We read with great interest the recent article by Sutter and colleagues reporting increased serum neurofilament light chain (NfL) levels in COVID‐19 patients compared to non–COVID‐19 intensive care unit (ICU) patients without infectious disease. 1 They postulated that infection with SARS‐CoV‐2 leads to neuronal injury in ICU‐patients and that NfL might be used to identify patients at risk for neurological complications.
We evaluated these findings in the broader context of infectious disorders by comparing the plasma NfL levels of critically ill patients with similar disease severity and pneumonia induced by SARS‐CoV‐2 versus bacterial pathogens. We excluded patients with neurological comorbidities or trauma and matched the study population investigated by Sutter et al for sex, age, and disease severity (COVID‐19 pneumonia vs bacterial pneumonia: mean age = 65 years, range = 42–89, 78% male, Sequential Organ Failure Assessment [SOFA] score = 4, range = 2–11 vs mean age = 67 years, range = 50–89, 90% male, SOFA score = 3, range = 0–5). Most notably, we observed that patients with COVID‐19 pneumonia had considerably lower NfL levels compared to patients with bacterial pneumonia at day 3 ± 1 after onset of sepsis (defined as infection‐related acute change in total SOFA score ≥ 2 points), which did not significantly increase over time (Fig A). Consistently with previous reports, we found a positive correlation between NfL levels and age as well as SOFA score in COVID‐19 patients (see Fig B, C).
FIGURE 1.
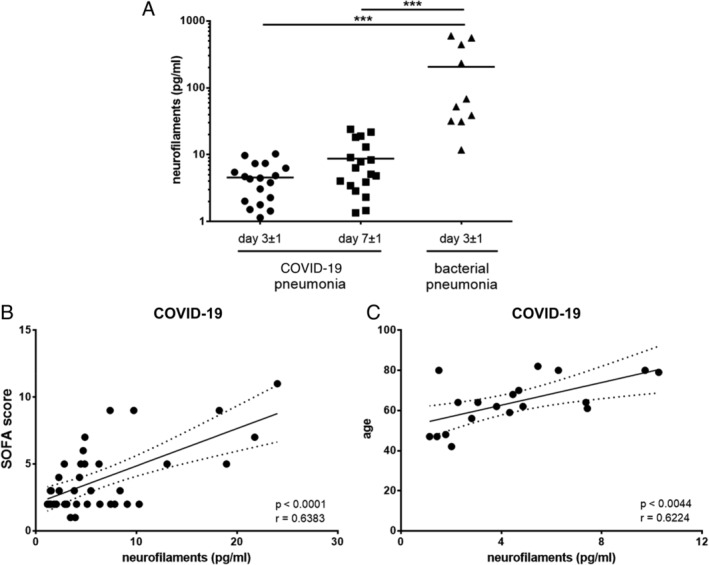
Neurofilament light chain (NfL) assay by Quanterix (Billerica, MA) HD‐X was used to measure NfL levels according to manufacturer's instructions. (A) Comparison of NfL levels in patients with COVID‐19 pneumonia at day 3 ± 1 and day 7 ± 1 (n = 18) and bacterial pneumonia at day 3 ± 1 (scatter dot blot with mean; n = 10) after onset of sepsis (analysis of variance on ranks, p < 0.001; Dunn multiple comparison test, ***p < 0.001). (B, C) Pearson correlation was used to assess correlations between neurofilaments and Sequential Organ Failure Assessment (SOFA) score (n = 36; day 3 ± 1 and 7 ± 1) or age (n = 18; day 3 ± 1) in COVID‐19 patients.
NfL value is a well‐established marker for neuronal injury. Corroborating our results, recent reports have shown low to intermediate NfL levels in COVID‐19 patients as compared to other infectious diseases, for example, bacterial pneumonia and sepsis. 2 , 3 Thus, with respect to changes of NfL levels, our own data and current evidence do not indicate commonly occurring neuronal damage in COVID‐19. However, differences in cohort composition, such as incidence of delirium or acute kidney injury, could explain the observations made by Sutter and colleagues. As recently reported, delirium itself is associated with NfL elevation and cognitive impairment independent of infection. 4 Furthermore, renal dysfunction might also have influenced NfL levels. 5
In conclusion, we agree with the authors' statement that prospective studies testing the cognitive outcome of COVID‐19 patients are needed to evaluate the prognostic value of NfL levels for neuronal injury during acute SARS‐CoV‐2 infection. Nonetheless, at this stage, we caution against interpreting the NfL data shown by Sutter et al. as indicating COVID‐19–specific neuronal damage.
Potential Conflicts of Interest
Nothing to report.
Acknowledgments
Plasma from a subgroup of an ongoing single‐center prospective cohort study was employed for analyses (NCT03620409; https://doi.org/10.1136/bmjopen-2019-036527). The clinical study was funded by the Federal Ministry of Education and Research (grant 03Z22JN12 to S.M.C.). This work was further supported by the Center for Sepsis Control and Care (FKZ 01EO1502), the Interdisciplinary Center of Clinical Research of the Jena Medical Faculty, and the Schilling foundation.
We thank the other members of the research group Translational Septomics for their support.
References
- 1. Sutter R, Hert L, De Marchis GM, et al. Serum neurofilament light chain levels in the intensive care unit: comparison between severely ill patients with and without coronavirus disease 2019. Ann Neurol 2021;89:610–616. [DOI] [PubMed] [Google Scholar]
- 2. Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID‐19. Neurology 2020;95:e1754–e1759. [DOI] [PubMed] [Google Scholar]
- 3. Ehler J, Petzold A, Wittstock M, et al. The prognostic value of neurofilament levels in patients with sepsis‐associated encephalopathy—a prospective, pilot observational study. PLoS One 2019;14:e0211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong TG, Vasunilashorn SM, Ngo L, et al. Association of plasma neurofilament light with postoperative delirium. Ann Neurol 2020;88:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akamine S, Marutani N, Kanayama D, et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep 2020;10:20350. [DOI] [PMC free article] [PubMed] [Google Scholar]


