Abstract
Background
It is unclear whether asthma and its allergic phenotype are risk factors for hospitalization or severe disease from SARS‐CoV‐2.
Methods
All patients over 28 days old testing positive for SARS‐CoV‐2 between March 1 and September 30, 2020, were retrospectively identified and characterized through electronic analysis at Stanford. A sub‐cohort was followed prospectively to evaluate long‐term COVID‐19 symptoms.
Results
168,190 patients underwent SARS‐CoV‐2 testing, and 6,976 (4.15%) tested positive. In a multivariate analysis, asthma was not an independent risk factor for hospitalization (OR 1.12 [95% CI 0.86, 1.45], p = .40). Among SARS‐CoV‐2‐positive asthmatics, allergic asthma lowered the risk of hospitalization and had a protective effect compared with non‐allergic asthma (OR 0.52 [0.28, 0.91], p = .026); there was no association between baseline medication use as characterized by GINA and hospitalization risk. Patients with severe COVID‐19 disease had lower eosinophil levels during hospitalization compared with patients with mild or asymptomatic disease, independent of asthma status (p = .0014). In a patient sub‐cohort followed longitudinally, asthmatics and non‐asthmatics had similar time to resolution of COVID‐19 symptoms, particularly lower respiratory symptoms.
Conclusions
Asthma is not a risk factor for more severe COVID‐19 disease. Allergic asthmatics were half as likely to be hospitalized with COVID‐19 compared with non‐allergic asthmatics. Lower levels of eosinophil counts (allergic biomarkers) were associated with a more severe COVID‐19 disease trajectory. Recovery was similar among asthmatics and non‐asthmatics with over 50% of patients reporting ongoing lower respiratory symptoms 3 months post‐infection.
Keywords: asthma, COVID‐19, eosinophils, SARS‐CoV‐2
Asthma is not a risk factor for more severe COVID‐19 disease. Allergic asthmatics are half as likely to be hospitalized compared with non‐allergic asthmatics and lower levels of eosinophil counts (allergic biomarkers) are associated with a more severe COVID‐19 disease trajectory. Recovery is similar among asthmatics and non‐asthmatics. Abbreviation: COVID, coronavirus disease 2019.

1. INTRODUCTION
The role of comorbid conditions in susceptibility to SARS‐CoV‐2 infection and the severity of its associated disease, COVID‐19, has been an area of ongoing investigation since the start of the pandemic. It is well known that viral infections are a common cause for asthma exacerbations requiring hospitalization, and previous studies have shown associations between infections with common respiratory viruses and coronaviruses (OC43 and 229E) and asthma exacerbations in both pediatric and adult patients. 1 , 2 Given that SARS‐CoV‐2 is a respiratory illness which causes viral pneumonia as a primary manifestation, experts initially suspected the virus might exert a similar effect and that those with underlying respiratory illnesses, such as asthma, might be at higher risk for poor outcomes from infection with the virus. As such, at the onset of the pandemic, both the Centers for Disease Control and Prevention and the World Health Organization advised that patients with asthma may be at greater risk for severe illness.
Despite this initial hypothesis, the published data regarding the effect of comorbid asthma on clinical outcomes have been discrepant. Although findings vary regionally, asthma prevalence in patients hospitalized with COVID‐19 appears to be lower than in the general populations of Brazil, China, Italy, Russia, Saudi Arabia, and Sweden. 3 , 4 , 5 , 6 In addition, several systematic reviews and multi‐site studies have reported no increased risk for COVID‐19 infection, severe COVID‐19 illness, or hospitalization in asthmatics after adjusting for potential confounders. 7 , 8 , 9 , 10 , 11 , 12 The mortality rate among asthmatics with COVID‐19 may even be lower than that reported in the general non‐asthmatic population with COVID‐19. 13 Conversely, in cohorts of COVID‐19 patients within the United States, recent studies have revealed the asthma prevalence to be up to 11% greater than the national average, 14 while also suggesting that asthma may be a risk factor for poor clinical outcomes. A retrospective, single‐center review of patients who tested positive for SARS‐CoV‐2 in Washington, D.C., documented an increased risk for intubation in patients with asthma as compared to those without asthma. 15 However, in a separate study from Denver, Colorado, there was no association between asthma and risk for intubation. 16 More recently, a large cohort study from the United Kingdom showed that asthmatics aged 16–49 were more likely to receive critical care. 17 However, the authors note that asthmatics did not necessarily have more clinically severe disease, so the increased rates of critical care for these patients may have been due to other factors, such as provider preference for closer monitoring given underlying respiratory disease. This same study also showed increased mortality for severe asthmatics, although the definition of severe asthma was based on patient self‐reports of maintenance medications taken in the 2 weeks prior to hospital admission.
Previous studies have also suggested that patients with a non‐allergic asthma phenotype may be at increased risk for severe COVID‐19 disease when compared to patients with allergic asthma. 18 , 19 The angiotensin‐converting enzyme 2 (ACE2) receptor has previously been identified as the cellular receptor for SARS‐CoV‐2, and reduced ACE2 expression has been reported in patients with allergic asthma, which would support a possible protective effect of an allergic asthma phenotype on COVID‐19 disease severity. 20 However, there has also been concern that higher expression of transmembrane protease serine 2 (TMPRSS2) found among asthmatics may facilitate SARS‐CoV‐2 cell entry given TMPRSS2's role in the necessary cleavage of the spike protein. 21 A single‐center retrospective analysis of patients presenting to the emergency department at a tertiary academic hospital in the Bronx, New York, supported the role of pre‐existing eosinophilia (AEC ≥150 cells/μl, a biomarker of allergic inflammation) as protective against hospitalization with COVID‐19 infection. 22 When a cohort of children under the age of 16 hospitalized with COVID‐19 in Wuhan were characterized, it was found that allergic status, including allergic rhinitis, drug allergy, atopic dermatitis, and food allergy, did not confer increased risk for COVID‐19 infection. 23 Furthermore, in a South Korean study, patients with non‐allergic asthma had a greater risk of SARS‐CoV‐2 test positivity and worse clinical outcomes than patients with allergic asthma. 18 Taken together, these findings suggest the nuanced role of asthma phenotypes in predicting clinical outcomes of COVID‐19. Therefore, the aim of this study was to evaluate all patients who tested positive for SARS‐CoV‐2 to determine the impact of asthma and asthma phenotypes on disease severity and outcomes in COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Data source
Our study was conducted at Stanford Health Care (SHC), an academic healthcare system that includes a tertiary and quaternary hospital, children's hospital, and affiliated clinics and acute care facilities. Patients who underwent FDA emergency use authorized SARS‐CoV‐2 nucleic acid amplification tests from either nasal, nasopharyngeal swab, or bronchoalveolar lavage from March 1, 2020, to September 30, 2020, were included. Electronic health record (EHR) data on the SARS‐CoV‐2‐positive patients were also obtained. This study was reviewed and approved with a waiver of consent by the Stanford Administrative Panel on Human Subjects in Medical Research.
Using an institutional informatics platform, we comprehensively characterized a cohort of patients who tested positive for SARS‐CoV‐2 and further categorized them by baseline asthma diagnosis using the International Classification of Disease (ICD‐9/10) diagnostic codes (Table S1) and hospitalization status. 24 Patients were categorized as having allergic asthma if they had at least one ICD‐10 code for a coexisting allergic disorder (Table S2). Patients younger than 28 days old and those without additional encounters or ICD‐10 codes within our EHR besides their SARS‐CoV‐2 diagnostic test were excluded from further analysis. Inpatients were defined as patients hospitalized at SHC within 14 days of a positive test for SARS‐CoV‐2.
Asthma severity was categorized according to the five steps in the GINA 2020 guidelines. 25 Each patient who was hospitalized was graded at peak COVID‐19 disease severity during their hospitalization and classified according to the five severity of illness categories from the National Institutes of Health (NIH) COVID‐19 Treatment Guidelines (Table S3). 26 Additionally, we identified patients who were enrolled in a prospective, longitudinal study (NCT# 04373148) to qualitatively assess clinical and immunological parameters in persons testing positive for SARS‐CoV‐2. Visits were conducted at prespecified intervals, and symptom surveys were administered.
2.2. Statistical analysis
We compared demographic characteristics between asthmatic and non‐asthmatic inpatients using the Wilcoxon rank‐sum test for continuous variables, chi‐square test, or Fisher's exact test for categorical variables. Binary logistic regression was used to determine the association between asthma and risk of hospitalizations among SARS‐CoV‐2‐positive patients, and ordinal logistic regression was used to determine the association between asthma and COVID‐19 disease severity among hospitalized patients. To quantitate the contributions of other known confounding factors to COVID‐19 hospitalizations and COVID‐19 disease severity, we used three models in each analysis: the first model (unadjusted) univariately analyzed the effects of asthma, the second model used a multivariate analysis adjusted for demographics (age, BMI, ethnicity), and the third model used a multivariate analysis adjusted for both demographics and known COVID‐19‐related comorbidities associated with more severe disease (diabetes, coronary heart disease, and hypertension). 27 , 28 Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each model. We evaluated the relationship between laboratory values at admission and COVID‐19 disease severity using ordinal regression models as an exploratory analysis. The interaction between each laboratory value and asthma status was also analyzed in the model. Bonferroni correction for multiple comparisons was applied for laboratory values and false discovery rate (FDR) was controlled at the 10% level. Absolute eosinophil counts from hospitalization were grouped by collection time into admission, during, and discharge values. Admission eosinophil counts were collected within 3 days prior to admission, discharge eosinophil counts were collected on the day of discharge, and during hospitalization, eosinophil counts were collected between admission and discharge counts. The median value was chosen to use as the per‐person count whether a patient had multiple values at any of the three timepoints. A mixed‐effect model was constructed to assess change in eosinophil counts during hospitalization in those patients that did not receive systemic steroids. In the sub‐cohort analysis among those enrolled in a prospective, longitudinal study (NCT# 04373148), the time from symptom onset to resolution was compared between asthmatic and non‐asthmatic patients using the Kaplan‐Meier curve and log‐rank test. All analyses were performed in R language (version 4.0.3) and packages stats (version 4.0.3), ordinal (version 2019.12.10).
3. RESULTS
3.1. Cohort characteristics
Between March 1, 2020 and September 30, 2020, 168,190 patients underwent SARS‐CoV‐2 diagnostic testing at SHC (Figure 1). 5,596 SARS‐CoV‐2‐positive patients were included in the analysis: 605 (10.8%) of patients were hospitalized at SHC, including 100 patients (16.5%) with a diagnosis of asthma. SARS‐CoV‐2‐positive outpatients were also included (4,991), and 498 (9.98%) were identified as asthmatic. In our cohort, cases and hospitalizations first peaked in March 2020 and then again in July 2020 (Figure S1).
FIGURE 1.
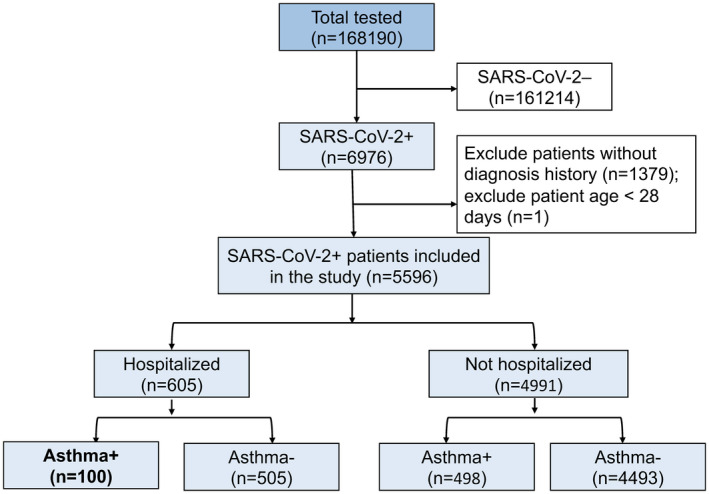
Consort diagram
Baseline characteristics for all patients including sex, smoking history, BMI, race, and ethnicity were comparable between hospitalized and non‐hospitalized patients with and without asthma (Table 1). Hospitalized asthmatics were significantly older than non‐hospitalized asthmatics, but there was no significant difference in age between hospitalized patients with and without asthma. Moreover, chronic obstructive pulmonary disease (COPD), chronic renal disease, obesity, and other chronic lung disease were significantly more prevalent among hospitalized asthmatics compared with the other groups (Table 1). Furthermore, there were no significant differences in COVID‐19 therapies received by inpatients with and without asthma, including antivirals, anti‐inflammatories, antibiotics, and steroids (Table S5).
TABLE 1.
Demographics and coexisting conditions of asthmatic COVID‐19 cohorts
| Characteristics | Hospitalized | Not Hospitalized |
p value (hospitalized COVID−19 Asthma+vs Asthma‐) |
|||
|---|---|---|---|---|---|---|
| COVID−19 Asthma+ | COVID−19 Asthma‐ | COVID−19 Asthma+ | COVID−19 Asthma‐ | |||
| Total N | 100 | 505 | 498 | 4493 | ||
| Age | ||||||
| Median (IQR)—years | 53.8 [31.1–69.9] | 50.5 [33.3–67.7] | 36.5 [20.7–56.4] | 36.9 [24.8–51.8] | .83 | |
| Distribution—No./Total no.(%) | 0–14 years | 7.0 (7.0%) | 29.0 (5.7%) | 71.0 (14.3%) | 441.0 (9.8%) | |
| 15–49 years | 38.0 (38.0%) | 219.0 (43.4%) | 262.0 (52.6%) | 2800.0 (62.3%) | ||
| 50–64 years | 21.0 (21.0%) | 116.0 (23.0%) | 101.0 (20.3%) | 800.0 (17.8%) | ||
| ≥65 years | 34.0 (34.0%) | 141.0 (27.9%) | 64.0 (12.9%) | 452.0 (10.1%) | ||
| Sex | .25 | |||||
| No./Total no.(%) | Female | 58 (58.0%) | 258 (51.1%) | 296 (59.4%) | 2336 (52.0%) | |
| Male | 42 (42.0%) | 247 (48.9%) | 202 (40.6%) | 2144 (47.7%) | ||
| Race and ethnicity | Total data available | 100 | 495 | 460 | 3493 | .62 |
| No./Total no.(%) | Asian | 9 (9.0%) | 52 (10.5%) | 35 (7.6%) | 290 (8.3%) | |
| Hispanic/Latino | 49 (49.0%) | 273 (55.2%) | 205 (44.6%) | 1861 (53.3%) | ||
| Non‐Hispanic Black | 3 (3.0%) | 18 (3.6%) | 37 (8.0%) | 123 (3.5%) | ||
| Non‐Hispanic White | 25 (25.0%) | 97 (19.6%) | 138 (30.0%) | 856 (24.5%) | ||
| Other | 14 (14.0%) | 55 (11.1%) | 45 (9.8%) | 363 (10.4%) | ||
| Smoking history | Total data available | 88 | 380 | 384 | 2045 | .41 |
| No./Total no.(%) | Current smoker | 3 (3.4%) | 22 (5.8%) | 19 (4.9%) | 79 (3.9%) | |
| Former smoker | 20 (22.7%) | 90 (23.7%) | 62 (16.1%) | 288 (14.1%) | ||
| Never smoker | 64 (72.7%) | 266 (70.0%) | 301 (78.4%) | 1662 (81.3%) | ||
| Passive smoke exposure | 1 (1.1%) | 2 (0.5%) | 2 (0.5%) | 16 (0.8%) | ||
| BMI classification | Total data available | 93 | 451 | 445 | 2459 | |
| No./Total no.(%) | Median (IQR)—BMI | 29 [25.2–35.3] | 27.8 [23.4–33.5] | 28.2 [23.9–34.3] | 27 [23–31.8] | .12 |
| Underweight (<18.5) | 7 (7.5%) | 29 (6.4%) | 33 (7.4%) | 183 (7.4%) | ||
| Healthy weight (18.5–24.9) | 15 (16.1%) | 125 (27.7%) | 102 (22.9%) | 698 (28.4%) | ||
| Overweight (25–29.9) | 32 (34.4%) | 122 (27.1%) | 126 (28.3%) | 745 (30.3%) | ||
| Obese (30–34.9) | 14 (15.1%) | 88 (19.5%) | 88 (19.8%) | 462 (18.8%) | ||
| Severely obese (35–39.9) | 12 (12.9%) | 50 (11.1%) | 42 (9.4%) | 198 (8.1%) | ||
| Morbidly obese (> = 40) | 13 (14.0%) | 37 (8.2%) | 54 (12.1%) | 173 (7.0%) | ||
| Coexisting disorder | ||||||
| No./Total no.(%) | Any | 85 (85.0%) | 363 (71.9%) | 262 (52.6%) | 1147 (25.5%) | .070 |
| Chronic obstructive pulmonary disease | 14 (14.0%) | 24 (4.8%) | 18 (3.6%) | 32 (0.7%) | .008 | |
| Cancer | 23 (23.0%) | 73 (14.5%) | 40 (8.0%) | 214 (4.8%) | .082 | |
| Cerebrovascular disease | 13 (13.0%) | 61 (12.1%) | 18 (3.6%) | 105 (2.3%) | .93 | |
| Chronic renal disease | 29 (29.0%) | 90 (17.8%) | 28 (5.6%) | 114 (2.5%) | .042 | |
| Coronary heart disease | 28 (28.0%) | 114 (22.6%) | 41 (8.2%) | 145 (3.2%) | .38 | |
| Diabetes | 38 (38.0%) | 178 (35.2%) | 69 (13.9%) | 324 (7.2%) | .73 | |
| Other endocrine system disease | 15 (15.0%) | 40 (7.9%) | 37 (7.4%) | 118 (2.6%) | .079 | |
| Hypertension | 58 (58.0%) | 238 (47.1%) | 150 (30.1%) | 575 (12.8%) | .094 | |
| Immunodeficiency | 9 (9.0%) | 33 (6.5%) | 11 (2.2%) | 49 (1.1%) | .59 | |
| Liver disease | 22 (22.0%) | 83 (16.4%) | 44 (8.8%) | 224 (5.0%) | .32 | |
| Obesity | 42 (42.0%) | 131 (25.9%) | 132 (26.5%) | 370 (8.2%) | .008 | |
| Other chronic lung disease | 20 (20.0%) | 38 (7.5%) | 29 (5.8%) | 54 (1.2%) | .003 | |
| Numbers of coexisting disorder above | 3 [1–5] | 2 [0–4] | 1 [0–2] | 0 [0–1] | .001 | |
| Mortality | 6 (6.0%) | 30 (5.9%) | NA | NA | 1 | |
p value was based on the Wilcoxon rank‐sum test, chi‐square test, or Fisher's exact test when appropriate.
3.2. Asthma phenotypes and COVID‐19 risk
In a univariate analysis, asthma was significantly associated with hospitalization for COVID‐19 (Figure 2A,B, unadjusted; OR 1.53 [95% CI 1.2, 1.93], p < .001). This association remained significant after adjusting for age, gender, and ethnicity (Figure 2B, adjusted for demographics; OR 1.55 [95% CI 1.21, 1.97], p < .001). However, after we adjusted for known comorbidities associated with more severe disease (ie, diabetes, obesity, coronary heart disease, and hypertension), 27 , 28 asthma was no longer a significant risk factor for hospitalization (Figure 2B, adjusted for demographics and coexisting conditions; OR 1.12 [95% CI 0.86, 1.45], p = .40). Twenty percent of hospitalized asthmatics were critical, compared to 21% of non‐asthmatic patients (Figure 3A). Additionally, asthma was not significantly associated with COVID‐19 disease severity among hospitalized inpatients in univariate (Figure 3B, unadjusted model; OR 1.14 [0.78, 1.67], p = .48) or multivariate analysis when adjusted for age, race, sex, and common comorbidities (Figure 3B, adjusted for demographics; OR 1.28 [0.84, 1.95], p = .24; adjusted for demographics and coexisting conditions; OR 1.21 [0.8, 1.85], p = .37).
FIGURE 2.
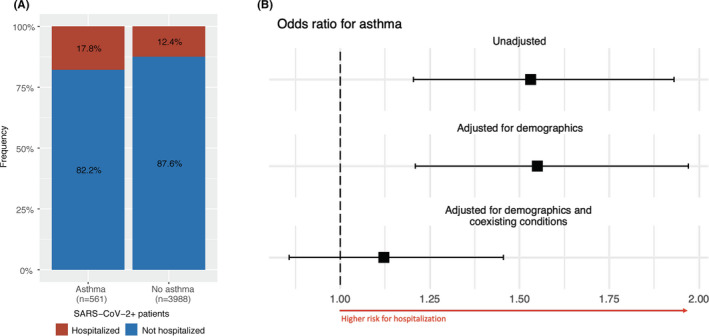
Association between asthma status and hospitalization. Legend: A, Numbers and percentages of hospitalizations stratified by asthma status. B, Forest plot indicating the odds ratio of asthma status on hospitalization in univariate analysis and adjusted odds ratios in multivariate analysis (adjusted for demographic data and adjusted for demographic data and other coexisting conditions). Medians and 95% CI are shown
FIGURE 3.
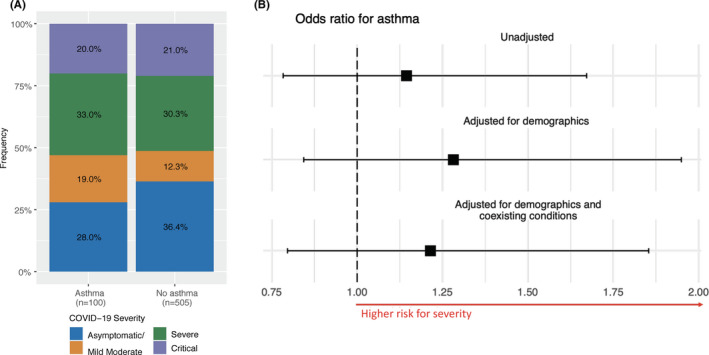
Association between asthma status and COVID‐19 severity among COVID‐19 hospitalized patients. Legend: A, The frequency of inpatients in each severity category stratified by asthma status. B, Forest plot indicating the odds ratio and 95% confidence interval (CI) of asthma status on COVID‐19 severity from logistic regression models in univariate analysis and multivariable analysis (adjusted for demographic data and adjusted for demographic data and other coexisting conditions)
In our asthmatic cohort, 167 patients (27.9%: 148 outpatients, 19 inpatients) were identified as having an allergic asthma phenotype (Figure 4A). Allergic asthma was significantly associated with a lower risk of hospitalization as compared to non‐allergic asthma in both univariate (Figure 4B, unadjusted; OR 0.55 [0.31, 0.92], p = .029) and multivariate analysis (Figure 4B, adjusted for demographics; OR 0.54 [0.3, 0.93], p = .031); adjusted for demographics and coexisting conditions; OR 0.52 [0.28, 0.91], p = .026). For hospitalized patients, allergic asthma status did not correlate with disease severity in any of the analyses (Figure 4C,D). To explore the effect of additional allergic conditions on COVID‐19 outcomes, we also modeled the effect of allergic rhinitis on risk of hospitalization and disease severity. We similarly found that patients with allergic rhinitis were less likely to be hospitalized after controlling for demographics and coexisting conditions including asthma (OR 0.58 [0.4, 0.83], p = .004), and there was no significant association with disease severity (OR 1.03 [0.57, 1.85], p = .93).
FIGURE 4.

Association between allergic asthma and hospitalization and COVID‐19 severity. Legend: A, The frequency of hospitalization between allergic and non‐allergic asthmatic among SARS‐CoV‐2‐positive patients. B, Forest plot showing the odds ratios and 95% CI of allergic asthma on hospitalization among asthmatic SARS‐CoV‐2‐positive patients from logistic regression models in univariate analysis and multivariable analysis (adjusted for demographic data and adjusted for demographic data and other coexisting conditions). C, The frequency of COVID‐19 severity between allergic and non‐allergic asthmatic among inpatients. D, Forest plot showing the odds ratios and 95% CI of allergic asthma severity among inpatients from ordinal regression models in univariate analysis and multivariable analysis (adjusted for demographic data and adjusted for demographic data and other coexisting conditions)
Eosinophil counts prior to COVID‐19 infection (historical eosinophils), an allergic biomarker, were available for 2,070 patients (398 inpatients and 1,672 outpatients) and significantly higher (Kruskal‐Wallis test, p < .0001) in asthmatics (Figure S2). Furthermore, when assessing absolute eosinophil counts (AECs) during hospitalization in patients without steroid, those with severe COVID‐19 disease had lower eosinophil levels during hospitalization as compared to patients with less severe disease, independent of asthma status (p = .0014, Figure 5).
FIGURE 5.
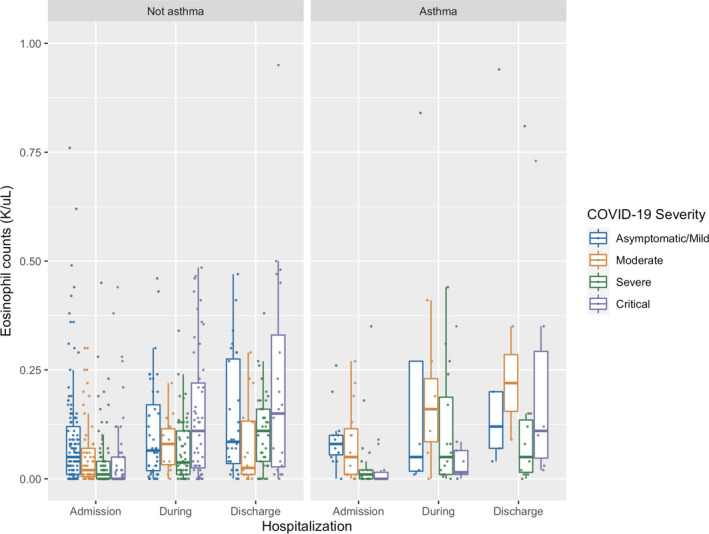
Eosinophil counts during hospitalization stratified by asthma status and COVID‐19 disease severity in patients without steroid usage. Legend: Admission eosinophil counts were collected within 3 days prior to admission, discharge eosinophil counts were collected on the day of discharge, and during hospitalization, eosinophil counts were collected in between admission and discharge counts
To further understand asthma phenotypes, we assessed outpatient controller or rescue medication use for SARS‐CoV‐2‐positive asthmatics with available records (368 patients, 61.6%: 307 outpatients, 61 inpatients); 213 patients were prescribed GINA step 1–2 therapies (57.9%). Among COVID‐19 hospitalized asthmatic inpatients, the majority of patients were prescribed GINA step 1 therapies (n = 25, 41%); four patients (6.6%) were prescribed GINA step 5 therapies. In asthmatics, there was no association between GINA classification and the risk for hospitalization (p = .22) or severity of illness (p = .62; Figure S3A,B).
Non‐allergic phenotypes of asthma were also assessed. There were 32 patients (14 hospitalized and 18 not hospitalized) in our cohort with a diagnosis of asthma/COPD overlap (Figure S4A). Patients with a diagnosis of both asthma/COPD were more likely to be hospitalized in univariate analysis (Figure S4B—unadjusted; OR 5.71 [2.77, 11.52], p < .0001) and multivariate analysis adjusted for demographics (OR 3.08 [1.46, 6.4], p = .0026). However, this relationship did not persist for either group in multivariate analysis after adjusting for demographics and comorbid conditions (OR 1.69 [0.76, 3.7], p = .19). For hospitalized patients, those with COPD only showed significantly increased odds of clinical severity in univariate analysis (Figure S4C,D—unadjusted; OR 3.9 [1.75, 9.02], p = .001), which did not persist when adjusting for demographics and comorbid conditions in multivariate analysis (p = .15).
Among COVID‐19 hospitalized patients in our cohort, we assessed the association between eighteen laboratory values collected at admission and COVID‐19 severity in asthmatic and non‐asthmatic inpatients. Among these, WBC count, lymphocyte count, monocyte count, lymphocyte/neutrophil ratio, procalcitonin, CRP, LDH, AST, ALT, procalcitonin, CK, and creatinine were significantly associated with COVID‐19 severity, regardless of asthma status (FDR < 0.1, Table S4).
3.3. Long‐term symptoms after COVID‐19 infection
To determine the long‐term effect of SARS‐CoV‐2 infection and time to resolution of symptoms in asthmatic and non‐asthmatic patients, we prospectively collected symptom data from a cohort of SARS‐CoV‐2‐positive patients (35 asthmatic and 76 non‐asthmatic) over recurring visits. The majority of patients (91%) reported initial symptoms from COVID‐19 infection (Figure 6A,B); less than 10% were asymptomatic (3 [8.6%] asthmatic and 7 [9.2%] non‐asthmatic patients). The percentage of patients reporting symptoms declined over an 8‐months follow‐up period in both groups. Most patients still reported symptoms at 30 days (96.9% in asthmatic and 98.6% in non‐asthmatic), 60 days (84.4% in asthmatic and 89.9% in non‐asthmatic), and 90 days (75% in asthmatic and 76.8% in the non‐asthmatic). The median follow‐up time was 141 days. There was no difference in time to resolution of symptoms between the groups (Figure 6C, median: 215 days vs 133 days, log‐rank test p = .40). Additionally, resolution of lower respiratory symptoms did not differ between asthmatics and non‐asthmatics (Figure 6D) with more than 50% of both groups still reporting lower respiratory symptoms 90 days after initial symptoms. Persistent symptoms for individual asthmatics were reported in all organ systems (Figure S5).
FIGURE 6.
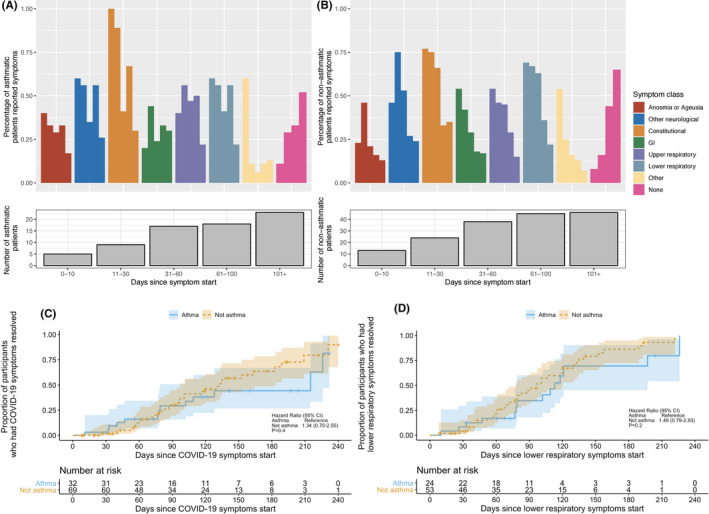
Longitudinal symptoms in asthmatic COVID‐19 patients. Legend: Longitudinal symptoms were followed in a subgroup of asthmatic and non‐asthmatic COVID‐19 patients. Patients were seen in visit windows including 0–10 days, 11–30 days, 31–60 days, 61–100 days, and 101+ days from symptom onset. Bar graph showing frequency of asthmatic A, and non‐asthmatic B, patients reporting symptoms in each symptom class over time by visit window. “None” indicates frequency of patients who reported not having any symptoms; number of patients seen in each visit window is shown below the bar graph. C, Kaplan‐Meier curves of time to all symptom resolution and corresponding 95% confidence interval bands by asthmatic and non‐asthmatic COVID‐19 patients. p value was based on the log‐rank test. D, Kaplan‐Meier curves of time to lower respiratory symptom resolution and corresponding 95% confidence interval bands by asthmatic and non‐asthmatic COVID‐19 patients. p value was based on the log‐rank test
4. DISCUSSION
We evaluated the role of asthma, asthma phenotypes, and GINA class on COVID‐19 infection severity in a diverse population of patients spanning from pre‐hospitalization to 8 months post‐hospitalization. Asthma does not independently increase the risk for hospitalization, severe disease, or long‐term symptoms in COVID‐19. More importantly, allergic asthma appears to protect against hospitalization.
Our study extensively evaluated 5,596 patients infected with the SARS‐CoV‐2 virus. Obesity, cardiovascular disease, and diabetes have been identified as risk factors for more severe COVID‐19 infection. 27 , 28 When controlling for these comorbid conditions in our cohort, we discerned that asthmatic patients did not have an increased risk for hospitalization. Using the NIH COVID‐19 severity guidelines, we rigorously characterized all patients hospitalized from March to September 2020. We believe this more meticulous phenotyping of clinical severity for patients in our cohort adds validity to our findings as conflicting findings from other studies may in part be due to the use of surrogate endpoints such as need for critical care or intensive care unit admission which are often affected by external factors. We determined that asthmatic patients hospitalized with COVID‐19 were older than those who were treated as outpatients; however, they did not have an increased risk for more severe COVID‐19 infection compared with non‐asthmatics with similar comorbid conditions.
The finding from our cohort that allergic asthmatics are half as likely to be hospitalized with COVID‐19 infection implies that certain asthma phenotypes may have a protective effect. The ACE2 receptor is upregulated in diabetes and hypertension, conditions known to increase the risk for severe COVID‐19 disease. 29 However, RNA experiments on nasal and bronchial epithelial cells from patients with allergic sensitization and allergic asthma showed downregulation of ACE2 expression, which may explain the potential protective effect of allergic asthma. 20 Patients with allergic rhinitis also had lower rates of hospitalization in our cohort, independent of asthma status, suggesting that allergic asthma may not be the only atopic condition with a potential protective effect.
Furthermore, one phenotype of allergic asthma is characterized by elevated eosinophil counts. In our cohort, asthmatics did have higher historical eosinophil counts than their non‐asthmatic counterparts, although we were unable to stratify further based on baseline allergic phenotype. Eosinophils, which are widely recognized as important markers for allergic inflammation, are also potent pro‐inflammatory leukocytes with antiviral properties through the release of RNAses and reactive nitrogen species. 21 Interestingly, eosinopenia has been noted to be a marker of early severe COVID‐19 disease, which may result from eosinophil exhaustion, viral inhibition of eosinophil production, or induction of eosinophil apoptosis. 4 , 30 , 31 In our study, patients with more severe COVID‐19 disease had lower eosinophil counts throughout hospitalization, independent of asthma status. This is intriguing and lends further evidence to the potential important role eosinophils may play in moderating COVID‐19 disease severity. Further studies are needed to elucidate the potentially protective role of eosinophils in COVID‐19 disease pathogenesis. This may also have implications for patients on biologics, which selectively inhibit the allergic inflammatory pathway leading to near complete eosinophil depletion. 32 The importance of baseline asthma severity on the risk for SARS‐CoV‐2 infection and COVID‐19 severity also warrants further investigation and may reflect associations between asthma therapies and COVID‐19 susceptibility and severity. Recent studies from South Korea and the United Kingdom used various methods to assess asthma severity based on medication use and showed a possible risk for worse COVID‐19 outcomes but also a potential protective effect from inhaled corticosteroids in some subgroups of patients. 33 , 34 , 35 These results, while seemingly conflicting, highlight the nuanced aspects of asthma phenotypes. In our trial, we classified asthma severity according to the GINA guidelines and did not find an association between severity as based on the GINA scale and COVID‐19 clinical outcomes. As GINA classification implies increased steroid therapy with more severe asthma, it is possible that asthma medications mitigate any potential association between GINA category and COVID‐19 severity. In vitro studies in airway epithelial cells have shown that certain inhaled glucocorticoids can reduce the replication of SARS‐CoV‐2. 36 Inhaled corticosteroids were also shown to downregulate expression of ACE2 and transmembrane protease serine 2 (TMPRSS2) genes in sputum cells; these genes are critical for coding receptors involved in SARS‐CoV‐2 viral entry. 37 , 38
To our knowledge, we are also the first group to compare the long‐term symptoms after COVID‐19 infection between asthmatics and non‐asthmatics. Between 10% and 80% of patients are estimated to experience prolonged symptoms from COVID‐19 up to 2 months after diagnosis. 39 , 40 The majority of patients in our study were followed for an average of 141 days, and most still reported symptoms at 60 and 90 days. There was no difference in time to resolution of any symptoms or importantly, in time to resolution of lower respiratory symptoms, between groups. These results suggest that patients with asthma are not at greater risk for long‐term symptoms of COVID‐19 as compared with their non‐asthmatic counterparts.
Our study has a few limitations. The data are generated from a single academic institution from ICD‐10 codes extracted from the medical record; however, we applied independent and consistent chart review, performing QA/QC on the data. Our approach and rigorous methodology enabled us the opportunity to confirm others' findings from larger cohorts. Moreover, we analyzed well‐characterized, detailed meta‐data on each patient from multiple timepoints to highlight the disease course from pre‐hospitalization to 8 months following symptom onset to assess clinical outcomes at many timepoints.
In conclusion, asthmatic patients do not have increased risk of hospitalization, more severe COVID‐19 disease, or long‐term respiratory symptoms. In fact, allergic asthmatics were protected from hospitalization with COVID‐19. Moreover, patients with more severe COVID‐19 disease had lower eosinophil levels during hospitalization than those with less severe disease, independent of asthma status. Additional, multi‐center studies are needed to evaluate the mechanisms by which eosinophils and atopic pathways may mitigate COVID‐19 disease severity.
CONFLICT OF INTEREST
LE, ZH, AL, TS, JF, SC, RO, MA, LB, BP, SJ, RP, RW, AB, GD, and NA report no conflicts of interest. WC reports research support from Regeneron.MD reports receiving grant funding from the Ping Li and Kim Li Endowment and the NIH. SS reports receiving grant funding from the NIH, Regeneron, DBV Technologies, Aimmune, Novartis, and CoFAR. KN reports grant funding from NIAID, NHLBI, NIEHS, and FARE, and support from the Sunshine Foundation, Parker Foundation, and Crown Foundation.SC receives grant funding from NIAID, CoFAR, Aimmune, DBV Technologies, Astellas, Regeneron, and FARE and support from the Maternal Child Health Research Institute, Sunshine Foundation, Parker Foundation, and Crown Foundation.
AUTHOR CONTRIBUTIONS
LE, ZH, WC, KN, MD, and RSC equally contributed to study design and data interpretation. ZH, JF, SC, and GD organized data collection, analysis, and figures. LE, WC, ZH, AL, SJ, SC, and RSC contributed to the literature search and writing of the original draft of the manuscript. RSC, WC, BP, AL, TS, SS, MA, LB, RP, RW, NA, KCN, AB, and RO contributed to participant data collection, participant visits, study resources, and critical revision. All authors had full access to all data and contributed to data collection and manuscript writing.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Sean N. Parker Center for Allergy and Asthma Research at the Stanford University School of Medicine, Sunshine Foundation, Crown Foundation, and Parker Foundation. We thank all patients and families who contributed to this analysis at our academic medical center. We are also especially thankful to all front‐line healthcare workers, essential personnel, and our clinical and research staff who made this research possible.
Eggert LE, He Z, Collins W, et al. Asthma phenotypes, associated comorbidities, and long‐term symptoms in COVID‐19. Allergy. 2022;77:173–185. 10.1111/all.14972
Eggert, He and Collins contributed equally and are first co‐authors.
REFERENCES
- 1. Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthmaexacerbations. Lancet. 2010;376(9743):826‐834. 10.1016/S0140-6736(10)61380-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections inexacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225. 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skevaki C, Karsonova A, Karaulov A, Xie M, Renz H. Asthma‐associated risk for COVID‐19 development. J Allergy Clin Immunol. 2020;146(6):1295‐1301. 10.1016/j.jaci.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 5. Bousquet J, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on asthma and COVID‐19 (June 2, 2020). Allergy. 2021;76(3):689‐697. 10.1111/all.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carli G, Cecchi L, Stebbing J, Parronchi P, Farsi A. Is asthma protective against COVID‐19? Allergy 2021;76(3):866‐868. 10.1111/all.14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID‐19. J Allergy Clin Immunol. 2020;146(2):307‐314.e4. 10.1016/j.jaci.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song J, Zeng M, Wang H, et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID‐19. Allergy. 2021;76(2):483‐496. 10.1111/all.14517 [DOI] [PubMed] [Google Scholar]
- 9. Kim S, Jung C, Lee JY, et al. Characterization of asthma and risk factors for delayed SARS‐CoV‐2 clearance in adult COVID‐19 inpatients in Daegu. Allergy. 2021;76(3):918‐921. 10.1111/all.14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Ao G, Qi X, Xie B. The association between COVID‐19 and asthma: a systematic review and meta‐analysis. Clin Exp Allergy. 2020;50(11):1274‐1277. 10.1111/cea.13733 [DOI] [PubMed] [Google Scholar]
- 11. Mendes NF, Jara CP, Mansour E, Araújo EP, Velloso LA. Asthma and COVID‐19: a systematic review. Allergy, Asthma Clin Immunol. 2021;17(1):5. 10.1186/s13223-020-00509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID‐19: prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021;203(7):893‐905. 10.1164/rccm.202008-3266OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heffler E, Detoraki A, Contoli M, et al. COVID‐19 in severe asthma network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2021;76(3):887‐892. 10.1111/all.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lovinsky‐Desir S, Deshpande DR, De A, et al. Asthma among hospitalized patients with COVID‐19 and related outcomes. J Allergy Clin Immunol. 2020;146(5):1027‐1034.e4. 10.1016/j.jaci.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenthal JA, Awan SF, Fintzi J, Keswani MSCIA, Ein D. Asthma is associated with increased risk of intubation but not hospitalization or death in coronavirus disease 2019. Ann Allergy, Asthma Immunol. 2021;126:93‐95. 10.1101/2020.05.24.20111971v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broadhurst R, Peterson R, Wisnivesky JP, et al. Asthma in COVID‐19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17(12):1645‐1648. 10.1513/AnnalsATS.202006-613RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Z, Hasegawa K, Ma B, et al. Association of asthma and its genetic predisposition with the risk of severe COVID‐19. J Allergy Clin Immunol. 2020;2:327‐329.e4. Accessed January 19, 2021. 10.1016/j.jaci.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID‐19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790‐798. 10.1016/j.jaci.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skevaki C, Karsonova A, Karaulov A, et al. SARS‐CoV‐2 infection and COVID‐19 in asthmatics: a complex relationship. Nat Rev Immunol. 2021;21(4):202‐203. 10.1038/s41577-021-00516-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS‐CoV‐2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203‐206.e3. 10.1016/j.jaci.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75(11):2829‐2845. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferastraoaru D, Hudes G, Jerschow E, et al. Eosinophilia in asthma patients is protective against severe COVID‐19 illness. J Allergy Clin Immunol Pract. 2021;9(3):1152‐1162.e3. 10.1016/j.jaip.2020.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du H, Dong X, Zhang J, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2021;76(2):510‐532. 10.1111/all.14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE: an integrated standards‐based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391‐395. [PMC free article] [PubMed] [Google Scholar]
- 25. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2020. www.ginasthma.org. Accessed January 19, 2021.
- 26. National Institutes of Health . Clinical spectrum, COVID‐19 treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/. Accessed January 19, 2021. [PubMed]
- 27. Chen Y, Gong X, Wang L, Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID‐19 diseases severity: a systematic review and meta‐analysis. medRxiv. 2020. 10.1101/2020.03.25.20043133 [DOI]
- 28. Kruglikov IL, Shah M, Scherer PE. Obesity and diabetes as comorbidities for COVID‐19: underlying mechanisms and the role of viral–bacterial interactions. Elife. 2020;9:e61330. 10.7554/eLife.61330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin‐converting enzyme‐2 receptor: a potential adhesion site for novel coronavirus SARS‐CoV‐2 (Covid‐19). J Clin Med. 2020;9(3):841. 10.3390/jcm9030841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID‐19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1‐7. 10.1016/j.jaci.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao L, Zhang Y, Yang X, Liu X. Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy. 2021;76(2):562‐564. 10.1111/all.14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: practical considerations on the organization of an allergy clinic—an EAACI/ARIA position paper. Allergy. 2021;76(3):648‐676. 10.1111/all.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi YJ, Park J‐Y, Lee HS, et al. Early view effect of asthma and asthma medication on the prognosis of patients with COVID‐19. Eur Respir J. 2021;57(3):2002226. 10.1183/13993003.02226-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID‐19‐related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106‐1120. 10.1016/S2213-2600(20)30415-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bloom C, Tom D, Docherty A, et al. Risk of adverse outcomes in patients with underlying respiratory conditions hospitalised with COVID‐19 using the ISARIC WHO clinical characterisation protocol: a national, multicentre prospective cohort. Lancet Respir Med. 2021. 10.1016/S2213-2600(21)00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuyama S, Kawase M, Nao N, et al. The inhaled steroid ciclesonide blocks SARS‐CoV‐2 RNA replication by targeting the viral replication‐transcription complex in cultured Cells. J Virol. 2020;95(1). 10.1128/JVI.01648-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters MC, Sajuthi S, Deford P, et al. COVID‐19–related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83‐90. 10.1164/rccm.202003-0821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS‐CoV‐2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147(2):510‐519.e5. 10.1016/j.jaci.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post‐acute covid‐19 in primary care. BMJ. 2020;370:m3026. 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


