Abstract
Introduction
Addressing the intersection between mental health and HIV is critical for the wellbeing of persons living with HIV (PLWH). This systematic review synthesized the literature on mental health interventions for PLWH in low‐ and middle‐income countries (LMICs) to determine intervention components and explore their relationship with intervention effectiveness.
Methods
We included only controlled clinical trials of interventions aiming to improve the mental health of PLWH. We conducted a search in the following databases: PubMed, CINAHL, PsycINFO and EMBASE for eligible studies describing the evaluation of interventions for mental health problems among PLWH in LMICs published through August 2020. Two reviewers independently screened references in two successive stages of title/abstract screening and then full‐text screening for references meeting title/abstract criteria.
Results
We identified a total of 30 eligible articles representing 6477 PLWH who were assigned to either the intervention arm (n = 3182) or control arm (n = 3346). The mental health interventions evaluated were psychological (n = 17, 56.67%), pharmacological (n = 6, 20.00%), combined psychological and pharmacological (n = 1, 3.33%) and complementary/alternative treatments (n = 6, 20.00%). The mental health problems targeted were depression (n = 22, 73.33 %), multiple psychological symptoms (n = 1, 3.33%), alcohol and substance use problems (n = 4, 13.33%), post‐traumatic stress disorder (n = 1, 3.33%) and HIV‐related neuro‐cognitive impairment (n = 2, 6.67%). Studies of interventions with significant effects had significantly a higher number of active ingredients than those without significant effects [3.41 (2.24) vs. 1.84 (1.46) Mean (SD)] [Mean difference = −1.56, 95% CI = −3.03 to −0.09, p = 0.037].
Conclusions
There continue to be advances in mental health interventions for PLWH with mental illness in LMICs. However, more research is needed to elucidate how intervention components lead to intervention effectiveness. We recommend scale up of culturally appropriate interventions that have been successfully evaluated in low‐ and middle‐income countries.
Keywords: mental health, psychotherapy, psychotropic, HIV/AIDS, anti‐retroviral therapy theory of change, low‐ and middle‐income countries
1. Introduction
Mental health problems in people living with HIV (PLWH) were recognized early in the AIDS epidemic as a key factor affecting HIV treatment outcomes in high‐income countries [1, 2]. However, mental health of PLWH has only recently received the attention it deserves in low‐ and middle‐income countries (LMICs) [3]. Despite the fact that mental health is a universal human right, the mental wellbeing of those living with HIV and mental illness is often neglected [4]. A call for global action to improve responses to non‐communicable diseases has increased focus towards mental health promotion, prevention and treatment of mental health conditions across the world [5].
The relationship between HIV and mental health is bidirectional. On one hand, pre‐existing mental health conditions increase the risk for HIV infections. Indeed, in some LMICs depression rates are more than 30% in PLWH [6]. In such cases, the risk for infection may be associated with poverty, transactional sex, sexual violence, sharing drug injection equipment, inconsistent condom use or with psychiatric symptoms that can impair cognition and judgment [7, 8]. On the other hand, PLWH are at increased risk of developing mental health conditions ranging from acute stress reactions to neurocognitive disorders [9, 10] which can undermine health‐seeking behaviours, reduce adherence to treatment [11] and lead to higher rates of mortality [12, 13, 14]. Also, some antiretrovirals can cause neuropsychiatric side effects for up to half of those using them [15]. Zidovudine and abacavir have been associated with mania and psychosis, whereas nevirapine and efavirenz have been associated with mood changes and vivid dreams [16]. HIV policy makers now acknowledge the importance of addressing the intersection between mental health and HIV, and the need to adopt a human rights‐based approach to improve the quality of life of PLWH [17].
In 2015, HIV care and treatment guidelines were updated to require the identification and management of depression among PLWH [18]. In 2018, the UNAIDS Programme Coordinating Board addressed the topic of “Mental Health and HIV/AIDS” for the first time in its history [19]. These developments indicate that it is now common knowledge that the HIV epidemic cannot end without addressing the mental health problems of PLWH [20]. Since mental health problems lie along a continuum that extends from mild distress to persistent and severe symptoms [21], mental health promotion, prevention and treatment of such conditions is crucial.
Systematic reviews of mental health interventions for PLWH, which come predominately from high‐income countries, show that these interventions lead to improvements in mental health, quality of life, adherence to medication and viral suppression [22, 23]. However, in LMICs, data are sparse. In past reviews of intervention trials for depression and anxiety among PLWH in LMICs, the majority were preliminary studies, and few demonstrated efficacy [24]. Mental health researchers recommended further development and adaptation of mental health interventions for resource‐limited settings to improve effectiveness.
Mental health interventions are complex. In pharmacological interventions, the component of the intervention responsible for therapeutic action is the active ingredient. However, in non‐pharmacological interventions, active ingredients may be more than the sum of the intervention components and include the context, expertise and behaviours of stakeholders, beneficiaries and providers [25]. The Medical Research Council guidance calls for more detailed and standardized descriptions of complex interventions in published reports to facilitate the exchange of knowledge and to encourage the synthesis of results from similar studies [26].
Integrating the theory of change into the Medical Research Council framework has been proposed as an effective way to evaluate such interventions as it takes into account multiple causal pathways [27]. It is, therefore, necessary to understand how interventions relate to and interact with components of the system to produce an effect. Previous researchers have used the Theory of Change within the Medical Research Council framework to provide a set of indicators to evaluate all stages of the causal pathway through which a mental health intervention may achieve impact [27]. These include involving stakeholders, training and supervision of intervention deliverers, creating community awareness about the intervention, caseload of the intervention deliverer, adherence to the intervention and the active ingredients in either the pharmacological or non‐pharmacological intervention used.
This systematic review synthesizes the literature on evaluated mental health interventions for PLWH, examines intervention components that may moderate causal mechanisms and tentatively explores their relationship to intervention effectiveness.
2. Methods
2.1. Search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline [28, 29]. We systematically searched the PubMed, CINAHL, PsycINFO and Embase databases for eligible studies describing the evaluation of interventions for mental health problems among PLWH in LMICs published through August 2020. We conducted our search by combining keywords and database‐specific subject headings for the following concepts: (1) The population: HIV, AIDS; (2) The outcome: mental health, psychology, depression, anxiety, substance use, alcohol use, drug use, smoking behaviour, suicide, post‐traumatic stress disorder and neuro‐cognitive impairment; (3) The study location: developing countries, low resource, middle income or low income; (4) Mental health intervention: anti‐depressive agents, anti‐psychotic agents, psychotherapy, counselling, exercise therapy, relaxation meditation; (5) Study designs: quasi‐experimental studies, controlled before‐after studies, randomized controlled trials and controlled clinical trials. The full search strategy with adapted terms for each database is included in Figure S1. The protocol for the systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020219483).
2.2. Eligibility criteria
We reviewed abstracts and full texts of retrieved articles according to the following inclusion criteria: (1) studies written in English; (2) conducted with adults living with HIV; (3) residing in LMICs based on the classification of the World Bank during the financial year in which the study was published; (4) Randomized controlled trials or quasi‐experimental studies that described the evaluation of mental health interventions in adults (≥18 years) living with HIV; (5) described a mental health treatment for mental health problems which lie along a continuum that extends from mild distress to persistent and severe symptoms. Studies describing mental health interventions were excluded if they were described as pilot studies, prospective cohort studies, conference abstracts or studies of children or adolescents (<18 years).
2.3. Selection process
Two reviewers independently screened all titles and abstracts and assessed full‐text articles against the inclusion criteria. A third reviewer was engaged to resolve discrepancies between the two reviewers at any point in the screening and assessment process. The number of included and excluded full‐text articles and reasons for exclusion are presented in the PRISMA flow chart (Figure 1).
Figure 1.
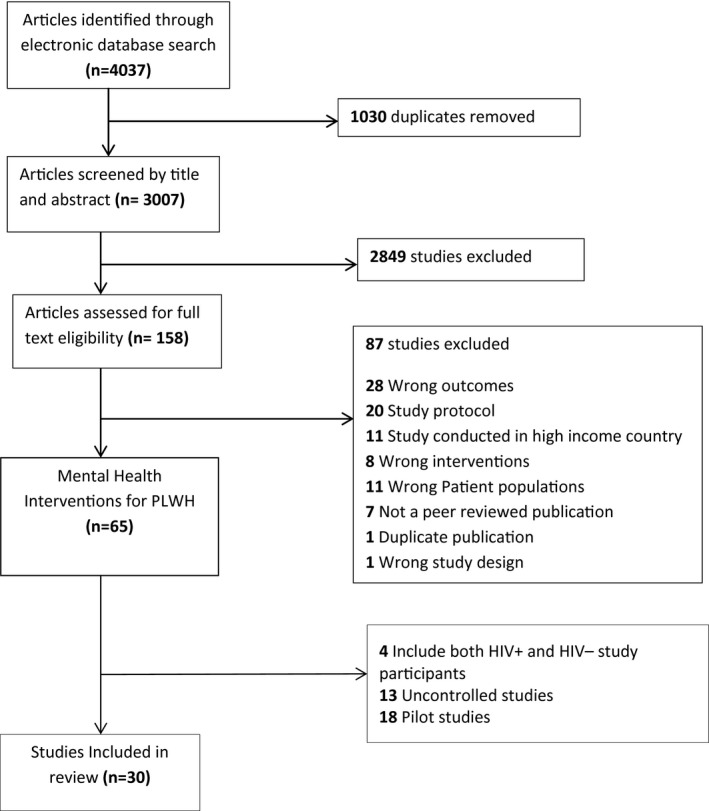
PRISMA flowchart. PLWH, people living with HIV.
2.4. Narrative synthesis
We conducted a narrative synthesis based on guidelines produced by Popay et al [31] for the Economic and Social Research Council UK Methods Programme (2006), selecting and using the techniques applicable to our research question and included studies. A flow chart summarizing the synthesis process is presented in Figure 2. First, we adopted the Theory of Change Framework proposed by De Silva et al [27] to guide our synthesis.
Figure 2.
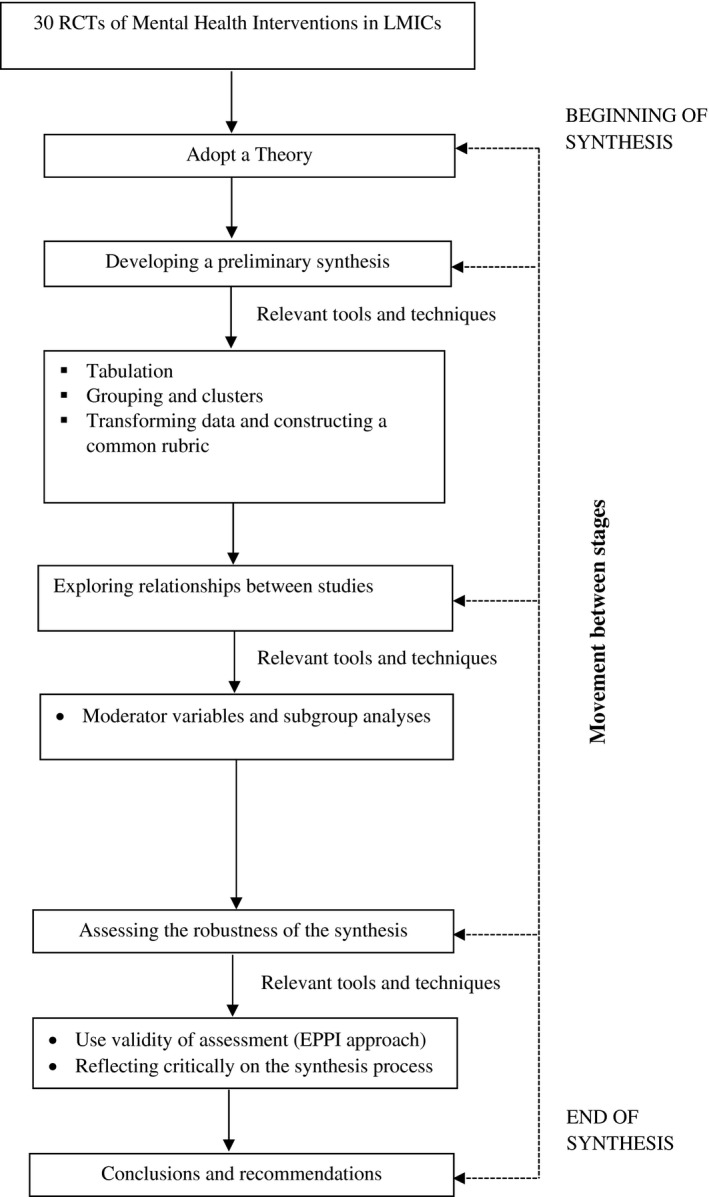
Narrative synthesis process. RCTs, randomized control trials; LMICs, low‐and‐middle‐income countries.
Second, data were extracted using a standardized data extraction tool that included the following elements: (1) location of study (country), income category of the country, study sample size, mean age of participants, gender of the study population, duration of follow‐up, type of mental health intervention, study design, and mental health problem targeted. We extracted data on the nature of study outcomes reported (e.g. immediate‐, short‐ and long‐term outcomes), indicators of intervention response reported (e.g. type of intervention deliverer, case load, treatment adherence, number and type of active ingredients) strategies to achieve outcomes (e.g. stakeholder & public involvement, community awareness, training and supervision of intervention deliverers). All data were extracted by two researchers and differences were resolved through discussions between the two reviewers or discussing with a third researcher.
Third, studies were clustered according to the characteristics in the data extraction tables, such as type of mental health intervention, targeted mental health problem, setting, gender, delivery format (individual vs. group approach), intervention effects (significant versus non‐significant), etc.
Fourth, we assessed relationships between the various study clusters and intervention effectiveness. Specifically, we assessed relationships between intervention type, intervention deliverer’s case load, treatment adherence and number of intervention active ingredients and intervention effectiveness. We also explored associations between stakeholder and public involvement, community awareness, training and supervision of intervention deliverers and intervention effectiveness.
Lastly, we examined the quality of the synthesis by assessing the methodological rigour of each study reviewed using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool [32]. Methodological rigour for the EPHPP tool produces a global rating of “‘strong,” “moderate” or “‘weak” for each study based on evaluations of two independent reviewers (EN‐M and CMS). Differences of opinion were resolved through discussions between the two reviewers or brought to a third independent individual to consider and make a final decision.
3. Results
3.1. Search and study selection
Our electronic search yielded 4,037 articles: 1,003 duplicate articles and 2,849 irrelevant articles were excluded. We assessed 158 full‐text articles for eligibility. Thirty controlled studies published through August 2020 were included in this systematic review. Figure 1 presents the flow chart of our study selection and the frequency of reasons for exclusion.
3.2. Characteristics of studies
Half of the reviewed studies were conducted in the African region (n = 15), and the other half in the Eastern Mediterranean Region (n = 7) and South‐East Asia Region (n = 8). The study’s total sample size ranged from 32 to 1140, intervention sample sizes ranged from 19 to 578 and control group sample sizes ranged from 13 to 562. The majority of studies (n = 22, 73.33 %) reported on interventions for depression [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55]. Major depressive disorder was confirmed using diagnostic interviews such as SCID or MINI in three studies [34, 35, 36]. One study (3.33 %) described an intervention for multiple psychological symptoms assessed with Symptom Check List‐90 (SCL‐90) [56]. Mental health Interventions for alcohol and substance use disorders were described in four studies (13.33 %) [57, 58, 59, 60], post‐traumatic stress disorder (PTSD) in one study (3.33 %) [61] and HIV neuro‐cognitive impairment in two studies (6.67 %) [62, 63]. Among the reviewed studies, four types of mental health interventions were described including psychological interventions (n = 17), pharmacological interventions (n = 6), combination of psychological and pharmacological (n = 1) and complementary and alternative interventions (n = 6). Detailed study characteristics are shown in Tables 1 and 2.
Table 1.
Characteristics of reviewed articles (N = 30)
| Total N = 30 | |
|---|---|
| N (%) | |
| Location of study | |
| Africa Region (AFRO) | 15 (50.00) |
| Eastern Mediterranean Region (EMRO) | 7 (23.33) |
| South‐East Asia Region (SEARO) | 2 (6.67) |
| Western Pacific Region(WPRO) | 6 (20.00) |
| Latin American Region (LAR) | 0 (0.00) |
| Incomes of countries | |
| Upper middle income | 17 (56.67) |
| Lower middle income | 8 (26.67) |
| Lower income | 5 (16.67) |
| Study design | |
| Cluster randomized clinical trial | 1 (3.33) |
| Individual randomized clinical trial | 25 (83.33) |
| Pre‐test & Post‐test design | 4 (13.33) |
| Gender of study population | |
| Females only | 8 (26.67) |
| Males only | 4 (13.33) |
| Both males & females | 18 (60.00) |
| Age of study participants | |
| Mean (SD) | 34.23 (4.60) |
| Median | 35 |
| Range | (26 to 41) |
| Type of mental health intervention | |
| Psychological intervention | 17 (56.67) |
| Pharmacological intervention | 6 (20.00) |
| Both psychological and pharmacological intervention | 1 (3.33) |
| Complementary /alternative intervention | 6 (20.00) |
| Outcomes | |
| Immediate outcomes | 14 (46.67) |
| Short‐term outcomes (<6 months) | 4 (13.33) |
| Long‐term outcomes (six to twelve months) | 12 (40.00) |
| Significant Intervention Effects | |
| Yes | 17 (56.67) |
| No | 13 (43.33) |
Table 2.
Mental Health interventions for Persons Living with HIV in Low‐ and Middle‐Income Countries
| Intervention |
Reference country |
Mental disorder | Sample | Study design | Intervention characteristics | Active ingredients | Intervention strategies | Key findings |
|---|---|---|---|---|---|---|---|---|
| Group Support Psychotherapy |
Nakimuli‐Mpungu et al; 2020 [35] Uganda |
Depression Tool:SRQ‐20 MINI |
Gender: M & F HIV:100% ART: 100% |
CRCT GSP = 578 GHE = 562 Follow‐up: twelve months |
Sessions: 8 Lay Health Workers C:IF Ratio = 10:1 Adherence: 80% |
Psych‐education Venting Social Support Positive coping skills Problem solving Livelihood skills |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: YES Acceptable: YES Fidelity: Assessed |
Participants in GSP group had significantly lower cases of major depression than did those in the lower GHE group |
| Schema Focused Group Therapy |
Jalali et al; 2019 [40] Iran |
Depression Tool: BDI‐II |
Gender: M(Prisoners) HIV:100% ART: NS |
Quasi Experimental SFGT = 21 CONTROL (WL) = 21 Follow‐up: Post‐test |
Sessions: 11 Specialist delivered C:IF Ratio = 10:1 Adherence: 100% |
Psych‐education Social support Positive coping skills Cognitive restructuring |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: NA Feasible: YES Acceptable: YES Fidelity: Assessed |
There was a significant difference in both pre‐ and post‐test scores in the depression between the experimental and waiting list control groups |
|
CBSM WeChat‐based mobile health intervention |
Guo et al; 2020 [47] China |
Depression Tool: CESD‐20 |
Gender: M& F HIV:100% ART:100% |
RCT CBSM = 150 CONTROL(WL) = 150 Follow‐up: Post‐test |
Sessions: 12 Self‐administered C:IF Ratio = NA Adherence:55% |
Psych‐education Positive coping skills Cognitive restructuring Relaxation Meditation Behavioural activation Physical activity |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NA Supervision IFs: NA Feasible: YES Acceptable: YES Fidelity: Assessed |
There was a significant difference in depression symptoms between the experimental and control groups |
| Group Coping Enhancement Programme |
Ye et al; 2018 [61] China |
PTSD, PTG Tool: PTGI‐21 & IES ‐ 15 |
Gender: M HIV:100% ART:47% |
RCT GCEP = 30 CONTROL = 30 Follow‐up: Post‐test |
Sessions: 11 Specialist delivered C:IF Ratio = 10:1 Adherence:80% |
Psych‐education Venting Positive coping skills Cognitive restructuring Social support |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: Assessed |
The intervention group reported more improvement in problem‐focused coping strategies, PTG, and PTSD than did the wait‐list control groups |
| Group Behavioural intervention |
Li et al; 2010 [53] Thailand |
Depression Tool: DST‐15 |
Gender: M& F HIV:100% ART: NS |
RCT CBI = 260 CONTROL = 247 Follow‐up: twelve months |
Sessions: 13 Specialist delivered C:IF Ratio = 130:1 Adherence: NS |
Cognitive restructuring Positive coping skills Physical activity Social support |
Stakeholders: YES Community aware: YES Training IFs: NS Supervision IFs: NS Feasible: NS Acceptable: Assessed Fidelity: NS |
The intervention did not have a significant effect on depression |
| Cognitive Behavioural Therapy |
Nobakht et al; 2018 [55] Iran |
Depression Tool: DASS‐21 |
Gender: F HIV:100% ART:93% |
RCT CBT = 33 CONTROL = 33 Follow‐up: Post‐test |
Sessions: 6 Specialist delivered C:IF Ratio = 30:1 Adherence:91% |
Psych‐education Venting Social support Positive coping skills Cognitive restructuring |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: YES Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was a significant reduction in depression among the intervention group compared to the control group |
| Group Cognitive Behavioural Therapy |
Papas et al; 2012 [58] Kenya |
Alcohol use disorder Tool: PDA, PDD |
Gender: M &F HIV:53% ART:66% HIV:100% ART: 61% |
RCT GCBT = 42 CONTROL = 33 Follow‐up: three months |
Sessions: 6 Specialist delivered C:IF Ratio = 21:1 Adherence: 93% |
Psych‐education Social support Positive coping skills Cognitive restructuring Livelihood skills Problem solving |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: Assessed Acceptable: Assessed Fidelity: Assessed |
Reported alcohol use at 3‐month post‐intervention alcohol was 69.4% in the CBT group and 37.5% in the control group |
| Single Brief Alcohol Reduction Intervention |
Wandera et al; 2017 [57] Uganda |
Alcohol use disorder Tool: AUDIT |
Gender: M & F HIV:100% ART: 77% |
RCT SBARI = 167 CONTROL = 170 Follow‐up: six months |
Sessions: 1 Specialist delivered C:IF Ratio = 83:1 Adherence:100% |
Psych‐education |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
The change in mean AUDIT scores was not statistically different between the intervention and control groups |
| Single Brief Alcohol Reduction Intervention |
Huis in ‘t Veld et al.2019 [59] South Africa |
Alcohol use disorder Tool: AUDIT |
Gender: M & F HIV:100% ART: 85% |
RCT SBARI = 267 CONTROL = 293 Follow‐up: twelve months |
Sessions: 1 Nurse delivered C:IF Ratio = 67:1 Adherence:100% |
Psych‐education |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: Assessed |
There was no significant difference in AUDIT scores between the intervention and control groups |
| Mindfulness‐Based Stress Reduction Intervention |
SeyedAlinaghi et al; 2012 [56] Iran |
Multiple Psychological Symptoms Tool: SCL‐90‐R |
Gender: M & F HIV:100% ART:0% |
RCT MBSR = 120 CONTROL = 125 Follow‐up: twelve months |
Sessions: 8 Specialist delivered C:IF Ratio = 120:1 Adherence: 73% |
Social support Relaxation Meditation Physical activity |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: NS Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant difference in mean SCL‐90R scores between the intervention and control groups |
| Group Rational‐Emotive‐Behaviour‐Based Therapy |
Surilena et al; 2014 [48] Indonesia |
Depression Tool: SRQ‐20 |
Gender: F HIV:100% ART: 100% |
RCT REBT = 72 CONTROL = 76 Follow‐up: Post‐test |
Sessions: 8 Specialist delivered C:IF Ratio = 72:1 Adherence: NS |
Psych‐education Cognitive restructuring Behavioural activation Social support Positive coping skills |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NS Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was a greater reduction in the SRQ‐20 mean scores in the intervention group compared to the control group. |
| Group Rational‐Emotive‐Behaviour‐Based Therapy |
Omeje et al; 2018 [60] Nigeria |
Alcohol Use Tool: AUDS;AIBS |
Gender: M & F HIV:100% ART: NS |
Quasi Experimental REBT = 61 CONTROL = 63 Follow‐up: one month |
Sessions: 20 Specialist delivered C:IF Ratio = NS Adherence:100% |
Psych‐education Cognitive restructuring Behavioural activation Social support Positive coping skills |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: YES Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
The intervention led to a significant reduction in AUDS % AIBS scores in the treatment group compared to those in the control group |
| Friendship Bench‐Problem Solving Therapy and Antidepressants |
Stockton et al; 2020 [43] Malawi |
Depression Tool: PHQ‐9 TCA |
Gender: M &F HIV:100% ART:100% |
Quasi Experimental FBPST = 134 CONTROL = 290 Follow‐up: six months |
Sessions: 6 LHW delivered C:IF Ratio = 67:1 Adherence: FB = 42% TCA = 31% |
Psych‐education Problem solving Medication |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: Assessed Acceptable: Assessed Fidelity: Assessed |
The programme did not have a significant effect on depression |
| Group Problem Solving Psychotherapy |
Kaaya et al; 2013 [50] Tanzania |
Depression Tool: HSCL‐15 |
Gender: F HIV:100% ART: 0% |
RCT PST = 168 CONTROL = 163 Follow‐up: Post‐test |
Sessions: 6 Specialist delivered C:IF Ratio = NS Adherence:56% |
Psych‐education Social Support |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant difference in depression symptoms between the intervention and control groups |
| Telephone Support |
Ross et al; 2013 [41] Thailand |
Depression Tool: CESD‐20 |
Gender: F (Pregnant) HIV:100% ART: NS |
RCT TS = 20 CONTROL = 20 Follow‐up: Post‐test |
Sessions: 8 Nurse delivered C:IF Ratio = 10 Adherence: NS |
Venting Social Support |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
Depression symptoms decreased significantly more in the intervention than in the control group |
| Community Home‐Based Social Support and Peer Counselling |
Pokhrel et al; 2018 [54] Nepal |
Depression Tool: CESD‐20 |
Gender: M & F HIV:100% ART: 100% |
RCT CSPC = 344 CONTROL = 338 Follow‐up: six months |
Sessions: 6 LHW and Specialist delivered C:IF Ratio = 114 Adherence: NS |
Social Support Positive coping skills |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
The intervention was more effective in reducing depression symptoms than the control group |
| Structured Support Groups |
Mundell et al; 2011[51] South Africa |
Depression Tool: CESD‐20 |
Gender: F HIV:100% ART:0% |
Quasi Experimental CSPC = 144 CONTROL = 217 Follow‐up: eight months |
Sessions: 10 Specialist delivered C:IF Ratio = 24 Adherence:50% |
Social Support Positive coping skills |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant difference in depressive symptoms between intervention and control groups |
| Accredited Social Health Activist (ASHA‐LIFE) Intervention |
Nyamathi et al; 2012 [39] India |
Depression Tool: CESD‐20 |
Gender: F HIV:100% ART:100% |
RCT ASHA = 34 CONTROL = 34 Follow‐up: six months |
Sessions: 6 LVW delivered C:IF Ratio = 9 Adherence: NS |
Positive coping skills Psych‐education |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
There was a greater reduction in the depression scores in the intervention group compared to the control group |
| Omega‐3 Fatty acids |
Ravi et al; 2016 [44] India |
Depression Tool: BDI, PHQ, HADS |
Gender: M & F HIV:100% ART:100% |
RCT Omega‐3 Fatty acids = 54 PLACEBO = 56 Follow‐up: two months |
Sessions: NA Deliverer = Specialist C:IF Ratio = NS Adherence: 93% |
Complementary/alternative treatment |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
Depression symptoms decreased significantly in the Omega‐3 fatty acids group compared to the placebo group |
| Yoga Intervention |
Kuloor et al; 2019 [37] India |
Depression Tool: HADS |
Gender: M & F HIV:100% ART:100% |
RCT Yoga = 30 CONTROL(WL) = 30 Follow‐up: Post‐test |
Sessions: 40 Delivered = NS C:IF Ratio = NS Adherence: 90% |
Relaxation Meditation Behavioural activation Physical activity |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NS Supervision IFs: NS Feasible: Assessed Acceptable: NS Fidelity: NS |
There was significantly more reduction in depression scores among participants in the intervention than in the control group |
|
Aerobic Exercise Physical Activity and Counselling |
Aweto et al; 2016 [46] Nigeria |
Depression Tool: BDI‐II |
Gender: M & F HIV:100% ART:100% |
RCT AE = 20 CONTROL = 20 Follow‐up: Post‐test |
Sessions: 18 Delivered : NS C:IF Ratio = NS Adherence: 90% |
Physical activity Relaxation |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NS Supervision IFs: NS Feasible: NS Acceptable: NS Fidelity: NS |
There was a significantly more reduction in depression scores in the intervention than in the control group |
| Physical Activity |
Daniels et al; 2018 [33] South Africa |
Depression Tool: BDI‐II |
Gender: F HIV:100% ART: 100% |
RCT PA = 30 CONTROL = 30 Follow‐up: Post‐test |
Sessions: 6 Delivered : Specialist C:IF Ratio = NS Adherence: NS |
Physical activity |
Stakeholders: YES Community aware: YES Training IFs: NS Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant difference in the reduction of depression scores between the intervention and control groups |
|
Medication (SSRI –Antidepressants Citalopram) |
Moosa et al; 2012 [34] South Africa |
Depression Tool: HAMD, SCID |
Gender: M & F HIV:100% ART: 100% |
RCT Citalopram = 19 IPT = 13 Follow‐up: Post‐test |
Sessions: NA Delivered : Specialist C:IF Ratio = 19 Adherence: 98% |
Medication |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NA Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant difference in the reduction of depression scores between the intervention and control groups |
|
Medication (Escitalopram) |
Hoare et al; 2014 [36] South Africa |
Depression Tool: MADRS, MINI |
Gender: M & F HIV:100% ART: 100% |
RCT Escitalopram = 51 PLACEBO = 51 Follow‐up: Post‐test |
Sessions: NA Treatment duration: six Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 100% |
Medication |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NA Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was no significant effect recorded for Escitalopram over placebo on the Montgomery‐Asberg Depression Rating Scale. |
|
Medication SARI –Antidepressants (Trazodone) |
Alikhani et al; 2020 [45] Iran |
Depression, and anxiety Tool: BDI‐II |
Gender: M HIV:100% ART: 100% |
RCT Trazodone = 25 PLACEBO = 50 Follow‐up: three months |
Sessions: NA Treatment duration: 12 Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 100% |
Medication |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NA Supervision IFs: NA Feasible: NS Acceptable: NS Fidelity: NS |
There was significantly more reduction in depression scores in the intervention than in the control group |
|
Medication ‐ Minocycline |
Nakasujja et al; 2013 [63] Uganda |
HIV Associated Neurocognitive Impairment Tool: Neuropsychological Test Battery |
Gender: M & F HIV:100% ART: 0% |
RCT Minocycline = 36 PLACEBO = 37 Follow‐up: six months |
Sessions: NA Treatment time: 24Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 71% |
Medication |
Stakeholders: YES Community aware: Not mentioned Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
Minocycline had no advantage for neurocognitive impairment over the placebo. |
|
Medication ‐ Minocycline |
Emadi‐Kouchak et al; 2016 [42] Iran |
Depression Tool: HDRS |
Gender: M & F HIV:100% ART: 100% |
RCT Minocycline = 25 PLACEBO = 25 Follow‐up: Post‐test |
Sessions: NA Treatment time: 6Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 92% |
Medication |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NS Supervision IFs: NS Feasible: NS Acceptable: NS Fidelity: NS |
There was significantly more reduction in depression scores in the intervention than in the control group |
| Medication ‐Lithium |
Decloedt et al; 2016 [62] South Africa |
HIV Associated Neurocognitive Impairment Tool: GDS |
Gender: M & F HIV:100% ART: 100% |
RCT Lithium = 32 PLACEBO = 34 Follow‐up: six months |
Sessions: NA Treatment time: 24Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 45% |
Medication |
Stakeholders: YES Community aware: YES Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
Lithium had no advantage for neurocognitive impairment over the placebo. |
| Nutrition Supplement‐(Fish oil Omega fatty acids) |
Opiyo et al; 2018 [49] Kenya |
Depression Tool: BDI‐II |
Gender: F HIV:100% ART: 0% |
RCT Omega3 Fatty acid = 109 CONTROL = 107 Follow‐up: 2 months |
Sessions: NA Treatment time: 8Weeks Delivered : Specialist C:IF Ratio = NS Adherence: 79% |
Complementary/alternative treatment |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: YES Supervision IFs: YES Feasible: NS Acceptable: NS Fidelity: NS |
Omega‐3 fatty acids had no advantage for depression over the placebo |
| Herbal supplement (Saffron Herbal Capsules) |
Jalali et al; 2018 [38] Iran |
Depression Tool: BDI‐II |
Gender: M HIV:29% ART: 28% |
RCT SHC = 109 CONTROL = 107 Follow‐up: Post‐test |
Sessions: NA Treatment time: 8Weeks Delivered : Specialist C:IF Ratio = NS Adherence: NS |
Complementary/alternative treatment |
Stakeholders: Not mentioned Community aware: Not mentioned Training IFs: NS Supervision IFs: NS Feasible: NS Acceptable: NS Fidelity: NS |
There was a significantly more reduction in depression scores in the intervention than in the control group |
ADS, Addiction Severity Index; AE, Aerobic Exercise; AER, Aerobic and Resistance Exercise; AIBS, Alcohol‐related Irrational Beliefs Scale; ART, Anti‐retroviral Therapy; ASHA, Accredited Social Health Activist; AUDIT, The Alcohol Use Disorders Identification Test; AUDS, The Alcohol Use Disorder Scale; BDI‐II, Beck Depression Inventory‐II; C:IF, Client to Intervention Facilitator ratio; CBI, Cognitive Behavioural Intervention; CBSM, Cognitive Behavioural Stress Management; CBT, Cognitive Behavioural Therapy; CBT‐AD, Cognitive Behavioural Therapy with Adherence and Depression; CESD‐20, The Center for Epidemiologic Studies for Depression tool; CRCT, Cluster Randomized Controlled Trial; CSPC, Community Home‐Based Social Support and Peer Counselling; DASS‐2, Depression Anxiety Stress Scale; DST, Depression Screening Test; DST‐15, Dexamethasone Suppression Test; EPDS, Edinburg Postnatal Depression tool; F, Female; FBPST, Friendship Bench‐Problem Solving Therapy; GCBT, Group Cognitive Behavioural Therapy; GCEP, Group Coping Enhancement Programme; GDS, Global Deficit Score; GHE, Group HIV Education; GSMT, Group Stress Management Training; GSP, Group Support Psychotherapy; HADS, Hospital Anxiety and Depression Scale; HDRS, The Hamilton Depression Rating Scale; HIV, Human Immunodeficiency Virus; HSCL‐15, Hopkins Symptom Checklist for Depression‐15; IC, Individual counselling; IES – 15, The Impact of Event Scale‐15; IPT, Interpersonal Psychotherapy; LHW, Lay Health Workers; M, Male; MBSR, Mindfulness‐Based Stress Reduction; MINI, The Mini‐International Neuropsychiatric Interview; NA, Not Applicable; NS, Not Assessed; PA, Physical Activity; PCL‐5, The Post‐traumatic Stress Disorder Checklist for DSM‐5; PDA, Percent days abstinent from alcohol; PDA, Percent drinking‐days Absent; PDD, Percent drinking days; PHQ‐9, The Patient Health Questionnaire −9; PSS‐10, The Perceived Stress Scale; PST, Problem‐Solving Therapy; PST‐AD, Problem Solving Therapy of Adherence and Depression; PTGI‐21, The Post‐traumatic Growth Inventory‐21; RCT, Randomized Controlled Trial; REBT, Rational‐Emotive‐Behaviour‐Based Therapy; SARI, Serotonin Antagonist and Reuptake Inhibitor; SBARI, Single Brief Alcohol Reduction Intervention; SCID, Severe combined immunodeficiency; SCL‐90‐R, Symptom Checklist‐90‐Revised; SFGT, Schema Focused Group Therapy; SHC, Saffron Herbal Capsules; SRQ‐20, The Self‐Reporting Questionnaire; SRQ‐20, The Self‐Reporting Questionnaire‐ 20; TCA, Tricyclic Antidepressants; TFSCI, Trauma‐Focused Stress and Coping Intervention; TS, Telephone Support; WL, Wait list.
3.3. Psychological interventions
The majority of studies described psychological interventions (n = 18). Of these, 13 targeted depression. These included six cognitive behavioural therapy (CBT) based interventions [35, 38, 47, 53, 55, 61], two problem solving‐based interventions [43, 50], one rational emotive behavioural therapy [48] and four psychosocial support groups [39, 41, 51, 54]. One study described a mindfulness‐based intervention targeting multiple psychological symptoms [56]. Other studies described trauma‐focused CBT for post‐traumatic stress disorder (n = 1) [61], brief alcohol interventions (n = 2) and rational emotive behavioural therapy (n = 1) targeting alcohol use problems [41, 57, 59, 60]. Only 11 (61.1%) of the 18 studies of psychological interventions reported significant effects.
3.4. Pharmacological interventions
This review found six studies which evaluated pharmacological interventions for depression (N = 4) and HIV‐related neuro‐cognitive impairment (N = 2). One study evaluated the effect of minocycline on depression [42], whereas three evaluated the effect of antidepressants, including trazodone [45], citalopram [34] and escitalopram [36] on depression. Two studies examined the treatment efficacy of minocycline and lithium for neurocognitive impairment [61, 62]. Only the two studies evaluating minocycline and trazadone reported significant effects. Other pharmacological interventions had no benefit over placebo. This review also revealed a single study which evaluated fluoxetine in combination with problem‐solving therapy where the intervention did not have significant effects [43].
3.5. Complementary and alternative interventions
This review revealed six studies which evaluated complementary and alternative interventions for depression. The interventions evaluated included omega‐3 fatty acids [49, 52], physical exercise [33] an herbal supplement [38] and yoga [37]. The study evaluating physical exercise and one study evaluating omega‐3 fatty acids did not register any advantage over placebo.
3.6. Association between intervention components and intervention effectiveness
We assessed the relationship between various intervention components and intervention effectiveness. The intervention components included qualifications of intervention deliverer (mental health specialist vs. non‐specialist vs. lay health worker), delivery approach (individual vs. group approach), case load per intervention deliverer, number of treatment sessions, adherence rate to treatment sessions and number of active ingredients per intervention. We found that studies of interventions with significant effects had significantly a higher number of active ingredients than those without significant effects [3.41 (2.24) vs. 1.84 (1.46) Mean (SD)] [Mean difference = −1.56, 95% CI = −3.03 to −0.09, p = 0.037]. Table S1 shows the relationship between specific active ingredients and intervention effectiveness of all reviewed studies.
Studies of interventions with significant effects had higher treatment adherence rates, lower case load per intervention deliverer than studies without significant effects; however, the differences did not attain statistical significance [85.47 (25.12) vs. 77.3 (24.88) Mean (SD)] [Mean difference = −8.16, 95% CI = −27.05 to 10.72, p = 0.384] and [23.29 (29.11) vs. 42.00 (46.62) Mean (SD)] [Mean difference = 18.71, 95% CI = −9.69 to 47.11, p = 0.188] respectively. Subgroup analyses indicate that among studies of psychological interventions, those where intervention deliverers had a low case load were more likely to have significant intervention effects than those where intervention deliverers had high case load [26.27 (35.23) vs. 70.14 (47.08) Mean (SD)] [Mean difference = 43.87, 95% CI = 2.77 to 84.96, p = 0.038]. The approach used to deliver interventions and qualifications of the intervention deliverer were not associated with intervention effectiveness. Table S2 in the supplementary file shows the detailed relationships between intervention components extracted and intervention effectiveness.
3.7. Assessing the quality of the synthesis
Of the 30 studies reviewed, 13 (43.33%) received a global rating of “strong” and 13 (43.33%) other studies received a global rating of “moderate” on the EPHP quality assessment tool. The remaining four studies (13.33%) were rated as “weak.” Overall, study quality was not associated with intervention effectiveness. However, subgroup analyses indicate that randomized controlled trials of strong quality were more likely to report non‐significant effects than those of moderate quality [72.43% vs. 18.18%, X2 = 4.89; p = 0.08]. Table S3 shows the quality assessment ratings of all reviewed studies.
4. Discussion
This review indicates that there have been advances in mental health treatments for PLWH in LMICs since the review by Collins and colleagues in 2006 [64] and the review by Sikkema and colleagues in 2015 [24]. Data presented indicate strong evidence that common mental health problems including depression, anxiety and PTSD are responsive to first‐line psychological treatments in LMICs. All reviewed RCTs of anti‐depressants for depression treatment, however, did not have any advantage over placebo.
Prior research in LMICs indicates significant challenges in anti‐depressant use. A large cluster randomized controlled trial of task‐shifting delivery models of antidepressants in Uganda [65] found that perfect adherence to antidepressants was the sole predictor of treatment adherence, yet perfect adherence was achieved by only 56% of treated PLWH. In Malawi, integration of depression management using antidepressants did not improve depression and was hindered by a nationwide stock out of antidepressants [66]. These findings coupled with prior evidence [67] and recommendations [68], support first‐line use of psychological interventions over pharmacological interventions in mental healthcare of PLWH in LMICs.
In this review, almost two‐thirds of psychological interventions evaluated were found to be effective for major depression, depression symptoms and alcohol use problems. However, only group support psychotherapy in Uganda [35], and the mobile health intervention in China [47] demonstrated sustained remission of depression. The scarcity of reported long‐term intervention effects calls for more large‐scale conclusive trials providing long‐term follow‐up data.
There was a low yield of research activity focusing on how and for whom interventions work. A study from China [62] suggests that interventions delivered via mobile health may work better for young single individuals than older married ones. This should stimulate more research on how mobile technologies could be used to increase accessibility to mental health services among the younger population in LMICs. A study from Uganda [34] suggests that a culturally sensitive group supports psychotherapy intervention delivered to gender‐specific groups leads to greater improvement among men than women 12‐month post‐treatment. In the reviewed studies, only 2664 men participated in the interventions compared to 3638 women. Interventions that attract men may make it possible to address issues like the perpetration of domestic violence and alcohol and drug use problems which are some of the drivers of the HIV epidemic [69, 70]. Provision of psychological treatments to both men and women would ensure holistic care for communities in LMICs.
The findings from this review indicated that active ingredients of mental health intervention may be critical for intervention effectiveness. Effective mental health interventions were more likely to have three or more active ingredients. The active ingredients associated with intervention effectiveness were cognitive restructuring, positive coping skills, venting (sharing personal problems) social support and behaviour activation. Future studies of intervention development should find simple and culturally appropriate ways of incorporating these active ingredients.
The Lancet Psychiatry Commission on psychological treatments research recommends that psychological treatments can be simplified and shaped in line with local cultural norms and conditions [71]. For example if one of the major maintaining factors of depression concerns lack of behaviour activation in daily life, then treatment strategies to increase behaviour activation can be formed in many different ways depending on what is the most relevant, acceptable, and affordable in the specific context or culture in which the problem exists.
This review showed that intervention adherence rates may be important for intervention effectiveness. Interventions tailored to the cultural context of the target population are more likely enhance treatment adherence rates than western interventions brought into a LMIC setting.
Although the approach used to deliver interventions and qualifications of the intervention deliverers were not associated with intervention effectiveness, interventions delivered in a group format and by lay health workers are more likely to ensure accessibility and sustainability of mental health interventions in LMICs. Given that our review also found that case load per intervention deliverer is critical to intervention effectiveness, counselling training programmes for lay health workers will be important in narrowing the mental health treatment gap in LMICs.
Contextual influences may also affect intervention effects. Only half of the studies reviewed described some form of stakeholder involvement and public involvement and these were not associated with significant intervention effects. A lack of stakeholder buys‐in may limit the extent to which the target population engages with activities required to deliver the intervention. The study integrating depression management with anti‐depressants in Malawi reports how Government officials failed to provide antidepressants at health facilities participating in intervention evaluations. Furthermore, the stakeholders failed to nominate community health workers who had a keen interest and were ready to commit time to deliver the interventions. Thus, therapy sessions had to be delivered by only one study employed community health worker [66].
Creating awareness through community sensitization meetings may go a long way in ensuring that community members support affected individuals to get to their therapy meetings. Involving the target population in intervention design is crucial in ensuring that the target population adheres to the intervention sessions. Including content and formats desired by the target population increases community ownership of the intervention. Nakimuli‐Mpungu et al [35] included gender‐specific groups, cultural rituals, livelihood skills – all requested for by the target population – moved therapy sessions to villages and provided a small financial incentive to intervention deliverers, thereby eliminating financial and transport barriers reported frequently by other studies.
In other studies, spiritual activities have been included into therapy sessions to make the intervention more meaningful to the target population and thereby enhance adherence to the intervention [53]. Recent research indicates that accommodating patient preference in mental health services maximizes treatment uptake and reduces financial costs of premature dropout and disengagement [72].
Several limitations need to be acknowledged, which mostly arise from the inherent risk of bias at review (e.g. incomplete retrieval of identified research and reporting bias). This emphasizes a need for a thoughtful interpretation of our results. One major problem we found was inconsistent reporting of interventions, with some studies providing sufficient detail about the mental health intervention, whereas others offered limited descriptions. Thus, our analyses of the association between specific intervention components with intervention effectiveness were limited by the description of the interventions provided in the reviewed articles. There is a risk that some interventions included components that were not reported and that some of the reported components were not received by all patients in the study. For example descriptions of stake holder or public involvement, training and supervision of intervention deliverers were not forthcoming for many studies.
Going forward, there is a need for conclusive trials of mental health interventions for PLWH with severe mental disorders. None of the reviewed studies described a mental health intervention for PLWH with severe mental disorders, including psychotic disorders. Second, this review did not find any definitive trials of mental health interventions among PLWH in Latin America. Given that the region still grapples with the HIV epidemic, there is an urgent need to address the mental health needs of PLWH in this region. Third, more research focused on intervention components such as intervention deliverers and active ingredients in mental health interventions would shed more light on which components lead to symptom remission and ultimately improve intervention effectiveness. Future studies could pool datasets across LMICs and use patient‐level to explore the mediating and moderating role of intervention components on the effects of mental health interventions in PLWH. Lastly, more evidence is needed for long‐term outcomes of mental health interventions for PLWH which were limited in the studies reviewed.
5. Conclusions
Sufficient evidence supports the presence of effective psychological treatments for common mental health problems in PLWH, including depression anxiety, and alcohol use disorders. Potential interventions using social media and mobile technologies should be explored given the COVID‐19 pandemic. Culturally appropriate, feasible and acceptable interventions that have been successfully piloted and fully evaluated in LMICs should be scaled up.
Evaluative research should be integral to national HIV care programmes, including access to adequate funding to encourage and permit the necessary studies. Because mental health treatment is critical for the success of the Treat All Policy, the implementation of proven, evidence‐based, and cost‐effective strategies should be the duty and responsibility of public health policy makers and healthcare providers.
Competing interest
All authors declare no competing interests.
Author’s contributions
EN‐M, JJ, CMS, MVI, BA, MR and SM conceptualized the study. MVI, MR and AVW managed the literature searches. EN‐M, BA and CMS conducted statistical analyses. EN‐M, CMS, BA and MVI wrote the initial manuscript. SM, JJ, JB, EM, AS and DC revised the manuscript critically for important intellectual content. All authors contributed to the final manuscript.
Supporting information
Table S1. Association between active ingredients and intervention effectiveness
Table S2. Relationship between intervention components and intervention effectiveness
Table S3. Quality Assessment of Quantitative studies of Mental Health interventions for Persons Living with HIV in LMIC
Figure S1. Search strategy
Acknowledgement
We acknowledge Augustine Mutale for his assistance in the preparation of this manuscript.
Disclaimer
The views expressed are the authors’ own and do not necessarily represent the views the United States government or any of its agencies.
Nakimuli‐Mpungu, E. , Musisi, S. , Smith, C. M. , Von Isenburg, M. , Akimana, B. , Shakarishvili, A. , Nachega, J. B. , Mills, E. J. , Chibanda, D. , Ribeiro, M. , V Williams, A. and Joska, J. A. Mental health interventions for persons living with HIV in low‐ and middle‐income countries: a systematic review. J Int AIDS Soc. 2021; 24(S2):e25722
PROSPERO Number: CRD42020219483.
Contributor Information
Etheldreda Nakimuli‐Mpungu, Email: ethelnakimuli@chs.mak.ac.ug.
Seggane Musisi, Email: segganemusisi@yahoo.ca.
Colin M Smith, Email: colin.smith@duke.edu.
Megan Von Isenburg, Email: megan.vonisenburg@duke.edu.
Benedict Akimana, Email: akimben@gmail.com.
Ani Shakarishvili, Email: shakarishvilia@unaids.org.
Jean B Nachega, Email: jbn16@pitt.edu.
Edward J Mills, Email: emills@mteksciences.com.
Dixon Chibanda, Email: dichi@zol.co.zw.
Marcelo Ribeiro, Email: marcelorabr@gmail.com.
Anna V Williams, Email: anna.v.williams@kcl.ac.uk.
John A Joska, Email: john.joska@uct.ac.za.
References
- 1. Brown GR, Rundell JR, McManis SE, Kendall SN, Zachary R, Temoshok L. Prevalence of psychiatric disorders in early stages of HIV infection. Psychosomat Med. 1992;54(5):588–601. [DOI] [PubMed] [Google Scholar]
- 2. Lyketsos CG, Hoover DR, Guccione M. Depression and survival among HIV‐infected persons. J Am Med Assoc. 1996;275(1):35–6. [DOI] [PubMed] [Google Scholar]
- 3. Chibanda D, Cowan FM, Healy JL, Abas M, Lund C. Psychological interventions for common mental disorders for people living with HIV in low‐and middle‐income countries: systematic review. Tropical Med Int Health. 2015;20(7):830–9. [DOI] [PubMed] [Google Scholar]
- 4. Patel V, Shekhar SS, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The lancet commission on global mental health & sustainable development. Lancet. 2018;pii:S0140. [DOI] [PubMed] [Google Scholar]
- 5. Political declaration of the third high‐level meeting of the general assembly on the prevention and control of non‐communicable diseases. New York: United Nations General Assembly; 2018. [Google Scholar]
- 6. Nakimuli‐Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub‐Saharan Africa: a systematic review. AIDS Behav. 2012;16(8):2101–18. 10.1007/s10461-011-0087-8 [DOI] [PubMed] [Google Scholar]
- 7. Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient‐reported barriers to adherence to antiretroviral therapy: a systematic review and meta‐analysis. PLoS Medicine. 2016;13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Passchier RV, Abas MA, Ebuenyi ID, Pariante CM. Effectiveness of depression interventions for people living with HIV in Sub‐Saharan Africa: A systematic review and metanalysis of psychological and immunological outcomes. Brain Behav Immun. 2018;73:261–73. [DOI] [PubMed] [Google Scholar]
- 9. Ciesla JA, Roberts JE. Meta‐analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. [DOI] [PubMed] [Google Scholar]
- 10. Patel P, Rose CE, Collins PY, Nuche‐Berenguer B, Sahasrabuddhe VV, Peprah E, et al. Noncommunicable diseases among HIV‐infected persons in low‐income and middle‐income countries: a systematic review and meta‐analysis. AIDS. 2018;32 Suppl 1:S5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low‐income and middle‐income countries: a systematic review. AIDS. 2012;26 Suppl 2:S117–S135. [DOI] [PubMed] [Google Scholar]
- 12. Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudfeld CR, Kaaya S, Gunaratna NS, Mugusi F, Fawzi WW, Aboud S, et al. Depression at antiretroviral therapy initiation and clinical outcomes among a cohort of Tanzanian women living with HIV. AIDS. 2017;31(2):263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Todd JV, Cole SR, Pence BW, Lesko CR, Bacchetti P, Cohen MH, et al. Effects of antiretroviral therapy and depressive symptoms on all‐cause mortality among HIV‐infected women. Am J Epidemiol. 2017;185(10):869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaida R, Truter I, Grobler C, Kotze T, Godman B. A review of trials investigating efavirenz‐induced neuropsychiatric side effects and the implications. Expert Rev Anti‐Infective Therapy. 2016;14(4):377–88. [DOI] [PubMed] [Google Scholar]
- 16. Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 2014;28(2):131–45. [DOI] [PubMed] [Google Scholar]
- 17. Mental health and human rights: Report of the United Nations High Commissioner for Human Rights. In: Rights UNHCfH, editor. A/HRC/34/32. New York: United Nations General Assembly; 2017.
- 18. WHO 2015 Policy brief: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection – What’s new. November 2015. Available from: https://www.who.int/hiv/pub/arv/policy‐brief‐arv‐2015/en
- 19. The 43rd UNAIDS programme Coordinating Board Meeting scheduled. 11‐13 December 2018. Geneva, Switzerland.
- 20. Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS (London, England). 2019;33(9):1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. Lancet. 2018;392(10157):1553–98. [DOI] [PubMed] [Google Scholar]
- 22. Shi Y, Zhao M, Chen S, Wang S, Li H, Ying J, et al. Effects of cognitive behavioral therapy on people living with HIV and depression: a systematic review and meta‐analysis. Psychol Health Med. 2019;24(5):578–94. [DOI] [PubMed] [Google Scholar]
- 23. Spaan P, van Luenen S, Garnefski N, Kraaij V. Psychosocial interventions enhance HIV medication adherence: a systematic review and meta‐analysis. J Health Psychol. 2018;25(10‐11):1326–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sikkema KJ, Dennis AC, Watt MH, Choi KW, Yemeke TT, Joska JA. Improving mental health among people living with HIV: a review of intervention trials in low‐and middle‐income countries. Global Mental Health. 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craig P, Dieppe P, Macintyre S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Br Med J. 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boutron I, Ravaud P. Reporting guidelines for nonpharmacological trials. In: Boutron I, Ravaud P, Moher D, editors. Randomized clinical trials of nonpharmacological treatments. Boca Raton, FL: Chapman & Hall/CRC Press; 2012. p. 199–210. [Google Scholar]
- 27. De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of change: a theory‐driven approach to enhance the Medical Research Council's framework for complex interventions. Trials. 2014;15(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ 2015; 350: g 7647. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Booth, A. , Clarke, M. , Dooley, G. , Ghersi, D. , Moher, D. , Petticrew, M. , and Stewart, L. , 2012. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Systematic reviews, 1 (1), pp.1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. Lancester, UK: Lancester University; [cited 2017 Nov 6]. 2006. [Google Scholar]
- 32. Effective Public Health Practice Project . Quality assessment tool for quantitative studies: Effective Public Health Practice Project, 1998. (accessed 30 August 2020)
- 33. Daniels AK, Van Niekerk RL. The impact of a therapeutic exercise intervention on depression and body self‐image in HIV‐positive women in sub‐Saharan Africa. Hiv/aids (Auckland, NZ). 2018;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moosa MY, Jeenah FY. Antidepressants versus interpersonal psychotherapy in treating depression in HIV positive patients. South Afr J Psychiatry. 2012;18(2):47–52. [Google Scholar]
- 35. Nakimuli‐Mpungu E, Musisi S, Wamala K, Okello J, Ndyanabangi S, Birungi J, et al. Effectiveness and cost‐effectiveness of group support psychotherapy delivered by trained lay health workers for depression treatment among people with HIV in Uganda: a cluster‐randomised trial. Lancet Global Health. 2020;8(3):e387–e398. [DOI] [PubMed] [Google Scholar]
- 36. Hoare J, Carey P, Joska JA, Carrara H, Sorsdahl K, Stein DJ. Escitalopram treatment of depression in human immunodeficiency virus/acquired immunodeficiency syndrome: a randomized, double‐blind, placebo‐controlled study. J Nerv Ment Dis. 2014;202(2):133–7. [DOI] [PubMed] [Google Scholar]
- 37. Kuloor A, Kumari S, Metri K. Impact of yoga on psychopathologies and quality of life in persons with HIV: a randomized controlled study. J Bodywork Movement Therapies. 2019;23(2):278–83. [DOI] [PubMed] [Google Scholar]
- 38. Jalali F, Hashemi SF. The effect of saffron on depression among recovered consumers of methamphetamine living with HIV/AIDS. Subst Use Misuse. 2018;53(12):1951–7. [DOI] [PubMed] [Google Scholar]
- 39. Nyamathi A, Ekstrand M, Salem BE, Sinha S, Ganguly KK, Leake B. Impact of Asha intervention on stigma among rural Indian women with AIDS. West J Nurs Res. 2013;35(7):867–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jalali F, Hasani A, Hashemi SF, Kimiaei SA, Babaei A. Cognitive group therapy based on schema‐focused approach for reducing depression in prisoners living with HIV. Int J Offender Therapy Comparat Criminol. 2019;63(2):276–88. [DOI] [PubMed] [Google Scholar]
- 41. Ross R, Sawatphanit W, Suwansujarid T, Stidham AW, Drew BL, Creswell JW. The effect of telephone support on depressive symptoms among HIV‐infected pregnant women in Thailand: an embedded mixed methods study. J Assoc Nurses AIDS Care. 2013;24(5):e13–24. [DOI] [PubMed] [Google Scholar]
- 42. Emadi‐Kouchak H, Mohammadinejad P, Asadollahi‐Amin A, Rasoulinejad M, Zeinoddini A, Yalda A, et al. Therapeutic effects of minocycline on mild‐to‐moderate depression in HIV patients: a double‐blind, placebo‐controlled, randomized trial. Int Clin Psychopharmacol. 2016;31(1):20–6. [DOI] [PubMed] [Google Scholar]
- 43. Stockton MA, Udedi M, Kulisewa K, Hosseinipour MC, Gaynes BN, Mphonda SM, et al. The impact of an integrated depression and HIV treatment program on mental health and HIV care outcomes among people newly initiating antiretroviral therapy in Malawi. PLoS One. 2020;15:e0231872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ravi S, Khalili H, Abbasian L, Arbabi M, Ghaeli P. Effect of omega‐3 fatty acids on depressive symptoms in HIV‐positive individuals: a randomized, placebo‐controlled clinical trial. Ann Pharmacother. 2016;50(10):797–807. [DOI] [PubMed] [Google Scholar]
- 45. Alikhani M, Ebrahimi A, Farnia V, Khazaie H, Radmehr F, Mohamadi E, et al. Effects of treatment of sleep disorders on sleep, psychological and cognitive functioning and biomarkers in individuals with HIV/AIDS and under methadone maintenance therapy. J Psychiatr Res. 2020;130:260–72. [DOI] [PubMed] [Google Scholar]
- 46. Aweto HA, Aiyegbusi AI, Ugonabo AJ, Adeyemo TA. Effects of aerobic exercise on the pulmonary functions, respiratory symptoms and psychological status of people living with HIV. J Res Health Sci. 2016;16(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- 47. Guo Y, Hong YA, Cai W, Li L, Hao Y, Qiao J, et al. Effect of a weChat‐based intervention (Run4Love) on depressive symptoms among people living with HIV in China: randomized controlled trial. J Med Internet Res. 2020;22:e16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Surilena S, Ismail RI, Irwanto I, Djoerban Z, Utomo B, Sabarinah S, et al. The effect of rational emotive behavior therapy (REBT) on antiretroviral therapeutic adherence and mental health in women infected with HIV/AIDS. Acta Medica Indonesiana. 2014;46(4):283–91. [PubMed] [Google Scholar]
- 49. Opiyo RO, Nyasulu PS, Koigi RK, Obondo A, Ogoyi D, Kogi‐Makau W. Effect of fish oil omega‐3 fatty acids on reduction of depressive symptoms among HIV‐seropositive pregnant women: a randomized, double‐blind controlled trial. Ann Gen Psychiatry. 2018;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaaya SF, Blander J, Antelman G, Cyprian F, Emmons KM, Matsumoto K, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV‐positive women on prenatal depression and disclosure of HIV status. AIDS Care. 2013;25(7):854–62. [DOI] [PubMed] [Google Scholar]
- 51. Mundell JP, Visser MJ, Makin JD, Kershaw TS, Forsyth BW, Jeffery B, et al. The impact of structured support groups for pregnant South African women recently diagnosed HIV positive. Women Health. 2011;51(6):546–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravi S, Khalili H, Abbasian L, Arbabi M, Ghaeli P. Effect of omega‐3 fatty acids on depressive symptoms in hiv‐positive individuals: a randomized, placebo‐controlled clinical trial. Ann Pharmacother. 2016;50(10):797–807. [DOI] [PubMed] [Google Scholar]
- 53. Li L, Lee SJ, Jiraphongsa C, Khumtong S, Iamsirithaworn S, Thammawijaya P, et al. Improving the health and mental health of people living with HIV/AIDS: 12‐month assessment of a behavioral intervention in Thailand. Am J Public Health. 2010;100(12):2418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pokhrel KN, Sharma VD, Pokhrel KG, Neupane SR, Mlunde LB, Poudel KC, et al. Investigating the impact of a community home‐based care on mental health and anti‐retroviral therapy adherence in people living with HIV in Nepal: a community intervention study. BMC Infect Dis. 2018;18(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nobakht A, Mohraz M, Rahimzadeh M, Tehranizadeh M, Behboodi‐Moghadam Z, Esmaelzadeh‐Saeieh S. The effect of cognitive behavioural therapy on depression, anxiety, and stress in women with HIV. HIV AIDS Rev. 2018;17(3):218–23. [Google Scholar]
- 56. SeyedAlinaghi S, Jam S, Foroughi M, Imani A, Mohraz M, Djavid GE, et al. Randomized controlled trial of mindfulness‐based stress reduction delivered to human immunodeficiency virus–positive patients in Iran: effects on CD4+ T Lymphocyte count and medical and psychological symptoms. Psychosom Med. 2012;74(6):620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wandera B, Tumwesigye NM, Nankabirwa JI, Mafigiri DK, Parkes‐Ratanshi RM, Kapiga S, et al. Efficacy of a single, brief alcohol reduction intervention among men and women living with HIV/AIDS and using alcohol in Kampala, Uganda: a randomized trial. J Int Assoc Providers AIDS Care. 2017;16(3):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Papas RK, Gakinya BN, Baliddawa JB, Martino S, Bryant KJ, Meslin EM, et al. Ethical issues in a stage 1 cognitive‐behavioral therapy feasibility study and trial to reduce alcohol use among HIV‐infected outpatients in western Kenya. J Empirical Res Human Res Ethics. 2012;7(3):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huis in‘t Veld D, Ensoy‐Musoro C, Pengpid S, Peltzer K, Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLoS One. 2019;14:e0220799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Omeje JC, Otu MS, Aneke AO, Adikwu VO, Nwaubani OO, Chigbu EF, et al. Effect of rational emotive health therapy on alcohol use among community‐dwelling, HIV‐positive patients. Medicine. 2018;97:e11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ye Z, Chen L, Lin D. The relationship between posttraumatic stress disorder symptoms and posttraumatic growth among HIV‐infected men who have sex with men in Beijing, China: the mediating roles of coping strategies. Front Psychol. 2018;9:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Decloedt EH, Freeman C, Howells F, Casson‐Crook M, Lesosky M, Koutsilieri E, et al. Moderate to severe HIV‐associated neurocognitive impairment: a randomized placebo‐controlled trial of lithium. Medicine. 2016;95:e5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, Katabira E, et al. Randomized trial of minocycline in the treatment of HIV‐associated cognitive impairment. Neurology. 2013;80(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS (London, England). 2006;20(12):1571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wagner G, Ghosh‐Dastidar B, Ngo V, Robinson E, Musisi S, Glick P, et al. A cluster randomized controlled trial of two task‐shifting depression care models on depression alleviation and antidepressant response among HIV clients in Uganda. Res Adv Psychiatry. 2016;3(1):12. [PMC free article] [PubMed] [Google Scholar]
- 66. Udedi M, Stockton MA, Kulisewa K, Hosseinipour MC, Gaynes BN, Mphonda SM, et al. Integrating depression management into HIV primary care in central Malawi: the implementation of a pilot capacity building program. BMC Health Serv Res. 2018;18(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta‐analytic review. J Clin Psychiatry. 2013;74(6):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. World Health Organization . Task shifting: global recommendations and guidelines [cited 2017 Oct 19]. Available from: http://www.who.int/healthsystems/task_shifting/en/
- 69. Eaton LA, Cain DN, Pitpitan EV, Carey KB, Carey MP, Mehlomakulu V, et al. Exploring the relationships among food insecurity, alcohol use, and sexual risk taking among men and women living in South African townships. J Primary Prevent. 2014;35(4):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. El‐Bassel N, Shaw SA, Dasgupta A, Strathdee SA. Drug use as a driver of HIV risks: re‐emerging and emerging issues. Curr Opin HIV AIDS. 2014;9(2):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, et al. The lancet psychiatry commission on psychological treatments research in tomorrow's science. Lancet Psychiatry. 2018;5(3):237–86. [DOI] [PubMed] [Google Scholar]
- 72. Windle E, Tee H, Sabitova A, Jovanovic N, Priebe S, Carr C. Association of patient treatment preference with dropout and clinical outcomes in adult psychosocial mental health interventions: a systematic review and meta‐analysis. JAMA Psychiatry. 2020;77(3):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between active ingredients and intervention effectiveness
Table S2. Relationship between intervention components and intervention effectiveness
Table S3. Quality Assessment of Quantitative studies of Mental Health interventions for Persons Living with HIV in LMIC
Figure S1. Search strategy


