Abstract
Introduction
Common mental disorders (CMDs) particularly depression and anxiety, are highly comorbid with HIV also in young people living with HIV (YLWH). In sub‐Saharan Africa (SSA) where most YLWH reside, there are limited summary data on CMDs among these youths, yet there are previous systematic reviews summarizing data on CMDs among adults living with HIV. We conducted a systematic literature review on the prevalence and correlates of CMDs among YLWH, aged 10 to 24 years, from SSA.
Methods
We searched African Index Medicus, African Journals Online and five other electronic databases (from database inception up to 31 December 2020) for relevant studies published in English. The key search terms applied were as follows: “Depression OR Anxiety”, “Young people”, “HIV infections” and “sub‐Saharan Africa”.
Results and discussion
Out of 3989 articles, 31 studies were included in the review. The prevalence of CMDs in YLWH widely varied ranging between 16.0% and 40.8% for major depression, 4.4% and 52.6% for depressive symptoms and 2.2% and 25.0% for anxiety symptoms. Anxiety disorder was estimated at 45.6%. Four of the five included studies with a comparison group of HIV‐negative young people reported significantly higher prevalence estimates of depressive disorders among YLWH. Several sociodemographic, psychosocial and HIV‐related correlates of CMDs were reported but most lacked consensus across studies. Nevertheless, female sex, older age, fewer schooling years, HIV‐positive status, bullying, sexual abuse, HIV‐related stigma, social support and poor antiretroviral therapy adherence were frequently reported (in ≥2 studies) as significant correlates of depressive symptoms among YLWH. Higher social support was the only frequent significant correlate of anxiety symptoms.
Conclusions
The burden of CMDs among YLWH from SSA is substantial and appears to be significantly higher when compared with HIV‐negative peers, particularly for depressive disorders. However, more comparative research is needed. Importantly, screening for CMDs at the youth HIV‐clinics should be prioritized especially for YLWH at high risk of CMDs, to facilitate early management or referral for treatment. Furthermore, youth‐friendly psychological interventions addressing CMDs in YLWH should urgently be piloted in SSA, incorporating contextual components that may directly or indirectly reduce symptoms of CMDs among YLWH, such as social support.
Keywords: young people, HIV infections, depression, anxiety, correlates, sub‐Saharan Africa
1. Introduction
Globally, there are over 1.8 billion young people aged 10 to 24 years, the majority residing in low‐ and middle‐income countries [1]. The term “young people” generally combines the overlapping terms of “adolescents,” that is individuals in the 10‐ to 19‐year age group and “youths,” that is the 15‐ to 24‐year age group [2] and will be used to refer to both age groups in this work. Young people represent a significant proportion of people living with HIV. As of 2018, 1.6 million young people aged 10 to 19 years were living with HIV globally [3], whereas 3.9 million young people aged 15 to 24 years lived with HIV worldwide by 2014 [4]. Most of these young people were infected through vertical transmission and live in sub‐Saharan Africa (SSA) [5, 6].
Common mental disorders (CMDs), herein referring to depressive and anxiety disorders or their symptoms, are very frequent in people living with HIV [7, 8, 9] and the risk is two to three times higher than the general population [10]. Among young people living with HIV (YLWH), global reviews [5, 11, 12] report the prevalence of comorbid CMDs as high as 44.0% for depressive disorders and 48.2% for anxiety disorders. In recent reviews on the burden of psychiatric disorders among YLWH aged 10 to 19 years in SSA [13, 14], depressive and anxiety symptoms ranged from 14% to 53% and 15% to 25% respectively. The burden of CMDs may also be higher in YLWH than their peers without HIV [15] or even other vulnerable groups of young people, such as those in juvenile detention [16]. The higher risk of CMDs in people living with HIV, including the youth, may be caused by side effects of antiretroviral therapy (ART) [17, 18], persistent HIV‐related stigma in the community [19, 20], the direct and indirect neurologic effects of HIV on the brain [21] and the fear of premature death [22]. There are detrimental consequences when CMDs co‐occur with HIV including worsened prognosis of HIV infection [23], increased risk of suicidality [24], non‐adherence to ART [25], poor quality of life [26] and alteration of economic productivity of people living with HIV [23].
Previous global reviews [5, 11, 12] and one recent review from SSA [13] have reported on factors associated with CMDs among YLWH, but there is little or no consensus across individual studies included in these reviews. Notably, most of the studies included in the global reviews have been conducted in Western countries (Europe and North America). Only four out of the 14 studies included in the review from SSA reported a few correlates of CMDs. Nevertheless, female sex [16, 27], older age [28, 29, 30], poor adherence to ART [31, 32], stressful life events [33, 34], parental or caregiver mental health status [15, 27, 35], maternal HIV‐positive status [15, 29], low cluster of differentiation‐4 (CD4) cell count [27, 36] and history of AIDS‐defining illness [36, 37] appear important correlates of CMDs in YLWH as they are reported by more than one study in the aforementioned reviews. Extrapolating findings from especially reviews of global nature to inform interventions seeking to address the mental health of YLWH from settings such as SSA may be problematic because of contextual differences (especially where very few of the studies included are from the setting targeted for intervention). With the increasing research effort towards an understanding of the mental health of people living with HIV from SSA [38], including the youth [39, 40, 41], there is a need for a greater understanding of context‐specific factors associated with CMDs among people living with HIV from this setting.
The increasing mental health issues among YLWH is an emerging public health concern with the potential of burdening the already busy healthcare systems and the scarce human resources in mental healthcare in resource‐limited settings like those of SSA [7, 23]. Hitherto, no study has extensively summarized data on the burden and contextual determinants of CMDs among YLWH from SSA, a region where most of these HIV‐positive young people reside. While there have been several global reviews trying to understand the burden of mental health problems among YLWH [5, 11, 12], most of the studies included have been conducted outside SSA, limiting the generalizability of their findings to the African context. Past systematic reviews on the burden of CMDs among people living with HIV from SSA [9, 38, 42] have only considered studies recruiting adults living with HIV sidelining YLWH who currently represent a considerable percentage of people living with HIV. The recent systematic reviews involving YLWH from SSA [13, 14] are limited to young people aged 10 to 19 years only and broadly focus on many psychiatric disorders. For the current systematic review, the overall objective was to summarize the available evidence on the prevalence and factors associated with CMDs among YLWH aged 10 to 24 years from SSA. The specific objectives of this review were:
To systematically summarize the existing literature on the prevalence of CMDs, specifically depressive and anxiety disorders, or their symptoms, among YLWH aged 10 to 24 years from SSA alongside information on measurement tools used and their contextual reliability and/or validity.
To systematically identify the factors associated with CMDs, specifically depressive and anxiety disorders or their symptoms, among YLWH aged 10 to 24 years from SSA.
The added value of this review is two‐fold. First, by extending the review age range, we include young people regarded as adults (societally and/or by law) who are expected to take care of themselves, most often outside the family context, with implications for an arrangement of mental health support. Second, although the recent reviews from SSA give important information on the prevalence and range of mental disorders in YLWH, the review by Olashore et al. [14] only addresses the association of these disorders with ART adherence. In both reviews [13, 14], their approach limits the possibility to understand which contextual factors importantly relate to CMDs, the most frequent of the psychiatric disorders. This review addresses this gap.
2. Methods
2.1. Search strategy
The Preferred Reporting Items for Systematic Review and Meta‐analysis (PRISMA) guidelines [43] informed the design and reporting of this systematic review. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42020160806. Structured electronic searches were initially conducted in African Index Medicus, African Journals Online, Google Scholar, PsycArticles, PsycInfo, PubMed and Web of Science Core collection databases between 3 and 24 December 2019. The search was later updated in January 2021 to capture publications up to 31st December 2020. Where applicable, databases were searched from the time of their inception. The key search terms included “Depression/Anxiety”, “Young people”, “HIV infections” and “sub‐Saharan Africa” which were combined by the Boolean operator “AND”. Synonyms for each of the key search terms were combined using the “OR” Boolean operator. Where applicable, Medical Subject Headings (MeSH) terms were used. (Additional file S1) provides the search string used in the PubMed database, which was modified to meet the specifications for other databases.
The search was restricted to studies published in the English language, where a database could allow this filter. All the retrieved references were exported and managed in EndNote version 7. An additional search for relevant articles was conducted by scanning the reference lists of included articles and any relevant systematic reviews captured in the initial search.
2.2. Screening of articles by inclusion and exclusion criteria
For potential inclusion, all the articles returned from the database searches were screened in two steps: i) based on title and abstract and ii) by full text. The first author (ET) screened the articles for eligibility. To reduce bias that may arise from human error, two other reviewers (CN and MKN) independently repeated 10% of the study screening at every stage through a systematic random selection of articles divided into two halves, each for the independent reviewers. Rates of agreement between each of the independent reviewers and the first reviewer were consistently high, and minor disagreements were settled through consensus. To be included for review, studies had to fulfil a pre‐determined inclusion and exclusion criteria as shown in Table 1.
Table 1.
Study selection criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Population | |
|
‐ Studies with HIV‐positive young people from SSA. ‐ Studies with participants aged 10 to 24 years or with mean/median age within this age bracket. |
‐ Studies involving HIV‐positive young people outside SSA. ‐ Studies involving HIV‐negative young people or with HIV‐positive participants outside the 10 to 24 age range. ‐ Studies with unspecified age range, mean or median age of participants. ‐ Studies with very specific subpopulations of young people e.g. pregnant women, out‐of‐school adolescents et cetera. |
| Outcome | |
|
‐ Studies on depression or depressive symptoms and associated factors. ‐ Studies on anxiety disorder or anxiety symptoms and associated factors. |
‐ Studies on mental disorders other than depression or anxiety, or their symptoms. ‐ Studies using measurement scales evaluating both anxiety and depression without providing separate data. ‐ Studies where reported measurement scales do not evaluate depression or anxiety, or their symptoms. |
| Study designs | |
|
‐ Cross‐sectional studies ‐ Case‐control studies ‐ Cohort studies |
‐ Review articles e.g. narrative, systematic, scoping ‐ Intervention studies ‐ Case studies/reviews ‐ Commentaries or editorials ‐ Conference proceedings, symposia abstracts, or workshop publications ‐ Qualitative studies ‐ Books or book sections ‐ Theses and dissertations |
Studies with a comparison group of HIV‐negative young people and providing disaggregated mental health data by HIV infection status were included. Studies duplicating similar project data to an already included main and more comprehensive article were excluded. SSA, sub‐Saharan Africa.
2.3. Data extraction
ET and MKN independently extracted the following data from included studies using a standardized Microsoft Excel data abstraction form: (i) Article details – name of the first author, title and publication year; (ii) Study details – country of origin, study design, study setting, data collection period and response rate (where reported) and the reported study limitations; (iii) Study participant characteristics – HIV‐related data (mode of acquisition, time since diagnosis, ART regimen and duration on ART treatment), sample size, sampling method, source of the sample, age (mean, median, or range) and sex (proportion of male vs. female); (iv) Outcome and measures – prevalence of depression and anxiety, or their symptoms, measurement tool used, cut off score applied (for screening tools), information on local tool validation, factors associated with depression and anxiety or their symptoms (alongside the reported measure of effect and precision estimate).
2.4. Quality assessment
ET and MKN independently assessed the risk of bias of each included study using the Newcastle–Ottawa Scale [44]. These authors then held a meeting to resolve any disagreements in quality rating. Using this tool, studies were assessed based on three domains: the selection of participants, comparability of study groups and the ascertainment of exposure (for case–control studies) or outcome of interest (for cohort and cross‐sectional studies). A star‐grading system was used, with each domain item receiving one or two stars if appropriate methods were reported. A maximum of nine stars was awarded for cohort and case–control studies, and a maximum of 10 stars for cross‐sectional studies. Studies were classified as unsatisfactory, satisfactory, good and very good if they had a total of 0 to 4, 5 to 6, 7 to 8 and 9 to 10 stars respectively.
2.5. Data analysis
Because of the heterogeneous nature of measurement tools used across included studies, data were narratively summarized. Data on the prevalence of CMDs among YLWH were summarized by each homogeneous measurement tool used, whereas data on correlates of CMDs were summarized by the investigated outcome (depression or anxiety). We manually calculated the odds ratio using the reported proportions from individual studies comparing the prevalence of CMDs between YLWH and their HIV‐negative peers. In this review, only factors significantly associated with either depression or anxiety, or their symptoms at p < 0.05 in the multivariable analysis were considered and extracted as correlates. Basic descriptive statistics (frequencies with percentages) were used to summarize data on the region of SSA where each of the included studies was conducted.
3. Results and Discussion
The electronic search yielded 3988 hits from the different databases (African Index Medicus, n = 11, African Journals Online, n = 147, Google Scholar, n = 2137, PsycArticles, n = 8, PsycInfo, n = 865, PubMed, n = 302 and Web of Science, n = 518). A scan of the reference lists of included articles and relevant systematic reviews captured by the search yielded one additional article. After removing duplicates and screening articles based on the eligibility criteria, we included 31 studies in the review. Figure 1 shows the PRISMA flowchart for the systematic review process. CMDs, common mental disorders.
Figure 1.
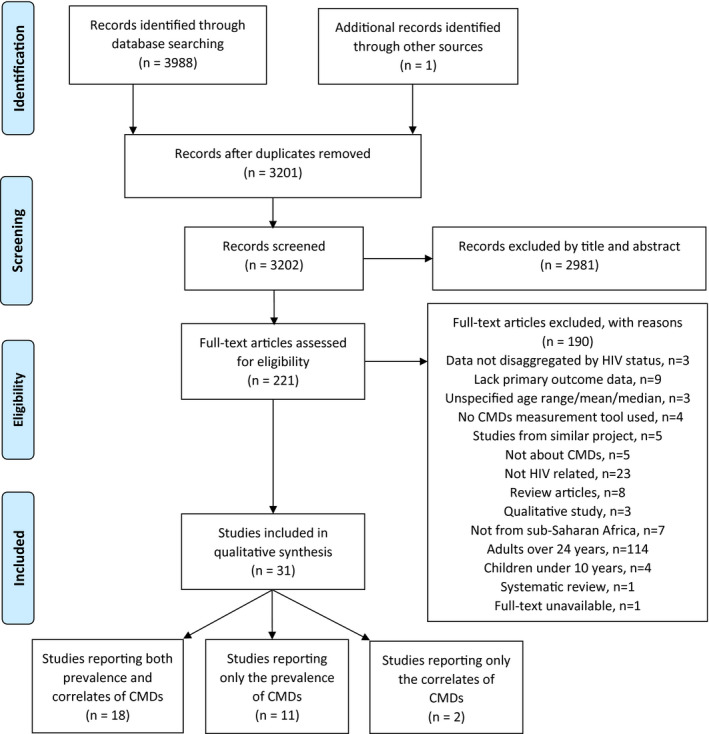
PRISMA flowchart for the systematic review process. CMDs, common mental disorders.
3.1. Characteristics of included studies
(Additional file S2) presents in detail the characteristics of the 31 included studies. The reviewed studies were mostly conducted in Eastern (n = 13; 41.9%) or Southern (n = 13; 41.9%) African countries except four studies [45, 46, 47, 48] that were conducted in a Western African country (12.9%) and one study (3.2%) from Central Africa [49]. The included studies enrolled a total of 9935 YLWH (individual study sample size ranging from 58 to 1088). Additionally, the studies with a comparison group enrolled 1000 HIV‐negative young people (individual study sample size ranging from 44 to 600).
Many of the studies (n = 29) were cross‐sectional in design and published after 2010, except two studies [47, 50]. A review of the literature on depression among HIV‐positive adults from SSA [38] also observed that most of the studies included were conducted after 2010. It is, therefore, encouraging to note that from the beginning of the last decade, there is an upsurge of research work towards an understanding of the mental health of people living with HIV in Africa with the potential to inform clinical practice and policy.
Many of the studies were conducted in urban settings of SSA (n = 20). Nearly all studies (n = 29) recruited YLWH from HIV‐specialized clinics except one study [51] that recruited from the community. In one study [52], this information could not be retrieved because the study was only available in abstract form (see study quality section for details). YLWH were exclusively on ART in most studies (n = 17). Eight studies [46, 53, 54, 55, 56, 57, 58, 59] recruited ART experienced (majority) and ART naïve participants, whereas six studies [45, 48, 60, 61, 62, 63] did not provide information on participant ART‐use status. In eight studies [52, 55, 59, 61, 64, 65, 66, 67] YLWH were exclusively perinatally HIV‐infected. Five studies [45, 49, 58, 68, 69] had a mixture of perinatally and behaviourally HIV‐acquired youths; the rest (18 studies) did not provide information on the mode of HIV infection. Outside Africa, certain characteristics of YLWH like mode of HIV infection (behavioural vs. perinatal) [8, 70] and ART‐use status (ART naïve vs. ART experienced) [38] may influence their mental health experiences. In this review, none of the studies recruiting a mix of participants provided disaggregated mental health data by any of these characteristics. An in‐depth investigation of this nature in the African context will require researchers working with a mixed sample of YLWH to collect and profile disaggregated mental health data by, for example mode of HIV infection or ART‐use status. Additional data on CMDs among YLWH residing in rural settings of SSA are also needed, so far, research focus has been biased towards urban settings.
3.2. Measurement tools for CMDs, their reliability and validity among YLWH from SSA
CMDs in YLWH from SSA were assessed using both diagnostic tools and symptom screeners. Diagnostic tools used in this study included the Mini‐International Neuropsychiatric Interview for children and adolescents (MINI‐KID) [71] used in three studies [45, 46, 64] to diagnose major depression and the tenth revision of the International Classification of Diseases (ICD‐10) symptom checklist [72] used by Musisi and Kinyanda [50] to diagnose major depression and anxiety disorder. The other studies used different types of CMD symptom screeners. Kinyanda et al. [55] used the 5th edition of the Diagnostic and Statistical Manual (DSM) of mental disorders referenced Child and Adolescent Symptom Inventory‐5 (CASI‐5) [73] and the fourth revision of the Youth Inventory (YI‐4R) [74] to assess symptoms of major depression, any anxiety disorder, generalized anxiety disorder, social and separation anxiety disorders. Buckley et al. [65] used the DSM (4th edition) referenced 84‐item Patient Health Questionnaire for Adolescents (PHQ‐A) [75] to assess symptoms of major depression and anxiety disorder (specifically panic disorder). Various screening tools based on different cut‐off scores were also used to measure depressive (Table 2) or anxiety symptoms (Table 3). These screening tools included the 9‐item patient health questionnaire [76] used in six studies [49, 53, 57, 60, 77, 78], the centre for epidemiologic studies depression scale [79] used in four studies [51, 54, 56, 62], the child depression inventory [80] used in four studies [58, 59, 81, 82], Beck’s depression inventory [83] used in three studies [48, 61, 84], the revised children’s depression rating scale [85] used by Kim et al. [28], the hospital anxiety and depression scale [86] used by Sale & Gadanya [47], Reynold’s adolescent depression Scale [87] used by Paul et al. [63], National Institute of Health toolbox – Sadness module [88] used by Molinaro et al. [52], the Beck’s youth inventory [89] used in two studies [66, 67] and the revised children’s manifest anxiety scale [90] used in two studies [58, 59]. Most studies did not report information on the reliability and/or validity of these measurement tools among YLWH. In some studies, where this information was not provided, authors pointed out that the tool they used was previously validated in the study country or provided a reference to the tool validation process (see Tables 2 and 3). Where reported, information on tool reliability and/or validity was mostly limited to Cronbach’s alpha, a measure of internal consistency of a scale, and values were above the acceptable threshold of 0.7.
Table 2.
Prevalence estimates for major depression and depressive symptoms among YLWH from SSA according to the measurement tool used
| Author, year | Country | Outcome of interest | Sample size (n) | Assessment tool used | Cut‐off score | Information on local tool validation | Prevalence estimates |
|---|---|---|---|---|---|---|---|
| The mini‐international neuropsychiatric interview for children and adolescents (MINI‐KID) | |||||||
| Adeyemo et al., 2020 [45] | Nigeria | Major Depression | 201 | MINI‐KID | NA | – |
16.9% |
| Ashaba et al., 2018 [64] | Uganda | Major Depression | 224 | MINI‐KID | NA | – | 16.0% |
| Ashaba et al. [46] | Nigeria | Major Depression |
75 HIV+ 75 HIV− |
MINI‐KID | NA | – |
20.0% among HIV+ 6.7% among HIV− p = 0.01 |
| International classification of diseases, tenth edition (ICD‐10) symptom checklist | |||||||
| Musisi & Kinyanda, 2009 [50] | Uganda | Major Depression | 82 | ICD‐10 | NA | – | 40.8% |
| The youth inventory fourth revision (YI‐4R) and the child and adolescent symptom inventory‐5 (CASI‐5) | |||||||
| Kinyanda et al., 2019 [55] | Uganda | Symptoms of Major Depression | 479 |
YI‐4R CASI‐5 |
NR |
Cronbach alpha of 0.88 and test–retest reliability of 0.2, p < 0.01 Cronbach alpha of 0.77 and test–retest reliability of 0.17, p < 0.01 |
5.2% |
| The patient health questionnaire for adolescents (PHQ‐A) | |||||||
| Buckley et al., 2020 [65] | South Africa | Symptoms of Major Depression |
81 HIV+ 81 HIV− |
PHQ‐A | NR | NR |
6.2% among HIV+ 7.4% among HIV− p = 0.99 |
| The 9‐item patient health questionnaire (PHQ‐9) | |||||||
| Dow et al., 2016 [53] | Tanzania | Depressive symptoms | 182 | PHQ‐9 | ≥10 | NR | 12.1% |
| Dyer et al., 2020 [60] | Kenya | Depressive symptoms | 479 | PHQ‐9 | ≥5 | NR | 10.0% |
| Ekat et al., 2020 [49] | DRC | Depressive symptoms | 135 | PHQ‐9 | ≥9 | NR | 38.5% |
| Gaitho et al., 2018 [77] | Kenya | Depressive symptoms | 270 | PHQ‐9 | ≥1 | NR | 52.6% |
| Haas et al., 2020 [78] | South Africa | Depressive symptoms | 1088 | PHQ‐9 | ≥10 | NR | 4.4% |
| Ramos et al., 2018 [57] | Tanzania | Depressive symptoms | 280 | PHQ‐9 | ≥10 | NR | 20.4% |
| The centre for epidemiologic studies depression scale (CES‐D) | |||||||
| Fawzi et al., 2016 [51] | Rwanda | Depressive symptoms | 193 | CES‐D | ≥30 | Not provided in this study. However, the authors note that they used a CES‐D previously validated in Rwanda | 26.0% |
| Filiatreau et al., 2020 [62] | South Africa | Depressive symptoms | 334 | CES‐D | ≥16 | Cronbach alpha of 0.76 | 27.5% |
| Kemigisha et al., 2019 [54] | Uganda | Depressive symptoms | 336 | CES‐D | ≥15 | Cronbach alpha of 0.85 | 45.8% |
| Okawa et al., 2018 [56] | Zambia | Depressive symptoms | 190 | CES‐D (10‐item) | ≥10 | Cronbach alpha of 0.74 | 25.3% |
| The child depression inventory | |||||||
| Lwidiko et al., 2018 [82] | Tanzania | Depressive symptoms |
300 HIV+ 600 HIV− |
CDI‐II | ≥12 | Cronbach alpha of 0.7 |
27.0% among HIV+ 5.8% among HIV− p < 0.001 |
| Cavazos‐Rehg et al., 2020 [81] | Uganda | Depressive symptoms | 675 | CDI‐S (Short form) | ≥3 | Authors claim to have used culturally adapted tools | 50.3% |
| West et al., 2018 [58] | South Africa | Depressive symptoms | 278 | CDI‐S (Short form) | ≥7 | Not provided in this study, but authors note that the tool was previously validated in South Africa | 7.6% |
| Woollett et al., 2017 [59] | South Africa | Depressive symptoms | 343 | CDI‐S (Short form) | ≥10 | Not provided in this study, but authors say they used measures previously validated among youth in South Africa (Cronbach alpha >0.70) | 14.0% |
| Beck’s depression inventory‐II (BDI‐II) | |||||||
| Abebe et al., 2019 [84] | Ethiopia | Depressive symptoms | 507 | BDI‐II | ≥21 | NR | 35.5% |
| Earnshaw et al., 2018 [61] | South Africa | Depressive symptoms | 250 | BDI‐II | ≥20 | Cronbach alpha of 0.9 | 33.8% |
| Yarhere & Jaja, 2020 [48] | Nigeria | Depressive symptoms | 58 | BDI‐II | ≥11 | NR | 44.8% |
| Beck’s youth inventory‐II (BYI‐II) | |||||||
| Hoare et al., 2019 [66] | South Africa | Depressive symptoms |
204 HIV+ 44 HIV− |
BYI‐II (Depression inventory) | NR | NR |
6.4% among HIV+ 2.3% among HIV− p < 0.01 |
| Kikuchi et al., 2017 [67] | Rwanda | Depressive symptoms | 475 | BYI‐II (Depression inventory) | >55 | Cronbach alpha of 0.84 | 22.1% |
| The revised children’s depression rating scale (CDRS‐R) | |||||||
| Kim et al., 2015 [28] | Malawi | Depressive symptoms | 562 | CDRS‐R | ≥55 | Not provided in this study. Authors provide a reference for information on tool validation | 18.9% |
| The hospital anxiety and depression scale (HADS) | |||||||
| Sale & Gadanya, 2008 [47] | Nigeria | Depressive symptoms | 162 | HADS (Depression scale) | ≥8 | NR | 39.5% |
| Reynolds adolescent depression scale‐second edition (RADS‐2) | |||||||
| Paul et al., 2015 [63] | Zambia | Depressive symptoms | 100 | RADS‐2 | ≥76 | NR | 19.0% |
| NIH toolbox sadness module | |||||||
| Molinaro et al., 2019 † [52] | Zambia | Depressive symptoms |
200 HIV+ 200 HIV− |
NIH | ≥60 | NE |
24.0% among HIV+ 13.0% among HIV− p = 0.03 |
This work was only available as a published abstract from an annual meeting with prevalence data within the abstract. DRC, Democratic Republic of Congo; NA, Not Applicable; NE, Not Extracted; NR, Not Reported.
Table 3.
Prevalence estimates for anxiety disorder or its symptoms among YLWH from SSA according to the measurement tool used
| Author, year | Country | Outcome of interest | Sample size | Assessment tool used | Cut‐off score | Information on local tool validation | Prevalence estimates |
|---|---|---|---|---|---|---|---|
| International classification of diseases, tenth edition (ICD‐10) symptom checklist | |||||||
| Musisi & Kinyanda, 2009 [50] | Uganda | Anxiety disorder | 82 | ICD‐10 | NA | – | 45.6% |
| The patient health questionnaire for adolescents (PHQ‐A) | |||||||
| Buckley et al., 2020 [65] | South Africa | Anxiety disorder symptoms (Panic disorder) |
81 HIV+ 81 HIV− |
PHQ‐A | NR | NR |
3.7% among HIV+ 2.5% among HIV− p = 0.99 |
| The revised children’s manifest anxiety scale (RCMAS) | |||||||
| West et al., 2018 [58] | South Africa | Anxiety symptoms | 278 | 14‐item RCMAS | ≥10 | Not provided in this study, but authors say tools were previously validated in South Africa | 6.7% |
| Woollet et al., 2017 [59] | South Africa | Anxiety symptoms | 343 | 28‐item RCMAS | NR | Not provided in this study, but authors say they used measures previously used with youth in South Africa (Cronbach alpha >0.75) | 25.0% |
| The youth inventory fouth revision (YI‐4R) and the child and adolescent symptom inventory‐5 (CASI‐5) | |||||||
| Kinyanda et al., 2019 [55] | Uganda |
Symptoms of: ‐Any anxiety disorder ‐GAD ‐SAD ‐SEAD |
479 |
YI‐4R CASI‐5 |
NR |
Cronbach alpha of 0.88 and test–retest reliability of 0.2, p < 0.01 Cronbach alpha of 0.77 and test–retest reliability of 0.17, p < 0.01 |
14.7% for Any anxiety disorder 7.2% for GAD 7.0% for SAD 5.4% for SEAD |
| The 7‐item generalized anxiety disorder scale (GAD‐7) | |||||||
| Haas et al., 2020 [78] | South Africa | Anxiety symptoms | 1088 | GAD‐7 | ≥10 | NR | 2.2% |
| Beck’s youth inventory‐II (BYI‐II) | |||||||
| Hoare et al., 2019 [66] | South Africa | Anxiety symptoms |
204 HIV+ 44 HIV− |
BYI‐II (Anxiety inventory) | NR | NR |
11.8% among HIV+ 9.1% among HIV− p = 0.61 |
GAD, Generalized Anxiety Disorder; NA, not applicable; NR, not reported; SAD, Social Anxiety Disorder; SEAD, Separation Anxiety Disorder.
For any meaningful epidemiological data that can inform appropriate interventions, there is a need for future studies involving YLWH in SSA to measure CMDs using culturally appropriate and locally validated tools. Where feasible, validated mental health diagnostic measures should be administered concurrently to check the diagnostic accuracy of these mental health screening tools.
3.3. Prevalence of CMDs among YLWH from SSA
Twenty‐nine studies reported the prevalence of either major depression or depressive symptoms [45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 77, 78, 81]. Of these, seven studies [50, 55, 58, 59, 65, 66, 78] additionally reported the prevalence of anxiety or its symptoms. No study investigated the prevalence of anxiety or its symptoms as a stand‐alone mental disorder among YLWH. Tables 2 and 3 present the prevalence of depressive and anxiety disorders (or their symptoms according to the screening tool used), as reported from the above studies.
In summary, wide‐ranging prevalence estimates of CMDs were reported among YLWH. The prevalence of major depression ranged between 16.0% and 40.8% [45, 46, 50, 64]. A prevalence of 5.2% for symptoms of major depression was reported by a Ugandan study [55]. When comparing YLWH and their HIV‐negative peers [65], the observed prevalence of symptoms of major depression was 6.2% and 7.4% respectively. Regardless of the screening tool used, depressive symptoms ranged between 4.4% and 52.6%. The prevalence of anxiety disorder among YLWH from Uganda was 45.6% [50]. When comparing YLWH and their HIV‐negative peers [65], the observed prevalence of symptoms of panic disorder was 3.7% and 2.5% respectively. Regardless of the screening tool used, anxiety symptoms ranged between 2.2% and 25.0%. Wide‐ranging CMD prevalence estimates have been documented in past reviews involving YLWH [11, 12, 91] but also adults living with HIV in SSA [9, 38]. Differences in study context and population (like the conceptualization of mental health issues, exposure levels to triggers of mental health problems), study respondents (self vs. others) and use of heterogenous measurement tools (including different cut‐off scores for similar measures) may contribute to the wide variation of the reported prevalence estimates. As a start point for possible quantification of the magnitude of CMDs among YLWH in SSA, researchers, perhaps from similar regions, should work towards the use of homogenous mental health measurement tools.
Compared to HIV‐negative peers, YLWH from SSA appear to be experiencing higher CMDs, particularly depressive disorders. However, the evidence is limited to draw any conclusions. Only five studies in this review [46, 52, 65, 66, 82] compared CMDs between YLWH and their HIV‐negative peers. Four of these studies reported significantly higher prevalence estimates of either major depression [46] or depressive symptoms [52, 66, 82] among YLWH (Table 2). In these studies, YLWH were 3.5‐ [46], 2.9‐ [66], 6.0‐ [82] and 2.1‐times [52] more likely to have higher depression compared to their HIV‐negative peers. Even though the odds of symptoms of major depression were 17% less likely among YLWH compared to HIV‐negative peers in the fifth study [65], there was no significant between‐group difference (Table 2). In the literature, individual empirical studies from other settings comparing for instance depressive symptoms among YLWH and their HIV‐negative peers report mixed results. Some observe significant group differences [92], whereas others observe insignificant differences [27, 93].
For anxiety, two of the five studies above [65, 66] also compared the prevalence of anxiety symptoms between YLWH and their HIV‐negative peers and reported slightly higher, but statistically insignificant, prevalence estimates among YLWH (Table 3). In these studies, even though insignificant, the odds of anxiety symptoms were 1.5 [65] and 1.3 times [66] higher in YLWH. In contrast, a study from Italy found significantly higher anxiety scores among YLWH compared to their HIV‐negative peers [92]. For a clearer insight as to whether YLWH from SSA are at an elevated risk of CMDs compared to their HIV‐negative peers, there is a need for more comparative research.
Despite the observed wide‐ranging prevalence estimates, this review generally shows that the burden of CMDs among YLWH from SSA is high, and that rates may be two to six times higher when compared with HIV‐negative youths, particularly for depressive disorders. However, caution must be taken when interpreting the reported prevalence estimates. Only four studies [45, 46, 50, 64] used a mental health diagnostic interview based on either DSM or ICD criteria. Two studies [55, 65] used DSM‐referenced checklists to assess symptoms of major depression and an anxiety disorder. The rest collected mental health data of YLWH using screening tools, some with unknown reliability and/or validity. Most importantly, screening and early management or referral for treatment of CMDs among YLWH from SSA are urgently needed at the HIV clinics servicing these youths, more so because CMDs co‐occurring with HIV are associated with worse HIV outcomes [23, 25].
Overall, fewer studies in this review focussed on anxiety compared to depression. This under‐investigation of anxiety disorders is of concern because previous research involving YLWH report higher rates of anxiety than depression [94]. Partly, the under‐investigation could be due to the paucity of adequately validated measurement tools of anxiety [95]. To allow more research focus on anxiety among YLWH in the African context, as has depression, there is a need for adequate validation of measurement tools for anxiety, taking into consideration contextual and cultural differences within the SSA setting.
3.4. Correlates of CMDs among YLWH from SSA
To the best of our knowledge, this is the first review from SSA to comprehensively collate information about factors associated with CMDs in YLWH. Recent reviews from SSA involving YLWH provide an overview of some of these factors because of broadly covering multiple psychiatric disorders [13, 14], focussing on only one correlate – ART adherence [14] or including HIV‐positive young people up to 19 years only [13, 14]. In this review, 19 studies reported the correlates of either major depression [64], symptoms of major depression [65] or depressive symptoms among YLWH [28, 48, 49, 53, 54, 56, 58, 61, 62, 66, 67, 69, 77, 78, 81, 82, 84]. Of these, four studies concurrently investigated correlates of anxiety symptoms [58, 66, 69, 78]. One study [68] independently focused on the correlates of anxiety symptoms. There was limited consensus across studies for most of the reported correlates. Generally, the factors reported to be significantly associated with CMDs among YLWH from these studies can be categorized into sociodemographic, psychosocial and HIV‐related clinical correlates and are presented as such in this paper.
3.5. Correlates of major depression or depressive symptoms among YLWH from SSA
Table 4 presents in detail the identified correlates of major depression or depressive symptoms among YLWH as reported from the studies above. In summary, none of the studies reported any sociodemographic correlate of major depression. The sociodemographic factors that significantly increased the risk for higher depressive symptoms among YLWH included: older age [49, 53, 69, 77, 84], female sex [28, 53], fewer schooling years [28, 49], longer distance to the clinic [54], and HIV‐positive status [66, 82]. Similarly, but in the inverse direction, Haas et al. [78] report younger age as significantly lowering the risk for depressive symptoms. There appeared to be a consensus between two or more studies regarding older age, female sex, fewer schooling years and HIV‐positive status as significant sociodemographic correlates of higher depressive symptoms, congruent with results from previous reviews involving YLWH [5, 11, 12]. In contrast, one included study [81] reported older youth age as protective against depressive symptoms and male sex as a risk indicator for higher depressive symptoms. Better overall health [69], residing in rural areas [82], not being in a romantic relationship [28], not failing a term or class [28] and higher height for age z‐scores [67] were the sociodemographic factors that significantly decreased the risk for depressive symptoms. However, there was a lack of consensus across studies for these correlates.
Table 4.
Sociodemographic, psychosocial and HIV‐related correlates of major depression or depressive symptoms among young people living with HIV from SSA
| Author, year | Outcome | Measure of effect (precision) | Sociodemographic correlates | Psychosocial correlates | HIV‐related correlates | |||
|---|---|---|---|---|---|---|---|---|
| Risk indicators | Protective indicators | Risk indicators | Protective indicators | Risk indicators | Protective indicators | |||
| Abebe et al., 2019 [84] | Depressive symptoms | AOR (95% CI) | ‐Older age: 2.20 (1.33 to 3.62) | NR |
‐Low social support: 2.74 (1.42 to 5.27) ‐HIV‐related stigma: 2.06 (1.35 to 3.14) |
NR |
‐Poor ART adherence: 1.73 (1.13 to 2.64) ‐History of opportunistic infections: 1.94 (1.15 to 3.27) |
NR |
| Ashaba et al., 2018 [64] | Major depression | AOR (95% CI) | NR | NR | ‐Bullying: 1.09 (1.00 to 1.20) | NR | NR | NR |
| Boyes et al., 2018 [69] | Depressive symptoms | β (Se) | ‐Older age: 0.07 (0.02) |
‐Better overall health: −0.18 (0.08) |
‐Internalized stigma: 0.29 (0.05) ‐Negative clinic interactions: 0.06 (0.02) ‐Emotional abuse 0.56 (0.14) ‐Sexual abuse: 0.83 (0.25) ‐Bullying victimisation: 0.04 (0.02) |
‐Self‐efficacy: −0.04 (0.02) ‐Higher social support: −0.11 (0.020) ‐Access to a clinic support group: −0.32 (0.10) ‐Positive parenting: −0.04 (0.01) |
‐ART side effects: 0.49 (0.12) | NR |
| Buckley et al., 2020 [65] | Symptoms of Major Depression | AOR (95% CI) | NR |
‐Living with someone with aggression or anger problems: 2.80 (1.05 to 7.44) ‐Ever witnessing violence at home: 4.34 (1.65 to 11.46) |
NR | NR | NR | |
| Cavazos‐Rehg et al., 2020 [81] | Depressive symptoms | AOR (95% CI) | ‐Male sex: 1.62 (1.15 to 2.27) | ‐Older age: 0.87 (0.77 to 0.98) | ‐Grandparent as primary caregiver: 1.83 (1.16 to 2.88) |
‐Higher social support (from friends): 0.96 (0.91 to 0.998) ‐Higher socio‐economic status (additional assets and employment): 0.85 (0.74 to 0.99) ‐Family cohesion: 0.94 (0.91 to 0.96) |
NR | NR |
| Dow et al., 2016 [53] | Depressive symptoms | MR (95% CI) |
‐Older age: 1.08 (1.03 to 1.14) ‐Female sex: 1.52 (1.11 to 2.09) |
NR | ‐HIV‐related stigma: 1.08 (1.04 to 1.11) | NR | ‐Poor ART adherence: 1.52 (1.07 to 2.18) | NR |
| Earnshaw et al., 2018 [61] | Depressive symptoms | ARR (95% CI) | NR |
‐Internalized stigma: 1.23 (1.13 to 1.34) ‐Associative stigma: 1.59 (1.37 to 1.84) |
NR | NR | NR | |
| Ekat et al., 2020 [49] | Depressive symptoms | APR (95% CI) |
‐Older age: 2.07 (1.06 to 4.04) ‐Stopping education: 1.60 (1.06 to 2.42) |
NR | NR | NR | ‐Poor ART adherence: 2.06 (1.23 to 3.45) | NR |
| Filiatreau et al., 2020 [62] | Depressive symptoms | APR (95% CI) | NR | NR |
‐History of physical violence: 2.02 (1.43 to 2.84) ‐History of sexual violence: 2.25 (1.58 to 3.19) ‐History of physical or sexual violence: 2.01 (1.43 to 2.83) ‐History of physical and sexual violence: 3.01 (2.06 to 4.39) |
NR | NR | NR |
| Gaitho et al., 2018 [77] | Depressive symptoms | AOR (95% CI) | ‐Older age: 2.34 (1.40 to 4.00) | NR | NR | NR | ‐Poor ART adherence: 1.84 (1.08 to 3.10) | NR |
| Haas et al., 2020 [78] | Depressive symptoms | AOR (95% CI) | NR |
‐Younger age: 10 to 12 years age group vs. 16 to 19 years age group = 0.05 (0.01 to 0.21) 13 to 15 years age group vs. 16 to 19 years group = 0.18 (0.08 to 0.40) |
‐Conflict in the household: 3.76 (1.97 to 7.17) | NR | NR | NR |
| Hoare et al., 2019 [66] | Depressive symptoms | β (95% CI) | ‐HIV+ status: 5.08 (1.35 to 8.82) | NR |
‐Stressful life events: 0.83 (0.57 to 1.08) ‐HIV‐related stigma: 9.93 (2.88 to 16.98) |
NR | NR | NR |
| Kemigisha et al., 2019 [54] | Depressive symptoms | AOR (95% CI) | ‐Travelling >30 minutes for routine clinic care: 1.66 (1.02 to 2.70) | NR | NR | NR | NR | NR |
| Kikuchi et al., 2017 [67] | Depressive symptoms | AOR (95% CI) | NR | ‐Higher height‐for‐age: 0.78 (0.62 to 0.99) | ‐Caregiver depression: 1.79 (1.13 to 2.7) | NR | ‐Taking efavirenz: 2.33 (1.21 to 4.50) | NR |
| Kim et al., 2015 [28] | Depressive symptoms | β (95% CI) |
‐Female sex: 2.13 (0.82 to 3.43) ‐Fewer years of schooling: 3.84 (1.71 to 5.98) |
‐Not failing a school term/class: −1.46 (−2.76 to −0.17) ‐Not being in a romantic relationship: −2.38 (−4.35 to −0.41) |
‐Being bullied for taking ART: 5.31 (3.19 to 7.43) |
‐No death in the family: −1.77 (−3.15 to −0.39) ‐Satisfaction with physical appearance: −0.93 (−1.74 to 0.11) |
NR |
‐No immune suppression: −2.58 (−4.29 to −0.87) |
| Lwidiko et al., 2018 [82] | Depressive symptoms | AOR (95% CI) | ‐HIV+ status: 1.96 (1.1 to 3.45) | ‐Rural residence: 0.61 (0.39 to 0.96) | ‐History of childhood deprivation: 4.76 (2.79 to 8.13) | NR | NR | NR |
| Okawa et al., 2018 [56] | Depressive symptoms | AOR (95% CI) | NR | NR |
‐Unsatisfactory relationship with family: 3.01 (1.20 to 7.56) ‐Unsatisfactory relationship with health workers: 2.68 (1.04 to 6.93) ‐HIV‐related stigma: 2.99 (1.07 to 8.41) |
NR | NR | NR |
| West et al., 2018 [58] | Depressive symptoms | APR (95% CI) | NR | NR | NR | ‐Higher social support: 0.25 (0.10 to 0.59) | NR | NR |
| Yarhere & Jaja, 2020 [48] | Depressive symptoms | β (t) | NR | NR |
‐Insomnia: 5.61 (2.94) ‐Suicidal thoughts: 4.64 (3.39) |
NR | NR |
Higher CD4 count: −0.001 (2.74) |
AOR, Adjusted odds ratio; APR, Adjusted prevalence ratio; ART, Antiretroviral therapy; CD4, Cluster of Differentiation‐4; CI, Confidence interval; MR, Mean ratio; NR, None reported; Se, Standard error; t, t statistic; β, Beta coefficients (adjusted).
Females living with HIV from SSA could be at a higher risk of depressive symptoms because of additional experiences of traumatic events such as sexual abuse and intimate partner violence, some of which may have had a role in their acquisition of HIV infection [96, 97]. Additionally, they are more likely to be stigmatized [98] and blamed for HIV transmission within families in patriarchal societies like those of SSA [99]. Older YLWH compared to younger ones are more likely to understand the threat posed by HIV infection to their own life [16], which may manifest as depressive symptoms. The awareness of HIV‐related cognitive deficits [41] by a YLWH manifesting in ways like grade retention or poor performance may explain the association between fewer years of schooling and higher depressive symptoms. Although two studies in this review observed significant associations between HIV‐positive status and higher depressive symptoms, Western empirical studies report non‐significant associations [27, 93]. In SSA where poverty is high [100], HIV‐related adjustments such as recommended intake of a balanced diet with ART use and meeting the regular transportation costs for clinic appointments may be additional challenges to most families of YLWH. Such challenges may lead to psychiatric manifestations among YLWH.
Bullying was the only reported psychosocial correlate of major depression in the study examining the relationship between psychosocial factors and major depression [64]. Psychosocial factors that significantly increased the risk for higher symptoms of major depression among YLWH included living with someone who has anger/aggression problems and ever witnessing violence at home [65]. Psychosocial factors that significantly increased the risk for higher depressive symptoms among YLWH were as follows: bullying victimization [69], bullying for taking ART [28], caregiver depression [67], grandparent as primary caregiver [81], low social support [84], HIV‐related stigma in its various forms such as perceived stigma [53, 56, 66, 84], internalized stigma [61, 69] and associative stigma [61], history of sexual [62, 69], emotional [69] and physical abuse [62] or a combination of physical and/or sexual abuse [62], conflict in the household [78], unsatisfactory relationship with family or health workers [56], insomnia and suicidal ideation [48], negative clinic interactions [69], history of childhood deprivation [82] and stressful life events [66]. On the other hand, the following psychosocial factors significantly lowered the risk for depressive symptoms in YLWH: access to a clinic support group [69], positive parenting [69], higher socio‐economic status [81], family cohesion [81], not being bereaved in the family [28], self‐efficacy [69], satisfaction with physical appearance [28] and higher social support [58, 69, 81]. Among the reported psychosocial correlates of depressive disorders in YLWH, bullying, HIV‐related stigma, history of sexual abuse and social support were consistently reported across two or more studies as significantly associated with depressive disorders, similar to previous review findings [5, 11, 12].
The negative effects of HIV‐related stigma among YLWH including social devaluation and experience of injustices like restrictions in interacting with other people and being denied equal opportunities of enrolling or staying in school [20], may explain why HIV‐related stigma (in its various forms) is associated with higher depressive symptoms in YLWH. HIV‐related stigma can lead to depression manifesting as low self‐worth, self‐isolation, loss of hope in future plans or aspirations and poor ART adherence [20, 84, 101]. As previously emphasized [19, 20], there is a need for continuously addressing HIV‐related stigma in multiple settings within the community. Bullying can lead to negative outcomes such as humiliation, self‐blame and shame [102] and coupled with living with HIV at a younger age, high levels of depressive symptomatology may be expected among YLWH. The finding that higher social support is associated with fewer depressive symptoms (or vice versa, low social support being associated with more depressive symptoms) supports the proposition of a buffering effect of social support against psychological distress as described in the “buffering hypothesis” [103]. This hypothesis postulates that any form of social support (social companionship, emotional, informational, or instrumental support) proffers protection to individuals facing stressful life events (in this case, HIV infection adversity) and assists them in coping with distress. The persistent psychological distress among sexually abused YLWH can be addressed through continued counselling and appropriate support mechanisms.
The following HIV‐related clinical factors significantly increased the risk for elevated depressive symptoms among YLWH: poor adherence to ART [49, 53, 77, 84], history of opportunistic infections [84], experiencing ART side effects [69] and taking efavirenz‐based ART [67]. On the other hand, better immunological stage [28] or increasing CD4 cell count [48] were associated with fewer depressive symptoms among YLWH. See Table 4 for effect sizes reported from individual studies. Of these correlates, only poor adherence to ART was consistently reported across four studies, a finding that has also been observed in past global reviews involving YLWH [5, 11, 12]. Non‐optimal adherence to ART can lead to viral non‐suppression [104], and patients may experience psychological distress when informed of a poor prognosis. This may explain the observed consistent significant association between poor ART adherence and elevated depressive symptoms among YLWH from SSA [49, 53, 77, 84]. However, depression can also be an antecedent to poor ART adherence [25], thus there is uncertainty on the temporality of the significant associations reported in the above four cross‐sectional studies.
3.6. Correlates of anxiety symptoms among YLWH from SSA
Table 5 presents in detail the identified correlates of anxiety symptoms among YLWH. The study that diagnosed anxiety disorder [50] did not report any correlation. Younger age [78] and better overall health among YLWH [69] were the only significant sociodemographic correlates of anxiety symptoms. The following psychosocial factors significantly increased the risk for anxiety symptoms among YLWH: internalized stigma [69], anticipated stigma [69], history of sexual abuse or emotional abuse in the past year [69], bullying victimization [69], poor parental monitoring [69], history of physical violence [78] and stressful life events [66]. Conversely, higher social support [58, 68], self‐efficacy [69], positive parenting [69] and access to a clinic support group [69] were the factors that significantly decreased the risk for anxiety symptoms among YLWH from SSA. Experiencing ART side effects was the only significant HIV‐related clinical correlate of anxiety symptoms among YLWH reported by one South African study [69].
Table 5.
Sociodemographic, psychosocial and HIV‐related correlates of anxiety symptoms among young people living with HIV from SSA
| Author (year) | Outcome | Measure of effect (precision) | Sociodemographic correlates | Psychosocial correlates | HIV‐related correlates | |||
|---|---|---|---|---|---|---|---|---|
| Risk indicators | Protective indicators | Risk indicators | Protective indicators | Risk indicators | Protective indicators | |||
| Besthorn et al. (2018) [68] | Anxiety symptoms | β (Se) | NR | NR |
NR |
‐Higher social support: −0.16 (0.06) | NR | NR |
| Boyes et al. (2018) [69] | Anxiety symptoms | β (Se) | NR |
‐Better overall health: −0.18 (0.08) |
‐Internalized stigma: 0.54 (0.07) ‐Past year emotional abuse: 1.17 (0.19) ‐History of sexual abuse: 1.08 (0.34) ‐Bullying victimisation: 0.15 (0.02) ‐Poor parental monitoring: 0.02 (0.01) ‐Anticipated stigma: 0.30 (0.08) |
‐Self‐efficacy: −0.06 (0.02) ‐Positive parenting: −0.05 (0.01) ‐Access to clinic support group: −0.43 (0.14) |
‐ART side effects: 0.51 (0.17) | NR |
| Haas et al., 2020 [78] | Anxiety symptoms | AOR (95% CI) | NR |
‐Younger age: 10 to 12 years age group vs. 16 to 19 years age group = 0.05 (0.01 to 0.37) 13 to 15 years age group vs. 16 to 19 years group = 0.19 (0.06 to 0.56) |
‐History of physical violence: 2.74 (1.09 to 6.85) | NR | NR | NR |
| Hoare et al. (2019) [66] | Anxiety symptoms | β (95% CI) | NR | NR | ‐Stressful life events: 0.72 (0.44 to 1.01) | NR | NR | NR |
| West et al. (2018) [58] | Anxiety symptoms | APR (95% CI) | NR | NR | NR | ‐Higher social support: 0.30 (0.13 to 0.71) | NR | NR |
APR, Adjusted prevalence ratio; ART, Antiretroviral therapy; CI, Confidence interval; NR, Not reported; Se, Standard error; β, Beta coefficients (adjusted).
Similar to what was observed for depressive symptoms, social support was also a significant correlate of anxiety symptoms reported across two studies [58, 68]. As earlier noted, social support may also provide a buffering effect against anxiety symptoms [103].
3.7. Quality of included studies
(Additional file S3) shows the quality scores of included studies according to their study designs, based on the Newcastle‐Ottawa risk of bias assessment tool. Two studies were graded to be of very good quality [55, 69], twelve of good quality [28, 78, 82, 84], nine of satisfactory quality [45, 49, 50, 51, 56, 61, 62, 68, 81] and seven of unsatisfactory quality [46, 47, 48, 57, 59, 60, 63]. One study [52], available only as an abstract publication, provided relevant mental health data for this review. This study was rated as unclear of high risk of bias because of a lack of feedback from authors when contacted (on three attempts) with a request for data that would enable assessment of the quality of the entire study.
Even though most of the studies reporting on correlates of CMDs among YLWH were of low risk of bias, they were cross‐sectional in design. This study design limits inferences on causality. Therefore, the summarized significant correlates of CMDs among YLWH in this review should be interpreted with caution. For better decisions on priority intervention areas, future studies from this setting should seek to substantiate the causal direction of the identified correlates, using, for instance longitudinal study designs.
3.8. Study limitations
This review has several limitations worth highlighting. First, the review does not provide pooled prevalence estimates of CMDs among YLWH from SSA because measurement tools used across studies were highly heterogeneous. Relatedly, because a meta‐analysis could not be performed, we are unable to report on publication bias. Second, the review deliberately focused on SSA. Even though the region has predominantly low‐ to middle‐income countries, findings may not necessarily be generalizable to other low‐ and middle‐income countries outside this context. Relatedly, across the different countries in SSA, there is diversity in aspects such as language, religious and cultural practices which may make the results ungeneralizable to some communities within the region. Lastly, the review search strategy was biased as only publications in English were considered. It may be that we left out important work reported in a language other than English.
3.9. Implications of the findings for future research, policy and practice
The limitations notwithstanding, this study has important implications for future research, policy and practice. There is a need to invest in mental health awareness as one of the primary prevention strategies aiming at preventing the occurrence of CMDs at high rates among YLWH in SSA. This can entail psychoeducating YLWH about CMDs, that is what they are, the signs and symptoms, when and where to seek help, and providing them with self‐help tips or quick guides through forums such as peer‐to‐peer meetings. The high burden of CMDs in YLWH from SSA highlights the urgent need to test youth‐friendly psychological and psychosocial interventions that address CMDs faced by African youths living with HIV. Adaptation of available interventions such as those identified in a scoping review by Okonji et al. [105] may be a good starting point. The high burden also calls for the integration of mental healthcare into the existing HIV care packages offered to YLWH in this setting. Successful integration requires training of primary health care personnel at the HIV clinics on how to manage CMDs (using, e.g. the World Health Organization’s mhGAP intervention guide [106]), adequate infrastructure (including the availability of psychotropic medication), followed by regular supervision and support from mental health specialists using a task‐shifting approach [107, 108].
Many of the included studies were cross‐sectional in design, did not compare the burden of CMDs between YLWH and their uninfected peers, and focused more on depression than anxiety. Alternative study designs that ascertain causal relationships are recommended for future investigations of factors associated with CMDs among YLWH in SSA. Where feasible, future studies seeking to understand CMDs in YLWH should include an appropriate comparison group of HIV‐negative peers to clearly describe the burden. Finally, more research on anxiety among YLWH from SSA is needed. Currently, this data remains limited.
4. Conclusions
According to this review, the prevalence of CMDs in YLWH from SSA is substantially high despite the wide variation of reported estimates. From studies that recruited a comparison group of HIV‐negative peers, it appears YLWH are at a higher risk of experiencing CMDs particularly depressive symptoms, but more comparative research is needed to draw definite conclusions. The mental health experience of YLWH in SSA is not any different compared to that of YLWH from other settings, all are reporting high rates of CMDs. However, some of the factors associated with CMDs among YLWH in SSA are context‐specific and may require contextualized intervention approaches. YLWH at an elevated risk of CMDs in SSA such as females, older youths, those with fewer schooling years, with a history of sexual abuse, reporting ART adherence issues, being bullied or experiencing HIV‐related stigma may benefit from early management or referral for treatment using a stepped care approach [109] if at least targeted screening for CMDs is done at the youth HIV clinics. Social support may lower the risk for CMDs among YLWH in SSA and can be an important component to consider when designing youth‐friendly intervention packages for YLWH with comorbid CMDs.
Competing interests
The authors declare no conflict of interest.
Authors’ contributions
MKN, AA and CRJCN conceived the study. ET, MKN, AA, CRJCN designed the study. CN, HK, PC contributed to the design of the study. ET did the initial screening of the articles, whereas MKN and CN independently checked the quality of data screening. ET and MKN independently extracted data and assessed the risk of bias for the included studies. ET and MKN wrote the first draft of the manuscript. AA, CN, CRJCN, HK and PC critically reviewed subsequent versions of the manuscript. All the authors have approved the submission of this final version.
Abbreviations
ART, antiretroviral therapy; CASI‐5, child and adolescent symptom inventory – fifth edition; CD4, cluster of differentiation‐4; CMDs, common mental disorders; DSM, diagnostic and statistical manual of mental disorders; ICD‐10, international classification of diseases – tenth edition; MINI‐KID, mini internatonal neuropsychiatric interview for children and adolescents; PRISMA, preferred reporting items for systematic reviews and meta‐analysis; PROSPERO, the international prospective register of systematic reviews; SSA, sub‐Saharan Africa; YI‐4R, Youth inventory – fourth revision; YLWH, young people living with HIV.
Funding
This work was funded by the Wellcome Trust International Master’s Fellowship to MKN (Grant number 201310/Z/16/Z). During this project, ET was supported by DELTAS Africa Initiative [DEL‐15‐003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107769/Z/10/Z) and the UK government. AA holds an award (Grant number MR/M025454/1) jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under MRC/DFID concordant agreement and is also part of the EDCTP2 programme supported by the European Union. The funders did not have a role in the design and conduct of the study. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Supporting information
Additional file S1. Search string used in PubMed database.
Additional file S2. Characteristics of included studies.
Additional file S3. Quality scores of the included studies.
Acknowledgements
We acknowledge the training department of the KEMRI‐Wellcome Trust Research Programme in conjunction with Pwani University, Kilifi, for offering ET the opportunity to work on this project as part of his post‐graduate diploma (PGD) in health research methods. We also acknowledge permission from the Director of KEMRI to publish this work.
Too, E. K. , Abubakar, A. , Nasambu, C. , Koot, H. M. , Cuijpers, P. , RJC Newton, C. and Nyongesa, M. K. Prevalence and factors associated with common mental disorders in young people living with HIV in sub‐Saharan Africa: A systematic review. J Int AIDS Soc. 2021; 24(S2):e25705
PROSPERO Number: CRD42020160806.
Contributor Information
Ezra K Too, Email: etoo@kemri-wellcome.org.
Amina Abubakar, Email: aabubakar@kemri-wellcome.org.
Carophine Nasambu, Email: cnasambu@kemri-wellcome.org.
Hans M Koot, Email: j.m.koot@vu.nl.
Pim Cuijpers, Email: p.cuijpers@vu.nl.
Charles RJC Newton, Email: cnewton@kemri-wellcome.org.
Moses K Nyongesa, Email: mkachama@kemri-wellcome.org.
References
- 1. UNFPA . The power of 1.8 Billion: adolescents, youth and the transformation of the future. united nations population fund. 2014. [cited 2020 Jul 3]. Available from: https://www.unfpa.org/sites/default/files/pub‐pdf/EN‐SWOP14‐Report_FINAL‐web.pdf
- 2. WHO . Orientation Programme on Adolescent Health for Health‐care Providers. World Health Organization. 2006. [cited 2020 Jul 7]. Available from: https://www.who.int/maternal_child_adolescent/documents/pdfs/9241591269_op_handout.pdf
- 3. UNICEF . Adolescent HIV prevention. United Nations Children's Fund. 2019. [cited 2020 Jul 5]. Available from: https://data.unicef.org/topic/hivaids/adolescents‐young‐people/
- 4. UNAIDS . Active involvement of young people is key to ending the AIDS epidemic by 2030. Joint United Nations Programme on HIV/AIDS. 2015. [cited 2020 Jul 3]. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2015/august/20150812_PACT
- 5. Evangeli M. Mental health and substance use in HIV‐infected adolescents. Curr Opin HIV AIDS. 2018;13(3):204–11. [DOI] [PubMed] [Google Scholar]
- 6. UNICEF . For every child, end AIDS: seventh stocktaking report, 2016. United Nations Children's Fund. 2016.
- 7. Chibanda D, Cowan F, Gibson L, Weiss HA, Lund C. Prevalence and correlates of probable common mental disorders in a population with high prevalence of HIV in Zimbabwe. BMC Psychiatry. 2016;16(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durteste M, Kyselyova G, Volokha A, Judd A, Thorne C, Cortina‐Borja M, et al. Anxiety symptoms and felt stigma among young people living with perinatally or behaviourally‐acquired HIV in Ukraine: a cross‐sectional survey. PLoS One. 2019;14:e0210412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lofgren SM, Bond DJ, Nakasujja N, Boulware DR. Burden of depression in outpatient HIV‐infected adults in sub‐saharan africa; systematic review and meta‐analysis. AIDS Behav. 2020;24(6):1752–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heron JE, Norman SM, Yoo J, Lembke K, O’Connor CC, Weston CE, et al. The prevalence and risk of non‐infectious comorbidities in HIV‐infected and non‐HIV infected men attending general practice in Australia. PLoS One. 2019;14:e0223224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc. 2017;20 Suppl 3:21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dessauvagie A, Jörns‐Presentati A, Napp A‐K, Stein D, Jonker D, Breet E, et al. The prevalence of mental health problems in sub‐Saharan adolescents living with HIV: a systematic review. Glob Ment Health (Camb). 2020;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olashore AA, Paruk S, Akanni OO, Tomita A, Chiliza B. Psychiatric disorders in adolescents living with HIV and association with antiretroviral therapy adherence in Sub‐Saharan Africa: a systematic review and meta‐analysis. AIDS Behav. 2021;25:1711–1728. [DOI] [PubMed] [Google Scholar]
- 15. Elkington KS, Robbins RN, Bauermeister JA, Abrams EJ, McKay M, Mellins CA. Mental health in youth infected with and affected by HIV: the role of caregiver HIV. J Pediatr Psychol. 2011;36(3):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mellins CA, Elkington KS, Leu C‐S, Santamaria EK, Dolezal C, Wiznia A, et al. Prevalence and change in psychiatric disorders among perinatally HIV‐infected and HIV‐exposed youth. AIDS Care. 2012;24(8):953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 2014;28(2):131–45. [DOI] [PubMed] [Google Scholar]
- 18. Kenedi CA, Goforth HW. A systematic review of the psychiatric side‐effects of efavirenz. AIDS Behav. 2011;15(8):1803–18. [DOI] [PubMed] [Google Scholar]
- 19. Ashaba S, Cooper‐Vince CE, Vořechovská D, Rukundo GZ, Maling S, Akena D, et al. Community beliefs, HIV stigma, and depression among adolescents living with HIV in rural Uganda. Afr J AIDS Res. 2019;18(3):169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kimera E, Vindevogel S, Reynaert D, Justice KM, Rubaihayo J, De Maeyer J, et al. Experiences and effects of HIV‐related stigma among youth living with HIV/AIDS in Western Uganda: a photovoice study. PLoS One. 2020;15:e0232359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bilgrami M, Okeefe P. Neurologic diseases in HIV‐infected patients. In: Biller J, Ferro J, editors. Handbook of clinical neurology. 121; Amsterdam, the Netherlands: Elsevier; 2014. p. 1321–44. [DOI] [PubMed] [Google Scholar]
- 22. Akena DH, Musisi S, Kinyanda E. A comparison of the clinical features of depression in HIV‐positive and HIV‐negative patients in Uganda. Afr J Psychiatry (Johannesbg). 2010;13(1):43–51. [DOI] [PubMed] [Google Scholar]
- 23. Abas M, Ali G‐C, Nakimuli‐Mpungu E, Chibanda D. Depression in people living with HIV in sub‐Saharan Africa: time to act. Trop Med Int Health. 2014;19(12):1392–6. [DOI] [PubMed] [Google Scholar]
- 24. Egbe CO, Dakum PS, Ekong E, Kohrt BA, Minto JG, Ticao CJ. Depression, suicidality, and alcohol use disorder among people living with HIV/AIDS in Nigeria. BMC Public Health. 2017;17(1):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low‐, middle‐ and high‐income countries: a systematic review and meta‐analysis. Curr HIV/AIDS Rep. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nyongesa MK, Mwangala PN, Mwangi P, Kombe M, Newton C, Abubakar AA. Neurocognitive and mental health outcomes and association with quality of life among adults living with HIV: a cross‐sectional focus on a low‐literacy population from coastal Kenya. BMJ Open. 2018;8:e023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gadow KD, Angelidou K, Chernoff M, Williams PL, Heston J, Hodge J, et al. Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr. 2012;33(6):456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim MH, Mazenga AC, Yu X, Devandra A, Nguyen C, Ahmed S, et al. Factors associated with depression among adolescents living with HIV in Malawi. BMC Psychiatry. 2015;15(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mellins CA, Brackis‐Cott E, Leu CS, Elkington KS, Dolezal C, Wiznia A, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus‐infected youth and seroreverters. J Child Psychol Psychiatry. 2009;50(9):1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, et al. A behavioral and cognitive profile of clinically stable HIV‐infected children. Pediatrics. 2006;117(3):763–70. [DOI] [PubMed] [Google Scholar]
- 31. Kang E, Delzell DA, Chhabra M, Oberdorfer P. Factors associated with high rates of antiretroviral medication adherence among youth living with perinatal HIV in Thailand. Int J STD AIDS. 2015;26(8):534–41. [DOI] [PubMed] [Google Scholar]
- 32. Mutumba M, Musiime V, Lepkwoski JM, Harper GW, Snow RC, Resnicow K, et al. Examining the relationship between psychological distress and adherence to anti‐retroviral therapy among Ugandan adolescents living with HIV. AIDS Care. 2016;28(7):807–15. [DOI] [PubMed] [Google Scholar]
- 33. Mutumba M, Bauermeister JA, Elkington KS, Bucek A, Dolezal C, Leu C‐S, et al. A prospective longitudinal study of mental health symptoms among perinatally HIV‐infected and HIV‐exposed but uninfected urban youths. J Adolesc Health. 2016;58(4):460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mutumba M, Bauermeister JA, Harper GW, Musiime V, Lepkowski J, Resnicow K, et al. Psychological distress among Ugandan adolescents living with HIV: examining stressors and the buffering role of general and religious coping strategies. Glob Public Health. 2017;12(12):1479–91. [DOI] [PubMed] [Google Scholar]
- 35. Malee KM, Tassiopoulos K, Huo Y, Siberry G, Williams PL, Hazra R, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care. 2011;23(12):1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166(6):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnoses on long‐term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. AIDS (London, England). 2009;23(14):1859–65. [DOI] [PubMed] [Google Scholar]
- 38. Bernard C, Dabis F, de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub‐Saharan Africa: a systematic review and meta‐analysis. PLoS One. 2017;12:e0181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abubakar A, Van de Vijver FJR, Hassan AS, Fischer R, Nyongesa MK, Kabunda B, et al. Cumulative psychosocial risk is a salient predictor of depressive symptoms among vertically HIV‐infected and HIV‐affected adolescents at the Kenyan Coast. Ann Glob Health. 2017;83(5–6):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Betancourt T, Scorza P, Kanyanganzi F, Fawzi MC, Sezibera V, Cyamatare F, et al. HIV and child mental health: a case‐control study in Rwanda. Pediatrics. 2014;134(2):e464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamau JW, Kuria W, Mathai M, Atwoli L, Kangethe R. Psychiatric morbidity among HIV‐infected children and adolescents in a resource‐poor Kenyan urban community. AIDS Care. 2012;24(7):836–42. [DOI] [PubMed] [Google Scholar]
- 42. Nakimuli‐Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub‐Saharan Africa: a systematic review. AIDS Behav. 2012;16(8):2101–18. [DOI] [PubMed] [Google Scholar]
- 43. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2013. p. 2012. [Google Scholar]
- 45. Adeyemo S, Adeosun II, Ogun OC, Adewuya A, David AN, Adegbohun AA, et al. Depression and suicidality among adolescents living with human immunodeficiency virus in Lagos. Nigeria. Child Adolesc Psychiatry Ment Health. 2020;14(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bankole KO, Bakare MO, Edet BE, Igwe MN, Ewa AU, Bankole IA, et al. Psychological complications associated with HIV/AIDS infection among children in South‐South Nigeria, sub‐Saharan Africa. Cogent Medicine. 2017;4(1):1372869. [Google Scholar]
- 47. Sale S, Gadanya M. Prevalence and factors associated with depression in HIV/AIDS patients aged 15–25 years at Aminu Kano Teaching Hospital, Nigeria. J Child Adolesc Ment Health. 2008;20(2):95–9. [DOI] [PubMed] [Google Scholar]
- 48. Yarhere I, Jaja T. Beck Depression Inventory scores for children with some chronic diseases (Type I diabetes mellitus, Sickle cell anaemia, and AIDS) on management in University of Port Harcourt Teaching Hospital. Afr J Diabetes Med. 2020;28(1). [Google Scholar]
- 49. Ekat MH, Yotebieng M, Leroy V, Mpody C, Diafouka M, Loubaki G, et al. Association between depressive symptoms and adherence among adolescents living with HIV in the Republic of Congo: a cross sectional study. Medicine. 2020;99:e21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Musisi S, Kinyanda E. Emotional and behavioural disorders in HIV seropositive adolescents in urban Uganda. East Afr Med J. 2009;86:46923. [DOI] [PubMed] [Google Scholar]
- 51. Fawzi MCS, Ng L, Kanyanganzi F, Kirk C, Bizimana J, Cyamatare F, et al. Mental health and antiretroviral adherence among youth living with HIV in Rwanda. Pediatrics. 2016;138(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Molinaro M, Mwanza‐Kabaghe S, Mweemba M, Matoka B, Mbewe EG, Kabundula P, et al. Evaluating the Relationship Between Depression and HIV‐associated Cognitive Impairment Among Children and Adolescents in Zambia. Neurology. 2019;92(15 Supplement):S7.001. [Google Scholar]
- 53. Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, O'Donnell K. Evaluating mental health difficulties and associated outcomes among HIV‐positive adolescents in Tanzania. AIDS Care. 2016;28(7):825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kemigisha E, Zanoni B, Bruce K, Menjivar R, Kadengye D, Atwine D, et al. Prevalence of depressive symptoms and associated factors among adolescents living with HIV/AIDS in South Western Uganda. AIDS Care. 2019;31(10):1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinyanda E, Salisbury TT, Levin J, Nakasujja N, Mpango RS, Abbo C, et al. Rates, types and co‐occurrence of emotional and behavioural disorders among perinatally HIV‐infected youth in Uganda: the CHAKA study. Soc Psychiatry Psychiatr Epidemiol. 2019;54(4):415–25. [DOI] [PubMed] [Google Scholar]
- 56. Okawa S, Mwanza Kabaghe S, Mwiya M, Kikuchi K, Jimba M, Kankasa C, et al. Psychological well‐being and adherence to antiretroviral therapy among adolescents living with HIV in Zambia. AIDS Care. 2018;30(5):634–42. [DOI] [PubMed] [Google Scholar]
- 57. Ramos JV, Mmbaga BT, Turner EL, Rugalabamu LL, Luhanga S, Cunningham CK, et al. Modality of primary HIV disclosure and association with mental health, stigma, and antiretroviral therapy adherence in Tanzanian youth living with HIV. AIDS Patient Care STDS. 2018;32(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. West N, Schwartz S, Mudavanhu M, Hanrahan C, France H, Nel J, et al. Mental health in south african adolescents living with hiv. AIDS Care. 2019;31(1):117–24. [DOI] [PubMed] [Google Scholar]
- 59. Woollett N, Cluver L, Bandeira M, Brahmbhatt H. Identifying risks for mental health problems in HIV positive adolescents accessing HIV treatment in Johannesburg. J Child Adolesc Ment Health. 2017;29(1):11–26. [DOI] [PubMed] [Google Scholar]
- 60. Dyer J, Wilson K, Badia J, Agot K, Neary J, Njuguna I, et al. The psychosocial effects of the COVID‐19 pandemic on youth living with HIV in Western Kenya. AIDS Behav. 2020;25(1):68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Earnshaw VA, Kidman RC, Violari A. Stigma, depression, and substance use problems among perinatally HIV‐infected youth in South Africa. AIDS Behav. 2018;22(12):3892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Filiatreau LM, Giovenco D, Twine R, Gómez‐Olivé FX, Kahn K, Haberland N, et al. Examining the relationship between physical and sexual violence and psychosocial health in young people living with HIV in rural South Africa. J Int AIDS Soc. 2020;23:e25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paul R, Maila B, Kusanthan T. A study to determine the levels of depression among HIV patients. Medical Sci Technol. 2015;4(3):256–60. [Google Scholar]
- 64. Ashaba S, Cooper‐Vince C, Maling S, Rukundo G, Akena D, Tsai A. Internalized HIV stigma, bullying, major depressive disorder, and high‐risk suicidality among HIV‐positive adolescents in rural Uganda. Glob Ment Health (Camb). 2018;5:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buckley J, Otwombe K, Joyce C, Leshabane G, Hornschuh S, Hlongwane K, et al. Mental health of adolescents in the era of antiretroviral therapy: is there a difference between HIV‐infected and uninfected youth in South Africa? J Adolesc Health. 2020;67(1):76–83. [DOI] [PubMed] [Google Scholar]
- 66. Hoare J, Phillips N, Brittain K, Myer L, Zar HJ, Stein DJ. Mental health and functional competence in the cape town adolescent antiretroviral cohort. J Acquir Immune Defic Syndr. 2019;81(4):e109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kikuchi K, Poudel KC, Rwibasira JM, Majyambere A, Mutabazi V, Nyonsenga SP, et al. Caring for perinatally HIV‐infected children: call for mental care for the children and the caregivers. AIDS Care. 2017;29(10):1280–6. [DOI] [PubMed] [Google Scholar]
- 68. Besthorn F, Kalomo EN, Lightfoot E, Liao ML. The relationship between social support and anxiety amongst children living with HIV in rural northern Namibia. Afr J AIDS Res. 2018;17(4):293–300. [DOI] [PubMed] [Google Scholar]
- 69. Boyes ME, Cluver LD, Meinck F, Casale M, Newnham E. Mental health in South African adolescents living with HIV: correlates of internalising and externalising symptoms. AIDS Care. 2018;31(1):95–104. [DOI] [PubMed] [Google Scholar]
- 70. Brown LK, Whiteley L, Harper GW, Nichols S, Nieves A. Psychological symptoms among 2032 youth living with HIV: a multisite study. AIDS Patient Care STDS. 2015;29(4):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI‐KID). J Clin Psychiatry. 2010;71(3):313–26. [DOI] [PubMed] [Google Scholar]
- 72. WHO . ICD‐10 symptom glossary for mental disorders : a glossary of symptoms used in the definition of criteria for the classification of mental and behavioural disorders in the 10th revision of the International Classification of Diseases (ICD‐10) / prepared by M. Isaac, A. Janca and N. Sartorius. Geneva: World Health Organization; 1994. [Google Scholar]
- 73. Gadow K, Sprafkin J. Child and adolescent symptom inventory‐5. Checkmate plus. Stony brook; 2013. [Google Scholar]
- 74. Gadow KD, Sprafkin J, Carlson GA, Schneider J, Nolan EE, Mattison RE, et al. A DSM‐IV‐referenced, adolescent self‐report rating scale. J Am Acad Child Adolesc Psychiatry. 2002;41(6):671–9. [DOI] [PubMed] [Google Scholar]
- 75. Johnson JG, Harris ES, Spitzer RL, Williams JBW. The patient health questionnaire for adolescents: Validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30(3):196–204. [DOI] [PubMed] [Google Scholar]
- 76. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gaitho D, Kumar M, Wamalwa D, Wambua GN, Nduati R. Understanding mental health difficulties and associated psychosocial outcomes in adolescents in the HIV clinic at Kenyatta National Hospital, Kenya. Ann Gen Psychiatry. 2018;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haas AD, Technau KG, Pahad S, Braithwaite K, Madzivhandila M, Sorour G, et al. Mental health, substance use and viral suppression in adolescents receiving ART at a paediatric HIV clinic in South Africa. J Int AIDS Soc. 2020;23:e25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 80. Kovacs M. Children’s Depression Inventory. A measure of depressive symptoms in children and adolescents. North Tonawanda: Multi‐Health Systems Inc; 1992.
- 81. Cavazos‐Rehg P, Xu C, Kasson E, Byansi W, Bahar OS, Ssewamala FM. Social and economic equity and family cohesion as potential protective factors from depression among adolescents living with HIV in Uganda. AIDS Behav. 2020;24(9):2546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lwidiko A, Kibusi SM, Nyundo A, Mpondo BC. Association between HIV status and depressive symptoms among children and adolescents in the Southern Highlands Zone, Tanzania: a case‐control study. PLoS One. 2018;13:e0193145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beck A, Steer R, Brown G. Manual for the Beck depression inventory‐II (BDI‐II). 1996.
- 84. Abebe H, Shumet S, Nassir Z, Agidew M, Abebaw D. Prevalence of depressive symptoms and associated factors among HIV‐positive youth attending ART follow‐up in Addis Ababa, Ethiopia. AIDS Res Treat. 2019;2019:4610458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Poznanski EO, Mokros HB. Children's depression rating scale, revised (CDRS‐R): Western Psychological Services Los Angeles. 1996.
- 86. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 87. Reynolds W. Reynolds Adolescent Depression Scale ‐ 2nd edition (RADS‐2); professional manual. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 88. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80 11 Suppl 3:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beck JS, Beck AT, Jolly JB, Steer RA. Beck Youth Inventories: second Edition for children and adolescents: manual: depression inventory for youth, anxiety inventory for youth, anger inventory for youth, disruptive behavior inventory for youth, self‐concept inventory for youth. San Antonio, Tex.; Boston: Psychological Corp.; Harcourt Brace; 2005.
- 90. Reynolds CR, Richmond BO. What I think and feel: a revised measure of children's manifest anxiety. J Abnorm Child Psychol. 1978;6(2):271–80. [DOI] [PubMed] [Google Scholar]
- 91. Scharko AM. DSM psychiatric disorders in the context of pediatric HIV/AIDS. AIDS Care. 2006;18(5):441–5. [DOI] [PubMed] [Google Scholar]
- 92. Bomba M, Nacinovich R, Oggiano S, Cassani M, Baushi L, Bertulli C, et al. Poor health‐related quality of life and abnormal psychosocial adjustment in Italian children with perinatal HIV infection receiving highly active antiretroviral treatment. AIDS Care. 2010;22(7):858–65. [DOI] [PubMed] [Google Scholar]
- 93. Le Prevost M, Arenas‐Pinto A, Melvin D, Parrott F, Foster C, Ford D, et al. Anxiety and depression symptoms in young people with perinatally acquired HIV and HIV affected young people in England. AIDS Care. 2018;30(8):1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Elkington KS, Cruz JE, Warne P, Santamaria EK, Dolezal C, Mellins CA. Marijuana use and psychiatric disorders in perinatally HIV‐exposed youth: does HIV matter? J Pediatr Psychol. 2016;41(3):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ali G‐C, Ryan G, De Silva MJ. Validated screening tools for common mental disorders in low and middle income countries: a systematic review. PLoS One. 2016;11:e0156939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Durevall D, Lindskog A. Intimate partner violence and HIV in ten sub‐Saharan African countries: what do the Demographic and Health Surveys tell us? Lancet Glob Health. 2015;3(1):e34–43. [DOI] [PubMed] [Google Scholar]
- 97. Spies G, Konkiewitz EC, Seedat S. Incidence and persistence of depression among women living with and without HIV in South Africa: a longitudinal study. AIDS Behav. 2018;22(10):3155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kamen C, Arganbright J, Kienitz E, Weller M, Khaylis A, Shenkman T, et al. HIV‐related stigma: implications for symptoms of anxiety and depression among Malawian women. Afr J AIDS Res. 2015;14(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Long C. “I Don't Know Who to Blame”: HIV‐Positive South African Women Navigating Heterosexual Infection. Psychol Women Q. 2009;33(3):321–33. [Google Scholar]
- 100. UNDP, OPHI . Global Multidimensional Poverty Index 2019:Illuminating Inequalities. United Nations Development Programme & Oxford Poverty and Human Development Initiative. 2019.
- 101. Denison JA, Banda H, Dennis AC, Packer C, Nyambe N, Stalter RM, et al. “The sky is the limit”: adhering to antiretroviral therapy and HIV self‐management from the perspectives of adolescents living with HIV and their adult caregivers. J Int AIDS Soc. 2015;18:19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Strøm IF, Aakvaag HF, Birkeland MS, Felix E, Thoresen S. The mediating role of shame in the relationship between childhood bullying victimization and adult psychosocial adjustment. Eur J Psychotraumatol. 2018;9:1418570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–57. [PubMed] [Google Scholar]
- 104. Martin DA, Luz PM, Lake JE, Clark JL, Veloso VG, Moreira RI, et al. Improved virologic outcomes over time for HIV‐infected patients on antiretroviral therapy in a cohort from Rio de Janeiro, 1997–2011. BMC Infect Dis. 2014;14:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Okonji EF, Mukumbang FC, Orth Z, Vickerman‐Delport SA, Van Wyk B. Psychosocial support interventions for improved adherence and retention in ART care for young people living with HIV (10–24 years): a scoping review. BMC Public Health. 2020;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. WHO . mhGAP Intervention Guide Mental Health Gap Action Programme Version 2.0 for mental, neurological and substance use disorders in non‐specialized health settings. World Health Organization. 2016. [PubMed]
- 107. Petersen I, Lund C, Stein D. Optimizing mental health services in low‐income and middle‐income countries. Curr Opin Psychiatry. 2011;24(4):318–23. [DOI] [PubMed] [Google Scholar]
- 108. WHO . Task shifting: Global recommendations and guidelines. World Health Organization. 2008.
- 109. Patel V, Simon G, Chowdhary N, Kaaya S, Araya R. Packages of care for depression in low‐and middle‐income countries. PLoS Med. 2009;6:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file S1. Search string used in PubMed database.
Additional file S2. Characteristics of included studies.
Additional file S3. Quality scores of the included studies.


