Abstract
Objectives
Though rare, neurological side effects of SARS-CoV-2 vaccinations are increasingly reported. Even if the first dosage goes uncomplicated, the second dose may be complicated by severe adverse reactions as in the following case.
Case report
A 52yo male developed sudden-onset reading difficulty and aphasia 7d after the second dose of an mRNA-based SARS-CoV-2 vaccine. He had a previous history of myocardial infarction, arterial hypertension, hyperlipidemia, and nephrolithiasis. Blood pressure was slightly elevated on admission. Blood tests revealed mildly elevated D-dimer, pre-diabetes and hyperuricemia. Cerebral magnetic resonance imaging revealed an intracerebral bleeding (ICB) in the left temporal lobe. Aphasia resolved almost completely within a few days. Blood pressure values were normal throughout hospitalisation. Whether there was a causal relation between the ICB and the vaccination remains speculative but cannot be definitively excluded.
Conclusions
A second dose of a SARS-CoV-2 vaccination may be followed by ICB. Though the pathophysiology of ICB remains unexplained a causal relation between ICB and the vaccination cannot be excluded. Risk factors for ICB should be carefully monitored in patients undergoing SARS-CoV-2 vaccination.
Keywords: Intracerebral bleeding, SARS-CoV-2, COVID-19, Aphasia, Side effect, Adverse reaction, Vaccination
Letter to the Editor
Though vaccination against SARS-CoV-2 is usually well tolerated, some patients experience side effects, including neurological adverse reactions.1 Neurological adverse reactions to SARS-CoV-2 vaccinations include exacerbation or new onset neuro-immunologic disease, such as myasthenia, Guillain-Barre syndrome, immune encephalitis, or multiple sclerosis2 but also ischemic stroke, or intra-cerebral bleeding (ICB). ICB may be due to hypocoagulability or immune-mediated thrombotic thrombocytopenia.3, 4 Even if patients do not experience side effects after the first dosage, they may develop severe neurological complications after the second dose, as in the following case.
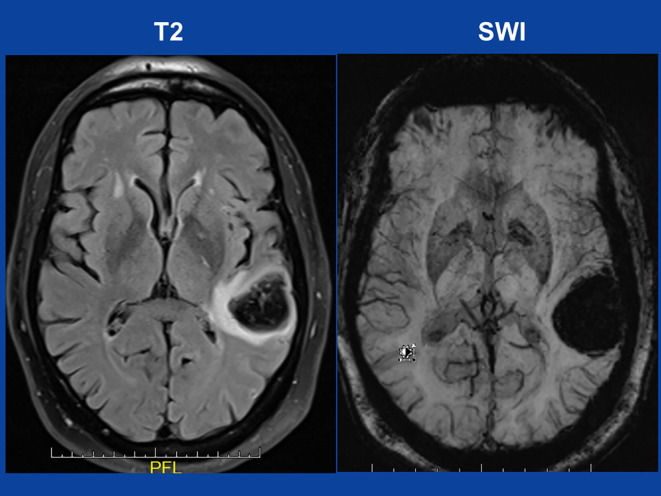
The patient is a 52yo non-smoking, HIV-negative male, who experienced sudden-onset difficulties with reading and speaking seven days after the second dose of an mRNA-based SARS-CoV-2 vaccine. He did not experience any side effects immediately after the first or second vaccination. His previous history was positive for myocardial infarction four months earlier treated with stent implantation into the left anterior descending, ramus circumflexus, and right coronary artery, arterial hypertension, hyperlipidemia, and nephrolithiasis. Myocardial infarction was complicated by systolic dysfunction and non-sustained (persistent) ventricular tachycardias being treated with a temporary life west. His current medication comprised sacubitril/valsartan, atorvastatin, bisoprolol, and acetyl-salicylic acid. Neurologic exam one day later (hospital day (hd)-1) revealed motor aphasia with paraphasias and a spontaneous Babinski sign on the right side. Blood pressure was 170/98 mmHg on admission. Blood tests were unremarkable except for slightly elevated D-dimer, hyperuricemia, and a HbA1c value of 5.8 (n, 4.0–5.7). The glucose tolerance test was indicative of pre-diabetes. Other coagulation parameters and the thrombocyte count were within normal limits. Cerebral MRI revealed a lobar bleeding in the left temporal lobe (Fig. 1 ). Cerebral CT angiography was normal. Aphasia resolved almost completely within a few days. On hd4 he was transferred to the rehabilitation unit with a therapy of sacubtril/valsartan, atorvastatin, and bisoprolol.
Fig. 1.
cerebral MRI on admission showing an ICB in the left temporal lobe on T2-weighted images (left) and on susceptibility-weighted imaging (SWI) sequencies (right).
The presented patient is interesting for ICB shortly after the second dose of an mRNA-based SARS-CoV-2 vaccine. Whether there was a causal relation between vaccination and the ICB remains speculative. Arguments for a causal relation are that ICB has been reported as a complication of a SARS-CoV-2 vaccination,5, 6 that ICB occurred time-linked to the vaccination, and that arterial hypertension can be a complication of a SARS-CoV-2 vaccination.7 A further argument in favour of a causal relation is that ICB has been repeatedly reported as a complication of infections with SARS-CoV-2.8 Whether the slightly elevated D-dimer indicates hypercoagulability followed by compensatory hypocoagulability remains speculative. Pathophysiological mechanisms explaining SARS-CoV-2 associated ICB could be endothelial cell dysfunction due to direct invasion of the virus, vaccination induced thrombocytopenia, hypocoagulability due to disseminated intravascular coagulopathy or increased fibrinolysis,9 or arterial hypertension due to affection of the autonomic innervation of the heart, Arguments against a causal relation are that the patient also had slightly elevated arterial hypertension on admission, that the latency between vaccination and ICB was 7 days, and that ICB after SARS-CoV2 vaccination has been only rarely reported. Possibly, the vaccination increased systolic blood pressure, or caused immune-mediated thrombocytopenia or hypocoagulability. The latency of 7 days argues against an immune mechanism as the T-cell response to the vaccination peaks not earlier than 14 days after vaccination.10
This case shows that the second dose of a mRNA-based SARS-CoV-2 vaccine may be followed by ICB. Though the pathophysiology of ICB remains unexplained a causal relation between ICB and the vaccination cannot be excluded. Infectiologists and neurologists should remain vigilant for complications of SARS-CoV-2 vaccination. Risk factors for ICB should be carefully monitored in patients undergoing SARS-CoV-2 vaccination
Funding
No funding was received for this study.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Finsterer J., Scorza F.A. SARS-CoV-2 vaccines are not free of neurological side effects. Acta Neurol Scand. 2021;144(1):109–110. doi: 10.1111/ane.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watad A., De Marco G., Mahajna H., et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althaus K., Möller P., Uzun G., et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021 doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fueyo-Rodriguez O., Valente-Acosta B., Jimenez-Soto R., et al. Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021;14(5):e242220. doi: 10.1136/bcr-2021-242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjørnstad-Tuveng T.H., Rudjord A., Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskr Nor Laegeforen. 2021;29:141. doi: 10.4045/tidsskr.21.0312. [DOI] [PubMed] [Google Scholar]
- 6.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meylan S., Livio F., Foerster M., Genoud P.J., Marguet F., Wuerzner G. CHUV COVID vaccination center. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021 Jun;77(6):e56–e57. doi: 10.1161/HYPERTENSIONAHA.121.17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leasure AC, Khan YM, Iyer R, et al. Intracerebral hemorrhage in patients with COVID-19: an analysis from the COVID-19 cardiovascular disease registry. Stroke 2021:STROKEAHA121034215. [DOI] [PMC free article] [PubMed]
- 9.Bunch C.M., Thomas A.V., Stillson J.E., et al. Thromboelastography-guided anticoagulant therapy for the double hazard of thrombohemorrhagic events in COVID-19: a report of 3 cases. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.931080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folegatti P.M., Ewer K.J., Aley P.K., et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]