Abstract
Background
Coronavirus disease 2019 (COVID‐19), which causes serious respiratory illnesses such as pneumonia and lung failure, was first reported in mid‐December 2019 in China and has spread around the world. In addition to causing serious respiratory illnesses such as pneumonia and lung failure, there have been conflicting reports about the presence of SARS‐CoV‐2 in the semen of patients who were previously diagnosed with COVID‐19 and possible implications for the male reproductive tract.
Objective
The goal for the present study was to review the current status of the literature concerning COVID‐19 and male reproduction.
Material and methods
An electronic literature search was done by using PubMed and Google Scholar databases. Relevant papers, concerning SARS‐COV‐2 and COVID‐19 and male reproduction, published between January 2020 and December 2020 were selected, analyzed and eventually included in the present literature review.
Results
SARS‐CoV‐2 may infect any cell type expressing angiotensin‐converting enzyme 2 (ACE2), including reproductive cells. Besides the presence of the SARS‐CoV‐2 receptor, the expression of host proteases, such as transmembrane serine protease 2 (TMPRSS2), is needed to cleave the viral S protein, allowing permanent fusion of the viral and host cell membranes. Here, we aimed to review the current status of the literature concerning COVID‐19 and male reproduction. The lack of co‐expression of ACE2 and TMPRSS2 in the testis suggests that sperm cells may not be at increased risk of viral entry and spread. However, the presence of orchitis in COVID‐19‐confirmed patients and compromised sex‐related hormonal balance among these patients intrigues reproductive medicine.
Discussion
SARS‐CoV‐2 may use alternate receptors to enter certain cell types, or the expression of ACE2 and TMPRSS2 may not be detected in healthy individuals.
Conclusion
COVID‐19 challenges all medical areas, including reproductive medicine. It is not yet clear what effects, if any, the COVID‐19 pandemic will have on male reproduction. Further research is needed to understand the long‐term impact of SARS‐CoV‐2 on male reproductive function.
Keywords: COVID‐19, male reproduction, SARS‐CoV‐2, testicles, viral
1. INTRODUCTION
In December 2019, an increase in serious pneumonia cases with no known cause was observed in Wuhan, China. Soon after, the number of cases rose dramatically, spreading throughout the world on all continents. The causative agent of the disease was identified as a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The disease caused by this agent was formally named coronavirus disease 2019 (COVID‐19) by the World Health Organization (WHO).
SARS‐CoV‐2 belongs to the β‐coronavirus cluster. Coronaviruses primarily cause enzootic infections in birds and mammals but, in the last few decades, have shown to infect humans as well. The outbreak of severe acute respiratory syndrome (SARS) in 2003 and Middle‐East respiratory syndrome (MERS) demonstrated the lethality of these viruses when they cross the species barrier and infect humans. 1 Current evidence indicates that SARS‐CoV‐2 was derived from bats. 2 , 3 , 4 , 5 Following SARS and MERS, COVID‐19 is the third known zoonotic disease caused by coronaviruses. 6
Coronaviruses are enveloped, non‐segmented positive‐sense RNA viruses belonging to the family Coronaviridae, which contain very large genomes for RNA viruses, with some viruses having the largest identified RNA genomes. Other common features within coronaviruses are (i) a highly conserved genomic organization, with a large replicase gene preceding structural and accessory genes; (ii) expression of many nonstructural genes by ribosomal frameshifting; (iii) several unique or unusual enzymatic activities encoded within the large replicase‐transcriptase polyprotein; and (iv) expression of downstream genes by synthesis of 3′ nested subgenomic mRNAs. 7
The coronaviral genome encodes four major structural proteins: the spike (S) protein, nucleocapsid (N) protein, membrane (M) protein, and envelope (E) protein, all of which are required to produce a structurally complete viral particle. 8 , 9 Individually, each protein primarily plays a role in the structure of the virus particle, but they are also involved in other aspects of the replication cycle. 1 The initial attachment of SARS‐CoV‐2 to the host cell is initiated by interactions between the S protein and its receptor. 7 Host proteases, such as transmembrane serine protease 2 (TMPRSS2), are needed to cleave the viral S protein, allowing permanent fusion of the viral and host cell membranes. 10 , 11 The accomplishment of these events drives the release of the viral RNA genome in the host cell and the subsequent start of the viral replication cycle.
A homolog of the angiotensin‐converting enzyme (ACE), designated ACE2, was identified as the receptor for SARS‐CoV‐1 12 and SARS‐CoV‐2. 5 Previous evidence demonstrated that SARS‐CoV‐2 had a ten times higher affinity to ACE2 than SARS‐CoV‐1, which was consistent with its higher efficiency of infection. 13 SARS‐CoV‐2 binding to ACE2 leads to downregulation of these receptors. 14 , 15 , 16 As a result, the activity of ACE2 is markedly attenuated. 17
Although ACE2 is ubiquitous, organs that express a high level of ACE2 are potential targets of SARS‐CoV‐2 infection. Therefore, the distribution and abundance of ACE2 in organs may be closely related to the clinical symptoms of COVID‐19. ACE2 is broadly distributed in the lungs, liver, intestine, and brain. This molecule is also enriched in the heart, kidneys, and testes. 18
Extensive literature reveals that the lungs of SARS patients are commonly the most affected organs, with severe degeneration of the epithelium. 19 , 20 , 21 Nevertheless, other organs are also known to be damaged by the virus. 22 , 23 , 24 , 25 , 26
As for human reproduction, concerns of potential vertical transmission have been raised. 27 Human embryos present all of the machinery needed for SARS‐CoV‐2 binding, internalization, and replication, suggesting that “in theory” viral infection may compromise embryonic and fetal development.
In males, ACE2 and TMPRSS2 are expressed in the testicular tissue, and the presence of SARS‐CoV‐2 in semen has been suggested. 28 However, information concerning the susceptibility of the male reproductive system to SARS‐CoV‐2 infection, sexual transmission, and possible effects on embryonic development remains inconsistent. Therefore, for the present study, a literature review was performed to determine whether male reproductive cells are vulnerable to SARS‐CoV‐2 infection and whether the infection may lead to decreased reproductive potential or transmission, resulting in deleterious effects on embryonic development and pregnancy outcomes.
2. OTHER VIRAL INFECTIONS IN THE MALE REPRODUCTIVE SYSTEM
Infectious and inflammatory conditions in the reproductive system may cause male infertility. Viral infections may impair male fertility by directly affecting spermatozoa, inducing sperm death, reducing sperm count, and decreasing motility by inducing inflammatory cytokines. Infections may also indirectly affect sperm production and the function of genital organs. 29
Several viruses may infect the testicles. Zika virus (ZIKV) can induce inflammation in the testis and epididymis, leading to testicular dysfunction and male infertility, as demonstrated in a mouse model. 30 In fact, ZIKV was detected in the semen of symptomatic men 31 and was shown to be sexually transmitted. 32 Mumps virus (MuV) has a high tropism for the testicles, and orchitis is a common complication. 33 Human immunodeficiency virus (HIV) infection also induces severe orchitis and results in male infertility. 34 HIV is detectable in semen shortly after infection and at all subsequent stages of the disease. 35
Hepatitis B virus (HBV) and hepatitis C virus (HCV) can invade the human male germ line. Transcription of HBV genes was shown to occur in human sperm cells and is regulated by host genes. 36 Moreover, HCV infection has mutagenic effects on the chromosomes in sperm cells and may lead to extensive hereditary effects owing to genetic alterations and chromosomal aberrations. 37 Human papillomavirus (HPV) was also found in most parts of the male reproductive system, including the testis, epididymis, ductus deferens, and semen. 38 , 39 , 40 Finally, both cytomegalovirus (CMV) 41 and herpes simplex virus (HSV) 42 were detected in human spermatozoa. While CMV was found to have no impact on male reproductive health, HSV detection in the ejaculate was directly correlated with reduced sperm motility and normal morphology. 43
Similar to other viruses that can enter the testis and cause orchitis and, in some cases, result in male infertility, 44 the virus that causes SARS may lead to orchitis, testis damage, and defects in spermatogenesis. 45 However, after performing in situ hybridization, using both sense and antisense RNA probes to determine if the SARS virus infected the testis directly, researchers did not observe positive staining in any of the SARS testis sections. In this study, specific positive signals were obtained in lung sections of individuals with SARS, which were stained as a positive control. 45
Temperature could be one reason for testis damage in SARS‐positive patients. Germ cells must develop at a temperature lower than 37°C. Persistent high fever may negatively affect spermatogenesis and increase oxidative stress. 46 It has been suggested that heat‐induced testicular cell degeneration may be mediated by apoptosis. 47 Although high fever is known to play an important role in viral orchitis, temperature might not be the only mechanism through which SARS affects testicular function. Xu et al 45 reported that the testes of non‐SARS‐infected patients with lasting high fever presented mild fibrosis and congestion, but there was no obvious germ cell loss or leukocyte infiltration.
The association with testicular damage in SARS and other types of viral orchitis could be attributed to endocrine dysfunction. The viruses per se might influence pituitary function. In HIV‐infected patients, hypogonadism was shown to be common secondary to hypothalamic‐pituitary‐gonadal axis dysfunction and associated with low LH and FSH levels and not with primary testicular failure. 48 Indeed, HIV has been found in pituitary cells and might account for damage to the hypothalamus and pituitary gland. 49
Hypogonadism has also been documented in HCV‐infected men. Although the etiology has not been established, systemic inflammation associated with suppression of the hypothalamic‐pituitary‐gonadal axis cannot be eliminated. 50 Hemorrhagic fever virus (HFV) 51 and HSV 52 are also known to affect the pituitary‐gonadal axis. Changes in several pituitary cell types have been observed in samples obtained from autopsies of SARS patients, 53 providing evidence of endocrine dysfunction in these patients, which may be correlated with defects in spermatogenesis.
3. THE SARS‐COV‐2 RECEPTOR AND ITS EXPRESSION IN THE MALE REPRODUCTIVE TRACT
ACE2, the functional host receptor for SARS‐CoV‐2, is part of the renin‐angiotensin‐aldosterone system (RAAS), the main network responsible for the regulation of systemic arterial pressure and electrolyte homeostasis. 54 Angiotensinogen, produced by the liver, is converted by renin in angiotensin I (Ang I). Subsequently, ACE catalyzes the conversion of Ang I to angiotensin II (Ang II), inducing increased blood pressure, promoting vasoconstriction and inflammation. 55 ACE2, in turn, cleaves Ang II to angiotensin (1–7), which exerts vasodilating, anti‐inflammatory, and anti‐fibrotic effects. 56 In addition, ACE2 cleaves Ang I into angiotensin (1–9), which is converted into angiotensin (1–7) by ACE. Therefore, ACE2 plays a crucial role in the RAAS system because the RAAS activation depends on the tissue ACE/ACE2 balance. 57
It has been proposed that high ACE2 levels might lead to an increased susceptibility to SARS‐CoV‐2 infection. 58 ACE2 is widely distributed in various human tissues; however, ACE2‐expressing organs do not equally participate in COVID‐19 pathophysiology, implying that other mechanisms are involved in orchestrating cellular infection resulting in tissue damage. 54 In fact, although ACE2 receptor is the best‐known host factor for SARS‐CoV‐2 entry, the involvement of another essential element, the TMPRSS2 protease, has been recognized.
Receptor recognition and membrane fusion occur through SARS‐CoV‐2 spike (S) protein. Virus entry requires S protein priming by cellular serine protease TMPRSS2, which involves S protein cleavage at S1/S2 and S2 subunits, 59 followed by viral release of the S1 subunit for post‐fusion confirmation. Subsequently, the S1 subunit binds to ACE2, whereas membrane fusion takes place via the S2 subunit. This mechanism is crucial for viral infection 10 , 59 , 60 , 61 , 62 , 63 (Figure 1).
FIGURE 1.
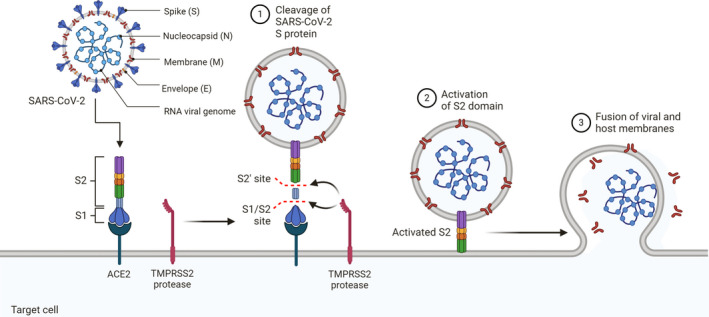
Mechanism of SARS‐CoV‐2 Viral Entry. The spike (S) protein of SARS‐CoV‐2 facilitates viral entry into target cells. Entry depends on binding of the surface unit, S1, of the S protein to the cellular receptor, the ACE2, which facilitates viral attachment to the surface of target cells. Entry requires S protein priming by cellular proteases, which involves S protein cleavage at the S1/S2 and the S2 sites and allows fusion of viral and cellular membranes, a process driven by the S2 subunit
Evidently, SARS‐CoV‐2 cell entry and pathologic effects mainly occur in cells of the respiratory tract, and further dissemination in the host, such as in the testis, may be related to local ACE2 and TMPRSS2 expression. Several tissues and cells have been described to possess an intrinsic RAAS that acts locally through different paracrine and autocrine mechanisms. 64 In the male reproductive system, components of this system have been observed in various organs and tissues, such as the testicles. 65 , 66 Members of the RAAS in the testes are regulated by steroids and gonadotropins. 67 , 68 Apparently, the local RAAS is isolated from the plasma RAAS by a testicular blood barrier that protects male fertility from substances such as ACE inhibitors. 69
As for ACE2, single‐cell RNA sequencing data on human testes showed predominant expression of ACE2 in spermatogonia and Leydig and Sertoli cells 70 ; however, at the protein level, ACE2 has been found to be expressed only in Leydig cells. 71 , 72 Men with severely impaired spermatogenesis have lower levels of ACE2 than fertile men, suggesting that this enzyme may modulate sperm formation. 73 , 74 , 75 ACE2 has also been reported to play key roles in the regulation of testosterone production and in the local vascular regulatory system. 76
TMPRSS2 is mainly expressed in the lung, salivary gland, thyroid, gastrointestinal tract, pancreas, kidney, and liver, according to RNA and protein expression data available at the Human Protein Atlas (HPA) database. Notably, it is also expressed in many male tissues, such as the ductus deferens, epididymis, seminal vesicle, and prostate. TMPRSS2 is highly expressed in prostate epithelial cells 77 and is androgen‐responsive. TMPRSS2 was also found to be released into semen in prostasomes. 78 More recently, it was suggested that spermatogonia express high levels of TMPRSS2. 77
When it comes to testis expression, available data are inconsistent. Ren et al, 79 revealed that not only TMPRSS2 but also ACE2 was highly expressed in genitourinary organs. The testis was also identified as a high‐risk organ because of the high expression level of TMPRSS2 and ACE2. On the other side, Stanley et al 80 suggested that sperm cells may not be at increased risk of ACE2‐ and TMPRSS2‐mediated viral entry and spread, given the lack of co‐expression in any testicular cell type.
However, clinical data identified SARS‐CoV‐2 infection of several organs, where ACE2 expression could not be detected in healthy individuals. These findings suggested that ACE2 expression levels may vary substantially between individuals or during an infection 81 or that SARS‐CoV‐2 can use alternate receptors to enter certain cell types (ie, the cell surface protein basigin (BSG, also known as CD147) 82 and CD26. 83
Several proteins have been recently identified to interact with SARS‐CoV‐2, including lectin receptors and multiple innate immune receptors, 84 heparan sulfate, 85 neuropilins, asialoglycoprotein receptor 1, and Kremen protein 1. 86 However, most of them lack virology‐related evidence to support their roles as SARS‐CoV‐2 entry factors.
It was suggested that the human tyrosine‐protein kinase receptor (AXL), a member of the TAM receptor family, which is abundantly expressed in Sertoli and Leydig cells, 87 not only interacts with the SARS‐CoV‐2 S glycoprotein but also facilities SARS‐CoV‐2 entry into human pulmonary epithelial cells in an ACE2‐independent manner. 88
Likewise, besides TMPRSS2, many proteases have been found to activate coronaviruses. Furin, 89 the endosomal cysteine proteases cathepsin L (CatB/L), 62 trypsin, 90 thermolysin, 91 elastase, 92 and factor Xa 93 have been shown to cleave SARS‐CoV‐1‐S. The amino acid homology between SARS‐CoV‐2‐S protein and SARS‐CoV‐1‐S protein is nearly 76% 10 ; however, apparently, TMPRSS2 is an essential host cell factor for SARS‐CoV‐2. 89
4. THE EFFECT OF THE SARS‐COV‐2 INFECTION ON THE MALE REPRODUCTIVE TRACT
The blood‐testicular or blood‐epididymis barrier, often described as Sertoli cell‐Sertoli cell tight junctions or tight junctions between the epithelium, is much more complex than just the tight junctions. These barriers consist of three components: anatomical, physiological, and immunological factors. Together, these components create a unique, anatomical, physiological, and immunological microenvironment, which is responsible for the proper development of germ cells into fully functional spermatozoa. 94 Nevertheless, several viruses, such as MuV, 95 ZIKV, 96 Ebola, 97 HBV, and HCV, 98 have been shown to disrupt the blood‐testis barrier and infect human testes.
Some viruses, such as ZIKV, may persist in the seminal fluid for a very long time. 99 Likewise, the Ebola virus has been detected in the semen of men after they have recovered from the disease, demonstrating the long‐term presence of the virus in semen. 100 These reports suggest that the blood‐testicular barrier may not be an efficient barrier to viruses.
4.1. Orchitis
Orchitis 101 and orchiepididymitis 102 were identified as complications of SARS‐CoV‐2. Immunohistochemistry demonstrated abundant IgG precipitation in the seminiferous epithelium of testes of individuals with SARS, indicating a possible immune response as the cause for the damage. 45
Additionally, thrombotic complications of SARS‐CoV‐2 may affect the genitourinary system, 101 with priapism reported in a critically ill patient, with acute respiratory distress syndrome and coagulopathic complications. 103 The high expression of ACE‐2 is the human testis 71 , 104 with viral binding may also lead to tissue inflammation and the development of orchi‐epididymitis with testicular pain. 105 In fact, testes from COVID‐19 patients exhibited significant seminiferous tubular injury, reduced Leydig cells, and mild lymphocytic inflammation. 106
4.2. Presence of SARS‐CoV‐2 on seminal fluid
A recent report by Li et al 28 described the presence of SARS‐CoV‐2 in semen samples from six patients, four of whom were at the acute stage of SARS‐CoV‐2 infection and the remaining two were recovering from the disease. Scientific evidence of this report is low due to the small sample size and lack of subsequent follow‐up. Moreover, the methodology used for the detection of the virus in the semen was unclear, and RT‐PCR was used to detect SARS‐CoV‐2 in nasal and pharyngeal swabs; however, it was not clear whether the same assay was used to detect SARS‐CoV‐2 in semen samples. Finally, the possible contamination of semen samples by other body fluids must also be considered, especially if samples were collected by masturbation.
The SARS‐CoV‐2 particle size ranges from 70 to 90 nm, 107 raising the question of whether it is possible that such a large virus would bypass the blood‐testis barrier. However, the MuV virus, a virus with larger dimensions than SARS‐CoV‐2, can disrupt the blood‐testis barrier and infect human testes. 95
As described previously, SARS‐CoV‐2 infection depends on the virus binding to its receptor ACE2 7 and fusion of the viral and host cell membranes by TMPRSS2. 108 The co‐expression of both the ACE2 and TMPRSS2 genes was reported in spermatogonia and prostate endocrine cells, 70 , 77 suggesting a potential vulnerability to SARS‐CoV‐2. 77 Nonetheless, the presence of SARS‐CoV‐2‐associated receptors does not guarantee infection.
In contrast to these findings, SARS‐CoV‐2 was not detected in the semen of a recovering patient with COVID‐19. 109 In this study, a semen sample was collected one week after the last positive nasopharyngeal swab and fifteen days after the onset of the disease. Despite the limitations of the nature of the study, a single case report, the absence of viral RNA amplification on the semen sample raises the question as to whether the virus was ever present or if it was present at the peak of the infection, and SARS‐CoV‐2 clearance in seminal fluid coincided with clinical recovery. It can also be speculated that the virus may be detected in the semen of patients experiencing a more severe disease or in samples collected during the acute phase.
Song et al 110 examined semen samples from COVID‐19‐confirmed patients at both the acute and recovery phases of the disease, and Guo et al 111 examined samples of a cohort of patients with a recent infection or recovering from COVID‐19. Comparable results with the abovementioned report were achieved, suggesting that SARS‐CoV‐2 is absent in the semen of men infected with COVID‐19; the articles also indicate the unlikely possibility of sexual transmission through the semen at about one month after the first detection. Song et al 110 also tested testicular specimens from a deceased COVID‐19 subject and did not detect the presence of viral RNA.
Investigating a larger group of patients, Pan et al 112 reported that SARS‐CoV‐2 was not detected in the semen of patients recovering from COVID‐19. The samples were tested 29–36 days after COVID‐19 diagnosis by qRT‐PCR. The authors also investigated the gene expression levels of ACE2 and TMPRSS2 in different cells of the testes and found that the expression of both genes was low. 112
Nora et al 113 also investigated the presence of SARS‐CoV‐2 in (i) 18 semen samples of patients in convalescence, obtained 8–54 days after the absence of COVID‐19 symptoms, (ii) two samples from patients with an active COVID‐19 infection, and (iii) 14 samples from negative controls. Consistent with the other studies previously mentioned, 109 , 110 , 112 in this trial, no RNA was detected by RT‐PCR.
Taken together, these studies indicated that it is unlikely that recovering subjects may still have and transmit SARS‐CoV‐2 through seminal fluid. Questions remain as to whether SARS‐CoV‐2 infection involves the testis and the seminal fluid under other circumstances. It may be hypothesized that more severe forms of the disease reflect a higher blood viral load and a higher chance to reach other organs and body fluids, including the testes and semen.
Until sexual transmission of SARS‐CoV‐2 from infected men to their partners is ruled out, patients need to be counseled to protect themselves and to consider all possible options to protect their pregnancy if motherhood is desirable.
4.3. Implication on seminal quality
The fact that SARS‐CoV‐2 shares the same receptor as SARS‐CoV‐1 and SARS‐CoV‐1 was determined to cause not only orchitis and testis damage but also defects in spermatogenesis, 45 suggests a possible implication of SARS‐CoV‐2 infection in the seminal quality. Testicular morphological changes in the testes of COVID‐19 patients indicate that SARS‐CoV‐2 infection may impair male germinal cell development and eventually lead to germinal cell loss. 114 In facts, scrotal discomfort has been described in COVID‐19‐diagnosed patients 110 ; moreover, moderate infection was associated with decreased sperm concentration 113 , 115 and motility. 113
Autopsied testicular and epididymal specimens of COVID‐19 patients showed the presence of interstitial edema, congestion, red blood cell exudation in testes, and epididymides. Thinning of seminiferous tubules was also observed. 115
TUNEL assays revealed that the number of apoptotic cells in COVID‐19 testes was significantly higher, 114 , 115 suggesting that SARS‐CoV‐2 damages the immune privilege and innate immune homeostasis of the testis and triggers a secondary autoimmune response contributing to the primary pathogenesis of viral orchitis and consequent testicular damage. 114
Oxidative stress by reactive oxygen species (ROS) is related to all the main changes observed in inflammatory and infectious diseases. 116 The spermatozoa are particularly susceptible to oxidative stress, leading to lipid peroxidation, resulting in disruption of membrane permeability and, thus, efflux of ATP, impairing flagellar movement. 116 , 117 The detrimental impact of oxidative stress on sperm parameters and fertility potential has been determined. 118 Sperm viability, motility, and fertilization potential are disrupted by oxidative stress in the reproductive tissues, evidenced by the presence of high levels of ROS in the semen of infertile men. 119 Therefore, it is suggested that the addition of sperm DNA fragmentation measurement to conventional diagnosing options such as semen analysis can play a crucial role in investigating the possibility of male fertility impairment caused by COVID‐19. 120
4.4. Hormonal milieu
Male subjects seem to not only be more susceptible to COVID‐19 than female subjects 121 , 122 but also their case fatality rate attributable to SARS‐CoV‐2 infection is also higher. 123 ACE2 expression levels have been demonstrated to be higher in male than in female patients, at least in the lungs 124 ; moreover, ACE2 is largely expressed in the testes, which show almost the highest ACE2 expression among various body tissues. 125
Salonia et al 126 speculated that a different hormonal situation could play an important role in the pathophysiology of COVID‐19. Even though ACE2 is expressed in Leydig cells, 125 in a testosterone‐independent manner, in these cells, the enzyme has been proposed to play a role in steroidogenesis. 127 When comparing sex‐related hormones of a cohort of men of reproductive age infected with SARS‐CoV‐2 with those of age‐matched healthy men, Ma et al 128 found a potential alteration of the androgenic hormonal milieu.
Evidence indicates that SARS‐CoV‐2 infection in male individuals per se causes acute‐stage hypogonadism, 129 the depletion of androgenic action triggering serious or an even fatal course of the disease. Patients with COVID‐19 without clinical testicular involvement have been found to present a reduced testosterone/LH ratio, indicating possible subclinical damage to male gonadal function. 128 Çayan et al 130 observed that the serum testosterone level at baseline has a significant increased risk for hospitalization in the intensive care unit and mortality in patients with COVID‐19. COVID‐19 might deteriorate the serum testosterone level in SARS‐CoV‐2‐infected male patients.
4.5. Transmission to the fetus
ACE2 is expressed in several human ovarian compartments, and it can be quantified in follicular fluid. 131 Nevertheless, RNA expression of TMPRSS2 in human cumulus cells was shown to be low or absent. 80 In contrast, a high level of ACE2 and TMPRSS2, found in the trophectoderm that gives rise to placenta, suggests that the developing placenta may be vulnerable to SARS‐CoV‐2 infection. 77 Tissues collected at the maternal‐fetal interface during the first semester of pregnancy, including both embryo‐derived cells (fetal placenta), maternal blood, and decidual cells, displayed a complex pattern in the expression of SARS‐CoV‐2‐associated receptors and factors. 132 Finally, by quantifying the fraction of each cell type co‐expressing different combinations of SARS‐CoV‐2 receptors with proteases, researchers suggested that the placenta is one of the most susceptible tissues to coronavirus infection. 77
Although it is unlikely that COVID‐19‐recovered subjects transmitted SARS‐CoV‐2 through seminal fluid, it is imperative to monitor these patients’ reproductive functions for any abnormalities that might impair future fertility. The possible presence of SARS‐CoV‐2 in seminal fluid must also be analyzed, with caution, in patients who have had different levels of viremia, are at different stages of the disease with different intervals between sample testing, and have recovered from systemic disease.
Evaluating the presence of SARS‐CoV‐2 in seminal samples is particularly important for patients planning parenthood. For those undergoing assisted reproduction treatments, semen washing may be a safe reproductive strategy to achieve pregnancy. As shown with other viruses present in seminal samples, semen washing removes spermatozoa, which are not vectors for the virus, from surrounding seminal fluid, and the virus‐negative sperm fractions may be used in assisted reproduction. 133 , 134 , 135
Stanley et al 80 examined the expression patterns of known viral host entry proteins to gain insights into the possible biological consequences of SARS‐CoV‐2 infection on reproduction. Transcripts of TMPRSS2 were either absent or existed at very low levels in cumulus cells. The authors suggested that assisted reproductive treatments, in which oocytes are collected and fertilized in vitro, may potentially reduce or eliminate exposure of susceptible cell types to infection when compared with natural conception. Therefore, it is possible that in vitro fertilization (IVF) may represent a safer reproductive strategy than natural conception at this time. Additionally, the association of sperm washing with IVF may be an alternative to significantly reduce the risk of viral transition to the fetus.
5. CONCLUSION
In conclusion, COVID‐19 challenges all medical areas, including reproductive medicine. The occurrence of COVID‐19 shows gender differences with men being more susceptible to SARS‐CoV‐2 infection and showing higher fatality rate than women. The male‐related susceptibility in COVID‐19 may be explained, in part, by the cell entry mechanisms of SARS‐CoV‐2. The ACE2, the cellular receptor for SARS‐CoV‐2, is largely expressed in the testes, which show almost the highest ACE2 expression among various body tissues. 125
Although the testes are immunologically privileged in case of viremia, some viruses can cross the blood‐testis barrier, causing local inflammation, persist after an acute infection, and theoretically replicate within the male reproductive tract, which seems to be the case of SARS‐CoV‐2.
In fact, a testicular involvement in COVID‐19 has been suggested. Previous studies demonstrated an association of SARS‐CoV‐2 and orchitis or orchiepididymitis. 101 , 102 SAR‐CoV‐2 may also disrupt male reproductive function through other mechanisms. It may activate oxidant‐sensitive pathways via inflammatory responses, inducing oxidative stress. Compromised sex‐related hormonal balance caused by acute‐stage hypogonadism or possible damage to male gonadal function is also a matter of debate. Finally, possible implications of varying levels of viremia for sexual transmission and consequently for embryonic infection, pregnancy outcome, and congenital disease cannot be excluded.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR'S CONTRIBUTION
EBJ designed the study and wrote specific sections; AS and AIJ wrote specific sections of the paper; and DB designed the study, wrote specific sections, and critically analyzed and edited the final manuscript.
Borges E Jr, Setti AS, Iaconelli A Jr, et al. Current status of the COVID‐19 and male reproduction: A review of the literature. Andrology. 2021;00:1–10. 10.1111/andr.13037
REFERENCES
- 1. Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019‐new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui DS, I Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mortola E, Roy P. Efficient assembly and release of SARS coronavirus‐like particles by a heterologous expression system. FEBS Lett. 2004;576(1–2):174‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lou Z, Sun Y, Rao Z. Current progress in antiviral strategies. Trends Pharmacol Sci. 2014;35(2):86‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baughn LB, Sharma N, Elhaik E, Sekulic A, Bryce AH, Fonseca R. Targeting TMPRSS2 in SARS‐CoV‐2 infection. Mayo Clin Proc. 2020;95(9):1989‐1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuba K, Imai Y, Ohto‐Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin‐angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Zhang HT, Xie YQ, et al. Morphological study of severe acute respiratory syndrome (SARS). Zhonghua Bing Li Xue Za Zhi. 2003;32(6):516‐520. [PubMed] [Google Scholar]
- 20. Tse GM, To KF, Chan PK, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol. 2004;57(3):260‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Zhou P, Wei Y, et al. Histopathologic changes and SARS‐CoV‐2 immunostaining in the lung of a patient with COVID‐19. Ann Intern Med. 2020;172(9):629‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jirak P, Larbig R, Shomanova Z, et al. Myocardial injury in severe COVID‐19 is similar to pneumonias of other origin: results from a multicentre study. ESC Heart Fail. 2021;8(1):37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panariello F, Cellini L, Speciani M, De Ronchi D, Atti AR. How does SARS‐CoV‐2 affect the Central Nervous System? A working hypothesis. Front Psychiatry. 2020;350:577436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Zhang Y, Wang F, et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID‐19). BMC Cardiovasc Disord. 2020;20(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez A, Orozco‐Aguilar J, Achiardi O, Simon F, Cabello‐Verrugio C. SARS‐CoV‐2/renin–angiotensin system: deciphering the clues for a couple with potentially harmful effects on skeletal muscle. Int J Mol Sci. 2020;21(21):7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y‐F, Zhang Z, Pan X‐L, et al. The chronic kidney disease and acute kidney injury involvement in COVID‐19 pandemic: a systematic review and meta‐analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamouroux A, Attie‐Bitach T, Martinovic J, Leruez‐Ville M, Ville Y. Evidence for and against vertical transmission for SARS‐CoV‐2 (COVID‐19). Am J Obstet Gynecol. 2020;223(1):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50(11):e13140. [DOI] [PubMed] [Google Scholar]
- 30. Ma W, Li S, Ma S, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2017;168(3):542. [DOI] [PubMed] [Google Scholar]
- 31. Mead PS, Duggal NK, Hook SA, et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med. 2018;378(15):1377‐1385. [DOI] [PubMed] [Google Scholar]
- 32. D'Ortenzio E, Matheron S, Yazdanpanah Y, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374(22):2195‐2198. [DOI] [PubMed] [Google Scholar]
- 33. Choi HI, Yang DM, Kim HC, et al. Testicular atrophy after mumps orchitis: ultrasonographic findings. Ultrasonography. 2020;39:266‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson JA, Ping LH, Dibben O, et al. HIV‐1 populations in semen arise through multiple mechanisms. PLoS Pathog. 2010;6(8):e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta P, Leroux C, Patterson BK, et al. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral Quasi species between blood and semen. J Infect Dis. 2000;182(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 36. Zhong Y, Liu DL, Ahmed MMM, et al. Transcription and regulation of hepatitis B virus genes in host sperm cells. Asian J Androl. 2018;20(3):284‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu Y, Ma M, Huang J, et al. Effects of hepatitis C virus infection on human sperm chromosomes. Clin Lab. 2016;62(3):373‐379. [DOI] [PubMed] [Google Scholar]
- 38. Gimenes F, Souza RP, Bento JC, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11(12):672‐687. [DOI] [PubMed] [Google Scholar]
- 39. Foresta C, Patassini C, Bertoldo A, et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One. 2011;6(3):e15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laprise C, Trottier H, Monnier P, Coutlee F, Mayrand MH. Prevalence of human papillomaviruses in semen: a systematic review and meta‐analysis. Hum Reprod. 2014;29(4):640‐651. [DOI] [PubMed] [Google Scholar]
- 41. Lippold S, Braun B, Kruger F, et al. Natural inhibitor of human cytomegalovirus in human seminal plasma. J Virol. 2019;93(6):e01855‐1–e01855‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurscheidt FA, Damke E, Bento JC, et al. Effects of herpes simplex virus infections on seminal parameters in male partners of infertile couples. Urology. 2018;113:52‐58. [DOI] [PubMed] [Google Scholar]
- 43. Klimova RR, Chichev EV, Naumenko VA, et al. Herpes simplex virus and cytomegalovirus in male ejaculate: herpes simplex virus is more frequently encountered in idiopathic infertility and correlates with the reduction in sperm parameters. Vopr Virusol. 2010;55(1):27‐31. [PubMed] [Google Scholar]
- 44. Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65(2):208‐231. first and second pages, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rao M, Zhao XL, Yang J, et al. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl. 2015;17(4):668‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu J, Xu Z, Jiang Y, Qian X, Huang Y. Cryptorchidism induces mouse testicular germ cell apoptosis and changes in bcl‐2 and bax protein expression. J Environ Pathol Toxicol Oncol. 2000;19(1–2):25‐33. [PubMed] [Google Scholar]
- 48. Ashby J, Goldmeier D, Sadeghi‐Nejad H. Hypogonadism in human immunodeficiency virus‐positive men. Korean J Urol. 2014;55(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sano T, Kovacs K, Scheithauer BW, Rosenblum MK, Petito CK, Greco CM. Pituitary pathology in acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1989;113(9):1066‐1070. [PubMed] [Google Scholar]
- 50. Brown TT. Hypogonadism in men with hepatitis C: what is a clinician to do? Clin Infect Dis. 2019;69(4):577‐579. [DOI] [PubMed] [Google Scholar]
- 51. Pekic S, Cvijovic G, Stojanovic M, Kendereski A, Micic D, Popovic V. Hypopituitarism as a late complication of hemorrhagic fever. Endocrine. 2005;26(2):79‐82. [DOI] [PubMed] [Google Scholar]
- 52. Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis. 2005;16(4):271‐281. [DOI] [PubMed] [Google Scholar]
- 53. Wei L, Sun S, Zhang J, et al. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem Cell Biol. 2010;88(4):723‐730. [DOI] [PubMed] [Google Scholar]
- 54. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme‐2 (ACE2), SARS‐CoV‐2 and pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol. 2020;251(3):228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Medina‐Enríquez MM, Lopez‐León S, Carlos‐Escalante JA, Aponte‐Torres Z, Cuapio A, Wegman‐Ostrosky T. ACE2: the molecular doorway to SARS‐CoV‐2. Cell Biosci. 2020;10(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Furuhashi M, Moniwa N, Takizawa H, Ura N, Shimamoto K. Potential differential effects of renin‐angiotensin system inhibitors on SARS‐CoV‐2 infection and lung injury in COVID‐19. Hypertens Res. 2020;43(8):837‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Silva ACSE, Teixeira MM. ACE inhibition, ACE2 and angiotensin‐(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res. 2016;107:154‐162. [DOI] [PubMed] [Google Scholar]
- 58. Swärd P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID‐19. Crit Care. 2020;24(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hartenian E, Nandakumar D, Lari A, Ly M, Tucker JM, Glaunsinger BA. The molecular virology of Coronaviruses. J Biol Chem. 2020;295(37):12910‐12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Choudhary S, Sreenivasulu K, Mitra P, Misra S, Sharma P. Role of genetic variants and gene expression in the susceptibility and severity of COVID‐19. Ann Lab Med. 2021;41(2):129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122‐4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84(24):12658‐12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shulla A, Heald‐Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85(2):873‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gianzo M, Subirán N. Regulation of male fertility by the renin‐angiotensin. System. 2020;21:7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pauls K, Metzger R, Steger K, Klonisch T, Danilov S, Franke F. Isoforms of angiotensin I‐converting enzyme in the development and differentiation of human testis and epididymis. Andrologia. 2003;35(1):32‐43. [DOI] [PubMed] [Google Scholar]
- 66. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin‐angiotensin systems. Physiol Rev. 2006;86(3):747‐803. [DOI] [PubMed] [Google Scholar]
- 67. Okuyama A, Nonomura N, Koh E, et al. Induction of renin‐angiotensin system in human testis in vivo. Arch Androl. 1988;21(2):29‐35. [DOI] [PubMed] [Google Scholar]
- 68. Dzau VJ, Ellison KE, Brody T, Ingelfinger J, Pratt RE. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987;120(6):2334‐2338. [DOI] [PubMed] [Google Scholar]
- 69. Nguyen G. Renin, (pro) renin and receptor: an update. Clin Sci. 2011;120(5):169‐178. [DOI] [PubMed] [Google Scholar]
- 70. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9(4):920‐1–920‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin‐converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):e1‐e9. [DOI] [PubMed] [Google Scholar]
- 72. Alenina N, Baranova T, Smirnow E, et al. Cell type‐specific expression of the Mas proto‐oncogene in testis. J Histochem Cytochem. 2002;50(5):691‐695. [DOI] [PubMed] [Google Scholar]
- 73. Younis JS, Abassi Z, Skorecki K. Is there an impact of the COVID‐19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 2020;318:E878‐E880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Douglas GC, O'Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. [DOI] [PubMed] [Google Scholar]
- 75. Reis AB, Araújo FC, Pereira VM, Dos Reis AM, Santos RA, Reis FM. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Histol. 2010;41(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 76. Pan PP, Zhan QT, Le F, Zheng YM, Jin F. Angiotensin‐converting enzymes play a dominant role in fertility. Int J Mol Sci. 2013;14(10):21071‐21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Singh M, Bansal V, Feschotte C. A single‐cell RNA expression map of human coronavirus entry factors. Cell Rep. 2020;32(12):108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen YW, Lee MS, Lucht A, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176(6):2986‐2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ren X, Wang S, Chen X, et al. Multiple expression assessments of ACE2 and TMPRSS2 SARS‐CoV‐2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID‐19. Infect Drug Resist. 2020;13:3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease (COVID‐19) and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016‐1035 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang X, Xu W, Hu G, et al. SARS‐CoV‐2 infects T lymphocytes through its spike protein‐mediated membrane fusion. Cell Mol Immunol. 2020;7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vankadari N, Wilce JA. Emerging WuHan (COVID‐19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thépaut M, Luczkowiak J, Vivès C, et al. DC/L‐SIGN recognition of spike glycoprotein promotes SARS‐CoV‐2 trans‐infection and can be inhibited by a glycomimetic antagonist. PLoS Pathog. 2020;17:e1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clausen TM, Sandoval DR, Spliid CB, et al. SARS‐CoV‐2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043‐1057. e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370(6518):856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao S, Zhu W, Xue S, Han D. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol. 2014;11(5):428‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang S, Qiu Z, Hou Y, et al. AXL is a candidate receptor for SARS‐CoV‐2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31:126‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS‐CoV‐2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yao YX, Ren J, Heinen P, Zambon M, Jones IM. Cleavage and serum reactivity of the severe acute respiratory syndrome coronavirus spike protein. J Infect Dis. 2004;190(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) spike glycoprotein‐mediated viral entry. Proc Natl Acad Sci USA. 2004;101(12):4240‐4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Matsuyama S, Ujike M, Morikawa S, Tashiro M, Taguchi F. Protease‐mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci USA. 2005;102(35):12543‐12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Du L, Kao RY, Zhou Y, et al. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem Biophys Res Commun. 2007;359(1):174‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mital P, Hinton BT, Dufour JM. The blood‐testis and blood‐epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84(5):851‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu H, Shi L, Wang Q, et al. Mumps virus‐induced innate immune responses in mouse Sertoli and Leydig cells. Sci Rep. 2016;6:19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission – continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(8):215‐216. [DOI] [PubMed] [Google Scholar]
- 97. Paz‐Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids – final report. N Engl J Med. 2018;379(13):1234‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Karamolahi S, Yazdi RS, Zangeneh M, Makiani MJ, Farhoodi B, Gilani MAS. Impact of hepatitis B virus and hepatitis C virus infection on sperm parameters of infertile men. Int J Reprod Biomed (Yazd). 2019;17(8):551‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao‐Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors ‐ final report. N Engl J Med. 2017;377(15):1428‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bridwell RE, Merrill DR, Griffith SA, Wray J, Oliver JJ. A coronavirus disease 2019 (COVID‐19) patient with bilateral orchitis: a case report. Am J Emerg Med. 2020;42:260.e3‐260.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gagliardi L, Bertacca C, Centenari C, et al. Orchiepididymitis in a boy with COVID‐19. Pediatr Infect Dis J. 2020;39(8):e200‐e202. [DOI] [PubMed] [Google Scholar]
- 103. Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID‐19). Am J Emerg Med. 2021;39(251):251.e5‐251.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019‐nCoV. Biochem Biophys Res Commun. 2020;525(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. La Marca A, Busani S, Donno V, Guaraldi G, Ligabue G, Girardis M. Testicular pain as an unusual presentation of COVID‐19: a brief review of SARS‐CoV‐2 and the testis. Reprod Biomed. 2020;41(5):903‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang M, Chen S, Huang B, et al. Pathological findings in the testes of COVID‐19 patients: clinical implications. Eur Urol Focus. 2020;6(5):1124‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kim JM, Chung YS, Jo HJ, et al. Identification of Coronavirus Isolated from a Patient in Korea with COVID‐19. Osong Public Health Res Perspect. 2020;11(1):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS‐CoV‐2 in semen and urine samples of a volunteer with positive naso‐pharyngeal swab. J Endocrinol Invest. 2020;43:1819‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song C, Wang Y, Li W, et al. Absence of 2019 Novel Coronavirus in Semen and Testes of COVID‐19 Patients. Biol Reprod. 2020;103:4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aitken RJ. COVID‐19 and human spermatozoa – potential risks for infertility and sexual transmission. Andrology. 2020;9:48‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nora H, Philippos E, Marcel A, et al. Assessment of SARS‐CoV‐2 in human semen ‐ a cohort study. Fertil Steril. 2020;114:233‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ma X, Guan C, Chen R, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS‐CoV‐2 infection in the testis and spermatogenesis damage in COVID‐19 patients. Cell Mol Immunol. 2021;18:487‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li H, Xiao X, Zhang J, et al. Impaired spermatogenesis in COVID‐19 patients. EClinicalMedicine. 2020;28:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kazhyna M, Davidovich I, Lis A. The European Journal of Contraception & Reproductive Health Care. Eur J Contracept Reprod Health Care. 2010;15(S1):181. [Google Scholar]
- 117. Sharma RK, Alsaad R, Bamajbuor FS, et al. Negative effects of oxidative stress (OS) on reproductive system at cellular level. In: Agarwal A, Sharma R, Gupta S, eds. Oxidative Stress in Human Reproduction: Shedding Light on a Complicated Phenomenon. 1st ed. New York: Springer; 2017:65–87. 10.1007/978-3-319-48427-3_4. [DOI] [Google Scholar]
- 118. Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9(4):331‐345. [DOI] [PubMed] [Google Scholar]
- 119. Agarwal A, Sharma RK, Nallella KP, Thomas AJ Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86(4):878‐885. [DOI] [PubMed] [Google Scholar]
- 120. Sengupta P, Dutta S. Does SARS‐CoV‐2 infection cause sperm DNA fragmentation? Possible link with oxidative stress. Eur J Contracept Reprod Health Care. 2020;25(5):405‐406. [DOI] [PubMed] [Google Scholar]
- 121. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hall KS, Samari G, Garbers S, et al. Centring sexual and reproductive health and justice in the global COVID‐19 response. Lancet. 2020;395(10231):1175‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS‐CoV‐2 receptor. Pharmacol Res. 2020;157:104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9(4):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Salonia A, Corona G, Giwercman A, et al. SARS‐CoV‐2, testosterone and frailty in males (PROTEGGIMI): a multidimensional research project. Andrology. 2020;9:19‐22. [DOI] [PubMed] [Google Scholar]
- 127. Douglas GC, O’Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. [DOI] [PubMed] [Google Scholar]
- 128. Ma L, Xie W, Li D, et al. Effect of SARS‐CoV‐2 infection upon male gonadal function: a single center‐based study. MedRxiv. 2020. [Google Scholar]
- 129. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. SARS‐CoV‐2: the endocrinological protective clinical model derived from patients with prostate cancer. Ther Adv Endocrinol Metab. 2020;11:2042018820942385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Çayan S, Saylam B, Uğuz M, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of Coronavirus disease 2019 (COVID‐19) in SARS‐CoV‐2 infected male patients: a cohort study. Aging Male. 2020;23:1493‐1503. [DOI] [PubMed] [Google Scholar]
- 131. Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM, Santos RA. Angiotensin‐(1–7), its receptor Mas, and the angiotensin‐converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95(1):176‐181. [DOI] [PubMed] [Google Scholar]
- 132. Vento‐Tormo R, Efremova M, Botting RA, et al. Single‐cell reconstruction of the early maternal‐fetal interface in humans. Nature. 2018;563(7731):347‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zafer M, Horvath H, Mmeje O, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV‐discordant couples: a systematic review and meta‐analysis. Fertil Steril. 2016;105(3):645‐655 e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fenizia C, Vittori C, Oneta M, et al. Human papillomavirus in spermatozoa is efficiently removed by washing: a suitable approach for assisted reproduction. Reprod Biomed Online. 2020;40(5):693‐699. [DOI] [PubMed] [Google Scholar]
- 135. Garrido N, Meseguer M, Bellver J, Remohi J, Simon C, Pellicer A. Report of the results of a 2 year programme of sperm wash and ICSI treatment for human immunodeficiency virus and hepatitis C virus serodiscordant couples. Hum Reprod. 2004;19(11):2581‐2586. [DOI] [PubMed] [Google Scholar]


