Abstract
Purpose
Biodegradable implants are of major interest in orthopaedics, especially in the skeletally immature population. Magnesium (Mg) implants are promising for selected surgical procedure in adults, but evidence is lacking. Thus, the aim of this study is to analyze the safety and efficacy of resorbable Mg screw in different orthopaedic procedures in skeletally immature patients. In addition, we present a systematic review of the current literature on the clinical use of Mg implants.
Methods
From 2018 until the writing of this manuscript, consecutive orthopaedic surgical procedures involving the use of Mg screws performed at our centre in patients < 15 years of age were retrospectively reviewed. In addition, a systematic review of the literature was performed in the main databases. We included clinical studies conducted on humans, using Mg-alloy implants for orthopaedic procedures.
Results
A total of 14 patients were included in this retrospective analysis. Mean age at surgery was 10.8 years (sd 2.4), mean follow-up was 13.8 months (sd 7.5). Healing was achieved in all the procedures, with no implant-related adverse reaction. No patients required any second surgical procedure. The systematic review evidenced 20 clinical studies, 19 of which conducted on an adult and one including paediatric patients.
Conclusion
Evidence on resorbable Mg implants is low but promising in adults and nearly absent in children. Our series included apophyseal avulsion, epiphyseal fractures, osteochondritis dissecans, displaced osteochondral fragment and tendon-to-bone fixation. Mg screws guaranteed stable fixation, without implant failure, with good clinical and radiological results and no adverse events.
Level of evidence
IV – Single cohort retrospective analysis with systematic review
Keywords: Magnesium, resorbable implant, paediatrics
Introduction
Osteosynthesis technique and orthopaedic implants have developed over the last decades with an increase of surgical indications in treating orthopaedic and traumatological conditions. The need for the removal of orthopaedic implants is controversial in the literature. In adults, it is usually indicated only in cases of intolerance or related complications. On the other hand, in skeletally immature patients, routine removal of orthopaedic implants is usually recommended for several reasons, in particular to avoid interference with growth. For this reason, a progressive interest has been shown on biodegradable materials, which do not require further surgery for their removal.1
Biodegradable implants may be subdivided into three groups. The first group is represented by polymers, such as poly-L-lactic acid or poly-lactic-co-glycolic acid. Poor mechanical properties and lack of osteoconductivity2 limit their use. The second category includes ceramics such as hydroxyapathite, which have shown high biocompatibility and osteoconductivity. However, ceramics have slow degradation rates and poor load-bearing capacity.3 Biodegradable metals have the greatest tensile and load-bearing properties than the other groups. Among them, magnesium (Mg) is one of the most interesting due to its biocompatibility, osteoconductivity and mechanical properties.4,5
Preclinical studies have shown that Mg alloys are well tolerated both by osteoblasts and growth plate chondrocytes6 whilst biomechanical testing showed higher pull-out forces of Mg screw as compared with polymeric ones.7 Moreover, the elastic modulus and density of Mg are much more similar to that of cortical bone, as compared with titanium and stainless steel.8 This would reduce the so-called stress shielding around the implant.9 The principal limitations of Mg implants have been shown to be the inability to control their degradation rate and the release of important amount of gas during corrosion.10 Moreover, fast degradation rate has been associated with implant failure and early loss of stability. In order to prevent these problems, Mg alloys have been introduced in clinical practice and a large number of alloying solutions are currently under investigation.
In addition, Mg++ has been shown to have an osteogenic effect by activating the Wnt pathway causing bone marrow stromal cells (BMSCs) to differentiate towards the osteoblast lineage.11 Lastly, Mg implants have been shown to produce less imaging artifacts during radiograph, CT and MRI than other materials (e.g. titanium (Ti) screws).12
Mg implants have shown to be interesting for some orthopaedic surgical procedure in adults, such as hallux valgus osteotomy fixation,13 ,14 anterior cruciate ligament (ACL) reconstruction,15 stabilization of bone grafting in osteonecrosis of the femoral head treatment16 and fixation of intraarticular fractures.17 Nevertheless, one of the most interesting settings for resorbable Mg alloys is paediatric surgery.
The aim of this study is to analyze the safety and efficacy of the clinical applications of resorbable Mg screws in different orthopaedic and traumatology procedures in skeletally immature patients, retrospectively analyzing a series of cases from our centre. We then conducted a systematic review of the literature to assess the actual evidence of the use of Mg implants in orthopaedic procedures focusing on the age of the patients, diagnosis, surgical approach, outcome and implant-related adverse reaction.
Materials and methods
Case series
After approval of the study by the local ethics committee, the database of Salesi Children’s Hospital was mined for the records of all surgical procedure performed from 01 January 2018 to 30 June 2020. The patients’ parents/ guardians provided their informed consent to the use of the children’s medical charts. Inclusion criteria were: age at surgery < 15 years, orthopaedic or traumatological pathologies with surgical indication treated by Mg screw implant and follow-up (FU) three or more months. Diagnosis, age at surgery, sex, surgical procedure, clinical evaluation and radiological exams, months of FU and eventual complications were recorded.
Potential implant-related adverse reactions were considered: abnormal discomfort at the site of the implant and presence of inflammatory signs at the site of the implant. Implant failure was considered as loss of integrity of the implant associated with: malunion, nonunion or delayed union of fractures, failure of the epiphysiodesis or failure of the tenodesis.
Review
The review process was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines18. The Medline-PubMed, Embase, Web of Science and Cochrane Systematic Review databases were searched for studies published in English up to 31 November 2020. The primary search keywords were: “magnesium orthopedics”, “magnesium screw”, “magnesium pin”, “magnesium implant orthopedics”. Papers were screened by title and abstract to identify relevant articles. Their reference lists were checked manually for additional articles. All the results were analyzed independently by two revisors. Exclusion criteria were considered: in vitro or in vivo preclinical study conducted on animals, clinical procedures not concerning the orthopaedic field (such as odontology, maxillo-facial surgery). Inclusion criteria were: clinical studies conducted on humans using Mg or Mg-alloy implants for orthopaedic procedures. The selection process is described in detail in Figure 1.
Fig. 1.
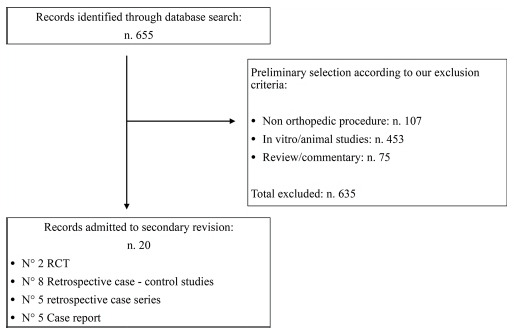
Selection protocol: abstract selection chart according to our inclusion criteria. Abstract inclusion and exclusion criteria are highlighted in the box.
Results
Retrospective analysis
A total of 14 patients were included in the retrospective analysis. The male/female ratio was 1:1 and mean age at surgery was 10.8 years (sd 2.4). Surgery was performed for different conditions: ten for epiphyseal fractures or apophyseal avulsion, two for epiphysiodesis, one for osteochondritis dissecans (OCD) and one for tendon-to-bone fixation. A total of seven procedures involved the lower limb, while seven involved the upper limb. Mean clinical FU was 13.8 months (sd 7.5; 6 to 26). All the procedures were performed using the same implant: 3.2-mm Herbert type, cannulated screw (MAGNEZIX; Syntellix AG, Hannover, Germany) of appropriate length using manufacturer’s instructions and proper surgical kit.
Clinical and radiological healing was achieved in all the procedures. No clinical evidence of either local or systemic implant-related adverse reaction was registered. Diagnosis, age at surgery, sex, surgical procedure, clinical and radiological FU and eventual complications are recorded in Table 1.
Table 1.
Diagnosis, age at surgery, sex, surgical procedure, clinical and radiological follow-up and eventual complication of the patients undergoing surgical procedure with magnesium resorbable screws between January 2018 and June 2020
| Diagnosis | Age (years) | Sex | Treatment | Follow-up, mths | Complications |
|---|---|---|---|---|---|
| Tibial spine avulsion fracture | 12 | F | ARIF (two cannulated screws)* | 26 | None |
| II toe F1 macrodactyly | 9 | F | Epiphysiodesis F1 (one cannulated screw)* | 26 | None |
| Clubfoot relapse | 10 | M | Partial tibialis anterior transposition with one interference screw* | 24 | None |
| Fracture-dislocation of patella | 13 | M | ORIF (three cannulated screws)* | 18 | None |
| Medial epicondyle avulsion | 10 | F | ORIF (two cannulated screws)* | 16 | None |
| Medial epicondyle avulsion | 11 | M | ORIF (two cannulated screws)* | 14 | Detachment of the screw head |
| Tibial distal epiphysis fracture | 12 | M | CRIF (one all-epiphyseal cannulated screw* + one trans-physeal Kirschner-wire) | 16 | None |
| Flat foot and hallux valgus | 8 | F | CNF + hallux proximal physis emiepiphysiodesis (one cannulated screw)* | 12 | None |
| Osteochondritis dissecans of the knee | 13 | F | Anterograde drilling + fixation (one cannulated screw)* | 12 | None |
| Medial epicondyle avulsion | 14 | M | ORIF (two cannulated screws)* | 6 | None |
| Tibial distal epiphysis fracture (Tillaux) | 13 | F | ORIF (two transphyseal cannulated screws)* | 6 | None |
| Medial epicondyle avulsion | 5 | M | ORIF (two cannulated screws)* | 6 | None |
| Medial epicondyle avulsion | 11 | F | ORIF (one cannulated screw)* | 6 | None |
| Medial epicondyle avulsion | 10 | M | ORIF (one cannulated screw)* | 6 | None |
resorbable MAGNEZIX screw (Syntellix AG; Hannover, Germany)
ARIF, arthroscopic reduction internal fixation; ORIF, open reduction internal fixation; CRIF, closed reduction internal fixation; CNF: calcanear notch filler
Case description of a patellar fracture dislocation and technical notes
A 13-year-old male was diagnosed with a fracture, a lateral dislocation of the left patella, which occurred playing amateur basketball. A radiograph was carried out before reduction of the patella and after the reduction a second radiograph and a preoperative CT scan were performed. The osteochondral fragment measured approximately 2.5 cm × 1.5 cm × 3.5 cm (Fig. 2).
Fig. 2.
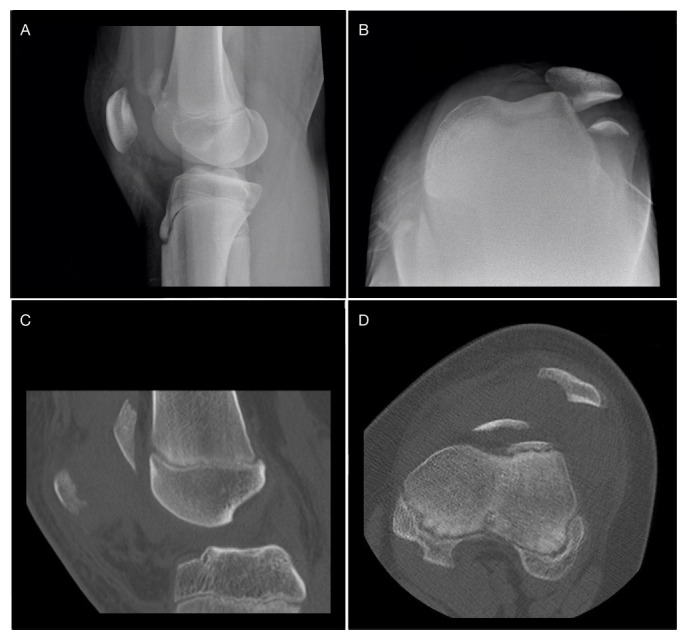
a) and b) Sagittal and axial radiograph showing fracture and lateral dislocation of the patella; c) and d) sagittal and axial CT slices after reduction of the patella, showing residual subluxation and a free osteochondral fragment along the trochlear groove.
The surgical procedure was performed by an arthrotomic open reduction and internal fixation with three MgYREZr cannulated screws (MAGNEZIX; Syntellix AG) and a medial patello-femoral ligament reconstruction. After patellar exposure the fracture was anatomically reduced and temporarily fixed with three guide wires. Using the manufacturer’s drill-bit, a hole was made and the 3.2-mm Herbert type screw of appropriate length was inserted with mild compression (Fig. 3)
Fig. 3.
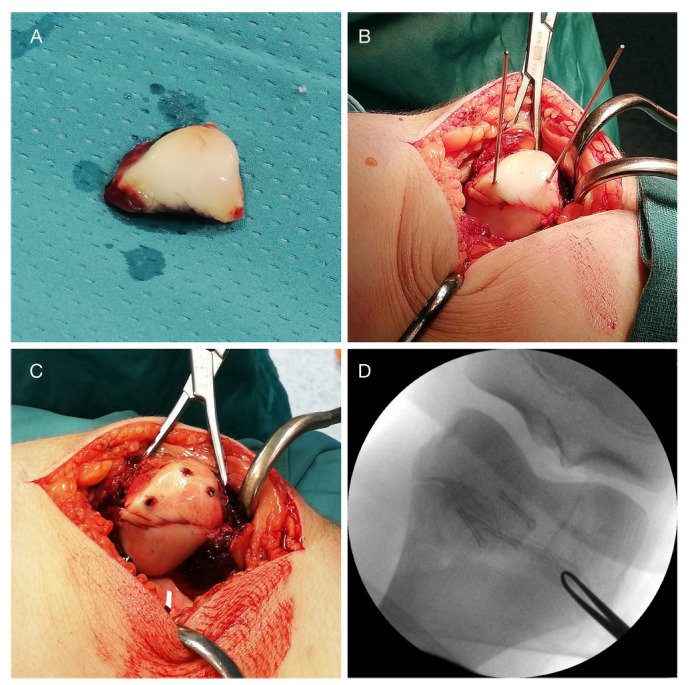
a) Free osteochondral fragment after removal from the trochlear groove; b) and c) fixation of the osteochondral fragments with guide wires and magnesium (Mg) cannulated screw according to the manufacturer instruction; d) intraoperative image intensifer confirms the position of the interfragmentary compressive Herbert type Mg screws.
The patient was discharged with a knee brace in fixed-extension position and non-weight-bearing prescription for four weeks. After the first month, he started with partial weight-bearing and physiotherapy. By the eighth week after the procedure the patient was full weight-bearing, with complete active and passive range of movement (ROM). Radiological imaging taken three months postoperatively are shown in Figure 4.
Fig. 4.
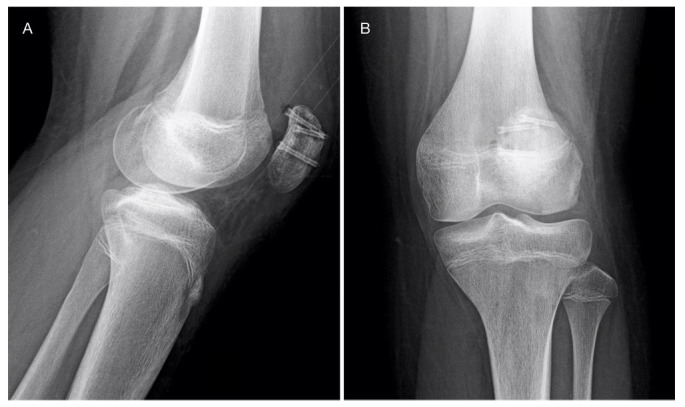
Radiographs taken at three months postoperatively show healing of the fracture and initial signals of magnesium screws resorption, with mild gas formation.
At 18 months after surgery the patient was subjectively well, with a Visual Analogue Score (VAS)19 of 0 during activities of daily living and fitness training at the gym. Active and passive ROM were complete, symmetric and pain-free, with a modified knee Hospital for Special Surgery (HSS) score of 95 (Fig. 5).20
Fig. 5.
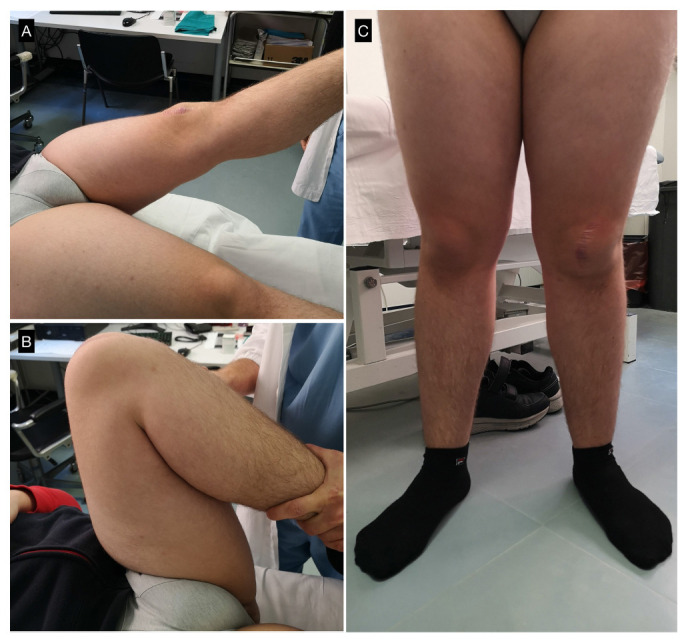
Clinical results at 18 months: a) and b) complete range of movement with painless full flexion and full extension; c) anteroposterior standing view of the lower limbs.
Systematic review
After duplicate removal, a total of 655 abstracts were found. Of these, 635 abstracts were excluded because they were either in vitro studies, animal model-based studies, odontology or maxillofacial surgery procedures. We found 20 clinical orthopaedic studies conducted on humans, including five case reports, five retrospective case series, five retrospective case-control studies and two randomized control trials (RCTs). In 19 out of the 20 studies, the patients were older than 20 years old. Only one study was conducted among a potential skeletally immature population.21
For all comparative studies (RCTs, case-control studies) treatment with Mg implants proved not to be inferior in terms of standard of care, and in one case yielded superior results.16 In all single cohort studies the authors reported good results without noteworthy complications. Only one case report revealed implant failure after Scapho-Trapezo-Trapezoideal arthrodesis, leading to revision surgery22 (Table 2).13,14,16,17,21-34
Table 2.
Main results from clinical studies included in our review
| Author | Design of study | Type of lesion | Patients | Procedure | Follow-up, mths | Results |
|---|---|---|---|---|---|---|
| Windhagen et al (2013) 23 | RCT | Hallux valgus CO | 13 vs 13 | CSF: Mg vs Ti | 6 | No SRD |
| Jungesblut et al (2020)21 | Retrospective case series | UOCD/OCF | 19 (mean age 13.7 yrs) | Fixation with Mg pins | 6 to 20 | One revision for intraarticular loose pin; 12 radiological healing |
| Turan et al (2020)24 | Case report | Radial styloid fr | 2 | CSF | 24 | Healing; no complications |
| Acar et al (2020)25 | Retrospective C-C study | MM O | 11 vs 11 | CSF: Mg vs Ti | 20.7 (sd 8.9) | No SRD; one Ti group IR |
| Atkinson et al (2019)26 | Retrospective C-C study | Hallux valgus CO | 11 vs 25 | CSF: Mg vs Ti | 19 (median) | No SRD |
| Aktan et al (2018)27 | Case report | Distal epiphysis humeral fr | 1 | CSF with Mg | 4 | Healing; no complications |
| Acar et al (2018)14 | Retrospective C-C study | Hallux valgus CO | 16 vs 15 | CSF: Mg vs Ti | 19.0 (sd 6.8) | No SRD |
| Gigante et al (2018)17 | Retrospective case series | Intercondylar eminence fr | 3 | CSF with Mg | 12 | Healing; no complications |
| Klauser et al (2019)28 | Retrospective C-C study | Hallux valgus CO | 100 vs 100 | CSF: Mg vs Ti | 3 | No SRD |
| Choo et al (2019)13 | Retrospective C-C study | Hallux valgus CO | 24 vs 69 | CSF: Mg vs Ti | 12 | No SRD |
| Acar et al (2018)29 | Case reports | Isolated LM fr | 1 | CSF with Mg | 24 | Healing; no complications |
| Kose et al (2018)30 | Retrospective, case series | MM fr | 11 | CSF with Mg | 17.3 (sd 4.1) | Healing; no complications |
| Plaass et (2018)31 | Retrospective C-C study | Hallux valgus CO | 13 vs 13 | CSF: Mg vs Ti | 36 | No SRD; one lost to FU, 11 partial FU |
| Plaass et al (2016)32 | Retrospective case series | Hallux valgus CO | 40 | CSF with Mg | 21.4 (mean) | One revision, six minor displacement |
| Biber et al (2016)33 | Case report | Capitulum humeri fr. | 1 | CSF with Mg | 12 | Healing; no complications |
| Wichelhaus et al (2016)22 | Case report | STT arthrodesis | 1 | CSF with Mg | 1.5 | Revision due to osteolysis and implant loosening |
| Zhao et al (2016)16 | RCT | ONFH | 24 vs 24 | VBG + Mg screw vs VBG without fixation | 12 | HHS > in Mg group; normal Mg, Ca, P levels |
| Yu et al (2015)34 | Retrospective case series | Femoral neck fr | 19 | CSF with Ti + iliac bone autograft fixed with Mg | 8 to 24 | One nonunion, 14 HHS > 90; three HHS > 80; one HHS < 80; no AVN |
RCT, randomized control trial; CO, chevron osteotomy; CSF, compression screw fixation; Mg, magnesium; Ti, titanium; SRD, statistically relevant difference; UOCD, undisplaced osteochondritis dissecans; OCF, osteochondral fragment; fr, fracture; C-C, case-control; MM, medial malleolar; O, osteotomy; IR, implant removal; LM, lateral malleolar; FU, follow-up; STT, scapho-trapezo-trapezoideal; ONFH, osteonecrosis of the femoral head; VBG, vascularized bone graft; HHS, Harris hip score; Ca, Calcium; P, Phosphate; AVN, avascular necrosis
Jungesblut et al,21 prospectively analyzed a cohort of 19 patients (mean age 13.7 years (sd 1.9; 11 to 17); ten female, nine male) after open or arthroscopic fixation of unstable OCD lesions or displaced osteochondral fragments with resorbable Mg pins. The aim of that study was to analyze the safety, efficacy and limitations of Mg pin-based fixation of unstable OCD lesions and displaced osteochondral fragments, analyzing clinical and radiological outcomes at a minimum of six months FU. Inclusion criteria were: 1) age < 18 years; 2) MRI-confirmed unstable OCD lesions or displaced osteochondral fragments; 3) fixation with Mg-based pins (MAGNEZIX); and 4) a minimum FU of six months. Complete radiographic healing was achieved in 12 patients, while in the others the process was still ongoing. One patient needed revision surgery due to implant failure with intraarticular migration of a pin 11 weeks after index surgery.
Discussion
The aim of the present preliminary study was to evaluate the safety and efficacy of the use of Mg-alloy based implants in orthopaedic procedures in skeletally immature patients. In addition, we conducted a systematic review of the current literature.
The first application of Mg in surgery started 187835 when it was used as a wire for a vessel anastomosis and later for orthopaedics and general surgery. Since the firsts applications and in more recent times, orthopaedic clinical use of those implants raised two main problems: the inability to control their degradation rate and the release of an important amount of gas during corrosion.10
In the context of body fluids, Mg alloys degrade with different corrosion reactions including microgalvanic and pitting corrosion. This is achieved with production of hydroxides, oxides and H2 gas species.36 Gas production has not been associated with clinically relevant consequences and has been directly related to the rate of corrosion: faster corrosion produces a greater amount of released gas. Gas analysis, performed by McBride,37 40 days after pure Mg band implantation has revealed 80% of nitrogen, 5.6% carbon dioxide, 6.5% oxygen, 7.3% hydrogen. In addition, Verbrugge38 found all the Mg corrosion product to be non-toxic and non-irritant.
On the other hand, rapid corrosion leading to implant failure before fracture healing was a concern and limited clinical application. Moreover, controlling degradation rate is of utmost importance because fast degradation produces a great amount of gas, inhibits osteogenesis and eventually destroys the near physis.39 Too slow a resorption rate may result in increased inflammatory response and fibrosis.1 Thus, a great number of different Mg alloys have been proposed and tested and studies are still ongoing.
Mg-based biodegradable materials may be divided into four groups: 1) pure Mg; 2) aluminum (Al) containing alloys; 3) Rare Earth Elements (REEs) containing alloys; 4) Al-free alloys.36 Every alloying element has a different contribution to improve mechanical properties, corrosion resistance or both. Al, Calcium (Ca), Manganese (Mn) and Lithium (Li) all improve corrosion resistance at various concentratio.40,41 Zinc (Zn), Zirconium (Zr) and Yttrium (Y) improve both corrosion resistance and mechanical properties.42,43
Castellani et al (2011) has demonstrated highly significantly greater maximum push-out force, ultimate shear strength and energy absorption to failure in a REEs-containing Mg alloy (WE43) rod implanted in Sprague-Dawley rats, with respect to standard Ti control. Moreover, tested Mg rods showed significantly higher bone-implant contact and bone volume per tissue volume. Systemic inflammatory response in tested animals was ruled out with blood sample analysis.15 Cheng et al (2016)15 have demonstrated that tendon graft fixed with a high purity Mg interference screw exhibited superior biomechanical properties and higher expression of collagen II as compared with the classic Ti screw, in a rabbit model of ACL rupture.
MgYREZr is a biodegradable, mainly made of WE43, Al-free and REEs-containing Mg alloy and is the first Mg biocompatible alloy approved for clinical use in orthopaedic surgery. While there is apparent controversy between stimulating and toxic effect of REEs on humans, recent studies have proposed a reconciliating point of view according to the hormesis phenomenon. According to this model, rare elements have got stimulating effects at low concentrations and inhibitory or toxic effects at increasing doses.36 Currently, MgYREZr is used to produce standard Herbert screws, pins and cortical bone screws with different length and diameter.
Only a few but promising studies are currently available on clinical application of these implants. The MgYREZr screw demonstrated similar efficacy in functional and radiological outcomes in modified Chevron osteotomy in hallux valgus,23 medial malleolar fracture44 and good clinical outcome in other isolated reports.29,33 All these studies, indeed, are conducted in adult populations, with the only exception being Jungesblut et al21, reporting on an average age of 13.7 years. Nonetheless, according to inclusion criteria we cannot tell if some of the patients had already reached skeletal maturity.
Our clinical department started using Mg screws in paediatric procedures after positive experiences with the treatment of intercondylar eminence fracture of the knee.17 Since 2018, MAGNEZIX screws have been used in 14 different procedures, as described in Table 1, ranging from orthopaedics to traumatology. Our series included apophyseal avulsion, epiphyseal fractures, OCD, displaced osteochondral fragment and tendon transposition using an interference screw. In all these cases Mg screws guaranteed stable fixation, without implant failure, with good clinical and radiological results. Implant corrosion was evident after two months, with minimum radiolucent space around the screws, as previously reported.21,30 None of the patients developed local pain, swelling or other clinical signs of discomfort or intolerance to the implant, thus there was no need for second surgery in any of the patients. It is worth underlining that implant removal represents nearly 35% of the annual surgical burden of our centre.
In patient number 5, six months after surgery there was evidence of a loose screw fragment beside the medial epicondyle of the elbow, perhaps corresponding to one of the screw head not completely tightening inside the cortical bone (Fig. 6). The patient was completely asymptomatic, and this was just an incidental finding on a routine control radiograph. Nonetheless, this could have been a major problem if the fragment were loose into an articular space, probably requiring surgical removal. On the other hand, the screw fragmentation confirms that the corrosion process is ongoing. Thus, we suggest that great attention be paid not to leave any part of these screws outside the cortical bone, especially in intraarticular procedures.
Fig. 6.
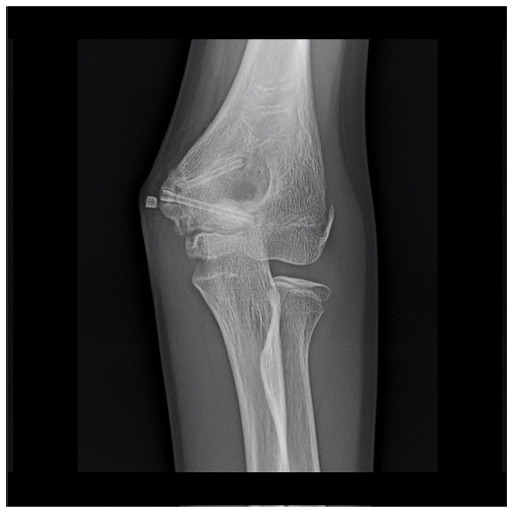
Elbow anteroposterior view of patient number 6 showing the screw fragment outside the cortical bone of the distal humerus, in the context of surrounding soft tissues. This was an incidental finding during routine follow-up and the patient was completely asymptomatic.
This manuscript presents a series of heterogeneous cases treated with the same fixation device. The short FU of some patients, the absence of a control group and the heterogeneity of cases included in the series are limitations. Nonetheless, this study is the first to present so many potential applications for this relatively new technique and clinical and radiological FU is long enough to assess healing of the lesions without implant-related complications in all patients. Moreover, the systematic review pointed out that, while in recent years evidence is growing in the adult setting, only one level IV study has been conducted among skeletally immature patients. Indeed, it is mandatory to add new evidence in this latter clinical setting, in order to transfer the promising results underlined in the present review.
In conclusion, this study confirmed that Mg resorbable screws are safe and effective for selected orthopaedic procedures in skeletally immature subjects, at least at short-term FU. No patient in our series required second surgery, or developed local or systemic signs of implant-related adverse reaction. This implant demonstrated safety towards the physis when implanted nearby. However, when inserted through the physis it displays the potential to bridge bone through it, leading to complete or partial epiphysiodesis. These would open to new potential applications in orthopaedic procedures of guided growth in developmental deformities.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding Statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical Statement
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ICMJE Conflict of Interest Statement
All authors declare they have no conflict of interest to state
Author Contributions
MB: Surgical procedures, Editing of the manuscript, Data collection, Revision of the databases..
MM: Surgical procedures, Editing of the manuscript.
VC: Surgical procedures, Editing of the manuscript.
DF: Surgical procedures, Editing of the manuscript.
ES: Data collection, Revision of the databases.
APG: Editing and revision of the manuscript.
References
- 1. Grün NG, Holweg PL, Donohue N, Klestil T, Weinberg AM. Resorbable implants in pediatric fracture treatment. Innov Surg Sci. 2018;3:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy 1998;14:726-737. [DOI] [PubMed] [Google Scholar]
- 3. Hamadouche M, Sedel L. Ceramics in orthopaedics. Bone Joint Res 2000;82:1095-1099. [DOI] [PubMed] [Google Scholar]
- 4. Castellani C, Lindtner RA, Hausbrandt P, et al. Bone-implant interface strength and osseointegration: biodegradable magnesium alloy versus standard titanium control. Acta Biomater 2011;7:432-440. [DOI] [PubMed] [Google Scholar]
- 5. Wagner FC, Polossek L, Yilmaz T, et al. Biodegradable magnesium vs. polylactide pins for radial head fracture stabilization: a biomechanical study. J Shoulder Elbow Surg 2021. 30:365-372 . [DOI] [PubMed] [Google Scholar]
- 6. Pichler K, Kraus T, Martinelli E, et al. Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. Int Orthop 2014;38:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ezechieli M, Ettinger M, König C, et al. Biomechanical characteristics of bioabsorbable magnesium-based (MgYREZr-alloy) interference screws with different threads. Knee Surg Sports Traumatol Arthrosc 2016;24:3976-3981. [DOI] [PubMed] [Google Scholar]
- 8. Kamrani S, Fleck C. Biodegradable magnesium alloys as temporary orthopaedic implants: a review. BioMetals 2019;32:185-193. [DOI] [PubMed] [Google Scholar]
- 9. Lucas GL, Cooke FW, Friis EA, et al. Stress shielding of bone. In: A Primer of Biomechanics. New York: Springer, 1999:79-88. [Google Scholar]
- 10. Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater 2010;6:1680-1692. [DOI] [PubMed] [Google Scholar]
- 11. Hung CC, Chaya A, Liu K, Verdelis K, Sfeir C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater 2019;98:246-255. [DOI] [PubMed] [Google Scholar]
- 12. Sonnow L, Könneker S, Vogt PM, Wacker F, von Falck C. Biodegradable magnesium Herbert screw - image quality and artifacts with radiography, CT and MRI. BMC Med Imaging 2017;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choo JT, Lai SHS, Tang CQY, Thevendran G. Magnesium-based bioabsorbable screw fixation for hallux valgus surgery - a suitable alternative to metallic implants. Foot Ankle Surg 2019;25:727-732. [DOI] [PubMed] [Google Scholar]
- 14. Acar B, Kose O, Turan A, Unal M, Kati YA, Guler F. Comparison of Bioabsorbable Magnesium versus Titanium Screw Fixation for Modified Distal Chevron Osteotomy in Hallux Valgus. Biomed Res Int. 2018;2018:5242806:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng P, Han P, Zhao C, et al. Magnesium inference screw supports early graft incorporation with inhibition of graft degradation in anterior cruciate ligament reconstruction. Sci Rep 2016;6:26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao D, Huang S, Lu F, et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016;81:84-92. [DOI] [PubMed] [Google Scholar]
- 17. Gigante A, Setaro N, Rotini M, Finzi SS, Marinelli M. Intercondylar eminence fracture treated by resorbable magnesium screws osteosynthesis: a case series. Injury 2018;49:S48-S53. [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hofmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huskisson EC. Measurement of pain. Lancet 1974; 2: 1127–31 [DOI] [PubMed] [Google Scholar]
- 20. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13-14. [PubMed] [Google Scholar]
- 21. Jungesblut OD, Moritz M, Spiro AS, Stuecker R, Rupprecht M. Fixation of Unstable Osteochondritis Dissecans Lesions and Displaced Osteochondral Fragments Using New Biodegradable Magnesium Pins in Adolescents Cartilage. 2020; doi: [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 22. Wichelhaus A, Emmerich J, Mittlmeier T. A Case of Implant Failure in Partial Wrist Fusion Applying Magnesium-Based Headless Bone Screws. Case Rep Orthop. 2016; doi: [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 23. Windhagen H, Radtke K, Weizbauer A, et al. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online 2013;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turan A, Kati YA, Acar B, Kose O. Magnesium bioabsorbable screw fixation of radial styloid fractures: case report. J Wrist Surg 2020;9:150-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acar B, Kose O, Unal M, et al. Comparison of magnesium versus titanium screw fixation for biplane chevron medial malleolar osteotomy in the treatment of osteochondral lesions of the talus. Eur J Orthop Surg Traumatol 2020;30:163-173. [DOI] [PubMed] [Google Scholar]
- 26. Atkinson HD, Khan S, Lashgari Y, Ziegler A. Hallux valgus correction utilising a modified short scarf osteotomy with a magnesium biodegradable or titanium compression screws - a comparative study of clinical outcomes. BMC Musculoskelet Disord 2019;20:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aktan C, Ertan MB, Turan A, Kose O. Fixation of small osteochondral fragments in a comminuted distal humerus fracture with magnesium bioabsorbable screws: a case report. Cureus 2018;10:e3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klauser H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg 2019;25:398-405. [DOI] [PubMed] [Google Scholar]
- 29. Acar B, Unal M, Turan A, Kose O. Isolated lateral malleolar fracture treated with a bioabsorbable magnesium compression screw. Cureus 2018;10:e2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kose O, Turan A, Unal M, Acar B, Guler F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: short-term clinical and radiological outcomes in eleven patients. Arch Orthop Trauma Surg 2018;138:1069-1075. [DOI] [PubMed] [Google Scholar]
- 31. Plaass C, von Falck C, Ettinger S, et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial. J Orthop Sci 2018;23:321-327. [DOI] [PubMed] [Google Scholar]
- 32. Plaass C, Ettinger S, Sonnow L, et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J Orthop Res 2016;34:2207-2214. [DOI] [PubMed] [Google Scholar]
- 33. Biber R, Pauser J, Geßlein M, Bail HJ. Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep Orthop. 2016; Vol 2016; doi:;??:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu X, Zhao D, Huang S, et al. Biodegradable magnesium screws and vascularized iliac grafting for displaced femoral neck fracture in young adults. BMC Musculoskelet Disord 2015;16:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huse EC. A new ligature. Chicago Med J 1878; 171-172. [PMC free article] [PubMed]
- 36. Agarwal S, Curtin J, Dufy B, Jaiswal S. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications Mater Sci Eng C Mater Biol Appl 2016;68:948-963. [DOI] [PubMed] [Google Scholar]
- 37. McBride ED. Magnesium screw and nail transfixion in fractures. South Med J 1938;31:508-515. [Google Scholar]
- 38. Verbrugge J. Le Matériel métallique résorbable en chirurgie osseuse. Paris, Masson; 1934.
- 39. Kraus T, Fischerauer S, Treichler S, et al. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater 2018;66:109-117. [DOI] [PubMed] [Google Scholar]
- 40. Ding Y, Wen C, Hodgson P, Li Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: a review. J Mater Chem B Mater Biol Med 2014;2:1912-1933. [DOI] [PubMed] [Google Scholar]
- 41. Liu C, Ren Z, Xu Y, Pang S, Zhao X, Zhao Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning. 2018; 13:e9216314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song GL, Atrens A. Corrosion mechanisms of magnesium alloys. Advanced engineering materials. Wiley-VCH Verlag, 1999;1:11-33 [Google Scholar]
- 43. Witte F, Hort N, Vogt C, et al. Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci 2008;12:63-72. [Google Scholar]
- 44. May H, Alper Kati Y, Gumussuyu G, et al. Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures. J Orthop Traumatol 2020;21:9;1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


