Abstract
Whether immunosuppression impairs severe acute respiratory syndrome coronavirus 2-specific T cell–mediated immunity (SARS-CoV-2-CMI) after liver transplantation (LT) remains unknown. We included 31 LT recipients in whom SARS-CoV-2-CMI was assessed by intracellular cytokine staining (ICS) and interferon (IFN)-γ FluoroSpot assay after a median of 103 days from COVID-19 diagnosis. Serum SARS-CoV-2 IgG antibodies were measured by ELISA. A control group of nontransplant immunocompetent patients were matched (1:1 ratio) by age and time from diagnosis. Post-transplant SARS-CoV-2-CMI was detected by ICS in 90.3% (28/31) of recipients, with higher proportions for IFN-γ-producing CD4+ than CD8+ responses (93.5% versus 83.9%). Positive spike-specific and nucleoprotein-specific responses were found by FluoroSpot in 86.7% (26/30) of recipients each, whereas membrane protein-specific response was present in 83.3% (25/30). An inverse correlation was observed between the number of spike-specific IFN-γ-producing SFUs and time from diagnosis (Spearman’s rho: –0.418; p value = .024). Two recipients (6.5%) failed to mount either T cell–mediated or IgG responses. There were no significant differences between LT recipients and nontransplant patients in the magnitude of responses by FluoroSpot to any of the antigens. Most LT recipients mount detectable—but declining over time—SARS-CoV-2-CMI after a median of 3 months from COVID-19, with no meaningful differences with immunocompetent patients.
KEYWORDS: clinical research/practice, complication: infectious, immunosuppression/immune modulation, infection and infectious agents, infection and infectious agents - viral, infectious disease, liver transplantation/hepatology, monitoring: immune
Abbreviations: CI, confidence interval; CMI, cell-mediated immunity; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; ICS, intracellular cytokine staining; IFN-γ, interferon-γ; IQR, interquartile range; LT, liver transplantation; M, SARS-CoV-2 membrane protein; mAb, monoclonal antibody; N, SARS-CoV-2 nucleoprotein; OD, optical density; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; S, SARS-CoV-2 spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2-CMI, SARS-CoV-2-specific T cell–mediated immunity; SD, standard deviation; SFU, spot forming unit; SOT, solid organ transplantation
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has disrupted liver transplantation (LT) programs worldwide. Although the clinical course and outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection seem to be better for LT recipients compared to other transplant groups,1 mortality rates among hospitalized patients during the first wave reached 20%.2 , 3
The understanding of the magnitude and durability of natural immunity following SARS-CoV-2 infection among immunocompromised patients is of paramount relevance to determine the individual susceptibility to reinfection. Previous studies have assessed SARS-CoV-2-specific T cell–mediated immunity (SARS-CoV-2-CMI) in kidney transplant (KT) recipients recovered from COVID-19, revealing that interferon (IFN)-γ-producing CD4+ and CD8+ T cells reactive to the spike (S) glycoprotein and other structural proteins may be detected for at least 6 months.4, 5, 6, 7 Specific data for LT recipients, however, are lacking. Variations in the magnitude of SARS-CoV-2-specific responses according to the type of immunosuppression, severity of COVID-19 and timing from transplantation are still largely unknown.
The present study was aimed at analyzing the frequencies of SARS-CoV-2-specific IFN-γ-producing T cell responses and their clinical correlates in a single-center cohort of LT recipients at various times from the diagnosis of COVID-19. To this end, we used two different methods: the intracellular cytokine staining (ICS) and a fluorescent ELISpot for cytokine secretion (IFN-γ FluoroSpot assay).
2. MATERIALS AND METHODS
2.1. Study population and setting
All adult LT recipients regularly seen at the University Hospital “12 de Octubre” (Madrid, Spain) with a diagnosis of COVID-19 confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) or rapid antigen detection test (Panbio™ COVID-19 Ag Rapid Test Device, Abbott Diagnostics) between March 15 and November 13, 2020, were eligible for inclusion. No exclusion criteria regarding clinical severity or timing from transplantation were applied. The assessment of SARS-CoV-2-CMI was performed at a single time point per patient (between December 9 and 21, 2020). The research was carried out in accordance with the ethical standards as laid down in the Declarations of Helsinki and Istanbul. The study protocol was approved by the institutional Research Ethics Committee (ref. 20/314) and all participants provided written informed consent.
The management of LT recipients with post-transplant COVID-19—including the use of antiviral agents, immunomodulatory therapies and supportive care—was in accordance with the national and local guidelines in place at each time, which have evolved throughout the study period according to the available evidence.8 , 9 Once recovered, patients were followed-up until January 2021 by phone calls or at the outpatient clinic according to routine practice, in order to assess the development of symptoms compatible with SARS-CoV-2 reinfection.
2.2. SARS-CoV-2-CMI assessment by ICS
Whole blood specimens were collected in sodium heparin tubes and shipped at room temperature to the Hospital Clínico Universitario (Valencia, Spain), where they were processed within 24 h. Enumeration of SARS-CoV-2-specific IFN-γ-producing CD69+ CD4+ and CD8+ T cells was performed by flow cytometry for ICS (BD FastimmuneTM kit, BD Biosciences, San Jose, CA) following a protocol originally designed for the assessment of cytomegalovirus (CMV)-specific cell-mediated immunity.10 , 11 In brief, heparinized whole blood (0.5 ml) was simultaneously stimulated for 6 h with two sets of 15-mer overlapping peptides (11-mer overlap) encompassing the SARS-CoV-2 S glycoprotein N-terminal 1- to 643-amino acid sequence (158 individual peptides) (PepMix™ SARS-CoV-2 Spike Glycoprotein, JPT peptide Technologies GmbH, Berlin, Germany) and the entire sequence of the membrane (M) protein (53 peptides) (Epitope Mapping Peptide Set [EMPS] SARS-CoV-2 VME1, JPT) at a concentration of 1 μg/ml per peptide and in the presence of costimulatory monoclonal antibodies (mAbs) targeting CD28 and CD49d (1 μg/ml). Brefeldin A (10 μg/ml) was added for the last 4 h of incubation. Next, blood was lysed (BD FACS lysing solution) and frozen at –80°C. On the day of testing, stimulated blood was thawed at 37°C, washed, permeabilized (BD permeabilizing solution) and stained with fluorochrome-labeled mAbs (anti-CD3-APC-Cy7, anti-CD69-PE, anti-CD4 or CD8-PerCP-Cy5.5, and anti-IFN-γ-FITC) for 1 h at room temperature. Samples mock-stimulated with phosphate-buffered saline (PBS)/dimethyl sulfoxide solution (without peptides) and costimulatory antibodies or stimulated with phytohemagglutinin (1 mg/ml) as non-specific mitogen (Sigma-Aldrich) were run in parallel in all experiments. Appropriate isotype controls were also used. Next, cells were washed, resuspended in 200 μl of 1% paraformaldehyde in PBS, and analyzed within 2 h on a FACScanto flow cytometer using BD FACSDivaTM software (BD Biosciences). CD3+/CD4+ and CD3+/CD8+ events were gated and analyzed for the T cell activation marker CD69+ and IFN-γ production. SARS-CoV-2-specific IFN-γ-producing CD69+ CD4+ and CD8+ T cells were enumerated by multiplying the corresponding percentages of T cells that produced IFN-γ upon peptide stimulation (after background subtraction) by absolute CD4+ and CD8+ T cell counts, and responses ≥0.1% were considered specific.
2.3. SARS-CoV-2-CMI assessment by the IFN-γ FluoroSpot assay
Whole blood specimens were processed at the Department of Immunology of the Hospital Universitario “12 de Octubre” within 24 h from sampling. Peripheral blood mononuclear cells (PBMCs) were freshly isolated by density-gradient centrifugation using Ficoll-Paque and seeded at 300 000 cells/well in IFN-γ FluoroSpotTM plates (MabTech, Nacka Strand, Sweden) with cell culture medium containing RPMI, 1% L-glutamine, 1% penicillin/streptomycin, 10% fetal bovine serum and anti-CD28 mAb (1 µg/ml). Test wells were performed in duplicate and supplemented with 15-mer overlapping peptides covering the S1 domain of the S glycoprotein (166 individual peptides) (SARS-CoV-2 S1 scanning pool, MabTech), the nucleoprotein (N protein) (102 peptides) (EMPS SARS-CoV-2 NCAP-1, JPT), and the M protein (53 peptides) (EMPS SARS-CoV-2 VME1, JPT) at a final concentration of 1 µg/ml. Negative control wells lacked peptides, and positive control wells included anti-CD3 mAb (MabTech). The fluorophore conjugates used were anti-BAM-490 and SA-550. Assays were incubated for 16–18 h at 37°C. The readout was performed following the manufacturer’s instructions for the Human IFN-γ/IL-2 FluoroSpotPLUS kit and spots were counted using an automated IRISTM FluoroSpot Reader System (both from MabTech). To quantify antigen-specific responses, spots of the negative control wells were subtracted from the mean spots test wells, and the results were expressed as IFN-γ-producing spot forming units (SFUs) per 106 PBMCs. Results were excluded if negative control wells had >80 SFUs/106 PBMCs or positive control wells had <400 SFUs/106 PBMCs. Reponses were considered positive if the results were at least three times higher than the mean of the negative control wells and above of the following antigen-specific cut-off values (which had been established by using a control group of 30 healthcare workers with no microbiological, serological or clinical evidence of SARS-CoV-2 infection): >25 SFUs/106 PBMCs for the S glycoprotein (S1 domain), >14 SFUs/106 PBMCs for the N protein, and >21 SFUs/106 PBMCs for the M protein.
2.4. SARS-CoV-2 serology
Serum SARS-CoV-2 IgG antibodies targeting the S1 protein were detected with the Euroimmun Anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) (Euroimmun AG, Lübeck, Germany) according to manufacturer’s instructions. Optical density (OD) values were measured at 450 nm using the PR 3100 microplate reader (Bio-Rad Life Science, Marnes-La-Coquette, France). Results were evaluated semi-quantitatively by calculating the ratio of the OD value of the sample over the OD value of the calibrator (relative OD), with the following cut-off values: ratio <0.8: negative; ratio ≥0.8 to <1.1: borderline; and ratio ≥1.1: positive.
2.5. Nontransplant controls
In order to analyze the impact of post-transplant immunosuppression on the development and magnitude of SARS-CoV-2-CMI measured by the IFN-γ FluoroSpot assay, we selected a group of immunocompetent patients diagnosed with COVID-19 that were hospitalized at our institution in March 2020. Nontransplant controls were matched in a 1:1 ratio with LT recipients according to age and time interval from the diagnosis of COVID-19 to the assessment of SARS-CoV-2-CMI. None of the controls had other causes of immunosuppression (i.e., active malignancy, HIV infection or chronic corticosteroid therapy).
2.6. Statistical analysis
Quantitative data were shown as the mean ± standard deviation (SD) or the median with interquartile range (IQR), and qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the χ2 test. The Mann-Whitney U test was applied for continuous variables. Correlations between continuous variables were evaluated using the Pearson’s correlation coefficient or the Spearman’s rho. The agreement between both methods used for assessing SARS-CoV-2-CMI (ICS and FluroSpot) was evaluated by the Kappa index. Statistical analysis was performed with SPSS version 20.0 (IBM Corp.).
3. RESULTS
3.1. Study population
Overall, 35 LT recipients regularly followed-up at our institution with a RT-PCR-confirmed diagnosis of SARS-CoV-2 infection between March 15 and November 13 were approached. Four of them refused to participate, and 31 patients (88.6%) were finally included ( Table 1). The median interval from the diagnosis of COVID-19 to the assessment of SARS-CoV-2-CMI of 103 days (IQR: 72.8–261.5). Mean tacrolimus and mycophenolic acid trough levels at that point were 4.9 ± 2.4 and 2.7 ± 1.5 ng/ml, respectively. Nineteen patients (61.3%) have a repeated SARS-CoV-2 RT-PCR after a median of 20 days (IQR: 14–66) from the initial positive testing. Eight of them (42.1%) had a persistently positive RT-PCR test for a median of 19 days (IQR: 11–26.5). A 46-year-old female patient that had undergone transplantation 6.3 years earlier and was on tacrolimus monotherapy developed a mildly symptomatic new episode of SARS-CoV-2 infection that was documented by RT-PCR after 5.5 months from the initial diagnosis of COVID-19.
TABLE 1.
Demographics and clinical characteristics of the study population (n = 31)
| Variable | |
|---|---|
| Age at diagnosis, years, mean ± SD | 61.7 ± 10.6 |
| Male gender, n (%) | 24 (77.4) |
| Ethnicity, n (%) | |
| Caucasian | 28 (90.3) |
| Latino | 3 (9.7) |
| Major chronic comorbidities, n (%) | |
| Hypertension | 13 (41.9) |
| Diabetes mellitus | 11 (35.5) |
| Obesity | 3 (9.7) |
| Heart disease | 2 (6.5) |
| Underlying cause of end-stage liver disease, n (%) | |
| HCV infection | 15 (48.4) |
| Alcoholic cirrhosis | 7 (22.6) |
| HBV infection | 3 (9.7) |
| HBV/HDV coinfection | 2 (6.5) |
| Drug-induced acute liver failure | 2 (6.5) |
| Othera | 2 (6.5) |
| Diagnosis of hepatocellular carcinoma, n (%) | 13 (41.9) |
| Time from transplantation to diagnosis of COVID–19, months, median (IQR) | 76.5 (15.2–185.0) |
| Immunosuppression at diagnosis of COVID–19, n (%) | |
| Prednisone | 8 (25.8) |
| Daily dose, mg, median (IQR) | 5 (5–15) |
| Tacrolimus | 19 (61.3) |
| Mofetil mycophenolate | 19 (61.3) |
| mTOR inhibitor | 4 (12.9) |
| Azathioprine | 1 (3.2) |
| Type of immunosuppressive regimen | |
| Mofetil mycophenolate monotherapy | 7 (22.6) |
| Tacrolimus, mofetil mycophenolate and prednisone | 6 (19.4) |
| Tacrolimus and mofetil mycophenolate | 6 (19.4) |
| Tacrolimus monotherapy | 6 (19.4) |
| mTOR inhibitor monotherapy | 4 (12.9) |
| Otherb | 2 (6.5) |
| Clinical severity of COVID–19, n (%) | |
| Outpatient management | 19 (61.3) |
| Hospitalization, no supplemental oxygen requirements | 5 (16.1) |
| Hospitalization, low-flow oxygen (FiO2 <40%) | 6 (19.3) |
| Hospitalization, high-flow oxygen (FiO2 ≥40%) | 1 (3.2) |
| Mode of diagnosis of SARS-CoV–2 infection, n (%) | |
| RT-PCR | 29 (93.5) |
| Rapid antigen detection test | 2 (6.5) |
| Radiological diagnosis of COVID–19 pneumonia, n (%) | 11 (35.5) |
| Laboratory values at presentation, mean ± SD | |
| Lymphocyte count, cells ×109 | 0.9 ± 0.5 |
| Lactate dehydrogenase, IU/L | 248.0 ± 68.9 |
| C-reactive protein, mg/dl | 4.9 ± 6.3 |
| Treatment, n (%) | |
| Hydroxychloroquine | 8 (25.8) |
| Azithromycin | 1 (3.2) |
| Lopinavir/ritonavir | 1 (3.2) |
| Remdesivir | 2 (6.5) |
| Low-to-intermediate-dose corticosteroids | 4 (12.9) |
| Methylprednisolone boluses | 3 (9.7) |
| Tocilizumab | 1 (3.2) |
| Follow-up from the diagnosis of COVID–19, days, median (IQR) | 183 (110.5–305.3) |
Abbreviations: COVID-19, coronavirus disease 2019; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; IQR, interquartile range; mTOR, mammalian target of rapamycin; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Autoimmune hepatitis and mushroom poisoning.
Everolimus and prednisone, and azathioprine, everolimus, and prednisone.
3.2. Post-transplant SARS-CoV-2-CMI assessed by ICS
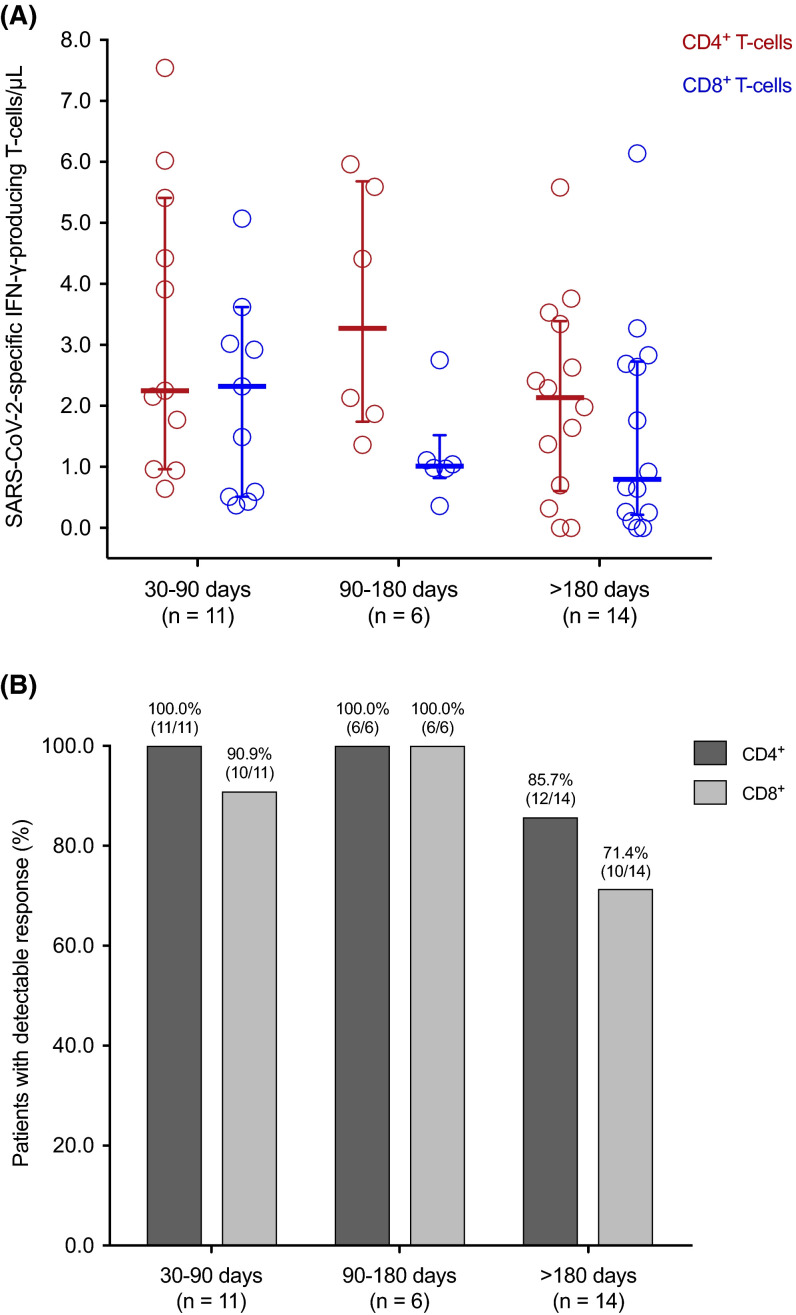
Any SARS-CoV-2-CMI (≥0.1%) was detectable by ICS in 90.3% (28/31) of patients. In detail, SARS-CoV-2-specific IFN-γ-producing CD4+ and CD8+ T cell responses were observed in 90.3% (28/31) and 83.9% (26/31), respectively. Absolute counts were 2.8 ± 2.0 CD4+ T cells/μl and 1.9 ± 2.1 CD8+ T cells/μl. One recipient—a 57-year-old female patient that had undergone LT 16 years earlier and was on tacrolimus—lacked any detectable T cell response after 290 days from the diagnosis of COVID-19.
When SARS-CoV-2-specific T cell counts were analyzed according to the interval from the diagnosis of COVID-19, there was a non-significant decrease over time for both CD4+ (3.3 ± 2.3 versus 2.1 ± 1.6 cells/μl from 30–90 days to >180 days; p value = .153) and CD8+ T cell subsets (2.7 ± 2.7 versus 1.6 ± 1.8 cells/μl, respectively; p value = .223) ( Figure 1).
FIGURE 1.
SARS-CoV-2-specific IFN-γ-producing T cell CD4+ (red) and CD8+ (blue) T cell counts (A) and proportion of patients with detectable (≥0.1%) responses (B) by the ICS method according to the time interval from the diagnosis of COVID-19 to the assessment. Horizontal bars and whiskers represent median values and interquartile ranges, respectively. IFN-γ, interferon-γ; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
3.3. Post-transplant SARS-CoV-2-CMI assessed by the IFN-γ FluoroSpot assay
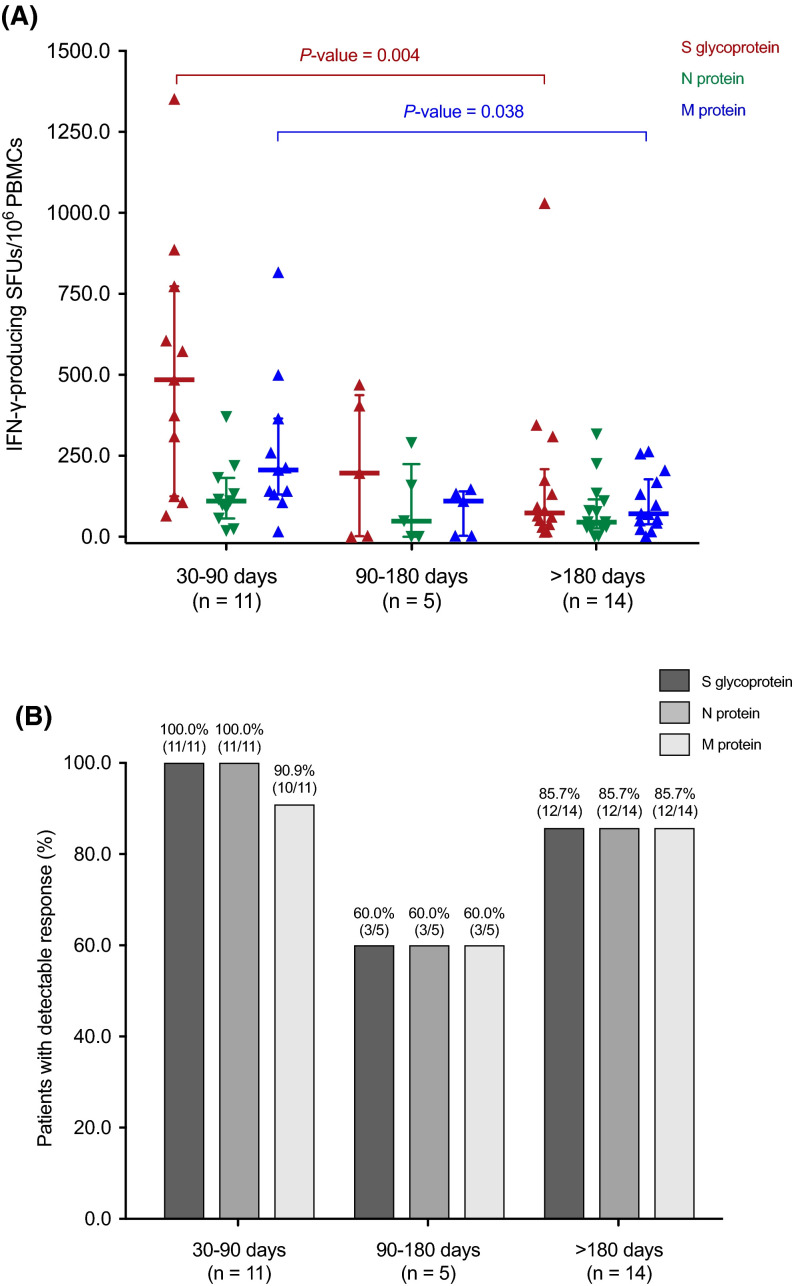
Any SARS-CoV-2-CMI was detectable by the IFN-γ FluoroSpot assay in 90.0% (27/20) of patients (one sample was not evaluable). In detail, positive S glycoprotein-specific (>25 SFUs/106 PBMCs) and N protein-specific (>14 SFUs/106 PBMCs) responses were present in 86.7% (26/30) each, whereas the rate of M protein-specific response (>21 SFUs/106 PBMCs) was 83.3% (25/30).
We also analyzed T cell responses in a continuous manner according to the time elapsed from COVID-19. The magnitude of S glycoprotein-specific (514 ± 387 versus 174 ± 267 SFUs/106 PBMCs; p value = .004) and M protein-specific responses (263 ± 226 versus 104 ± 87 SFUs/106 PBMCs; p value = .038) decreased from the first (30–90 days) to the last time interval analyzed (>180 days), respectively ( Figure 2). An inverse correlation was accordingly found between the number of S glycoprotein-specific IFN-γ-producing SFUs per 106 PBMCs and the interval from COVID-19 diagnosis (Spearman’s rho: –0.418; p value = .024), reflecting a decrease in the magnitude of response over time.
FIGURE 2.
SARS-CoV-2-specific IFN-γ-producing T cell responses reactive to the S glycoprotein (red), the N protein (green), and the M protein (blue) (A) and proportion of patients with positive (>25 S-reactive, >14 N-reactive and >21 M-reactive SFUs/106 PBMCs) responses (B) by the IFN-γ FluoroSpot assay according to the interval from the diagnosis of COVID-19 to the assessment. Horizontal bars and whiskers represent median values and interquartile ranges, respectively. Comparisons were performed with the Mann-Whitney U test. IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot forming unit
3.4. Agreement between ICS and IFN-γ FluoroSpot assay
We restricted this analysis to T cell responses against the S glycoprotein and the N protein by the FluoroSpot assay, since these were the viral antigens used for stimulation in the ICS method. We found a moderate agreement between positive (≥0.1%) SARS-CoV-2-specific IFN-γ-producing CD4+ T cell responses by ICS and S-specific T cell responses (>25 SFUs/106 PBMCs) by FluoroSpot (kappa: 0.526; p value = .001). The agreement with the M-specific (>21 SFUs/106 PBMCs) T cell response was only slight (kappa: 0.211; p value = .190). No agreement was found between CD8+ T cell responses and the results of the FluoroSpot assay to either S (kappa: 0.040; p value = .827) or M antigens (kappa: 0.010; p value = .273).
3.5. SARS-CoV-2 ELISA IgG serology
SARS-CoV-2 IgG positivity was observed in 90.3% (27/31) of the patients. Two LT recipients (6.5%) were negative for IgG, whereas one further patient (3.2%) had a borderline result in the ELISA. The two recipients with negative IgG titers also lacked SARS-CoV-2-specific T cell responses by the IFN-γ FluoroSpot assay. One of them—a 54-year old male patient that had undergone transplantation 2.5 months before COVID-19 diagnosis and was on triple therapy with tacrolimus, mofetil mycophenolate and prednisone—had no detectable SARS-CoV-2-specific IFN-γ-producing CD4+ T cells by ICS either. There was a significant correlation between the semi-quantitative results of the IgG ELISA (relative OD) and the number of M protein-specific (r: 0.380; p value = .038) ( Figure 3) but not S glycoprotein-specific (r: 0.064; p value = .749) IFN-γ-producing SFUs per 106 PBMCs. No correlation was observed with the SARS-CoV-2-specific CD4+ T cell count measured by ICS (r: 0.110; p value = .555) either.
FIGURE 3.
Correlation (Spearman’s rho) between the semiquantitative results of SARS-CoV-2 IgG ELISA and the number of M protein-specific IFN-γ-producing SFUs per 106 PBMCs by the IFN-γ FluoroSpot assay. IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot forming unit
3.6. Clinical correlates of SARS-CoV-2-CMI
The potential correlations between the magnitude of SARS-CoV-2-specific T cell responses and clinical variables, including the severity of COVID-19 and the type of immunosuppression, were also investigated. No significant differences in SARS-CoV-2-specific IFN-γ-producing CD4+ and CD8+ T cell counts enumerated by ICS were observed according to the presence of diabetes mellitus, the requirement of hospitalization or oxygen therapy, or the use of tacrolimus, an antiproliferative agent (i.e., mofetil mycophenolate or azathioprine) or prednisone at the time of SARS-CoV-2-CMI assessment (Figure S1). No apparent impact on SARS-CoV-2-specific T cell responses by FluoroSpot was noted for the presence of diabetes or clinical severity either (Figure S2). On the other hand, M protein-specific responses were significantly lower among patients receiving tacrolimus (median: 101.3 versus 157.5 SFUs/106 PBMCs; p value = .052) or prednisone (median: 16.7 versus 141.7 SFUs/106 PBMCs; p value = .009) (Figure S3). Patients receiving prednisone were less likely to exhibit S glycoprotein-specific (57.1% [4/7] versus 95.7% [22/23]; p value = .031), N protein-specific (57.1% [4/7] versus 95.7% [22/23]; p value = .031), and M protein-specific (42.9% [3/7] versus 95.7% [22/23]; p value = .006) T cell responses. A similar association was also observed between the use of tacrolimus and M protein-specific response (72.2% [13/18] versus 100.0% [12/12]; p value = .066). Finally, no significant correlations were observed between SARS-CoV-2-CMI and other clinical and laboratory variables, including time from transplantation and patient age (Table S1) or immunosuppressive drug levels (Table S2).
3.7. Comparison with nontransplant controls
Finally, we compared SARS-CoV-2-specific T cell responses by the IFN-γ FluoroSpot assay in LT recipients and a nontransplant control group of 30 immunocompetent patients matched by age and time from COVID-19 (Table S3). As expected, the interval from the initial diagnosis of SARS-CoV-2 to the SARS-CoV-2-CMI measurement was similar both groups (158.0 ± 94.9 versus 162.5 ± 29.9 days, respectively; p value = .809).
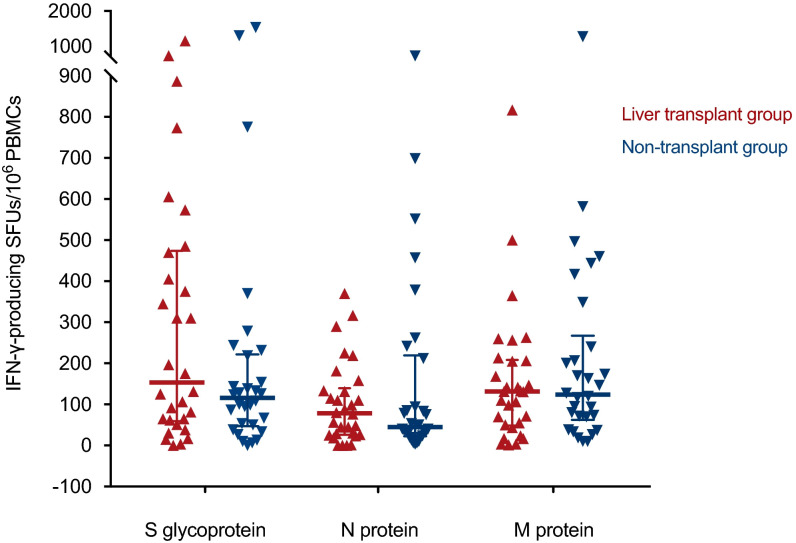
We observed no significant differences between LT recipients and nontransplant patients in the proportion of detectable T cell responses to the S glycoprotein (86.7% [26/30] versus 86.7% [26/30], respectively; p value = 1.000), the N protein (86.7% [26/30] versus 83.3% [25/30]; p value = 1.000), or the M protein (83.3% [25/30] versus 90.0% [27/30]; p value = .706). When the results of the FluoroSpot assay were analyzed as continuous variable, the number of SFUs per 106 PBMCs for any of these antigens was also comparable ( Figure 4; Table 2).
FIGURE 4.
SARS-CoV-2-specific IFN-γ-producing T cell responses measured by the IFN-γ FluoroSpot assay in 30 LT recipients (red) and 30 nontransplant patients (blue) matched (1:1 ratio) by age and time interval from the diagnosis of COVID-19. Horizontal bars and whiskers represent median values and interquartile ranges, respectively. IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot forming unit
TABLE 2.
SARS-CoV-2-specific T cell responses assessed by the IFN-γ FluoroSpot assay in LT recipients and nontransplant patients matched by age and time interval from the diagnosis of COVID-19
| SARS-CoV–2-specific IFN-γ-producing SFUs per 106 PBMCs, median (IQR) | LT group (n = 30) |
Nontransplant group (n = 30) |
p value |
|---|---|---|---|
| S glycoprotein | 153 (59–473) | 115 (47–221) | .297 |
| N protein | 78 (26–139) | 45 (22–219) | .836 |
| M protein | 131 (48–208) | 123 (62–267) | .579 |
Abbreviations: IFN-γ, interferon-γ; IQR, interquartile range; LT, liver transplantation; M, membrane protein; N, nucleoprotein; PBMC, peripheral blood mononuclear cell; S, spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SFU, spot forming unit.
Comparisons were performed with the Mann-Whitney U test.
4. DISCUSSION
In the present cross-sectional study, the functionality and magnitude of SARS-CoV-2-CMI have been analyzed by means of two methods in a cohort of 31 LT recipients at different time intervals from the diagnosis of COVID-19. The clinical severity of SARS-CoV-2 infection was also variable, with about two thirds of the recipients being managed as outpatients. The main finding is that the majority of LT recipients recovered from COVID-19 had detectable SARS-CoV-2-CMI (90.3% by ICS and 90.0% by ELISpot-based IFN-γ FluoroSpot assay) after a median of 103 days, and exceeding 6 months in some patients. In addition, anti-SARS-CoV-2 S1 protein IgG was detected in 90.3% of patients. No differences were evident in the proportion of positive SARS-CoV-2-specific T cell responses or in the number of IFN-γ-producing SFUs targeting each of the structural proteins investigated between LT recipients and nontransplant patients matched by age and time from diagnosis. These findings collectively indicate that immunosuppression has no meaningful impact on the long-term ability to mount specific T cell–mediated immunity elicited by natural SARS-CoV-2 infection after LT.
Only a few studies have analyzed the kinetics of SARS-CoV-2-CMI in solid organ transplant (SOT) recipients following COVID-19,4, 5, 6, 7 , 12 , 13 and none of them were focused on the LT population. By means of an ELISpot assay, Candon et al. characterized SARS-CoV-2-specific T cells in a small group of KT recipients at a shorter interval from symptom onset than in our cohort (median of 38.5 days). All recipients with confirmed SARS-CoV-2 infection displayed CD4+ and CD8+ T cells reactive to at least 6 of 9 viral peptides, with frequencies similar to the nontransplant patients on hemodialysis.4 In a larger cohort, we previously reported that SARS-CoV-2-CMI was present by ICS in 57.1% and 57.9% of KT recipients at months 4 and 6 after the diagnosis, respectively.5 In other study, SARS-CoV-2-specific CD4+ polyfunctional T cell responses were detected in 9 out of 10 patients—most of them KT recipients—during the acute phase of COVID-19.6 Hartzell et al. reported that stable KT recipients on tacrolimus-based immunosuppression hospitalized due to COVID-19 exhibited changes in peripheral blood immune cell phenotypes similar to those observed in the general population.12
All-cause mortality rates among SOT recipients hospitalized due to COVID-19 during the first pandemic wave have been reported to exceed that of the general population.1 , 3 Although outcomes for LT recipients seem to be relatively better as compared to other transplant groups, the pooled estimate for in-hospital mortality in a recent meta-analysis reached 20%.14 However, it has been recently proposed that the more severe course observed for post-transplant COVID-19 could be attributed to the higher burden of comorbidities and more common presence of risk factors for poor outcome (such as older age or acute kidney injury) rather than to the transplant status itself.15, 16, 17, 18 The absence of significant differences in between LT recipients and matched nontransplant patients in the magnitude of SARS-CoV-2-CMI would support this notion. Thieme et al. also reported that SARS-CoV-2-specific M- and S-reactive polyfunctional CD8+ T cell counts and neutralizing antibody-titers did not differ between SOT recipients and nontransplant controls.6 In a mixed cohort of SOT recipients, Favà et al. demonstrated the development of robust serological and functional responses comparable to nontransplant patients during early convalescence.7 Studies performed in other types of immunocompromised hosts have reached similar conclusions.19 Overall, these findings emphasize the need of taking into account not only the seroconversion rate but also the development of T cell–mediated immunity in studies assessing the immunogenicity of SARS-CoV-2 vaccines in the SOT population.20
While there is a long-standing technical and clinical experience with the quantification of CMV-specific T cell responses in the SOT setting,21 the optimal methodological approach for the assessment of post-transplant SARS-CoV-2-CMI remains to be established. Thus, we have applied two different techniques that offer complementary results. The ICS method is able to phenotype T cell responses (CD4+ versus CD8+), whereas the IFN-γ FluoroSpot assay informs on the viral antigen-specificity of such responses. In accordance with our previous study performed in KT recipients,5 the results of the FluoroSpot assay correlate with CD4+ but not CD8+ T cell responses enumerated by ICS. In addition, the categorical agreement—albeit moderate—was higher for the immunodominant S glycoprotein. It should be noted, however, that both methods are not entirely comparable in terms of sample analyzed (whole blood versus PBMCs) and duration of antigen stimulation (6 versus 16–18 h). On the other hand, there were differences in the S peptides used for in vitro stimulation. Specific T cells enumerated by ICS were reactive to both subdomains (S1 and S2) of the viral glycoprotein, whereas the FluoroSpot assay only detected responses against the S1 subdomain. It could be hypothesized that the ICS method would be able to detect a larger T cell repertoire elicited by both natural infection and vaccination.
In line with studies performed in the general population,22 we observed a significant decline over time in the S glycoprotein-specific T cell response assessed by the IFN-γ FluoroSpot assay (which was not mirrored by the results obtained for other antigens or with the ICS method). In addition, the high proportion of detectable SARS-CoV-2-CMI by ICS after a median of 3.4 months contrasts with that observed by our group with the same method among KT recipients, which only reached 57.1% and 19.0% for SARS-CoV-2-specific CD4+ and CD8+ T cell responses, respectively, at month 4.5 Differences in the type and amount of immunosuppression may account for such discrepancy. Indeed, most of the KT recipients in our previous study were receiving a triple regimen based on a calcineurin inhibitor, an antiproliferative agent and prednisone,5 whereas 54.8% of LT recipients were on monotherapy (mainly mofetil mycophenolate). Consistent with this, patients receiving prednisone or tacrolimus or prednisone at the time of immune assessment exhibited lower SARS-CoV-2-specific T cell responses by the FluoroSpot assay. The deleterious impact of tacrolimus-containing regimens on the capacity of mounting SARS-CoV-2-CMI was also observed in the setting of KT.5 Interestingly, we observed no apparent effect of the clinical severity of COVID-19 (i.e. requirement of hospitalization or oxygen therapy) on the magnitude of T cell responses. Some 23, 24, 25 but not all authors 22 , 26 have found a positive correlation between clinical severity at early phases of infection and SARS-CoV-2-specific T cell counts in nontransplant patients, although studies differ in the timing of evaluation. Increasing evidence shows that subjects with asymptomatic SARS-CoV-2 infection are able to mount specific T cell responses comparable to those observed in patients recovering from COVID-19.22 It is to be assumed that this concept also extends to LT recipients, since most of the patients in our cohort had only mild clinical manifestations and were managed as outpatients.
Although this is the largest study to date investigating SARS-CoV-2-CMI after SOT (and the only one focused on LT recipients and applying different methods), some limitations should be acknowledged. The number of patients included was relatively low, particularly for the more severe courses of disease. Therefore, no multivariate analysis was performed and the clinical associations observed (i.e., type of immunosuppression) should be taken with caution. Despite matching by age and time from diagnosis, LT recipients and nontransplant controls differed in some clinical characteristics, since all the patients in the latter group required hospital admission (as compared to only 38.7% in the LT group). Thus, the lack of apparent differences in the magnitude of SARS-CoV-2-CMI could be at least partially explained by the characteristics of the control cohort, since immunocompetent patients unable to mount robust T cell–mediated responses early after infection are more likely to progress to severe symptoms (P. Almendro-Vázquez, Instituto de Investigación Sanitaria Hospital “12 de Octubre”, personal communication, 2021). Nevertheless, a sensitivity analysis restricted to hospitalized LT recipients and their corresponding controls (12 pairs) did not reveal differences between both groups in the size of T cell responses (data not shown). Finally, the timing for the evaluation of SARS-CoV-2-CMI was variable, and the cross-sectional design precluded the analysis of post-transplant kinetics over time.
In conclusion, SARS-CoV-2 infection—even if mild and not requiring hospitalization or oxygen therapy—elicited specific T cell–mediated immunity detectable in nine out of ten LT recipients after a median of 103 days from the episode of COVID-19. The magnitude of post-transplant SARS-CoV-2 T cell responses did not significantly differ from that observed in nontransplant patients hospitalized due to COVID-19. Further studies are needed to characterize the functionality and durability of natural or vaccine-induced SARS-CoV-2-CMI among LT recipients.
ACKNOWLEDGMENTS
This work was supported by the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science and Innovation (COVID-19 Research Call COV20/00181)—co-financed by European Development Regional Fund “A way to achieve Europe”. M.F.R. holds a research contract “Miguel Servet” (CP18/00073), P.T. a research contract “Juan Rodés” (JR19/00049) and R.L.G. a research contract “Rio Hortega” (CM19/00120), all from the ISCIII, Spanish Ministry of Science and Innovation.
DISCLOSURE
The authors of thismanuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information Instituto de Salud Carlos III, Grant/Award Number: COV20/00181, CM19/00120, JR19/00049 and CP18/00073
Footnotes
Mario Fernández-Ruiz and Beatriz Olea contributed equally to this work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Fig S1
Fig S2
Fig S3
Supplementary Material
REFERENCES
- 1.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21(5):1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study [published online ahead of print]. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097 [DOI]
- 4.Candon S, Guerrot D, Drouot L, et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21:854–863. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Ruiz M, Olea B, Giménez E, et al. SARS-CoV-2-specific cell-mediated immunity in kidney transplant recipients recovered from COVID-19. Transplantation. 2021;105(6):1372–1380. doi: 10.1097/TP.0000000000003672. [DOI] [PubMed] [Google Scholar]
- 6.Thieme CJ, Anft M, Paniskaki K, et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression [published online ahead of print]. Transplantation. 2021. 10.1097/TP.000000000000375 [DOI] [PMC free article] [PubMed]
- 7.Favà A, Donadeu L, Sabé N, et al. SARS-CoV-2-specific serological and functional T-cell Immune responses during acute and early COVID-19 convalescence in solid organ transplant patients [published online ahead of print]. Am J Transplant. 2021. 10.1111/ajt.16570 [DOI] [PMC free article] [PubMed]
- 8.Spanish Ministry of Health, Centro de Coordinación de Alertas y Emergencias Sanitarias. Documento técnico de manejo clínico del COVID-19: atención hospitalaria. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Protocolo_manejo_clinico_ah_COVID-19.pdf. Accessed January 17, 2021.
- 9.Loinaz C, Marcacuzco A, Fernández-Ruiz M, et al. Varied clinical presentation and outcome of SARS-CoV-2 infection in liver transplant recipients: Initial experience at a single center in Madrid, Spain. Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13372. e13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solano C, Benet I, Clari MA, et al. Enumeration of cytomegalovirus-specific interferon-γ CD8+ and CD4+ T cells early after allogeneic stem cell transplantation may identify patients at risk of active cytomegalovirus infection. Haematologica. 2008;93:1434–1436. doi: 10.3324/haematol.12880. [DOI] [PubMed] [Google Scholar]
- 11.Giménez E, Albert E, Torres I, et al. SARS-CoV-2-reactive interferon-γ-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J Med Virol. 2020;93:375–382. doi: 10.1002/jmv.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartzell S, Bin S, Benedetti C, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant. 2020;20:3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phadke VK, Scanlon N, Jordan SC, Rouphael NG. Immune responses to SARS-CoV-2 in solid organ transplant recipients. Curr Transplant Rep. 2021;1-13. 10.1007/s40472-021-00322-5 [DOI] [PMC free article] [PubMed]
- 14.Jayant K, Reccia I, Virdis F, et al. COVID-19 in hospitalized liver transplant recipients: An early systematic review and meta-analysis. Clin Transplant. 2021;35(4) doi: 10.1111/ctr.14246. e14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of coronavirus infectious disease-19 (COVID-19) in solid organ transplant recipients [published online ahead of print]. Transplantation. 2021. 10.1097/TP.0000000000003670 [DOI] [PMC free article] [PubMed]
- 16.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linares L, Cofan F, Diekmann F, et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247251. e0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington P, Harrison CN, Dillon R, et al. Evidence of robust memory T-cell responses in patients with chronic myeloproliferative neoplasms following infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Br J Haematol. 2021;193(4):692–696. doi: 10.1111/bjh.17402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sester M, Leboeuf C, Schmidt T, Hirsch HH. The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant. 2016;16:1697–1706. doi: 10.1111/ajt.13684. [DOI] [PubMed] [Google Scholar]
- 22.Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med. 2021;218(5) doi: 10.1084/jem.20202617. e20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183 doi: 10.1016/j.cell.2020.08.017. 158-68.e14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183 doi: 10.1016/j.cell.2020.09.038. 996-1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.