Abstract
Objectives
The immunogenicity of the Comirnaty® vaccine against coronavirus disease 2019 (COVID-19) has not been adequately studied in elderly people with comorbidities. We assessed antibody and T-cell responses targeted to the S protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) following full vaccination in nursing-home residents.
Methods
Sixty nursing-home residents (44 female, age 53–100 years), of whom ten had previously been diagnosed with COVID-19, and 18 healthy controls (15 female, age 27–54 years) were recruited. Pre- and post-vaccination blood specimens were available for quantification of total antibodies binding the SARS-CoV-2 S protein and for enumeration of SARS-CoV-2 S-reactive IFN-γ CD4+ and CD8+ T cells by flow cytometry.
Results
The seroconversion rate in (presumably) SARS-CoV-2-naïve nursing-home residents (41/43, 95.3%) was similar to that in controls (17/18, 94.4%). A booster effect was documented in post-vaccination samples of nursing-home residents with prior COVID-19. Plasma antibody levels were higher (p < 0.01) in recovered nursing-home residents (all 2500 IU/mL) than in individuals across the other two groups (median 1120 IU/mL in naïve nursing-home residents and 2211 IU/ml in controls). A large percentage of nursing-home residents had SARS-CoV-2 S-reactive IFN-γ CD8+ (naïve 31/49, 63.2%; recovered 8/10, 80%) or CD4+ T cells (naïve 35/49, 71.4%; recovered 7/10, 70%) at baseline, in contrast to healthy controls (3/17, 17.6% and 5/17, 29%, respectively). SARS-CoV-2 IFN-γ CD8+ and CD4+ T-cell responses were documented in 88% (15/17) and all control subjects after vaccination, respectively, but only in 65.5% (38/58) and 22.4% (13/58) of nursing-home residents. Overall, the median frequency of SARS-CoV-2 IFN-γ CD8+ and CD4+ T cells in nursing-home residents decreased in post-vaccination specimens, whereas it increased in controls.
Conclusion
The Comirnaty COVID-19 vaccine elicits robust SARS-CoV-2 S antibody responses in nursing-home residents. Nevertheless, the rate and frequency of detectable SARS-CoV-2 IFN-γ T-cell responses after vaccination was lower in nursing-home residents than in controls.
Keywords: Comirnaty®COVID-19 vaccine, Nursing-home residents, SARS-CoV-2, SARS-CoV-2 S antibodies, SARS-CoV-2 S T cells
Introduction
The Comirnaty® (Pfizer–BioNTech) coronavirus disease 2019 (COVID-19) vaccine—a nucleoside-modified messenger RNA that encodes the full-length transmembrane S glycoprotein locked in its perfusion conformation—elicits high levels of serum neutralizing antibodies (NtAbs), targeting mainly the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD), and strong TH1-skewed functional CD4+ and CD8+ T-cell responses in experimental models and humans [[1], [2], [3], [4]]. The efficacy of the vaccine has been shown to approach 95% in preventing COVID-19 across a wide range of age groups [5]. Real-world data have confirmed the efficacy of the vaccine in protecting against the occurrence of severe clinical forms of the disease [6,7]. Nevertheless, information on the immunogenicity and efficacy of this vaccine in elderly people with comorbidities and frailty is scarce [8,9]; such people have been prioritized for vaccination worldwide due to their increased risk of developing severe clinical forms of COVID-19 [10]. To gain further insight into this issue, we assess here SARS-CoV-2-S-targeted antibody and functional T-cell responses after vaccination with Comirnaty in a cohort of nursing-home residents, most displaying one or more comorbidities, and either presumably SARS-CoV-2-naïve or with documented prior SARS-CoV-2 infection.
Material and methods
Participants and study design
A total of 60 participants (44 female) in the study, selected from two nursing homes affiliated to the Clínico-Malvarrosa Health Department, Valencia, Spain—which together provide care for 226 residents—were enrolled in the current study. A total of 18 healthy individuals (15 female) with no history of SARS-CoV-2 infection at baseline who worked in the microbiology laboratory (out of 50 potential participants) served as controls. Both patients and controls were randomly selected by creating an aleatory list using the Excel 2020 (Microsoft, Redmond, Washington, USA) INDEX function. Pre- and post-vaccination blood specimens from participants were collected in sodium heparin tubes (Beckton Dickinson, UK Ltd, UK). Informed consent was obtained from participants. The study was approved by the Hospital Clínico Universitario INCLIVA Research Ethics Committee (February, 2021).
Antibody assays
The following immunoassays were used in the current study. (a) Roche Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics, Pleasanton, CA, USA), an electrochemiluminescence sandwich immunoassay (ECLIA) that quantifies total (IgG and IgM) antibodies directed against RBD. The assay is calibrated with the first WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody [10]. (b) Elecsys® Anti-SARS-CoV-2 (Roche Diagnostics), a qualitative ECLIA detecting IgG and IgM antibodies against SARS-CoV-2 nucleoprotein. Both assays were run on cobas® e601 modular analyser (Roche Diagnostics, Rotkreuz, Switzerland). Plasma specimens were further diluted (1/10) for antibody quantification when appropriate. (c) LIAISON® SARS-CoV-2 TrimericS IgG assay (Diasorin S.p.A, Saluggia, Italy), run on a DiaSorin LIAISON platform (DiaSorin, Stillwater, USA). Immunoassays were performed and interpreted following the instructions of the respective manufacturers. Cryopreserved (–20°C) plasma specimens were thawed and assayed in singlets within 1 month after collection. Baseline and follow-up specimens from a given participant were analysed in the same run.
T-cell immunity assay
SARS-CoV-2-S-reactive IFNγ-producing CD8+ and CD4+ T cells were enumerated in whole blood by flow cytometry for intracellular cytokine staining (ICS) (BD Fastimmune, BD-Beckton Dickinson and Company-Biosciences, San Jose, CA, USA) as previously described [11,12] (see legend to Supplementary Material Fig. S1, which shows representative flow cytometry plots).
Statistical methods
Frequency comparisons for categorical variables were carried out using the Fisher exact test. Differences between medians were compared using the Mann–Whitney U-test or the Wilcoxon test for unpaired and paired data, as appropriate. The Spearman rank test was used for correlation analyses between continuous variables. Two-sided exact p-values were reported; p < 0.05 was considered statistically significant. The analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA).
Results
Participants and sampling
The median age of the participants was 87.5 years (range 53–100) for nursing-home residents and 48.5 years (range 27–60 years) for controls. As shown in the Supplementary Material Table S1, 51/60 subjects (84%) had one or more comorbidities at enrolment (median 4, range 1–7). Baseline blood specimens were collected within 1 week before the first vaccine dose in both nursing-home residents and controls. Post-vaccination specimens were drawn at a median of 17.5 days (range 14–35 days) or 15 days (range 13–35 days) after the second dose in nursing-home residents and controls, respectively.
SARS-CoV-2-specific antibodies in nursing-home residents and controls
No serological evidence of prior SARS-CoV-2 infection was found in 49/59 nursing-home residents (83%) at baseline. Pre-vaccination plasma was not available from one patient; 17% of nursing-home residents (10/59) had suffered from COVID-19 (nursing-home residents recovered).
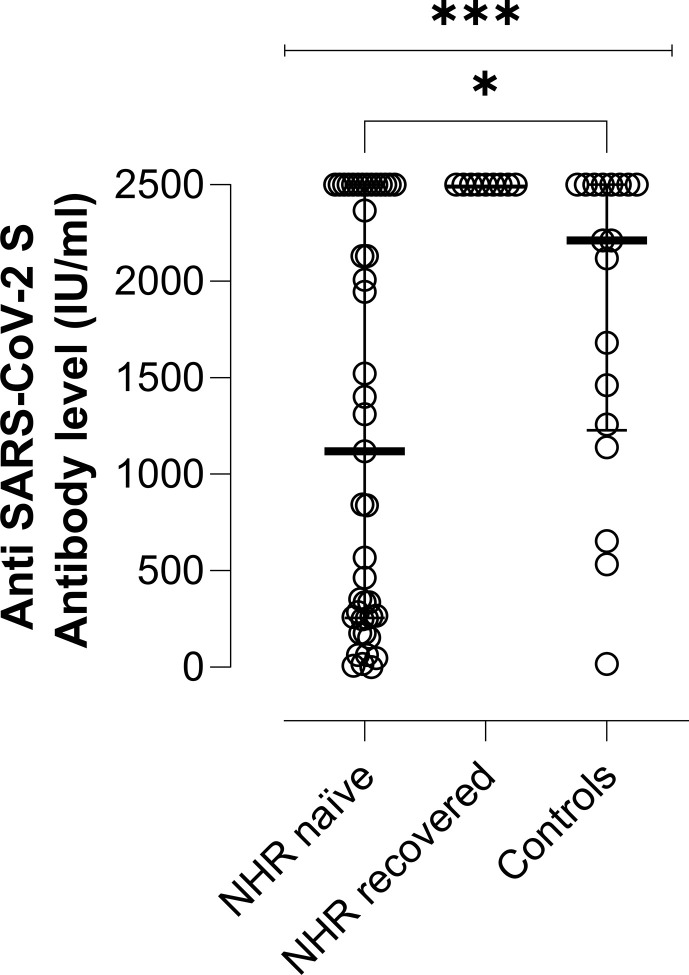
Plasma collected after the second vaccine dose was available for 43 of the 49 nursing-home residents with no documented prior infection. One of the remaining six patients died before receiving the second dose. Forty-one out of the 43 subjects tested positive by Roche SARS-CoV-2-S immunoassay. All but one specimen tested negative by SARS-CoV-2-N immunoassay, suggesting that one nursing-home resident had presumably contracted SARS-CoV-2 infection between the first and second vaccine doses. Therefore, the overall seroconversion rate in this nursing-home resident group was 95.3% (41/43). Of interest, plasma specimens from nursing-home residents and controls testing negative were run with LIAISON® SARS-CoV-2 TrimericS IgG assay, which also returned negative results. All ten recovered nursing-home residents had detectable SARS-CoV-2 S- and N-binding antibodies at baseline; one patient died before receiving the second vaccine dose. A booster effect was observed in all nine individuals following full-dose vaccination, with a median 33-fold (range 10–600-fold) increase in antibody levels. Seventeen out of 18 controls had SARS-CoV-2 S-binding antibodies after the second vaccine dose, while none tested positive for N-specific antibodies. Accordingly, the seroconversion rate in this subgroup was 94.4% (17/18). As shown in Fig. 1 , plasma levels of SARS-CoV-2 S antibodies following complete vaccination were higher (p < 0.01) in recovered nursing-home residents (all 2500 IU/mL) than in those presumably SARS-CoV-2-naïve (median 1120 IU/mL; range 1.08–2500) or controls (median 2211 IU/mL; range, 18.4–2500). In turn, controls displayed higher SARS-CoV-2 S antibody levels than SARS-CoV-2-naïve nursing-home residents (p 0.05).
Fig. 1.
Severe acute respiratory syndrome coronavirus 2 S protein (SARS-CoV-2 S) plasma antibody levels as measured by Roche Elecsys® Anti-SARS-CoV-2 S immunoassay in nursing-home residents (NHRs) with or without documented prior SARS-CoV-2 infection and in healthy controls following complete vaccination. The limit of detection of the assay is 0.4 IU/mL and its quantification range is between 0.8 and 250 IU/mL. Plasma specimens were further diluted (1/10) for antibody quantification when appropriate. Bars represent median levels and the asterisks indicate a significant difference in antibody levels across groups (p < 0.01).
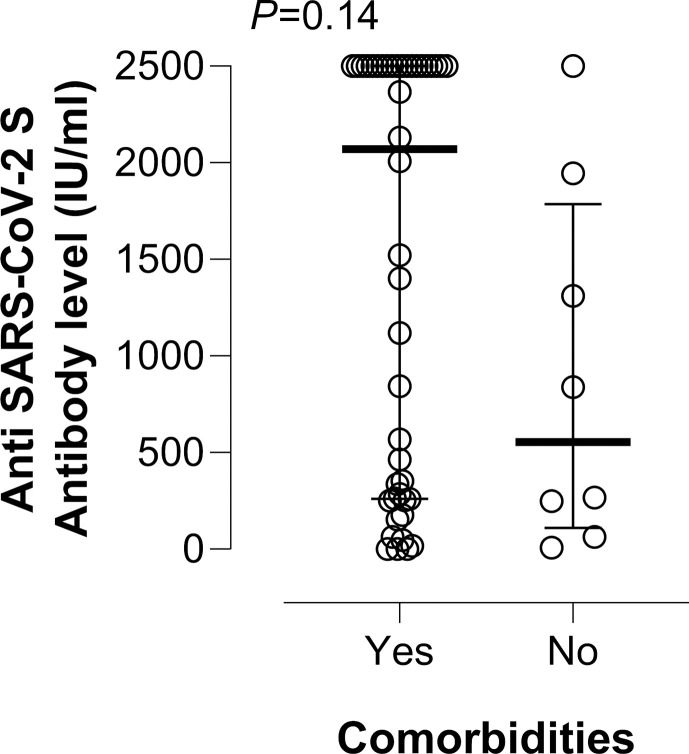
Among nursing-home residents with no documented prior infection, the seroconversion rate was comparable (p > 0.99) in individuals presenting either with comorbidities (33/35, 94%) or without (9/9, 100%). Moreover, having a comorbidity did not impact significantly (p 0.14) on SARS-CoV-2 S antibody levels in this population group (Supplementary Material Fig. S2).
SARS-CoV-2 S-specific T cells in nursing-home residents and controls
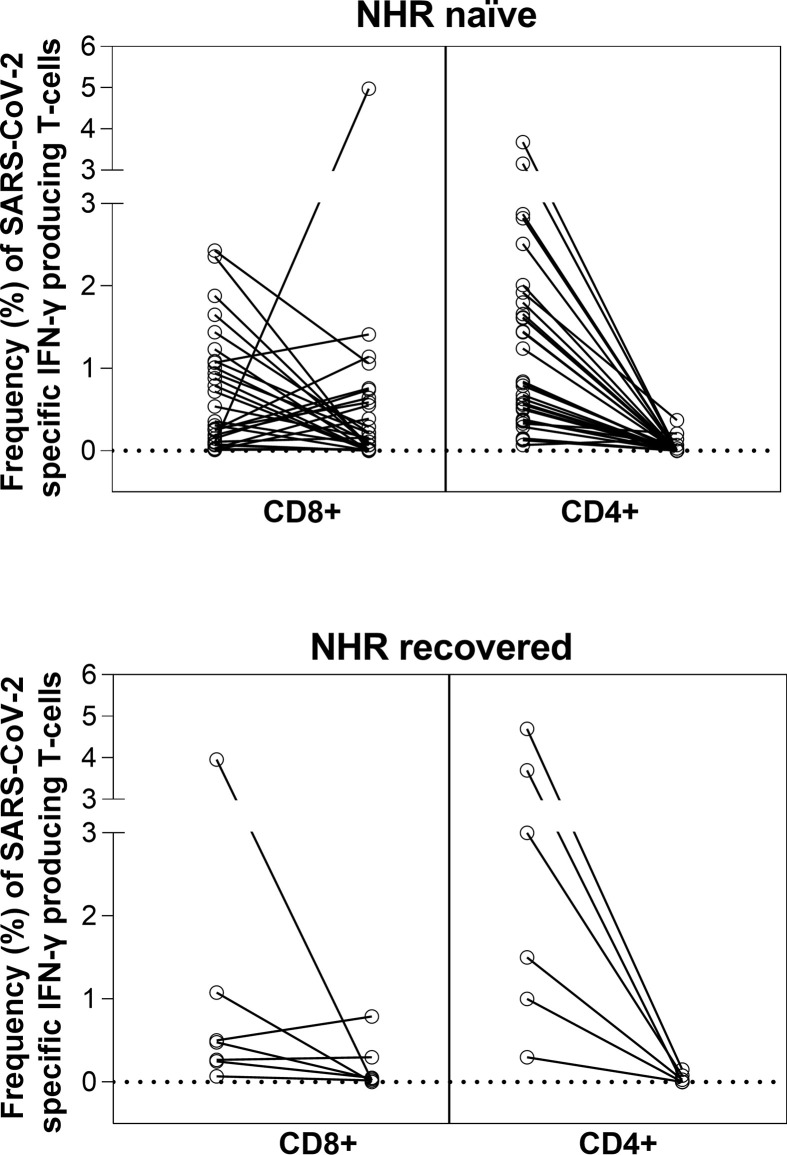
Analysis of pre-vaccination blood specimens revealed the presence of SARS-CoV-2 S-reactive IFN-γ CD8+ and CD4+ T cells in 31/49 (63%) and 35/49 (71.4%) of naïve nursing-home residents and in 8/10 (80%) and 7/10 (70%) of nursing-home residents with a history of SARS-CoV-2 infection; these figures were substantially lower in healthy controls (3/17, 17.6% for CD8+ and 5/17, 29% for CD4+ T cells) (Table 1 ). Following the second vaccine dose, all control subjects had detectable SARS-CoV-2 S-reactive IFN-γ CD4+ T cells and 15/17 (88%) had both IFN-γ CD4+ and CD8+ T cells. Conversely, the percentage of nursing-home residents exhibiting detectable SARS-CoV-2 IFN-γ CD8+ or CD4+ T cell responses (or both), independently of their baseline SARS-CoV-2 infection status, dropped consistently after vaccination (except for CD8+ T cells in nursing-home residents without prior infection), as shown in Table 1. In fact, the percentage of responders was lower in nursing-home residents than in controls (p 0.32 for CD8+ and p <0.001 for CD4+). Among nursing-home residents, the percentage of participants displaying detectable SARS-CoV-2 CD8+ and CD4+ T cells following vaccination was comparable across presumably SARS-CoV-2-naïve and recovered participants (p 0.99 for CD8+ and p 0.20 for CD4+). Interestingly, both loss and de novo acquisition of detectable SARS-CoV-2 IFN-γ CD8+ or CD4+ T-cell responses were observed in some nursing-home residents, particularly in CD8+ T cells (Fig. 2 ).
Table 1.
Detection of SARS-CoV-2-S-reactive T cells in pre- and post-vaccination blood specimens from nursing-home residents and controls
| Study group | SARS-CoV-2 S IFN-γ-producing T cells in pre/post-vaccination peripheral-blood specimens |
|||||
|---|---|---|---|---|---|---|
| No. of subjects with detectable CD8+ T-cell response (%) |
No. of subjects with detectable CD4+ T-cell response |
No. of subjects with detectable CD8+ and CD4+ T-cell responses |
||||
| Pre-vaccination | Post-vaccination | Pre-vaccination | Post-vaccination | Pre-vaccination | Post-vaccination | |
| Nursing-home residents with no documented prior SARS-CoV-2 infection | 31 (63) | 32 (65) | 35 (71) | 9 (18) | 25 (51) | 5 (11) |
| Nursing home residents with prior SARS-CoV-2 infectiona | 8 (80) | 6 (66) | 7 (70) | 4 (44) | 7 (70) | 2 (22) |
| Healthy controls | 3 (17.6) | 15 (88) | 5 (29) | 17 (100) | 2 (11) | 15 (88) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
One patient died before receiving the second vaccine dose.
Fig. 2.
Individual kinetics of severe acute respiratory syndrome coronavirus 2 S protein- (SARS-CoV-2 S-)reactive IFN-γ-producing CD8+ or CD4+ T-cell levels in presumably SARS-CoV-2-naïve (A) and recovered (B) nursing-home residents (NHRs).
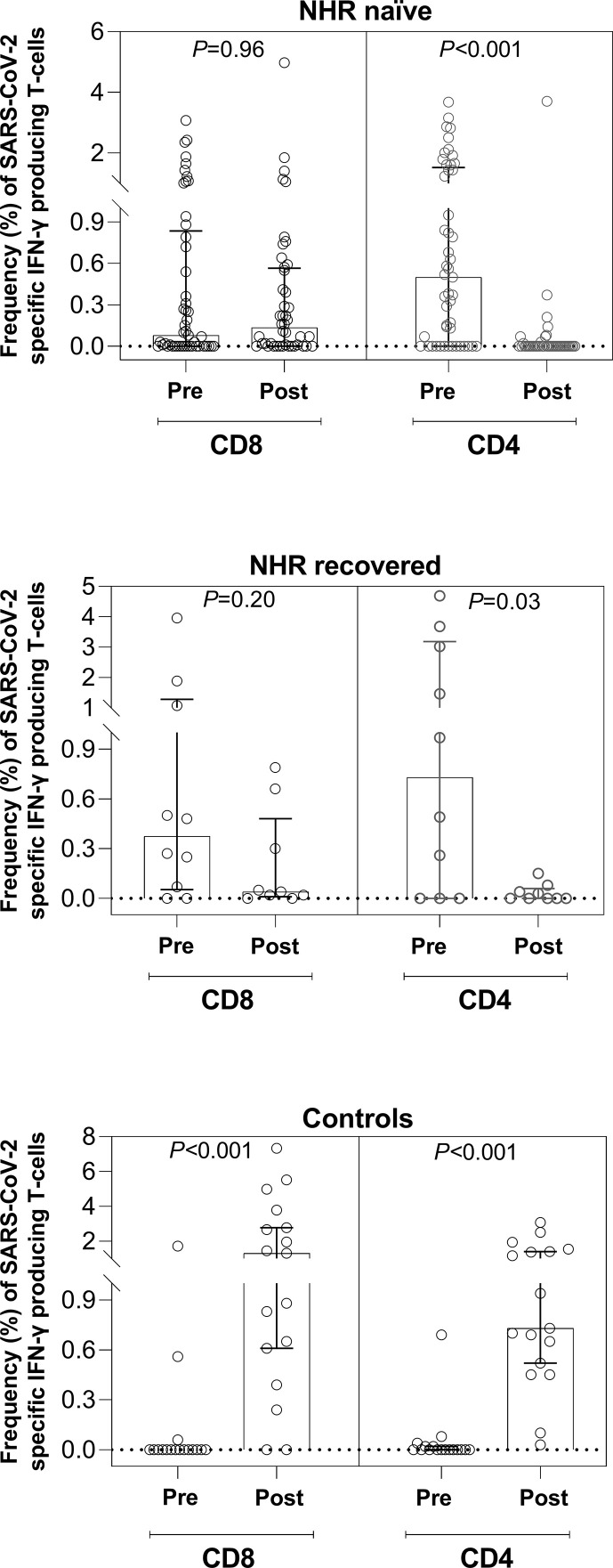
As shown in Fig. 3 and Table 2 , overall the magnitude of SARS-CoV-2 IFN-γ CD8+ or CD4+ T-cell responses in nursing-home residents, irrespective of their SARS-CoV-2 infection status, decreased in post-vaccination specimens, although statistical significance was reached only for SARS-CoV-2 IFN-γ CD4+ T cells (p < 0.001 for SARS-CoV-2-naïve and p 0.03 for recovered participants). The opposite was observed for healthy controls (p < 0.001 for both T-cell subsets). Moreover, median SARS-CoV-2-reactive T-cell frequency in post-vaccination specimens was higher in controls than in nursing-home residents (p < 0.001 for both CD8+ and CD4+ T cells), whereas SARS-CoV-2-naïve and recovered individuals had comparable median frequencies (p 0.72 for CD8+ and p 0.18 for CD4+).
Fig. 3.
Box plots depicting pre- and post-vaccination severe acute respiratory syndrome coronavirus 2 S protein- (SARS-CoV-2 S-)reactive IFN-γ-producing CD8+ or CD4+ T-cell levels in presumably SARS-CoV-2-naïve (A) or recovered (B) nursing-home residents (NHRs) and controls (C). The p values for comparisons are shown.
Table 2.
Frequency of SARS-CoV-2-S-reactive T cells in pre- and post-vaccination peripheral-blood specimens from nursing-home residents and healthy controls
| Study group | SARS-CoV-2 S IFN-γ-producing T cells in pre/post-vaccination peripheral blood specimens |
|||
|---|---|---|---|---|
| CD8+ median % (range)/no. of specimens analysed |
CD4+ median % (range)/no. of specimens analysed |
|||
| Pre-vaccination | Post-vaccination | Pre-vaccination | Post-vaccination | |
| Nursing home residents with no documented prior SARS-CoV-2 infection | 0.08 (0–3.08)/49 | 0.14% (0–4.98)/43 | 0.50 (0–3.68)/49 | 0 (0–3.71)/43 |
| Nursing home residents with prior SARS-CoV-2 infection | 0.38 (0–3.96)/10 | 0.04 (0–0.79)/9 | 0.73 (0–4.69)/10 | 0 (0–0.15)/9 |
| Healthy controls | 0 (0–1.72)/18 | 1.09 (0–7.33)/18 | 0 (0–0.69)/18 | 0.73 (0.03–3.08)/18 |
SARS-CoV-2 S, severe acute respiratory syndrome coronavirus 2 S protein; IFN, interferon.
Interestingly, neither SARS-CoV-2 IFN-γ CD8+ nor CD4+ T-cell frequencies in post-vaccination specimens correlated with SARS-CoV-2 S antibody levels, as measured by ECLIA, in naïve nursing-home residents (ρ 0.05, p 0.74 and ρ –0.06, p 0.73, respectively). A correlation analysis in recovered nursing-home residents could not be performed as SARS-CoV-2 antibody levels of 2500 IU/mL were measured in all samples.
Overall, the rate and magnitude of detectable SARS-CoV-2 S-reactive IFN-γ CD8+ and CD4+ T-cell responses following vaccination were comparable in nursing-home residents with or without comorbidities (Supplementary Material Table S2).
Discussion
Here, SARS-CoV-2 S-targeted antibody and functional T-cell responses after vaccination with Comirnaty were evaluated in a cohort of nursing-home residents and controls prior to and 2–3 weeks after vaccination with Comirnaty. To that purpose, total antibodies binding SARS-CoV-2 RBD by means of an ECLIA normalized to the first WHO international standard [11], which strongly correlate with neutralizing antibody titres [14,15], were quantified. In turn, SARS-CoV-2 S-reactive IFN-γ-producing CD8+ and CD4+ T cells were enumerated using a whole-blood flow cytometry assay [12,13]. Most nursing-home residents recruited (84%, median age 87.5 years) had one or more comorbidities, and were either with or without a SARS-CoV-2 diagnosis by serological and molecular assay prior to vaccination. The main findings of the study are summarized as follows.
First, overall, the SARS-CoV-2 S seroconversion rate was similar in nursing-home residents with no evidence of prior SARS-CoV-2 infection (41/43, 95.2%) and controls (17/18, 94.4%). Nevertheless, median antibody levels were higher in controls than in naïve nursing-home residents (p 0.05). This observation concurs with data reported in the phase I vaccine trial by Walsh et al. [2], but is in contrast to the findings of Collier et al. [9] who found no age-related differences in post-vaccination antibody titres; however, they used a neutralization assay instead of an RBD-binding total antibody ECLIA.
Second, a dramatic booster effect was documented in all nursing-home residents previously infected by SARS-CoV-2; in fact, these subjects reached significantly higher antibody levels than were measured in those presumed to be naïve and in controls (p < 0.01).
Third, while detectable SARS-CoV-2 S-reactive IFN-γ CD8+ and/or CD4+ T-cell responses were documented in post-vaccination specimens from all control subjects, they were present in 18% (9/49) to 66% (6/9) of nursing-home residents, depending upon the T-cell subset considered and whether or not subjects had a prior history of SARS-CoV-2 infection. The difference in the rate of detectable responses between controls and nursing-home residents was particularly dramatic for SARS-CoV-2-S CD4+ T cells (p < 0.001). Among nursing-home residents, the percentage of participants exhibiting detectable SARS-CoV-2 CD8+ or CD4+ T cells following vaccination was comparable across presumably SARS-CoV-2-naïve and recovered individuals (p 0.99 for CD8+ and p 0.20 for CD4+). Moreover, in contrast to controls, a decrease in the frequency of SARS-CoV-2 S-reactive IFN-γ T cells, most notably CD4+ T cells, was noticed in post-vaccination specimens from most nursing-home residents, regardless of their SARS-CoV-2 infection status. Interpreting the T-cell response data presented herein is confounded by difficulty in ascertaining the true infection status of participants, regarding which a differential effect of the second Comirnaty dose on T- and B-cell immunity was reported in COVID-19-naïve and recovered individuals, with the latter exhibiting poorer responses [16,17], perhaps due to the development of immunological anergy. These observations [16,17], if confirmed, would lend support to the use of a single booster dose for previously infected individuals. In effect, functional SARS-CoV-2 S IFN-γ T cells detected in pre-vaccination specimens may well have been seasonal coronavirus cross-reactive T cells (see [18] for a review). Circulation of seasonal coronaviruses in nursing-home facilities and repeated exposure of residents is common over the winter season. Nevertheless, we cannot rule out that some of the current study participants, nursing-home residents in particular, could have been asymptomatically infected and failed to mount durable antibody responses despite robustly expanding SARS-CoV-2-specific T cells [19].
As stated above, a major finding of the current study is that, regardless of the true SARS-CoV-2 infection status of participants, overall nursing-home residents displayed poorer SARS-CoV-2 S T-cell responses (in particular CD4+ T-cell responses) than healthy controls after vaccination. In contrast, Collier et al. [9] found no age-related (<80 versus >80 years old) differences in T-cell response, as measured by a CD3+ IFN-γ Fluorospot, after a full vaccination dose; however, the authors admitted that they were unable to adjust for confounders such as immune suppression and comorbidities and had no information on the pre-vaccination SARS-CoV-2 infection status of the participants. Our finding could be explained partly by the detrimental impact of age-related immunosenescence on immune responses to vaccines [20]. Nevertheless, the vaccine elicited a seemingly robust humoral response in most nursing-home residents who were presumably naïve or recovered, with SARS-CoV-2 S antibody levels showing no correlation with SARS-CoV-2 T-cell frequencies in SARS-CoV-2-naïve subjects. The apparent contraction of SARS-CoV-2-S-reactive IFN-γ T cells in recovered nursing-home residents following the second vaccine dose was also observed by Camara et al. [16], who hypothesized that this second dose may functionally exhaust SARS-CoV-2 S-specific T cells. This may also apply to cross-reactive T cells. In this sense, CD4+ T-cell responses against common cold coronaviruses (CCCs) were decreased in SARS-CoV-2-infected healthcare workers, suggesting that exposure to SARS-CoV-2 might somehow interfere with CCC responses [21]. Whether this might also be the case following vaccination needs to be defined.
Fourth, comorbidities did not appear to have a major impact on either the seroconversion rate or the magnitude of antibody or T-cell responses following the second vaccine dose in nursing-home residents.
The current study has several limitations that must be emphasized. First, the number of participants was relatively limited. Second, frequencies instead of absolute numbers of SARS-CoV-2 S-reactive T cells are reported throughout the study; the latter would have been advisable since discrete lymphopenia is not unusual in the elderly. Third, the possibility that nursing-home residents displayed SARS-CoV-2 S-reactive T cells with functional specificities other than IFN-γ production was not explored. Fourth, a whole-blood flow cytometry assay was used to assess T-cell immunity; it is uncertain whether employing isolated peripheral-blood mononuclear cells instead would have increased sensitivity. Fifth, serial determinations may have provided more accurate information on the T-cell responses elicited by the vaccine across comparison groups. Sixth, no attempt was made to differentiate between truly SARS-CoV-2-specific and cross-reactive T cells [22,23]. Seventh, further sample dilutions to precisely measure antibody levels in some participants was not performed.
In summary, we were able to document robust SARS-CoV-2 S antibody responses in healthy controls and nursing-home residents following two doses of the Comirnaty COVID-19 vaccine, Nevertheless, our data point to differential vaccine efficacy between nursing-home residents and controls in terms of eliciting SARS-CoV-2 IFN-γ T-cell responses, in particular of the CD4+ T-cell subset. In this context, the potentially detrimental effect of pre-existing bona fide or cross-reactive SARS-CoV-2 immunity seen in nursing-home residents merits further investigation.
Author contributions
EA, EG, MJA, PA, MJR, IT, DH and BO: methodology and data validation. PB, MJB and CR: in charge of implementing public health policies to combat SARS-CoV-2 epidemic at nursing home residences affiliated to the Health Department Clínico-Malvarrosa. DN: conceptualization, supervision, writing the original draft. All authors reviewed the original draft.
Transparency declaration
The authors declare no conflicts of interest. Ignacio Torres holds a Río Hortega Contract (CM20/00090) from the Health Institute Carlos III. Eliseo Albert holds a Juan Rodés Contract (JR20/00011) from the Health Institute Carlos III. Estela Giménez holds a Juan Rodés Contract (JR18/00053) from the Health Institute Carlos III. No public or private funds were received for this work.
Acknowledgements
We are grateful to all personnel who work at nursing-home residences affiliated to the Health Department Clínico-Malvarrosa and at Clinic University Hospital, in particular those at the microbiology laboratory, for their commitment in the fight against COVID-19.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.06.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 2.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 4..
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;S0140–6736:947–948. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockman M.A., Mwimanzi F., Sang Y., Ng K., Agafitei O., Ennis S., et al. Weak humoral immune reactivity among residents of long-term care facilities following one dose of the BNT162b2 mRNA COVID-19 vaccine. medRxiv. 2021:21253773. doi: 10.1101/2021.03.17.21253773. [DOI] [Google Scholar]
- 9.Collier D.A., Ferreira I.A.T.M., Datir R., Meng B., Bergamaschi L., Lim E., et al. Age-related heterogeneity in neutralising antibody responses to SARS-CoV-2 following BNT162b2 vaccination. medRxiv. 2021 doi: 10.1101/2021.02.03.21251054. [DOI] [Google Scholar]
- 10.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattiuzzo G, Bentley EM, Hassall M, Routley S, Richardson S, Bernasconi V et al. Establishment of the WHO international standard and reference Panel for anti-SARS-CoV-2 antibody. WHO/BS/2020.2403, 10 December 2020.
- 12.Giménez E., Albert E., Torres I., Remigia M.J., Alcaraz M.J., Galindo M.J., et al. SARS-CoV-2-reactive interferon-gamma-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J Med Virol. 2021;93:375–382. doi: 10.1002/jmv.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Ruiz M., Olea B., Giménez E., Laguna-Goya R., Trujillo H., Caravaca-Fontán F., et al. SARS-CoV-2-specific cell-mediated immunity in kidney transplant recipients recovered from COVID-19. Transplantation. 2021;105:1372–1380. doi: 10.1097/TP.0000000000003672. [DOI] [PubMed] [Google Scholar]
- 14.Higgins V., Fabros A., Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021 doi: 10.1128/JCM.03149-20. JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poljak M., Oštrbenk Valenčak A., Štamol T., Seme K. Head-to-head comparison of two rapid high-throughput automated electrochemiluminescence immunoassays targeting total antibodies to the SARS-CoV-2 nucleoprotein and spike protein receptor binding domain. J Clin Virol. 2021;137:104784. doi: 10.1016/j.jcv.2021.104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camara C., Lozano-Ojalvo D., Lopez-Granados E., Paz-Artal E., Pion M., Correa-Rocha R., et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naïve and COVID-19 recovered individuals. bioRxiv. 2021:436441. doi: 10.1101/2021.03.22.436441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanovic M.I., Cornelius A.R., Wilson J.P., Karmacharya T., Gray-Gaillard S.L., Allen J.R., et al. Poor antigen-specific responses to the second BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. medRxiv. 2021:21251311. doi: 10.1101/2021.02.07.21251311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrotri M., van Schalkwyk M.C.I., Post N., Eddy D., Huntley C., Leeman D., et al. T cell response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira B., Xu X.N., Akbar A.N. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front Immunol. 2020;11:583019. doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva Antunes R., Pallikkuth S., Williams E., Esther D.Y., Mateus J., Quiambao L., et al. Differential T cell reactivity to endemic coronaviruses and SARS-CoV-2 in community and health care workers. J Infect Dis. 2021:jiab176. doi: 10.1093/infdis/jiab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dykema A.G., Zhang B., Woldemeskel B.A., Garliss C.C., Cheung L.S., Choudhury D., et al. Functional characterization of CD4+ T-cell receptors cross-reactive for SARS-CoV-2 and endemic coronaviruses. J Clin Invest. 2021:146922. doi: 10.1172/JCI146922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogbe A., Kronsteiner B., Skelly D.T., Pace M., Brown A., Adland E., et al. T cell assays differentiate clinical and subclinical SARS-CoV-2 infections from cross-reactive antiviral responses. Nat Commun. 2021;12:2055. doi: 10.1038/s41467-021-21856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.