003). Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 284 G472–G480. [DOI] [PubMed] [Google Scholar]
- Zizza P., Iurisci C., Bonazzi M., Cossart P., Leslie C. C., Corda D., et al. (2012). Phospholipase A2IVα regulates phagocytosis independent of its enzymatic activity. J. Biol. Chem. 287 16849–16859. 10.1074/jbc.m111.309419 [DOI] [PMC free article] [PubMed] [Google Scholar]