CONFLICT OF INTEREST
Dr. O'Mahony reports grants from Science Foundation Ireland, during the conduct of the study; grants from GSK; and personal fees from PrecisionBiotics, outside the submitted work. None of the other authors report any conflicts of interest.
To the Editor,
The current pandemic caused by the SARS‐CoV‐2 virus has so far infected more than 130 million people worldwide, resulting in approximately 3 million deaths. While the current clinical and public health priorities are designed to limit severe acute and fatal episodes of the disease, and to quickly roll out vaccines to the general population, it has become apparent that there may also be significant detrimental long‐term effects following SARS‐CoV‐2 infection that impact daily functioning and quality of life. 1 The mechanisms underpinning the post‐acute sequelae of SARS‐CoV‐2 infection's long‐lasting symptoms can include direct effects of the infection (eg endothelial damage, lung fibrosis) or indirect effects associated with changes in the microbiome or abnormalities in inflammatory and immune signalling pathways stimulated by the infection. 2 , 3
In order to examine the potential long‐term immune changes that occur following elimination of the primary infection, we examined the levels of 52 cytokines and growth factors (using MSD multiplex kits) in the serum of patients who attended follow‐up post‐COVID infection clinics at Cork University Hospital, Cork, Ireland (the Clinical Research Ethics Committee of the Cork Teaching Hospitals approved this study and all patients provided informed consent). We choose cytokines that are representative of a wide range of different types of immune responses and immune processes including Th1, Th2, Th9, Th17, Tregs, acute phase responses, vascular remodelling and angiogenesis, growth factors, adhesion molecules, chemokines and innate proinflammatory responses. All patients had been hospitalized for PCR‐proven SARS‐CoV‐2 infection (median in‐patient stay of 5.5 days, range 1 day to 24 days) during the first wave of the pandemic in Ireland (March‐May 2020). Thirty‐eight serum samples were obtained from twenty‐four patients (median age 53.5 years, 11 female) at 3–9 months following hospital discharge (two samples at least 1 month apart were obtained from 14 patients, while 1 sample only was obtained from 10 patients). Clinical severity ranged from mild to critical during hospitalization, and the most common symptoms at follow‐up clinics were fatigue and/or dyspnoea (Table S1). Sera obtained prior to the pandemic from twenty‐nine healthy volunteers (median age 43.2 years, 14 female) were analysed in parallel.
Of the 52 analytes measured, 19 were significantly elevated in post‐COVID patient sera compared to healthy controls (Table S2). These 19 mediators are illustrated as dot plots in Figures 1 and 2. One group of mediators, C‐reactive protein (CRP), serum amyloid A (SAA), interleukin‐1 receptor antagonist (IL‐1RA), IL‐6, IL‐8, IL‐15, IL‐16, monocyte chemotactic protein (MCP)‐1 and MCP‐4, can be broadly categorized as being associated with ongoing inflammatory responses (Figure 1A). 4 These mediators remained as elevated in samples taken 6–9 months following hospital discharge as those levels observed 3–6 months following discharge (Table S2). A second group of mediators, vascular endothelial growth factor (VEGF‐A), soluble tyrosine‐protein kinase receptor Tie‐2 (Tie‐2), soluble intercellular adhesion molecule (ICAM‐1) and basic fibroblast growth factor (bFGF), can be generally associated with endothelial dysfunction, remodelling and angiogenesis (Figure 1B). 5 The remaining elevated mediators are associated with patterns of lymphocyte polarization. Elevated IL‐4, macrophage‐derived chemokine (MDC) and thymic stromal lymphopoietin (TSLP) sera levels indicate activation of TH2 responses (Figure 2A), while IL‐17A, macrophage inflammatory protein (MIP)‐3α and IL‐12/23p40 indicate ongoing TH17 activity (Figure 2B). Other indicators of TH2‐associated activities are just outside statistical significance (IL‐5, p = .112; Table S2). While TH1 responses are well described to be upregulated during acute infection, 6 the levels of these mediators (eg IFN‐γ, IP‐10) decrease following elimination of the virus and are at control levels in our cohort of post‐COVID patients (Table S2).
FIGURE 1.
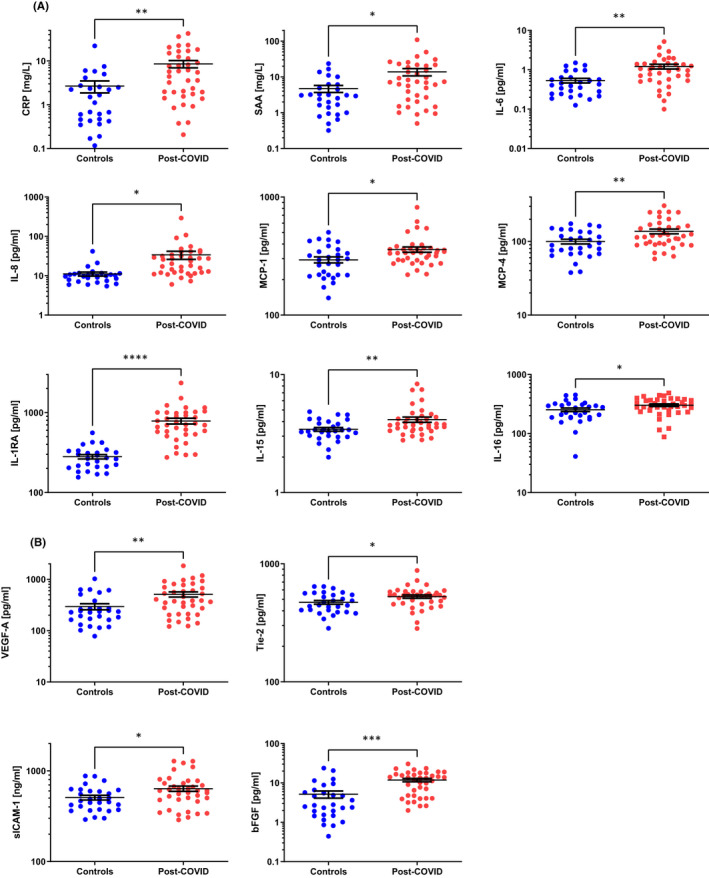
Proinflammatory and endothelial mediators in post‐COVID patients. Proinflammatory mediators (A) and angiogenesis‐associated factors (B) are elevated in sera from patients 3–9 months post‐COVID hospital discharge compared to levels in sera from healthy controls. Results are illustrated on a log scale, and the mean ± standard error is indicated for each group. Depending on the data distribution, unpaired t‐tests or Mann‐Whitney tests were used to determine statistical significance. *p < .05; **p < .01; ***p < .001; and ****p < .0001
FIGURE 2.
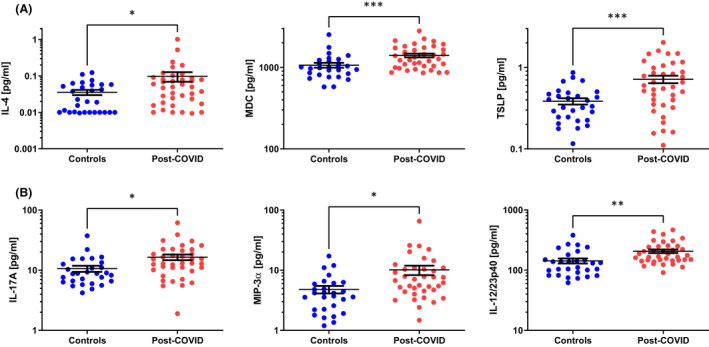
TH2 and TH17 cytokines in post‐COVID patients. Sera from patients 3–9 months post‐COVID hospital discharge are significantly different compared to healthy controls for TH2 cytokines (A) and TH17 cytokines (B). Results are illustrated on a log scale, and the mean ± standard error is indicated for each group. Depending on the data distribution, unpaired t‐tests or Mann‐Whitney tests were used to determine statistical significance. *p < .05; **p < .01; and ***p < .001
Our data suggest that there are long‐term immunological consequences following SARS‐CoV‐2 infection, at least in those who had acute symptoms severe enough to require hospitalization (summarized in Figure S1). While the relatively low number of patients included in our study at this stage does not allow us to perform subgroup analysis, it is possible that these immune mediators may associate with clinically meaningful disease variables and ultimately may be of therapeutic value, if findings are replicated in future studies. Of particular interest is the elevation in TH2‐associated mediators. Could this response be a component of the mucosal repair mechanisms that occur following viral damage, or does this indicate new TH2‐associated pathological immune activity that might underpin an increased risk of developing allergy or asthma? Clearly, the potential immune mechanisms underpinning the emerging post‐COVID clinical entities will become increasingly more important to understand as the healthcare systems adapt to caring for large numbers of COVID‐19 survivors during the coming months and years.
Supporting information
Fig S1
Supplementary Material
REFERENCES
- 1. Amenta EM, Spallone A, Rodriguez‐Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID‐19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12):ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sokolowska M, Lukasik ZM, Agache I, et al. Immunology of COVID‐19: mechanisms, clinical outcome, diagnostics, and perspectives‐A report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy. 2020;75(10):2445‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL‐1 to IL‐38), interferons, transforming growth factor β, and TNF‐α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984‐1010. [DOI] [PubMed] [Google Scholar]
- 5. Bonaventura A, Vecchié A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID‐19. Nat Rev Immunol. 2021;21(5):319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasrija R, Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID‐19 disease. Int Immunopharmacol. 2021;90:107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


