Abstract
According to preliminary data, seroconversion after mRNA SARS-CoV-2 vaccination might be unsatisfactory in Kidney Transplant Recipients (KTRs). However, it is unknown if seronegative patients develop at least a cellular response that could offer a certain grade of protection against SARS-CoV-2. To answer this question, we prospectively studied 148 recipients of either kidney (133) or kidney-pancreas (15) grafts with assessment of IgM/IgG spike (S) antibodies and ELISpot against the nucleocapside (N) and the S protein at baseline and 2 weeks after receiving the second dose of the mRNA-1273 (Moderna) vaccine. At baseline, 31 patients (20.9%) had either IgM/IgG or ELISpot positivity and were considered to be SARS-CoV-2-pre-immunized, while 117 (79.1%) patients had no signs of either cellular or humoral response and were considered SARS-CoV-2-naïve. After vaccination, naïve patients who developed either humoral or cellular response were finally 65.0%, of which 29.9% developed either IgG or IgM and 35.0% S-ELISpot positivity. Factors associated with vaccine unresponsiveness were diabetes and treatment with antithymocytes globulins during the last year. Side effects were consistent with that of the pivotal trial and no DSAs developed after vaccination. In conclusion, mRNA-1273 SARS-CoV-2 vaccine elicits either cellular or humoral response in almost two thirds of KTRs.
KEYWORDS: clinical research / practice, infection and infectious agents - viral, infectious disease, kidney transplantation / nephrology, vaccine
Abbreviations: ATG, antithymocyte globulins; DSAs, donor-specific antibodies; eGFR, estimated glomerular filtration rate; IFN-γ, Interferon- γ; KTRs, kidney transplant recipients; MSIP, membrane ELISPot plates; PBMCs, peripheral blood mononuclear cells; PCR, polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SOT, solid-organ transplant; TMB, tetramethylbenzidine
1. INTRODUCTION
Since the beginning of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, there have been over 130 million reported cases and over 3 million deaths.1 The speed of the SARS-CoV-2 expansion causing a world pandemic has led to the rapid development of numerous vaccines, several of which are already approved for emergency use in humans in the United States and Europe.2 Among the mRNA vaccines, the BNT162b2 (Comirnaty) (Pfizer/BioNTech)3 and mRNA-1273 (Moderna)4 mRNA vaccines have data on immunocompetent people showing 94.1%–95% efficacy in preventing Coronavirus Disease 2019 (COVID-19).
Kidney transplant recipients (KTR) are among the most vulnerable groups of patients to develop severe COVID-19 with higher reported morbidity and mortality compared to the general population.5 There were neither solid-organ transplant (SOT) recipients nor immunosuppressive patients in the phase 3 trials of the Moderna and Pfizer vaccines. Despite the lack of information on the safety and immunogenicity of new mRNA vaccines against COVID-19 in this population and considering that the potential benefits of the vaccine likely outweigh the risks, both the European Society for Organ Transplantation and the American Society for Transplantation recommend vaccination of SOT recipients.
Previous studies in the setting of influenza vaccination have shown that the influenza vaccine efficacy is not optimal in KTRs,6 , 7 and further studies have shown that additional doses or higher doses are needed to increase immunogenicity.8 , 9 The authors of a recent study of solid-organ transplant recipients receiving mRNA vaccines (48% received the mRNA-1273 vaccine) could show that only 17% of the patients developed a humoral response (anti-S1 or antireceptor-binding domain) at a median of 20 days after the first vaccine dose.10
Our hypothesis is that the elicited humoral and cellular immune responses to mRNA-1273 SARS-CoV-2 vaccination in KTRs could be lower than the reported response in the general population due to both immunosuppressive therapy and primary underlying co-morbid conditions. The primary objective of our study was to evaluate cellular and humoral responses in KTRs who received the mRNA-1273 (Moderna) vaccine.
2. MATERIAL AND METHODS
One-hundred and sixty-six (166) patients who were actively followed-up at the Hospital Clínic of Barcelona after kidney transplantation were initially screened. A total of 148 of these patients were recipients of a kidney graft and 18 recipients of both pancreas and kidney grafts. Exclusion criteria for receiving the vaccine and entering the study included age <18 years, transplantation within the last 3 months, having received antithymocyte globulins (ATG) or Rituximab in the last 3 months for rejection, and active SARS-CoV-2 infection. History of previous COVID-19 was not an exclusion criterion, and patients were considered for vaccination 3 months after the infection episode. Finally, 162 patients received the first dose of the mRNA-1273 SARS-CoV-2 vaccine (Moderna), as one patient received another vaccine and three refused to participate in the study. Thirteen patients out of this population were excluded from the final analysis, since data were incomplete in 12 cases and 1 patient was excluded due to COVID-19 3 weeks after the first dose. So,the final population included 148 patients (133 recipients of a kidney and 15 recipients of kidney-pancreas grafts). All of these patients received the two doses of the vaccine, and complete data were available. A study flow-chart is depicted in Figure S1.
After signing the informed consent, blood samples were withdrawn from patients at baseline and 2 weeks after the second dose of the mRNA-1273 SARS-CoV-2 vaccine (100 mcg administered in the deltoid region, 4 weeks apart from the first dose). In patients who tested to be IgG positive, another blood sample was withdrawn at 2–3 weeks after the first dose. The choice of the time-points was based on the previous experience of the phase-1 trial.11 The Institutional Ethics Committee approved the study (code HCB/2021/0222).
At all the time-points, we studied the antibody response against the S protein (IgM/IgG) and the cellular response to both the nucleocapside (N) and spike (S) proteins of SARS-CoV-2 virus by means of the ELISpot technique.
Patients were further categorized as either SARS-CoV-2-naïve or SARS-CoV-2-pre-immunized according to the baseline status before receiving the vaccine. If patients proved to have either cellular or humoral response at baseline, they were defined as “pre-immunized,” considering this baseline immunity to derive from previous exposure to SARS-CoV-2. In all the other cases patients were defined as “naïve.”
The objective of the study was to determine the biological response to the vaccine in SARS-CoV-2 naïve patients, defined as positive if patients developed 2 weeks after the second dose either antibodies (IgM or IgG) or cellular response to the S protein, assessed through the ELISpot technique. No-response to vaccine was defined as negativity of both antibodies and ELISpot assay 2 weeks after the second dose of the vaccine. Results on patients who proved to be pre-immunized to SARS-CoV-2 are presented apart.
Secondary outcomes included the analysis of all the baseline factors associated with no-response to the vaccine for either cellular or humoral response or both. Safety analysis included phone interview with patients 48–72 h after each dose in order to assess the patients’ reported short-term side-effects, defined on a semiquantitative scale as none/mild/moderate/severe. As a safety measure, also donor-specific antibodies were assessed at baseline and 2 weeks after the second dose by Luminex technique; an allele was considered positive if the MFI was greater than 1500 and 4 times higher than the Lowest Reactive Antigen (LRA) of the same locus.12
2.1. Quantification of antibodies to SARS-CoV-2 by Luminex
In order to establish seroprevalence, we used a serological assay based on the Luminex technique that has the benefit of a higher dynamic range than other assays, favoring the quantification of immunoglobulin levels. We measured antibodies against the Receptor-Binding Domain (RBD) of the spike glycoprotein of SARS-CoV-2 by Luminex.13 Crude median fluorescent intensities (MFI) were exported using the xPONENT software. Assay cutoff was calculated as the mean plus 2 standard deviations of log10-transformed MFIs of a donor pool of 30 negative samples obtained before the COVID-19 pandemic. The data used for the calculations were the ratio of the raw MFI of the particular individual with the raw MFI obtained from the donor pool, and a value ≥1 was considered to be positive. Sensitivity of the assay using samples from participants previously diagnosed with COVID-19 and with more than 10 days since the onset of symptoms was 97% for IgG and 75% for IgM, with specificities of 100% for IgG and IgM.
2.2. IFN-γ ELISpot
Stimulation was conducted with 2 × 105 PBMCs in X-VIVOTM 15 medium (Lonza) supplemented with 10% heat inactivated AB serum and PepTivator® SARS-CoV-2 Prot_S and N peptide pools1 (1 µg/ml, Miltenyi Biotec). The diluent was PBS+DMSO 20% with final concentration of DMSO 1%. In the negative control of the ELISpot, the X-VIVO 15 medium was employed with DMSO 20% to a final concentration of 1%. Negative control wells lacked peptides, and positive control wells included mAb CD3-2 of Kit. Cells were incubated overnight (16–20 h) at 37℃ 5% CO2 in precoated anti-IFN-γ MSIP white plates (mAb 1-D1K, Mabtech). Plates were then washed five times with PBS (Sigma-Aldrich) and incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated anti-IFN-γ detection antibody (1 μg/ml; clone mAb-7B6-1; Mabtech). After five further washes with PBS, tetramethylbenzidine (TMB) substrate was added and spots were counted using an automated ELISpot Reader System (Autoimmun Diagnostika GmbH).
To quantify positive peptide-specific responses, spots of the unstimulated wells were subtracted from the peptide-stimulated wells and the results expressed as Spot forming units SFU/2x105 PBMCs. We determined SARS-CoV-2-specific spots by spot increment, defined as stimulated spot numbers ≥6 SFU/2 × 105 PBMCs. This cutoff was defined calculating the mean ±2 standard deviations in a group of healthy donors obtained prior to the start of the pandemic of SARS-CoV-2. Spot counting was done automatically and re-evaluated manually in all cases.
2.3. Statistical analysis
Description of baseline characteristics was tabulated by groups defined as pre-immunized and naïve to SARS-CoV-2. Continuous variables have been described as mean with standard deviation or median and interquartilic range [25th; 75th percentiles], according to data distribution and differences between groups were analyzed by means t-test for independent or Mann-Whitney U test, respectively. Categorical variables have been described as either absolute frequencies or percentages and analyzed by Fisher’s Exact test. Estimation of vaccine no-response risk was assessed by odds ratio (OR) and their 95% confidence intervals (95% CI) by means of univariate logistic regression models taking into account the following independent variables: age, sex, diabetes, type of transplant (kidney-pancreas versus kidney), treatment with anti-thymocyte globulins (ATG) during the last year, lymphopenia defined as <1000/mm3, time from transplantation <1 year, eGFR (CKD-EPI), baseline immunosuppression, according to individual drug or the combination received by the patient, type of donor, BMI, ethnicity, and blood type. In order to establish independent factors predicting lack of response to the vaccine, variables that were associated with the chosen outcome with a p ≤ .010 were finally entered into a multivariable logistic model. Changes in the ELISpot and antibody titres through time points were assessed by Wilcoxon signed-rank test for related samples. Differences in the ELISpot and antibody titres between groups were analyzed by Mann-Whitney U test for independent samples. In all statistical analyses, we applied a two-sided type I error of 5%. To perform all the analysis, the software SPSS v.25 (IBM Corp., Armonk, NY) has been used. Figures were designed with GraphPad v.5 (GraphPad Software).
3. RESULTS
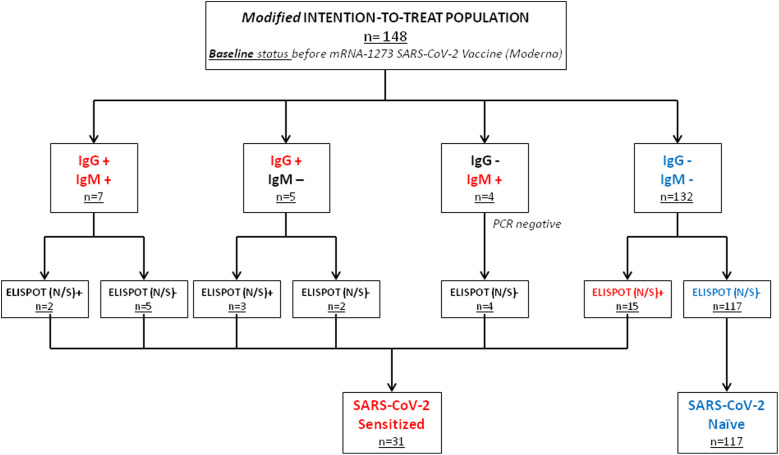
Of the final population including 148 patients, 31 were SARS-CoV-2-pre-immunized (n = 31) at baseline, for presenting either positive S protein antibodies (IgM or IgG, n = 16), or a N/S protein positive ELISpot (n = 15). Of these patients, only five had a history of proven infection assessed by PCR swab and two more had close contact with positive cases in the family. The other 117 patients were negative for both S protein antibodies and ELISpot tests and were therefore considered as SARS-CoV-2 naïve and none of them had history of clinically evident COVID-19 ( Figure 1). Patients who were IgM positive but IgG negative (n = 4, all ELISpot negative) were tested with a PCR 48 h after the analysis in order to rule out acute infection, and in all cases the PCR was negative.
FIGURE 1.
Study population according to the baseline status. Patients are highlighted in red if they had evidence of previous immunization against SARS-CoV-2 and in blue in the absence of previous immunization
3.1. Baseline characteristics
Mean age of the studied population was 57.62 ± 14.32, being significantly higher in the SARS-CoV-2-naïve group, with a predominance of male sex (70.9%). There was also a higher proportion of kidney-pancreas recipient in the SARS-CoV-2-pre-immunized group. In all the other baseline parameters no differences were observed between the two groups ( Table 1). The median time from transplant to vaccine was 1.65 [0.79–4.94] years, with 27.7% of patients having received the vaccine during the first year after kidney transplantation. Treatment with antithymocyte globulins (ATG) was employed (either for rejection or induction) during the last year in 11.5% of patients. Only three patients (2.0%) received Rituximab in the context of either pretransplant desensitization or antibody-mediated rejection treatment during the last year, all in the SARS-CoV-2-naïve group. Blood analysis at baseline revealed mean eGFR assessed by the CKD-EPI formula to be 49.07 ± 20.06 ml/min/1.73 m2. Patients with lymphopenia represented 29.1% of the entire population. Regarding immunsuppression, patients were receiving tacrolimus with either mycophenolate or mTOR inhibitors in 50.0% and 28.4% of cases, respectively. In 8.1% of cases immunosuppression was based on belatacept, while in all the other cases (13.6%) other combinations were employed ( Table 2).
TABLE 1.
Baseline demographic and clinical characteristics of the final population
| Total (n = 148) |
SARS-CoV–2 pre-immunized (n = 31) |
SARS-CoV–2 naïve (n = 117) |
p-value | |
|---|---|---|---|---|
| Age (year) | 57.62 ± 14.32 | 52.42 ± 14.81 | 59.00 ± 52.42 | .022 |
| Sex (%female) | 29.1% | 28.2% | 32.3% | .661 |
| Diabetes (%yes) | 21.6% | 12.9% | 23.9% | .226 |
| BMI | 25.60 ± 4.23 | 25.48 ± 3.61 | 25.64 ± 4.39 | .857 |
| Ethnicity (%) | .058 | |||
| Caucasic | 90.5% | 80.6% | 93.2% | |
| Hispanic | 7.4% | 16.1% | 5.1% | |
| African | 2.0% | 3.2% | 1.7% | |
| Blood type (%)a | .964 | |||
| A | 49.3% | 51.6% | 48.7% | |
| B | 2.7% | 3.2% | 2.6% | |
| O | 43.9% | 45.2% | 43.6% | |
| AB | 1.4% | - | 1.7% | |
| Type of donor (%) | .257 | |||
| Living | 32.4% | 25.8% | 34.2% | |
| DBD | 41.2% | 48.4% | 39.3% | |
| DCD II | 6.8% | 12.9% | 5.1% | |
| DCD III | 19.6% | 12.9% | 21.4% | |
| Type of transplantation | .017 | |||
| Kidney | 89.9% | 77.4% | 93.2% | |
| Kidney-pancreas | 10.1% | 22.6% | 6.8% | |
| Time from transplant (years) | 1.65 [0.79–4.94] | 1.83 [1.04–7.46] | 1.62 [0.71–4.49] | .532 |
| Transplant <1 year (%) | 27.7% | 22.6% | 29.1% | .652 |
| Dialysis vintage (months) | 17 [4–37.5] | 13 [0.75–40.5] | 17 [5–38] | .685 |
| Previously transplanted (yes) | 23.0% | 19.4% | 23.9% | .810 |
| Any rejection (%yes) | 20.3% | 29.0% | 17.9% | .209 |
| Baseline cPRA I+II (%) | 0 [0–24] | 0 [0–7] | 0 [0–34] | .752 |
| eGFR CKD-EPI (ml/min) | 49.07 ± 20.06 | 52.48 ± 22.56 | 48.16 ± 19.34 | .288 |
| Leukocytes (/mm3) | 6263 ± 2038 | 6261 ± 1979 | 6263 ± 2062 | .995 |
| Hb (g/dl) | 13.31 ± 1.79 | 13.10 ± 1.78 | 13.37 ± 1.80 | .476 |
| Lymphocytes (/mm3) | 1400 ± 745 | 1371 ± 767 | 1408 ± 742 | .809 |
| Lymphopenia (<1000/mm3) (%yes) | 29.1% | 32.3% | 28.2% | .661 |
| Treated during the last year with (%yes) | ||||
| Antithymocyte globulins (ATG) | 11.5% | 6.5% | 12.8% | .527 |
| Rituximab | 2.0% | — | 2.6% | 1 |
Missing value in four cases.
TABLE 2.
Baseline immunosuppression according to the individual drug or the combination received by the patient
| Total (n = 148) |
SARS-CoV–2 pre-immunized (n = 31) |
SARS-CoV–2 naïve (n = 117) |
p-value | |
|---|---|---|---|---|
| Tacrolimus (%yes) | 84.5% | 87.1% | 83.8% | .785 |
| Trough levels (ng/ml) | 7.09 ± 2.51 | 6.75 ± 2.11 | 7.19 ± 2.62 | .432 |
| Cyclosporine (%yes) | 3.4% | — | 4.3% | .584 |
| Trough levels (ng/ml) | 83.42 ± 38.30 | — | 83.42 ± 38.30 | — |
| Mycophenolate (%yes) | 62.8% | 67.7% | 61.5% | .676 |
| Dose (mg/daily)a | 785 ± 286 | 771 ± 307 | 790 ± 282 | .796 |
| mTOR inhibitors (%yes) | 32.4% | 32.3% | 32.5% | 1 |
| Trough levels (ng/ml) | 4.44 ± 1.84 | 4.54 ± 2.20 | 4.41 ± 1.77 | .852 |
| Prednisone (%yes) | 79.7% | 80.6% | 79.5% | 1 |
| Dose (mg/daily) | 5.06 ±1.74 | 4.54 ± 2.20 | 4.41 ± 1.77 | .160 |
| Azathioprine (%yes) | 2.7% | — | 3.4% | .580 |
| Belatacept (%yes) | 8.1% | 12.9% | 6.8% | .277 |
| Eculizumab (%yes) | 1.4% | — | 1.7% | 1 |
| According to combination | .163 | |||
| Tacrolimus + Mycophenolate | 50.0% | 58.1% | 47.9% | |
| Tacrolimus + mTOR inhibitors | 28.4% | 25.8% | 29.1% | |
| Belatacept-based | 8.1% | 12.9% | 6.8% | |
| Other | 13.5% | 3.2% | 16.2% |
Normalized to the dose of mycophenolic acid.
3.2. Humoral response after the mRNA-1273 SARS-CoV-2 vaccine
Of the 117 SARS-CoV-2-naïve patients, 35 patients (29.9%) developed either IgG or IgM 2 weeks after the second dose of the mRNA-1273 vaccine. Twenty-seven patients (23.1%) developed only IgG, five patients (4.3%) both IgG and IgM, and three (2.6%) only IgM. The factors associated with absence of humoral response at the univariable analysis were increasing age (p = .036 for 10-year increase starting from 50 years) and baseline immunosuppression, with possible protection provided by the combination Tacrolimus +mTOR inhibitors (OR [95% CI] 0.35 [0.13–0.89], p = .029). At multivariable analysis, only baseline immunosuppression, with a similar estimation of OR, was still significantly associated with no-response to vaccine ( Table 3, left).
TABLE 3.
Univariable and multivariable analysis on factors associated with vaccine no-response according to antibodies or ELISpot results 2 weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine
| Vaccine no-response (Abs) |
Vaccine no-response (ELISpot) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | p-value | Multivariable | p-value | Univariable | p-value | Multivariable | p-value | |
| Age | ||||||||
| <50 years | .036 | Ref | .192 | Ref | .069 | Ref | .229 | |
| 51–60 years | 4.27 [1.30–14.02] | .016 | 3.10 [0.80–11.94] | .100 | 2.59 [0.85–7.92] | .094 | 1.38 [0.38–4.99] | .616 |
| 61–70 years | 3.83 [1.22–12.04] | .021 | 3.44 [0.92–12.83] | .065 | 1.12 [0.36–3.47] | .838 | 0.36 [0.08–1.54] | .170 |
| >70 years | 3.64 [1.20–11.04] | .022 | 3.21 [0.87–11.80] | .078 | 3.28 [1.10–9.79] | .033 | 0.94 [0.24–3.66] | .935 |
| Sex (female) | 1.85 [0.71–4.80] | .202 | 1.22 [0.54–2.75] | .618 | ||||
| Diabetes (yes) | 1.77 [0.64–4.89] | .265 | 3.51 [1.42–8.67] | .006 | 5.65 [1.67–19.04] | .005 | ||
| Type of transplant (kidney-pancreas vs. kidney) |
0.69 [0.15–3.07] | .629 | 0.38 [0.07–2.00] | .257 | ||||
| Previous Tx (yes) | 2.33 [0.80–6.76] | .117 | 0.90 [0.38–2.12] | .810 | ||||
| Baseline immunosuppression | ||||||||
| TAC + MPA | Ref | .088 | Ref | .067 | .178 | |||
| TAC + mTORi | 0.35 [0.13–0.89] | .029 | 0.28 [0.09–0.82] | .020 | 1.95 [0.80–4.59] | .140 | ||
| Belatacept | 2.23 [0.25–19.65] | .469 | 1.73 [0.15–19.75] | .658 | 0.38 [0.07–2.02] | .260 | ||
| Other | 0.47 [0.15–1.48] | .201 | 0.34 [0.09–1.22] | .100 | 0.78 [0.26–2.30] | .662 | ||
| ATG <1 year | 7.00 [0.88–55.47] | .065 | 5.86 [0.63–53.96] | .119 | 6.10 [1.61–22.98] | .008 | 5.62 [0.89–35.53] | .065 |
| Lymphopenia (yes) | 1.39 [0.55–3.50] | .477 | 3.96 [1.65–9.45] | .002 | 2.80 [1.01–7.77] | .047 | ||
| Time from Tx <1yr | 1.85 [0.71–4.80] | .202 | 2.93 [1.27–6.78] | .012 | 1.23 [0.37–4.06] | .732 | ||
| eGFR (ml/min/1.73m2) | ||||||||
| >60 | Ref | .070 | Ref | .041 | Ref | .077 | ||
| 45–60 | 0.50 [0.17–1.47] | .214 | 0.42 [0.12–1.43] | .166 | 3.12 [1.03–9.45] | .044 | 4.50 [1.25–16.18] | .021 |
| 30–45 | 0.90 [0.32–2.49] | .848 | 0.68 [0.21–2.23] | .535 | 3.12 [1.11–8.75] | .030 | 3.67 [1.13–11.97] | .030 |
| <30 | 8.69 [1.02–73.99] | .048 | 5.45 [0.56–52.89] | .153 | 5.80 [1.72–19.57] | .005 | 4.11 [0.98–17.09] | .052 |
Bold values highlight statistical significance.
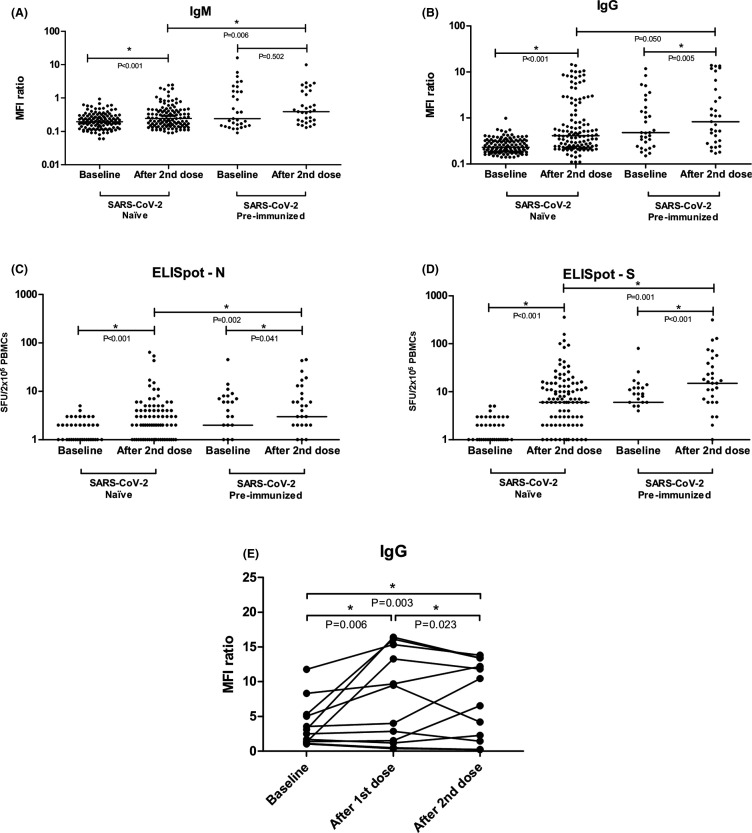
In naïve patients IgM increased from 0.20 [0.13–0.29] to 0.25 [0.15–0.43] (p < .001) and IgG from 0.23 [0.18–0.32] to 0.41 [0.24–1.67] (p < .001) (Figure 3A,B).
FIGURE 3.
Changes in IgM and IgG concentration (A,B), N-ELISpot (C), and S-ELISpot (D) before and after vaccination with mRNA-1273 SARS-CoV-2 vaccine. Changes in the IgG titer in patients who were IgG(+) at baseline (E). Differences are analyzed by means of the Mann-Whitney test between groups and of the Wilcoxon signed-rank test for related samples within the same group along different time-points and an asterisk (*) identifies statistical significance. Bars identify medians. The Y-axis in Figure 3A–D is 10-logarithmic based, so patients with “0” SFU/2x105 PBMCs are not displayed
3.3. Cellular response after the mRNA-1273 SARS-CoV-2 vaccine
Of the final population of 117 SARS-CoV-2 naïve patients, 64 patients (54.7%) developed S-ELISpot positivity 2 weeks after the second dose of the mRNA-1273 vaccine. Fifteen patients (12.8%) also developed N-ELISpot positivity, all of which were also S-ELISpot positive. Factors that were associated with absence of cellular response to the S protein (S-ELISpot negativity) were increasing age (>70 years with OR [95% CI] 3.28 [1.10–9.79], p = .033), diabetes (OR [95% CI] 3.51 [1.42–8.67], p = .006), receiving ATG during the last year (OR [95% CI] 6.10 [1.61–22.98], p = .008), lymphopenia (OR [95% CI] 3.96 [1.65–9.45], p = .002), time from transplant <1 year (OR [95% CI] 2.93 [1.27–6.78], p = .012) and decreasing eGFR starting from <60 ml/min/1.73m2 (p = .041). At multivariable analysis, the factors that were still associated with S-ELISpot no-response were diabetes (OR [95% CI] 5.65 [1.67–19.04], p = .005), lymphopenia (OR [95% CI] 2.80 [1.01–7.77], p = .047) and decreasing eGFR (Table 3, right).
Spots for the S protein significantly increased in the naïve group from 0 [0–1] to 6 [1–13] (p < .001) (Figure 3D). Unexpectedly, spots for the N protein increased significantly from 0 [0–1] to 1 [0–3.5] too (p < .001) due to the positivity of 15 cases (Figure 3C). Representative samples of S-positive patients are displayed in Figure S2.
3.4. Discordance between humoral and cellular response
Development of both humoral (either IgG or IgM) and cellular response (S-ELISpot positivity) was observed in 23 patients (19.6%). In IgG-positive population (n = 35), 21 patients also developed (17.9%) S-ELISpot positivity. N-ELISpot positivity was observed in six cases of the IgG-positive population. Patients who were IgM positive but IgG negative (n = 3) were S-ELISpot positive in two cases and N-ELISpot negative in all cases. In patients who were either IgG or IgM positive, spots for the S protein were higher than in seronegative patients (8 [3–18] vs. 1 [5–11], p = .042). Patients who were negative for both IgG and IgM (n = 82) were S-ELISpot positive in 41 cases. Of these 41 cases IgM(–)/IgG(–) and S-ELISPOT(+), there were nine cases who were positive for N-ELISpot. In the other IgM(–)/IgG(–)/SELISpot(–) 41 cases, N-ELISpot also tested negative.
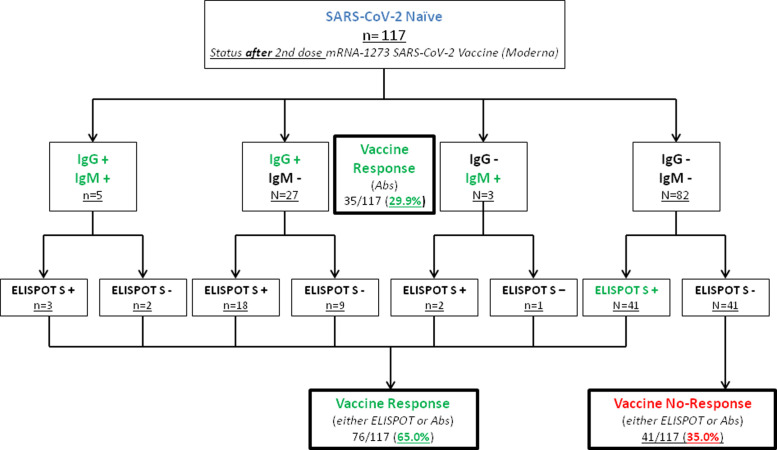
Patients who presented any response to the mRNA-1273 vaccine (either IgG/IgM or S-ELISpot positivity) were finally 76 (65.0%), while 41 did not develop any kind of response (35.0%) ( Figure 2).
FIGURE 2.
Development of humoral and cellular response in SARS-CoV-2-naïve patients after administration of the mRNA-1273 vaccine. Patients are highlighted in green according to the development or either antibodies or S-ELISpot positivity, identifying vaccine responders. Patients without response to the vaccine are highlighted in red
Considering vaccine nonresponders as patients who were both IgG/IgM and S(ELISPot)-negative (n = 41) after the second dose of the mRNA-1273 vaccine, the factors that were associated with an absence of response at univariable analysis were diabetes (OR [95% CI] 3.41 [1.41–8.22], p = .006), receiving ATG during the last year (OR [95% CI] 10.07 [2.64–38.31], p = .001), lymphopenia (OR [95% CI] 3.82 [1.64–8.89], p = .001), time from transplant <1 year (OR [95% CI] 3.51 [1.52–8.08], p = .003) and eGFR <30 ml/min/1.73 m2 (OR [95% CI] 4.95 [1.48–16.46], p = .009). At multivariable analysis the factors that were finally associated with vaccine no-response were still diabetes (OR [95% CI] 4.65 [1.41–15.31], p = .037) and treatment with ATG during the last year (OR [95% CI] 7.23 [1.12–46.51], p = .037) ( Table 4).
TABLE 4.
Univariable and multivariable analysis on factors associated with global vaccine no-response, defined as the negativity of both antibodies and ELISpot assay two weeks after the second dose of mRNA-1273 SARS-CoV-2 vaccine
| Vaccine no-response (neither Abs nor ELISpot) |
||||
|---|---|---|---|---|
| Univariable | p-value | Multivariable | p-value | |
| Age | ||||
| 50 years | Ref | .062 | .394 | |
| 51–60 years | 4.76 [1.30–17.46] | .018 | 2.50 [0.57–11.03] | .224 |
| 61–70 years | 2.35 [0.62–8.83] | .203 | 0.83 [0.16–4.29] | .826 |
| 70 years | 4.58 [1.29–16.26] | .018 | 1.26 [0.26–6.15] | .769 |
| Sex (female) | 1.55 [0.67–3.56] | .296 | ||
| Diabetes (yes) | 3.41 [1.41–8.22] | .006 | 4.65 [1.41–15.31] | .037 |
| Type of transplant (kidney-pancreas vs. kidney) |
0.59 [0.11–3.10] | .541 | ||
| Previous Tx (yes) | 1.27 [0.53–3.05] | .590 | ||
| Baseline immunosuppression | ||||
| TAC + MPA | Ref | .673 | ||
| TAC + mTORi | 1.26 [0.52–3.02] | .605 | ||
| Belatacept | 0.60 [0.11–3.25] | .554 | ||
| Other | 0.64 [0.20–2.04] | .455 | ||
| ATG <1yr | 10.07 [2.64–38.31] | .001 | 7.23 [1.12–46.51] | .037 |
| Lymphopenia (yes) | 3.82 [1.64–8.89] | .001 | 2.73 [0.96–7.71] | .058 |
| Time from Tx <1 year | 3.51 [1.52–8.08] | .003 | 1.14 [0.33–3.93] | .830 |
| eGFR (ml/min/1.73 m2) | ||||
| >60 | Ref | .024 | Ref | .161 |
| 45–60 | 1.11 [0.32–3.83] | .864 | 1.13 [0.26–4.91] | .866 |
| 30–45 | 2.83 [0.98–8.15] | .054 | 3.18 [0.92–10.95] | .066 |
| <30 | 4.95 [1.48–16.46] | .009 | 3.30 [0.78–14.01] | .105 |
Bold values highlight statistical significance.
3.5. Relative changes in antibodies concentration and spots in the pre-immunized population
In pre-immunized patients IgM increased from 0.24 [0.15–1.59] to 0.39 [0.20–1.60] (p = .502) and IgG increased from 0.48 [0.24–1.69] to 0.83 [0.30–4.19] (p = .005) ( Figure 3A,B). Comparing the naïve group with the pre-immunized group at 2 weeks after the second dose, IgM was significantly higher in the pre-immunized group (p = .006), as well as IgG (p = .050). In patients who were IgG positive at baseline (n = 12), we also determined the relative increase between the first and the second doses. It appeared that the second dose effectively increased the IgG titer from 6.75 [1.26–14.82] after the first dose to 8.49 [1.65–13.10] after the second dose (p = .023) (Figure 3E).
Spots for the S protein significantly increased in the pre-immunized group from 6 [0–12] to 15 [6–39] (p < .001) (Figure 3D). The N-spots also increased in the pre-immunized group from 2 [0–7] to 3 [1–9] (p = .041). Comparing the naïve group with the pre-immunized group at 2 weeks after the second dose, S-ELISpot was significantly higher in the pre-immunized group (p = .001) as well as N-ELISpot (p = .002).
Within the pre-immunized group, patients with a prior history of infection (n = 5) had higher IgG at baseline (5.04 [1.79–8.53] vs. 0.40 [0.23–1.14], p = .006) as well as after the second dose (13.41 [2.23–13.62] vs. 0.63 [0.28–1.74], p = .026) in comparison with patients without a history of COVID-19. No differences were observed for IgM. In patients with a positive history of infection, N-ELISpot positivity was observed at baseline in 3/5 cases (60.0%) versus 7/26 (26.9%) of patients without history of COVID-19 (p = .296). S-ELISpot at baseline was positive in 4/5 patients (80.0%) with history of COVID-19 versus 14/26 (53.8%) in patients without it (p = .368). After receiving vaccination, the five patients with previous COVID-19 had N-ELISpot positivity in three cases (60.0%) and S-ELISpot positivity in all cases (100.0%), while patients without previous history of infection had N-ELISpot positivity in 9/26 cases (34.6%, p = .350) and S-ELISpot positivity in 20/26 cases (76.9%, p = .553).
3.6. Safety analysis indicates reasonable and expectable side effects after the mRNA-1273 vaccine and no detection of DSAs
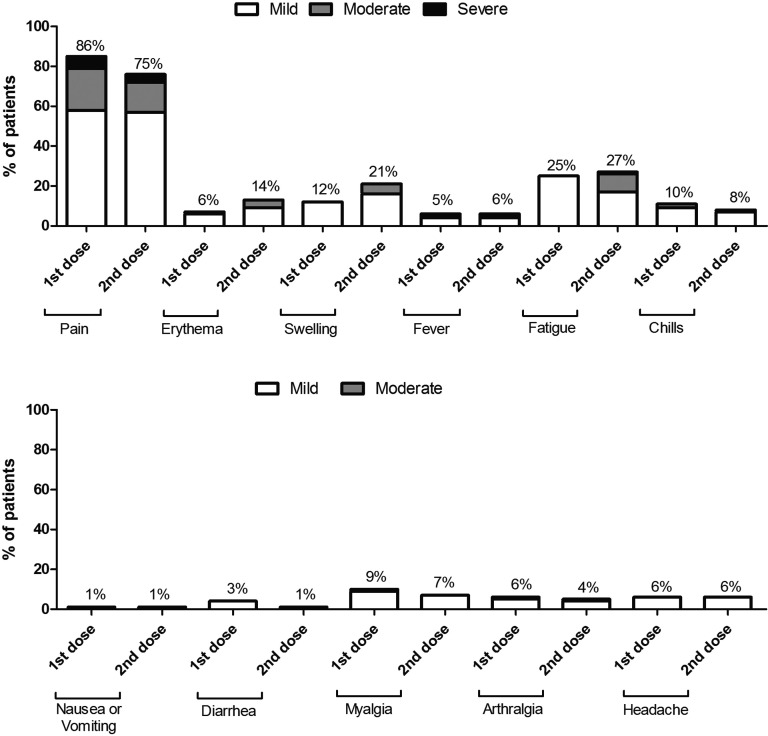
Patient-reported side effects on a semiquantitative scale (none/mild/moderate/severe) were consistent with the pivotal trial ( Figure 4), with pain in the injection site being the most commonly reported affecting 86% and 75% of the included population after the first and the second doses, respectively. The second most commonly reported side effect was fatigue that affected 25% and 27% of population after the first and the second doses, respectively. There were no significant differences between the first and the second doses for all the reported side effects. Patients took analgesics in 19.3% and 26.8% of cases after the first and the second dose, respectively.
FIGURE 4.
Side effects reported by patients after receiving the two doses of the mRNA-1273 SARS-CoV-2 vaccine
DSAs were present in five cases at baseline (3.4% of the entire population), and no cases of de-novo DSAs were observed after the second dose.
4. DISCUSSION
KTRs are at especially high risk of unfavorable outcome in case of infection with SARS-CoV-2. The reported mortality rate is up to 25% in these patients.14 Since treatment alternatives are still scarce, as of yet the only possible strategy beyond masks and social distancing is an effective and safe vaccine. Although the current vaccine strategies—with the exception of attenuated virus—seem to be safe, there are little data available in KTRs in terms of both safety and effectiveness.
We show herein that the mRNA-1273 SARS-Cov-2 vaccine is safe in KTRs and that the SARS-CoV-2 vaccine is associated with the same side effect profile as in the pivotal study.15 Main side effects were pain at the injection side and fatigue. Importantly, no de-novo DSAs appeared after receiving the second dose.
In SARS-CoV-2 mRNA vaccine studies in the general population seroconversion were observed in practically all patients.3 , 11 However, as expected, in our cohort the response rate was lower than in the general population, a finding that is coherent with the available data in the field. Considering only humoral response, S-specific antibodies were developed only by 29.9% of patients in our population. Grupper et al. reported a 37.5% antibody response rate after the second dose of the BNT162b2 vaccine.16 Boyarsky et al. recently reported a higher seroconversion of 54% in patients receiving a mRNA vaccine, either mRNA-1273 (Moderna) or BNT162b2 (Pfizer).17
A point of novelty in our study is the assessment of cellular response through the ELISpot technique that, to our knowledge, is currently unknown in KTRs. A strong T cell response is part of the consequences of coronavirus infections and seems to play an important role in terms of long-term immunological memory.18 Especially, in a population with a reduced antibody response information about the T cell response should be part of the assessment and furthermore it could be part of an individualized management strategy.19 Taking into account the percentage of patients who had a positive S-ELISpot after the second dose, the percentage of patients who developed either a humoral or a cellular response increased to 65% and half of antibody-negative patients had actually developed a positive ELISpot (Figure 2). This finding highlights that patients may be actually protected against SARS-CoV-2 despite the absence of S antibodies. To which extent cellular immunity, in the absence of detectable antibodies, is able to prevent severe infection or death from SARS-CoV-2, it is yet to be determined and only clinical follow-up of these patients will give the final answer. Moreover, we observed that 15 patients had also developed ELISpot positivity for the N-protein; this raises the question whether these particular patients became immunized by direct virus contact/infection without presenting symptoms in the meantime of vaccination process, independently of the potential immunization to the S protein that could have been developed after vaccination. Another option could be that the SARS-CoV2 N-responses could come from cross-reactivity with N proteins from other members of the coronavirus family.
It is well known that the response rate to viral vaccines is less intensive in patients with immunosuppression.6 , 7 For example, the Hepatitis B vaccine response rate is 40% in liver transplant recipients,20 while in stem-cell transplanted patients only 51.9% achieve a response.21
Further studies are necessary in order to evaluate if a third vaccine dose could increase the level of protection from the vaccine in the SOT population. Moreover, at this point, it seems especially reasonable to vaccinate the family members and caregivers of solid organ transplant recipients as part of a cocoon strategy. Cocooning is a well-known principle for vaccinations if the target population cannot be vaccinated or are at risk of having a low response rate.22 In any case, these results highlight the need to reach herd immunity as fast as possible in order to protect the SOT population.
To our knowledge, this is the first study that identifies diabetes mellitus in solid organ transplant recipients as a “risk factor” for not developing an immunogenic response to the vaccine of SARS-CoV-2. In the setting of the hepatitis B virus vaccine, Schillie et al. observed that diabetes mellitus patients seemed to have a reduced response to the hepatitis B vaccines. These authors stated that diabetic patients showed an appropriate humoral response to vaccination in general, but impaired cellular response may account for less robust antibody production after hepatitis B vaccination.23 These authors propose as possible causes of this phenomenon less circulating helper T cells, an alteration of the CD4-to-CD8 lymphocyte ratio, and reduced lymphocyte blastogenesis as well as impaired antigen presentation.
Maintenance immunosuppression did not seem to have any influence on the immunological response, with the exception of mTOR inhibition associated with a more favorable humoral response. A preliminary study observed that mycophenolate was associated with less humoral response,10 but firm conclusions are far from being made with the available data. On the other side, having received ATG during the last year proved to be associated with vaccine nonresponse (antibodies or S-ELISpot) (OR [CI] 7.23 [1.12–46.51], p = .037), thus highlighting the profound immunosuppression given by this drug (Table 4). It has to be highlighted that also lymphopenia, independently of ATG, was associated with S-ELISpot nonresponse (Table 3, right). One may argue that the two variables are associated with each other, as ATG typically causes profound lymphopenia early after transplantation and is associated with immune-senescence at the long-term.24 However, the percentage of patients at baseline with lymphopenia (28.6%) was higher than that of patients who received ATG during the last year (11.6%); this highlights that different mechanisms apart from ATG are implicated after kidney transplantation, including maintenance immunosuppression and comorbidities and that lymphopenia per se represents a risk factor for not developing a cellular response.
In our multivariate analysis, a glomerular filtration rate below 30 ml/min barely missed statistical significance as an independent risk factor for no immunological response. In a recently published study with a mRNA vaccine in dialysis patients, Grupper et al. observed a robust, although less intense, antibody response in 96% of cases. Therefore, it could be speculated that in our pharmacologically immunosuppressed patients, the most important factor is the immunosuppression and not impaired renal function.25
The limitations of our study include a low number of patients in order to draw solid conclusions about the real protective effect of the vaccine. However, a low rate of seroconversion or of cellular response might be surrogate parameters for less efficacy. Moreover, our study lacks a healthy control group. However, we figured that the already published data on healthy individuals are convincing enough in order to get relevant results without a control group, especially considering that in a situation of scarcity a control group would be difficult if it consists of individuals who do not belong to risk populations. Another possible limitation of our study is the absence of serial measurements after vaccination. Long-term data on safety are also needed and will be followed-up.
In conclusion, the mRNA SARS-CoV-2 vaccine provoked an immune response in 65% of patients who received immunosuppression due to a kidney or kidney-pancreas transplant. This is a lower response rate than in the general population. New strategies need to be developed in order to adequately protect this vulnerable group.
ACKNOWLEDGMENTS
Natalia Egri is a recipient of a “Contracte Clínic De Recerca Emili Letang - Josep Font.” We would like to thank Dr. Josep M Campistol for his valuable suggestions and support for the development of the study and revision of the manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
ENDNOTE
1The PepTivator® SARS-CoV-2 Prot_S is a pool of peptides, consisting mainly of 15-mer sequences with 11 amino acids overlap, covering the immunodominant sequence domains of the spike glycoprotein (“S”) of SARS-Coronavirus 2 (GenBank MN908947.3, Protein QHD43416.1). PepTivator® SARS-CoV-2 Prot_N is a pool of peptides, consisting mainly of 15-mer sequences with 11 amino acids overlap, covering the complete sequence of the nucleocapsid phosphoprotein (“N”) of SARS-Coronavirus 2 (GenBank MN908947.3, Protein QHD43423.2).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Fig S1
Fig S2
REFERENCES
- 1.World Health Organization. WHO coronavirus dashboard. https://covid19.who.int/. Accessed April 14, 2021.
- 2.US Department of Health and Human Services. BARDA’s expanding COVID-19 medical countermeasure portfolio. https://www.medicalcountermeasures.gov/app/barda/coronavirus/COVID19.aspx?filter=vaccine. Accessed April 14, 2021.
- 3.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — Preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza a H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52(2):248–256. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 7.Birdwell KA, Ikizler MR, Sannella EC, et al. decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54(1):112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordero E, Roca-Oporto C, Bulnes-Ramos A, et al. Two doses of inactivated influenza vaccine improve immune response in solid organ transplant recipients: results of TRANSGRIPE 1–2, a randomized controlled clinical trial. Clin Infect Dis. 2017;64(7):829–838. doi: 10.1093/cid/ciw855. [DOI] [PubMed] [Google Scholar]
- 9.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 10.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA - J Am Med Assoc. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson LA, Anderson EJ, Rouphael NG, et al. mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—Preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meneghini M, Melilli E, Martorell J, et al. Combining sensitive crossmatch assays with donor/recipient human leukocyte antigen eplet matching predicts living-donor kidney transplant outcome. Kidney Int Rep. 2018;3:926–938. doi: 10.1016/j.ekir.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagud-Marrahi E, Cofan F, Torregrosa J-V, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single center cohort of kidney recipients. Am J Transplant. 2020;20(10):2058–2059. doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021. 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed]
- 17.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021. Published online May 5, 2021. 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed]
- 18.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 19.Babel N, Anft M, Blazquez-Navarro A, et al. Immune monitoring facilitates the clinical decision in multifocal COVID-19 of a pancreas-kidney transplant patient. Am J Transpl. 2021;20(11):3210–3215. doi: 10.1111/ajt.16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loinaz C, de Juanes JR, Gonzalez EM, et al. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology. 1997;44(13):235–238. [PubMed] [Google Scholar]
- 21.Shalabi RA, Borg MA, Hughes TE, et al. Outcome of repeated vaccination to Hepatitis B virus in patients failing to respond to vaccination following allogeneic hematopoietic stem cell transplantation (HSCT): If at first you don’t succeed, try try again. Blood. 2019;134(S1):2012. [Google Scholar]
- 22.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Inf Dis. 2011;52(2):157–162. doi: 10.1093/cid/ciq001. [DOI] [PubMed] [Google Scholar]
- 23.Schillie SF, Spradling PR, Murohy TV. Immune response of hepatitis B Vaccine among persons with diabetes. Diabetes Care. 2012;35:2690–2697. doi: 10.2337/dc12-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crepin T, Carron C, Roubiou C, et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant. 2015;15(4):1028–1038. doi: 10.1111/ajt.13092. [DOI] [PubMed] [Google Scholar]
- 25.Grupper A, Sharon N, Finn T, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;6 doi: 10.2215/CJN.03500321. CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.