Abstract
Background
When the novel coronavirus disease 2019 (COVID-19) appeared, concerns about its course in patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) arose. This study aimed to evaluate the incidence, severity and risk factors of the more severe COVID-19 course among MS and NMOSD patients.
Methods
From March 1, 2020, to February 28, 2021, 12 MS centres, representing 70% of the Czech MS and NMOSD population, reported laboratory-confirmed COVID-19 cases via the Czech nationwide register of MS and NMOSD patients (ReMuS). The main outcome was COVID-19 severity assessed on an 8-point scale with a cut-off at 4 (radiologically confirmed pneumonia) according to the World Health Organisation´s (WHO) COVID-19 severity assessment.
Results
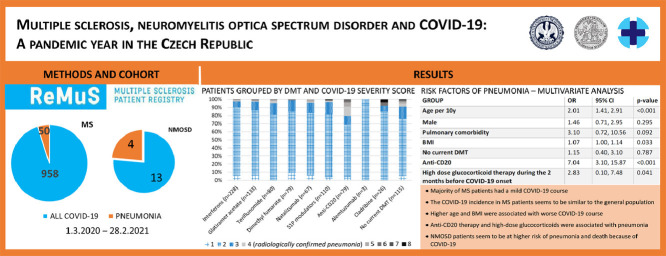
We identified 958 MS and 13 NMOSD patients, 50 MS and 4 NMOSD patients had pneumonia, 3 MS and 2 NMOSD patients died. The incidence of COVID-19 among patients with MS seems to be similar to the general Czech population. A multivariate logistic regression determined that higher body mass index (BMI [OR 1.07, 95% CI, 1.00-1.14]), older age (OR per 10 years 2.01, 95% CI, 1.41-2.91), high-dose glucocorticoid treatment during the 2 months before COVID-19 onset (OR 2.83, 95% CI, 0.10-7.48) and anti-CD20 therapy (OR 7.04, 95% CI, 3.10-15.87) were independent variables associated with pneumonia in MS patients. Increase odds of pneumonia in anti-CD20 treated MS patients compared to patients with other disease-modifying therapy (same age, sex, BMI, high-dose glucocorticoid treatment during the 2 months before COVID-19 onset, presence of pulmonary comorbidity) were confirmed by propensity score matching (OR 8.90, 95% CI, 3.04-33.24). Reports on COVID-19 infection in patients with NMOSD are scarce, however, data available up to now suggest a high risk of a more severe COVID-19 course as well as a higher mortality rate among NMOSD patients. In our cohort, 4 NMOSD patients (30.77%) had the more severe COVID-19 course and 2 patients (15.39%) died.
Conclusion
: The majority of MS patients had a mild COVID-19 course contrary to NMOSD patients, however, higher BMI and age, anti-CD20 therapy and high-dose glucocorticoid treatment during the 2 months before COVID-19 onset were associated with pneumonia. Based on this study, we have already started an early administration of anti-SARS-CoV-2 monoclonal antibodies and preferential vaccination in the risk group of patients.
Keywords: Multiple sclerosis, Neuromyelitis optica spectrum disorder, COVID-19, B-cell depleting therapies, Disease-modifying therapies
Graphical abstract
1. Introduction
When COVID-19 appeared in Wuhan in December 2019 and started to spread rapidly all around the world, concern about its course in patients with autoimmune diseases including MS and neuromyelitis optica spectrum disorder (NMOSD) arose. The effect of immunomodulatory drugs and other possible risk factors on the course of this new enemy had to be evaluated as soon as possible. The results would influence clinical decisions that would have a long-term impact due to the chronicity of the diseases and the long-lasting effects of some treatments.
Already reported data indicates that the majority of MS patients have a mild COVID-19 course. Risk factors associated with worse clinical severity seem to be similar to that of the general population (Louapre et al., 2020a; Mares and Hartung, 2020; Möhn et al., 2020, NF IMPULS [WWW Document], URL; Salter et al., 2021; Sharifian-Dorche et al., 2021; Sormani et al., 2021). However, the effect of immunomodulatory therapies on the course of COVID-19 has not been satisfactorily elucidated yet. As for NMOSD, reports on COVID-19 infection are scarce, but the available data (Alonso et al., 2021, Český statistický úřad, n.d.; Ciampi et al., 2020; Creed et al., 2020; Fan et al., 2020; Louapre et al., 2020b; Sahraian et al., 2020; Zeidan et al., 2020a) indicates a high proportion of hospitalized patients and a high mortality rate among these patients.
The aim of this study was to evaluate the incidence, severity and risk factors of the more severe course of COVID-19 among MS and NMOSD patients when Czechia is one of the countries with the highest number of COVID-19 infected people per capita. The large homogenous cohort based on the Czech nationwide registry of MS and NMOSD patients (ReMuS) may help to clarify COVID-19 characteristics among MS and NMOSD patients and help with clinical decisions about prevention, treatment and vaccination.
2. Methods
2.1. Data collection
This was a multicenter, retrospective, observational cohort study of patients with MS or NMOSD with laboratory-confirmed infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The data were collected via registry ReMuS from March 1, 2020, to February 28, 2021.
ReMuS was established by the Endowment Fund IMPULS (“NF IMPULS,” n.d.). The guarantor of expertise of this registry is the Section for Neuroimmunology and Liquorology of the Czech Neurological Society. The estimated number of MS patients in Czechia is 20,000, ReMuS represents all 15 Czech MS centres and approximately 80% of Czech MS patients. The ReMuS is based on informed consent, thus it is possible to use retrospective data for scientific and research purposes without requiring new approvals. All patients signed an informed consent form for inclusion in ReMuS.
2.2. Population of interest
Inclusion criteria for analysis were (1) MS or NMOSD, (2) a COVID-19 diagnosis based on a positive result of a SARS-CoV-2 polymerase chain reaction test (PCR) or positive antigen test or positive serological test and (3) known outcome of acute illness (return to normal activities or end of self-isolation in asymptomatic cases; or death). Data were collected by healthcare professionals retrospectively from the first contact until an outcome, taking as baseline the day of symptoms appearance or positive laboratory test (if earlier).
2.3. Variables assessed
The collected data set was based on the Global Data Sharing initiative COVID-19 core data set (Peeters et al., 2020) and the experience of MS clinicians and epidemiologists. Data on demographics, MS, immunomodulatory treatment, COVID-19 course, comorbidities and basic laboratory parameters were obtained (Tables 1 and 5). The primary endpoint was the participant´s clinical status at the most severe point of their COVID-19 course (on an 8-point ordinal scale), referred to as the COVID-19 severity score, where 1 indicated the asymptomatic patient; 2 the symptomatic patient; 3 patient with suspected pneumonia defined by both dry cough and shortness of breath; 4 patient with radiologically confirmed pneumonia (chest X-ray/CT scan); 5 need of supplemental oxygen; 6 need of non-invasive ventilation or high flow oxygen therapy (HFOT); 7 need of invasive ventilation or extracorporeal membrane oxygenation (ECMO); and 8 death.
Table 1.
Patients distribution, demographic and clinical characteristics overall and by COVID-19 course.
| No. (%)1 | |||
|---|---|---|---|
| Characteristic | Overall (n = 958)2 | Mild COVID-19 (n = 874) | More severe COVID-19 (n = 50) |
| Demographic | |||
| Age, mean (SD), y | 43.46 (10.94) | 42.88 (10.79) | 51.95 (10.81) |
| Male | 272 (28.42) | 238 (27.26) | 21 (42.00) |
| BMI, mean (SD)3 | 25.98 (5.17) | 25.84 (5.10) | 28.25 (5.38) |
| Ever smoker (former + current smoker)4 | 154 (18.44) | 143 (18.19) | 11 (23.40) |
| Pregnancy5 | 5 (0.73) | 5 (0.94) | 0 |
| Healthcare professional | 80 (8.29) | 74 (8.51) | 4 (8.00) |
| COVID-19 | |||
| COVID-19 reinfection patients | 5 (0.52) | 4 (0.46) | 1 (2.00) |
| Vaccination before infection | 3 (0.31) | 3 (0.34) | 0 |
| COVID-19 duration, mean (SD), d | 16.08 (8.55) | 15.58 (7.33) | 27.16 (17.18) |
| Confirmed by PCR | 857 (89.46) | 790 (90.39) | 47 (94.00) |
| Confirmed by antigen test | 16 (1.67) | 13 (1.49) | 3 (6.00) |
| Confirmed by serological test | 49 (5.11) | 48 (5.49) | 0 |
| COVID-19 onset in Czechia | 958 (100.00) | 874 (100.00) | 50 (100.00) |
| Hospitalisation | 43 (4.49) | 9 (1.03) | 34 (68.00) |
| Duration of hospitalisation (SD), d | 9.02 (8.57) | 9.78 (15.58) | 8.82 (5.33) |
| ICU stay | 10 (1.04) | 0 | 10 (20.00) |
| Duration of ICU stay (SD), d | 5.5 (3.91) | 0 | 5.5 (3.91) |
| COVID-19 symptoms | |||
| New/worsening neurological symptoms | 122 (13.25) | 106 (12.33) | 10 (30.30) |
| Consequent relapse treated with glucocorticoids | 26 (2.82) | 19 (2.21) | 6 (18.18) |
| Fever (>38°C) | 376 (40.52) | 327 (37.63) | 41 (82.00) |
| Dry cough | 398 (42.89) | 346 (39.77) | 44 (88.00) |
| Fatigue | 708 (76.05) | 651 (74.66) | 48 (96.00) |
| Pain (joints, bones, muscles) | 452 (49.08) | 411 (47.40) | 33 (71.74) |
| Sore throat | 178 (19.41) | 168 (19.42) | 9 (18.75) |
| Shortness of breath | 202 (21.72) | 157 (18.03) | 39 (78.00) |
| Nasal congestion | 347 (37.80) | 331 (38.44) | 13 (26.53) |
| Chills | 269 (29.43) | 231 (26.83) | 34 (69.39) |
| Loss of smell or taste | 501 (54.28) | 483 (55.52) | 15 (31.91) |
| Headache | 473 (51.36) | 437 (50.52) | 30 (61.22) |
| Gastrointestinal symptoms | 209 (22.55) | 185 (21.29) | 24 (48.98) |
| Anorexia | 97 (10.54) | 82 (9.51) | 15 (30.61) |
| Nausea | 90 (9.77) | 76 (8.81) | 14 (28.57) |
| Vomiting | 36 (3.91) | 29 (3.36) | 7 (14.29) |
| Diarrhoea | 112 (12.16) | 100 (11.59) | 12 (24.49) |
| Abdominal pain | 57 (6.20) | 50 (5.80) | 7 (14.29) |
| Tromboembolism | 4 (0.44) | 1 (0.12) | 3 (6.00) |
| Conjunctivitis6 | 16 (2.24) | 16 (2.41) | 0 |
| Skin rash7 | 15 (2.13) | 14 (2.13) | 1 (0.02) |
| Comorbidities | |||
| One or more comorbidities | 303 (31.63) | 260 (29.75) | 27 (54.00) |
| Cardiac comorbidity | 44 (4.69) | 35 (4.07) | 8 (16.00) |
| Hypertension | 112 (11.85) | 91 (10.53) | 14 (28.00) |
| Diabetes | 20 (2.12) | 16 (1.86) | 3 (6.00) |
| Chronic liver disease | 25 (2.65) | 23 (2.67) | 1 (2.00) |
| Chronic kidney disease | 3 (0.32) | 3 (0.35) | 0 |
| Chronic neurological and neuromuscular disease | 55 (5.78) | 41 (4.73) | 4 (8.00) |
| Pulmonary comorbidity | 27 (2.87) | 22 (2.55) | 5 (10.00) |
| Imunodeficiency disease | 10 (1.06) | 10 (1.16) | 0 |
| Malignancy | 17 (1.80) | 14 (1.63) | 2 (4.00) |
| Other relevant comorbidity | 94 (9.96) | 80 (9.33) | 4 (8.00) |
| Prothrombotic mutation8 | 12 (66.67) | 10 (62.50) | 2 (100.00) |
| MS | |||
| MS duration, mean (SD), y | 12.52 (11.25) | 12.19 (10.74) | 17.74 (17.55) |
| EDSS < 4 | 712 (75.42) | 662 (76.98) | 29 (58.00) |
| EDSS >= 4 | 232 (24.58) | 198 (23.02) | 21 (42.00) |
| High dose glucocorticoid during the 2 months before COVID-19 onset9 | 63 (6.98) | 53 (6.35) | 9 (19.15) |
| Other recently used immunosuppressive drug | 39 (4.14) | 35 (4.08) | 2 (4.00) |
| Last absolute white blood cell count before COVID-19 (x10^9/l) (SD)10 |
6.77 (2.23) | 6.75 (2.20) | 7.19 (2.58) |
| Last absolute lymphocyte cell count before COVID-19 (x10^9/l) (SD)11 |
2.06 (2.11) | 2.07 (2.15) | 1.20 (1.11) |
| DMT at the date of COVID-19 onset | 838 (87.66) | 766 (87.94) | 40 (80.00) |
| Interferons | 237 (24.82) | 222 (25.49) | 6 (12.00) |
| Glatiramer acetate | 135 (14.14) | 130 (14.93) | 4 (8.00) |
| Teriflunomide | 82 (8.59) | 76 (8.73) | 4 (8.00) |
| Dimethyl fumarate | 86 (9.01) | 78 (8.96) | 1 (2.00) |
| Natalizumab | 74 (7.75) | 64 (7.35) | 3 (6.00) |
| Fingolimod | 109 (11.41) | 102 (11.71) | 4 (8.00) |
| Siponimod | 1 (0.10) | 1 (0.11) | 0 |
| Ponesimod | 3 (0.31) | 3 (0.34) | 0 |
| Ocrelizumab | 76 (7.96) | 60 (6.89) | 14 (28.00) |
| Rituximab | 5 (0.52) | 3 (0.34) | 2 (4.00) |
| Alemtuzumab | 3 (0.31) | 3 (0.34) | 0 |
| Cladribine | 26 (2.72) | 24 (2.76) | 2 (4.00) |
| No DMT | 118 (12.36) | 105 (12.06) | 10 (20.00) |
| Duration of ocrelizumab/rituximab treatment (SD), y | 1.81 (2.04) | 1.56 (1.70) | 3.00 (2.80) |
| Time from last dost of ocrelizumab/rituximab (SD), d | 113.45 (64.48) | 114.86 (65.15) | 108 (61.52) |
| MS centre | |||
| Ceske Budejovice | 54 (5.64) | 52 (5.95) | 2 (4.00) |
| Hradec Kralove | 62 (6.47) | 38 (4.35) | 2 (4.00) |
| Jihlava | 30 (3.12) | 30 (3.43) | 0 |
| Olomouc | 34 (3.55) | 33 (3.78) | 1 (2.00) |
| Ostrava | 92 (9.60) | 84 (9.61) | 8 (16.00) |
| Pilzen | 73 (7.62) | 70 (8.00) | 3 (6.00) |
| Prague 10 | 25 (2.61) | 23 (2.63) | 0 |
| Prague 2 | 248 (25.89) | 225 (25.74) | 21 (42.00) |
| Prague 4 | 37 (3.86) | 34 (3.89) | 3 (6.00) |
| Prague 5 | 146 (15.24) | 140 (16.02) | 6 (12.00) |
| Teplice | 119 (12.42) | 114 (13.04) | 4 (8.00) |
| Zlin | 38 (3.97) | 31 (3.55) | 0 |
All data not available for all individuals, the percentage corresponds to the proportion of patients with known value;
34 patients have unknown COVID-19 course,
data were missing for 21.29% of patients,
data were missing for 12.84% of patients,
data were missing for 7.3% of patients,
data were missing for 25.57% of patients,
data were missing for 26.41% of patients,
data were missing for 98.12% of patients,
data were missing for 5.74% of patients,
data were missing for 30.69% of patients and patients treated with S1P modulators (fingolimod, siponimod, ponesimod) were excluded due to the mechanism of this drug´s action,
data were missing for 32.15% of patients and patients treated with S1P modulators (fingolimod, siponimod, ponesimod) were excluded due to the mechanism of this drug´s action, SD = standard deviation, BMI = body mass index, PCR = polymerase chain reaction, ICU = intensive care unit, EDSS = expanded disability status scale
Table 5.
Characteristics of patients with neuromyelitic optica spectrum disorder (NMOSD) and COVID-19
| Sex, Age, yr | NMOSD duration, yr | EDSS | Antibody status | Therapy | HDG2 | COVID-19 severity score | Comorbidities |
|---|---|---|---|---|---|---|---|
| F, 30 | 0.93 | 3.5 | Seronegative | Rituximab, prednisone 17,5 mg/day | No | 2 | Former smoker |
| M, 70 | 14.53 | 6.5 | AQP4+ | Prednisone 20 mg/day | No | 8 | Cardiovascular comorbidity, hypertension, rheumatoid arthritis |
| F, 52 | 10.08 | 2.0 | AQP4+ | Inebilizumab | No | 4 | No |
| F, 42 | 2.68 | 3.5 | Seronegative | Rituximab | No | 2 | Pulmonary comorbidity |
| M, 47 | 17.41 | 3.5 | Seronegative | Rituximab | No | 4 | Pulmonary comorbidity |
| F, 40 | 16.46 | 1.5 | AQP4+ | Prednisone 5mg/day | No | 3 | Thyreopathy |
| M, 44 | 5.67 | 1.5 | AQP4+ | Rituximab | No | 2 | Psoriasis, former smoker |
| F, 67 | 5.72 | 6.0 | AQP4+ | Rituximab | No | 8 | Hypertension, malignancy |
| F, 60 | 15.32 | 4.5 | Seronegative | No therapy | No | 2 | Pulmonary comorbidity, obesity |
| M, 45 | 5.76 | 1.5 | AQP4+ | Rituximab | No | 2 | Psoriasis |
| F, 19 | 0.41 | 1.5 | Seronegative | Azthioprine 75 mg/day, prednisone 5 mg/day | No | 2 | No |
| F, 55 | 9.57 | 3.0 | AQP4+ | Azathioprine 100 mg/day | No | 2 | No |
| M, 63 | 1.34 | 2.0 | Seronegative | No therapy | No | 2 | Malignancy |
HDG2 = high dose glucocorticoid during the previous 2 months, F = female, M = male, COVID-19 severity score: 2 = symptomatic patient, 3 = patient with suspected pneumonia defined by both dry cough and shortness of breath, 4 = patient with radiologically confirmed pneumonia (chest X-ray or CT scan), 8 = death
2.4. Statistical analyses
The whole analysis was applied to MS patients. Cohort characteristics were summarized using mean (SD) for continuous variables and frequencies (%) for categorical variables. Data were compared between patients without radiologically confirmed pneumonia (mild COVID-19; corresponding to COVID-19 severity scores 1-3) and patients with radiologically confirmed pneumonia, resp. COVID-19 severity scores of 4 or more (more severe COVID-19). Our “more severe COVID-19 course” corresponded to a “moderate”, “severe” and “very severe COVID-19 course” according to the World Health Organisation (WHO) definition (World Health Organisation, 2020). We did not use any data imputations. Comparison between groups was made using t-test and χ2 as appropriate. Two-sided P values were considered statistically significant at .05.
Univariate logistic regression models were performed on relevant identified variables to assess their association with COVID-19 outcome (mild vs more severe COVID-19). Because of the prior comparison between mild and more severe COVID-19 groups as well as prior reports of an association between B-cell depleting antibodies with an increased risk of worse COVID-19 courses (Sormani et al., 2021), we categorized disease-modifying treatment (DMT) into 3 groups: rituximab and ocrelizumab (anti-CD20), no current DMT and other DMT (reference). Subsequently, a multivariate logistic regression model was performed to determine which variables were independently associated with more severe COVID-19. Variable selection was based on univariate logistic regression. Associations were reported using odds ratios (ORs) and 95% CIs.
Additional analyses were focused on the anti-CD20. We performed 1:2 propensity score matching. Patients treated with anti-CD20 were matched with patients treated with other DMT by determined variables from a previous multivariate logistic regression model. Association with more severe COVID-19 was reported using OR and 95% CI. We also investigated the effect of the duration from the last anti-CD20 infusion and the effect of the time since therapy started.
Sensitivity analyses were run by repeating all analyses using a leave one out procedure, rerunning the analysis excluding one of the largest MS centres (Prague 2, Prague 5, Teplice) at a time. Data analyses were performed in R version 4.0.2.
Due to the small sample size of the NMOSD patient cohort, we used just descriptive statistics for them.
3. Results
3.1. Population
As of February 28, 2021, 958 MS laboratory-confirmed COVID-19 patients with known infection outcome and 13 NMOSD patients were reported. The characteristics of MS patients are shown in Table 1. Briefly, almost half of the patients were reported by MS centres in Prague (47.60%), mean age was 43.36 years (SD 10.94), most patient´s Expanded Disability Status Scale (EDSS) was lower than 4 (75.42%) and the mean MS duration was 12.52 years (SD 11.25). In a majority of patients, COVID-19 infection was confirmed by PCR (89.46%). Approximately one third of patients had one or more comorbidities. Not all data were available for all individuals, but we did not use any data imputations. We could not have classified 34 patients as mild or more severe COVID-19 because of their unknown pneumonia status.
3.2. Incidence
The estimated number of MS patients in Czechia is 20,000. At the time of this analysis, ReMuS contained information about 13,471 patients from 12 participating MS centres with at least one visit to an MS centre in the previous 12 months, thus our patient base represented almost 70% of Czech MS patients. As of February 28, 2021, 1004 laboratory-confirmed COVID-19 patients with MS were reported (ongoing COVID-19 included). That means approximately 7.45% of MS patients had COVID-19. At the same time, there were 1,240,302 laboratory-confirmed COVID-19 cases in Czechia (11.59% of the Czech population) (Komenda et al., 2021), (Český statistický úřad). The incidence of COVID-19 among patients with MS seems to be similar (or lower) to the general Czech population.
3.3. COVID-19 symptoms
The number of patients with particular COVID-19 symptoms is shown in Table 1. The most common symptom was fatigue. New or worsening neurological symptoms were reported in 122 (13.25%) patients, 26 (21.31%) of them had a consequent relapse treated with glucocorticoids. All symptoms, as well as consequent relapse, were more common among patients with more severe COVID-19, except for sore throat, nasal congestion and loss of smell or taste.
3.4. Risk factors for a more severe COVID-19
The distribution of COVID-19 severity scores according to the different DMT in MS patients is presented in Table 2 . A total of 50 patients (5.41%) had a more severe COVID-19. Supplemental oxygen was necessary for 9 patients (0.98%), non-invasive ventilation or HFOT for 12 (1.30%), invasive ventilation for one (0.11%) and 3 patients died (0.33%).
Table 2.
Patients grouped by DMT and COVID-19 severity score
| DMT/SEVERITY SCORE | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Interferons | 11 | 194 | 17 | 4 | 0 | 2 | 0 | 0 |
| Glatiramer acetate | 7 | 108 | 14 | 2 | 0 | 2 | 0 | 0 |
| Teriflunomide | 0 | 65 | 11 | 3 | 0 | 1 | 0 | 0 |
| Dimethyl fumarate | 7 | 60 | 11 | 0 | 0 | 1 | 0 | 0 |
| Natalizumab | 4 | 52 | 8 | 1 | 2 | 0 | 0 | 0 |
| S1P modulators | 4 | 86 | 16 | 2 | 0 | 1 | 0 | 1 |
| Anti-CD20 | 3 | 51 | 9 | 9 | 4 | 2 | 1 | 0 |
| Alemtuzumab | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladribine | 0 | 22 | 2 | 0 | 0 | 1 | 0 | 1 |
| No current DMT | 5 | 83 | 17 | 4 | 3 | 2 | 0 | 1 |
| Total (n = 920) | 41 | 724 | 105 | 25 | 9 | 12 | 1 | 3 |
DMT = disease modifying therapy, Anti-CD20 = ocrelizumab and rituximab, S1P modulators = fingolimod, siponimod and ponesimod, 1 = asymptomatic patient, 2 = symptomatic patient, 3 = patient with suspected pneumonia defined by both dry cough and shortness of breath, 4 = patient with radiologically confirmed pneumonia (chest X-ray or CT scan), 5 = need of supplemental oxygen, 6 = need of non-invasive ventilation or high-flow oxygen therapy, 7= need of invasive ventilation or extracorporeal membrane oxygenation, 8 = death
Risk factors for more severe COVID-19 in univariate analysis are reported in Table 3 . Variables associated with increased risk of more severe COVID-19 in the univariate logistic regression model that were considered for retention in the multivariate logistic regression model are reported in Table 4 . From the variables with significant mutual correlations, the one with the highest predictive value was always selected for the multivariate analysis. Among selected variables, older age (OR per 10 years 2.01, 95% CI, 1.41-2.91), higher BMI (OR 1.07, 95% CI, 1.00-1.14), anti-CD20 therapy (OR 7.04, 95% CI, 3.10-15.87) and high-dose glucocorticoid treatment during the 2 months before COVID-19 onset (OR 2.83, 95% CI, 0.10-7.48) were shown to be independent variables associated with a more severe COVID-19 course.
Table 3.
Risk factors of more severe COVID-19 course – univariate analysis
| GROUP | n | OR | 95% CI | p-value |
|---|---|---|---|---|
| Age per 10y | 924 | 2.16 | 1.63, 2.84 | <0.001 |
| Male | 923 | 1.93 | 1.07, 3.44 | 0.026 |
| One or more comorbidities | 924 | 2.77 | 1.56, 4.96 | <0.001 |
| Cardiac comorbidity | 910 | 4.49 | 1.84, 9.86 | <0.001 |
| Pulmonary comorbidity | 912 | 4.24 | 1.37, 10.9 | 0.005 |
| Diabetes | 912 | 3.37 | 0.77, 10.6 | 0.060 |
| Ever smoker (former/current) | 833 | 1.37 | 0.65, 2.68 | 0.37 |
| BMI | 744 | 1.08 | 1.02, 1.13 | 0.003 |
| EDSS | 910 | |||
| EDSS < 4 | — | — | ||
| EDSS >= 4 | 2.42 | 1.34, 4.32 | 0.003 | |
| DMT category | 921 | |||
| A. DMT except for anti-CD20 | — | — | ||
| B. No current DMT | 2.79 | 1.24, 5.84 | 0.009 | |
| C. Anti-CD20 | 7.44 | 3.70, 14.6 | <0.001 | |
| MS duration, y | 920 | 1.04 | 1.01, 1.07 | 0.003 |
| High dose glucocorticoid therapy during the 2 months before COVID-19 onset | 882 | 3.49 | 1.52, 7.32 | 0.002 |
OR = odds ratio, CI = confidence interval, BMI = body mass index, EDSS = expanded disability status scale, DMT = disease modifying therapy, Anti-CD20 = ocrelizumab and rituximab
Table 4.
Risk factors of more severe COVID-19 course – multivariate analysis
| GROUP | OR | 95% CI | p-value |
|---|---|---|---|
| Age per 10y | 2.01 | 1.41, 2.91 | <0.001 |
| Male | 1.46 | 0.71, 2.95 | 0.295 |
| Pulmonary comorbidity | 3.10 | 0.72, 10.56 | 0.092 |
| BMI | 1.07 | 1.00, 1.14 | 0.033 |
| No current DMT | 1.15 | 0.40, 3.10 | 0.787 |
| Anti-CD20 | 7.04 | 3.10, 15.87 | <0.001 |
| High dose glucocorticoid therapy during the 2 months before COVID-19 onset | 2.83 | 0.10, 7.48 | 0.041 |
n = 738, OR = odds ratio, CI = confidence interval, BMI = body mass index, DMT = disease modyfing drug, Anti-CD20 = ocrelizumab and rituximab
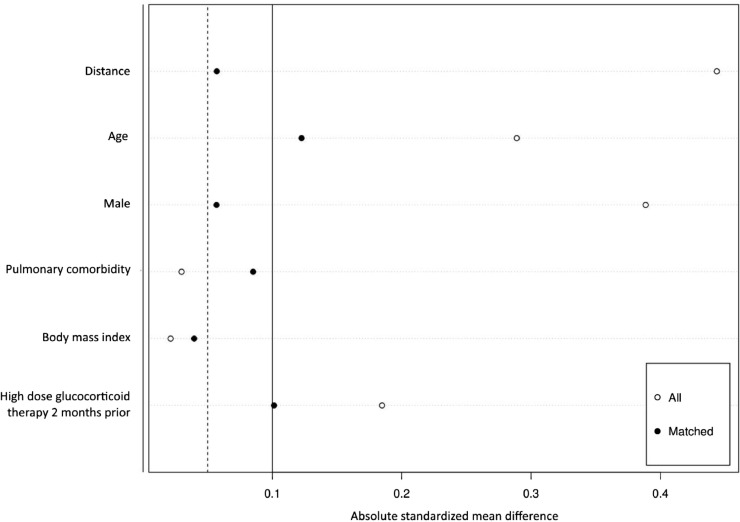
In 1:2 propensity score matching (Figure 1 ), patients treated with anti-CD20 compared to the same sex, age, presence of pulmonary comorbidity, BMI and high-dose glucocorticoid treatment during the 2 months before COVID-19 onset, patients treated with other DMT had an almost 9-fold increased odds of a more severe COVID-19 course (OR 8.90, 95% CI 3.04-33.24, p<.00001). Exploratory analyses revealed no association between COVID-19 severity and the time passed since the last anti-CD20 infusion as well as the duration of anti-CD20 treatment.
Figure 1.
Absolute standardized mean difference of covariates before and after matching
Sensitivity analyses run by repeating all the analyses using a leave one out procedure rerunning the analysis excluding one of the largest MS centres one at a time, showed risk factors that were consistent with the entire cohort.
3.5. Patients with NMOSD
Characteristics of 13 laboratory-confirmed patients with COVID-19 and NMOSD are presented in Table 5 . Although no statistical analysis can be performed because of the low number of patients in this cohort, we observed a high prevalence of more severe COVID-19 as well as a high fatality rate among these patients. Four NMOSD patients (30.77%) had the more severe COVID-19 course and 2 patients (15.39%) died.
4. Discussion
We present a large homogenous cohort of MS and NMOSD patients with COVID-19 collected via the Czech nationwide registry ReMuS. Our patient base represents almost 70% of Czech MS patients. The data collection was initiated when the first case of COVID-19 was spotted in Czechia on March 1, 2020, the majority of patients was, however, infected from October 2020 to January 2021 (88.67%). Czechia was one of the countries with the highest number of COVID-19 infected people per capita, but at that time the availability of COVID-19 laboratory tests was quite sufficient. Unlike the previous studies (Louapre et al., 2020a; Salter et al., 2021; Sormani et al., 2021) we didn´t have to include suspected COVID-19 cases. That´s the probable reason we have a higher proportion of asymptomatic patients. We also didn´t use any data imputations and all values were obtained from healthcare professionals.
As opposed to previous publications, our data analysis was based on the WHO definition (World Health Organisation, 2020) of COVID-19 severity and reflected the presence of pneumonia, not hospitalization. This can eliminate the bias derived from hospitalisation because of lack of self-sufficiency or because of preventive COVID-19 treatment (e.g., convalescent plasma). Moreover, some patients avoid hospitalisation in crowded hospitals during the pandemic, despite pneumonia or poor health. The severity of hospitalized patients also varies over time. At the beginning of the pandemic, almost every patient was isolated in a hospital. The presence of pneumonia also plays an important role in the long-term impact of COVID-19(George et al., 2020).
First, corresponding to previous publications, we confirmed that the majority of MS patients have a mild COVID-19 course. In our cohort, there was even a little lower proportion of patients with more severe COVID-19 outcome and a considerably lower mortality rate in comparison to the North American Registry of Patients with MS (COViMS) (3.3%) (Salter et al., 2021), the French MS registry (Covisep) (3.5%) (Louapre et al., 2020a), Italian cohort (Musc-19) (1.54%), review of German authors (4.00%) (Möhn et al., 2020), review of Canadian and Iranian authors (1.8%) (Sharifian-Dorche et al., 2021) and the general Czech population (1.8%) (Komenda M. et al., 2021). This could be caused by a different definition of a more severe COVID-19 outcome as well as a higher proportion of asymptomatic and minimally symptomatic patients in our cohort. The other reason is possibly low representation of high-risk older patients without DMT, with comorbidities and long MS duration, who are not followed in ReMuS (the mean age in COViMS is 47.7, in ReMuS 43.46; one and more comorbidities are present in half of the patients in COViMS compared to a third in ReMuS). We also included only laboratory-confirmed cases. In older patients with higher EDSS, there is a lower probability of undergoing a laboratory test due to their limited mobility. Some patients also may have behaved more cautiously and adhered more strictly to public health recommendations due to their chronic illness. Furthermore, the mortality rates are not consistent among publications probably because of the low numbers of deaths.
Second, we identified independent risk factors for COVID-19 in multivariate analysis. Similar to data from other MS registries and the general population, we confirmed higher age and BMI as independent risk factors of more severe COVID-19 (Goumenou et al., 2020; Louapre et al., 2020a; Möhn et al., 2020; Salter et al., 2021; Sharifian-Dorche et al., 2021; Sormani et al., 2021). In agreement with the COViMS (Salter et al., 2021), Musc-19 (Sormani et al., 2021) and with data in other autoimmune diseases (Gianfrancesco et al., 2020), we confirmed that high-dose glucocorticoid treatment during the 2 months before COVID-19 onset is associated with a worse disease course. This was not unexpected, as glucocorticoids affect the immune system, reducing responsiveness to infections.
However, the 4 largest publications (Louapre et al., 2020a; Möhn et al., 2020; Salter et al., 2021; Sormani et al., 2021) are not entirely consistent on the question of the effect of immunomodulatory therapies on the course of COVID-19, especially anti-CD20. The Covisep registry (Louapre et al., 2020a), as well as the German review of 873 patients (Möhn et al., 2020), reported no association of anti-CD20 therapies with worse COVID-19 outcomes. Its small sample size and inconsistency may have limited the ability to detect associations. On the other hand, the increased frequency of more severe COVID-19 in people treated with anti-CD20 compared to other DMT was observed in Musc-19 (Sormani et al., 2021) as well as in our study. In our cohort, 81 MS patients were treated with antiCD20 and 16 of them contracted pneumonia. We proved that assumption via multivariate logistic regression and propensity score matching. Nevertheless, none of the antiCD20 treated MS patients died.
The most common adverse events in patients treated with anti-CD20 therapy are viral respiratory infections. Anti-CD20 therapy binds to CD20 on the surface of B-cells, effectively depleting them, and interferes with antibody development. Therefore, B-cell depletion could potentially compromise antiviral immunity, including the development of SARS-CoV-2 antibodies. (Mehta et al., 2020; Syed, 2018) Serological studies following COVID-19 infection as well as COVID-19 vaccination in patients treated with anti-CD20 therapy will be crucial to determine the characteristics of the immune response to COVID-19 to help with clinical decisions about treatment and vaccination. We did not observe a link between time from last infusion of anti-CD20 or duration of anti-CD20 treatment. This can be due to the small sample size, but it is consistent with the idea that the immunological effect of anti-CD20 treatment may last longer than 6 months.
We should also mention the higher proportion of patients with consequent relapse after COVID-19 infection among those with a more severe COVID-19 course in our cohort (Table 1). Longitudinal studies are needed to evaluate the long-term consequences of COVID-19, relapse rate included.
Information on COVID-19 and pregnancy is still scarce, even more so when it comes to pregnancy in MS patients. Pregnant women do not appear to be at greater risk of COVID-19 infection. While the risk of critical care appears increased relative to the general population, there does not appear to be a significantly higher mortality rate. (Yam et al., 2020) In our cohort, 5 pregnant MS patients with COVID-19 were reported, all with a mild COVID-19 course (2 in the first trimester, 2 in the third trimester, one unknown).
To date, there are only a few published cases of COVID-19 in patients with NMOSD (Alonso et al., 2021; Ciampi et al., 2020; Creed et al., 2020; Fan et al., 2020; Louapre et al., 2020b; Sahraian et al., 2020; Zeidan et al., 2020b). From 46 confirmed or highly suspected patients with COVID-19, 20 (43.48%) had a mild infection course, 20 (43.48%) required hospitalisation and 6 (13.04%) patients died from COVID-19. A total of 5 deaths occurred in patients under treatment with rituximab, and one with prednisone. That corresponds to our findings. In our cohort, above 30% of patients had a more severe COVID-19 course and approximately 15% of patients died. Reports on COVID-19 infection in patients with NMOSD are scarce, so it is not possible to draw definite conclusions regarding the severity of the infection in these patients. Further research to combine NMSOD cases globally is needed. However, data available up to now suggest a high risk of a more severe COVID-19 course as well as a higher mortality rate among NMOSD patients.
Limitations of this analysis include low representation of children as well as older patients without DMT, with comorbidities and long MS duration, who are not followed in ReMuS. In older patients with higher EDSS, there is also less probability to undergo a laboratory test because of their limited mobility. Not all patients underwent a CT scan or chest X-ray so more severe cases could be underestimated. Otherwise, there can be an improved outcome in comparison to the general population due to greater adherence to public health recommendations because of MS diagnosis. Finally, although the multivariate analysis and propensity score matching adjusted for the effect of DMT on COVID-19 severity for the main confounding factors, we cannot exclude that some residual confounding can partly explain the observed associations.
5. Conclusion
Overall, this study confirms that a majority of MS patients have a mild COVID-19 course. Anti-CD20 therapy, high-dose glucocorticoid during the 2 months before COVID-19 onset and risk factors known in the general population such as higher BMI and age were associated with more severe COVID-19 course. These findings are in agreement with previous studies as well as knowledge about infections in MS patients. It will be important to look at longitudinal and serological studies to evaluate the long-term consequences of COVID-19 and the characteristics of the immune response in MS patients. Contrarily, it seems that NMOSD patients are at higher risk of worse COVID-19 course, even though the COVID-19 reports are scarce.
The results from this analysis are very important for clinical practice. Based on this study, we have already started an early administration of anti-SARS-CoV-2 monoclonal antibodies and preferential vaccination in the group of patients treated by anti-CD20. We continue to collect this data and plan to publish it soon.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: D.S. was supported by the Czech Ministry of Education project Progres Q27/LF1 and received financial support for conference travel from Novartis, Biogen and Bayer. D.H. was supported by the Czech Ministry of Education project Progres Q27/LF1 and received compensation for travel, speaker honoraria, and consultant fees from Biogen Idec, Novartis, Merck, Bayer, Sanofi Genzyme, Roche and Teva, as well as support for research activities from Biogen Idec. R.A. received speaker honoraria, advisory board fees, research support or conference travel support from Biogen, Merck, Sanofi, Roche and Novartis. M.P. received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Merck, Novartis, Biogen, Sanofi-Genzyme and Roche. I.S. received travel fees from Biogen, Roche, Teva and consultant fees for Novartis (advisory board). M. Valis was supported by grant projects of the Ministry of Health of the Czech Republic (FN HK 00179906) and the Charles University in Prague, Czechia (PROGRES Q40), as well as by the project PERSONMED – Centre for the Development of Personalized Medicine in Age-Related Diseases, Reg. No. CZ.02.1.01-0.0-0.0-17 048- 0007441, co-financed by the European Regional Development Fund (ERDF). The other authors have nothing to disclose. I.W. received financial support for conference travel from Merck, Biogen, Teva, Roche and Novartis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CreDIT authorship contribution statement
Dominika Stastna: Conceptualization, Data curation, Investigation, Methodology, Project administration, Analysis, Writing – original draft
Ingrid Menkyova: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing - review & editing
Jiri Drahota: Conceptualization, Analysis, Writing - review & editing
Aneta Mazouchova: Analysis, Writing - review & editing
Jana Adamkova: Investigation, Writing - review & editing
Radek Ampapa: Investigation, Writing - review & editing
Marketa Grunermelova: Investigation, Writing - review & editing
Marek Peterka: Investigation, Writing - review & editing
Eva Recmanova: Investigation, Writing - review & editing
Petra Rockova: Investigation, Writing - review & editing
Matous Rous: Investigation, Writing - review & editing
Ivana Stetkarova: Investigation, Writing - review & editing
Martin Valis: Investigation, Writing - review & editing
Marta Vachova: Investigation, Writing - review & editing
Ivana Woznicova: Investigation, Writing - review & editing
Dana Horakova: Conceptualization, Investigation, Methodology, Supervision, Writing - review & editing
Acknowledgements
The authors are very grateful to all employees of MS centres participating in the data collection. Without their hard work and dedication, this study would never have been possible. Special acknowledgements are also due to the scientific team of MS centre in Prague 2 for key ideas and advice which were essential to the data analysis and to Tomas Fogl for language editing.
References
- Alonso R., Silva B., Garcea O., Diaz P.E.C., dos Passos G.R., Navarro D.A.R., Valle L.A.G., Salinas L.C.R., Negrotto L., Luetic G., Tkachuk V.A., Míguez J., de Bedoya F.H.D., Goiry L.G., Sánchez N.E.R., Burgos M., Steinberg J., Balbuena M.E., Alvarez P.M., López P.A., Ysrraelit M.C., León R.A., Cohen A.B., Gracia F., Molina O., Casas M., Deri N.H., Pappolla A., Patrucco L., Cristiano E., Tavolini D., Nadur D., Granda A.M.T., Weiser R., Cassará F.P., Sinay V., Rodríguez C.C., Lazaro L.G., Menichini M.L., Piedrabuena R., Escobar G.O., Carrá A., Chertcoff A., Pujols B.S., Vrech C., Tarulla A., Carvajal R., Mainella C., Becker J., Peeters L.M., Walton C., Serena M.A., Nuñez S., Rojas J.I. COVID-19 in multiple sclerosis and neuromyelitis optica spectrum disorder patients in Latin America. Mult. Scler. Rel. Disord. 2021;51 doi: 10.1016/j.msard.2021.102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Český statistický úřad, n.d.Obyvatelstvo | ČSÚ [WWW Document]. URL https://www.czso.cz/csu/czso/obyvatelstvo_lide (accessed 4.17.21).
- Ciampi E., Uribe-San-Martín R., Soler B., Fernández R., García P., Navarrete-Asenjo C., Miguel Tirapegui J., Torres R., Polanco J., Suárez F., José Cuello M., Cárcamo C. COVID-19 in MS and NMOSD: A multicentric online national survey in Chile. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M.A., Ballesteros E., Jr L.J.G., Imitola J. Mild COVID-19 infection despite chronic B cell depletion in a patient with aquaporin-4-positive neuromyelitis optica spectrum disorder. Mult. Scler. Rel. Disord. 2020;44 doi: 10.1016/j.msard.2020.102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M., Qiu W., Bu B., Xu Y., Yang H., Huang D., Lau A.Y., Guo J., Zhang M.N., Zhang X., Yang C.S., Chen J., Zheng P., Liu Q., Zhang C., Shi F.D. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:787. doi: 10.1212/NXI.0000000000000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., Gibbons M.A., Hart N., Jenkins R.G., McAuley D.F., Patel B.v., Thwaite E., Spencer L.G. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- Gianfrancesco M., Hyrich K.L., Hyrich K.L., Al-Adely S., Al-Adely S., Carmona L., Danila M.I., Gossec L., Gossec L., Izadi Z., Jacobsohn L., Katz P., Lawson-Tovey S., Lawson-Tovey S., Mateus E.F., Rush S., Schmajuk G., Simard J., Strangfeld A., Trupin L., Wysham K.D., Bhana S., Costello W., Grainger R., Hausmann J.S., Hausmann J.S., Liew J.W., Sirotich E., Sirotich E., Sufka P., Wallace Z.S., Wallace Z.S., Yazdany J., MacHado P.M., MacHado P.M., MacHado P.M., Robinson P.C., Robinson P.C. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea A.O., Petrakis D., Tsoukalas D., Kostoff R., Rakitskii V., Spandidos D.A., Aschner M., Calina D. Covid 19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020 doi: 10.3892/mmr.2020.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenda M., Karolyi M., Bulhart V., Žofka J., Brauner T., Hak J., Jarkovský J., Mužík J., Blaha M., Kubát J., Klimeš D., Langhammer P., Daňková Š., Májek O., Bartůňková M., Dušek L. COVID 19: Přehled aktuální situace v ČR [WWW Document]. Onemocnění aktuálně [online] 2021. https://onemocneni-aktualne.mzcr.cz/covid-19 URL. (accessed 4.17.21) [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.-A., Vukusic S., Lubetzki C., de Sèze J. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020;77 doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Maillart E., Papeix C., Zeidan S., Biotti D., Lepine Z., Wahab A., Zedet M., Labauge P., Tilikete C., Pique J., Tourbah A., Mathey G., Dimitri Boulos D., Branger P., Kremer L.D., Marignier R., Collongues N., de Seze J. Outcomes of coronavirus disease 2019 in patients with neuromyelitis optica and associated disorders. Eur. J. Neurol. 2020;14612 doi: 10.1111/ene.14612. [DOI] [PubMed] [Google Scholar]
- Mares J., Hartung H.-P. Multiple sclerosis and COVID-19 164. 2020. pp. 217–225. [DOI] [PubMed] [Google Scholar]
- Mehta P., Porter J.C., Chambers R.C., Isenberg D.A., Reddy V. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N., Konen F.F., Pul R., Kleinschnitz C., Prüss H., Witte T., Stangel M., Skripuletz T. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. J. Clin. Med. 2020;9:4067. doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NF IMPULS [WWW Document], URLhttp://www.multiplesclerosis.cz/. (accessed 4.18.21).
- Peeters L.M., Parciak T., Walton C., Geys L., Moreau Y., de Brouwer E., Raimondi D., Pirmani A., Kalincik T., Edan G., Simpson-Yap S., de Raedt L., Dauxais Y., Gautrais C., Rodrigues P.R., McKenna L., Lazovski N., Hillert J., Forsberg L., Spelman T., McBurney R., Schmidt H., Bergmann A., Braune S., Stahmann A., Middleton R., Salter A., Bebo B.F., Rojas J.I., van der Walt A., Butzkueven H., van der Mei I., Ivanov R., Hellwig K., Sciascia do Olival G., Cohen J.A., van Hecke W., Dobson R., Magyari M., Brum D.G., Alonso R., Nicholas R., Bauer J., Chertcoff A., de Sèze J., Louapre C., Comi G., Rijke N. COVID-19 in people with multiple sclerosis: a global data sharing initiative. Mult. Scler. J. 2020;26:1157–1162. doi: 10.1177/1352458520941485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraian M.A., Azimi A., Navardi S., Rezaeimanesh N., Naser Moghadasi A. Evaluation of COVID-19 infection in patients with Neuromyelitis optica spectrum disorder (NMOSD): A report from Iran. Mult. Scler. Rel. Disord. 2020 doi: 10.1016/j.msard.2020.102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., Sahraian M.A., Fadda G., Osherov M., Sharifian-Dorche A., Karaminia M., Saveriano A.W., la Piana R., Antel J.P., Giacomini P.S. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: a systematic review. Mult. Scler. Rel. Disord. 2021 doi: 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani, Maria P., de Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia, Mario A., Patti F., Salvetti M., Nozzolillo A., Bellacosa A., Protti A., di Sapio A., Signori A., Petrone A., Bisecco A., Iovino A., Dutto A., Repice A.M., Conte A., Bertolotto A., Bosco A., Gallo A., Zito A., Sartori A., Giometto B., Tortorella C., Antozzi C., Pozzilli C., Mancinelli C.R., Zanetta C., Cordano C., Cordioli C., Scandellari C., Guaschino C., Gasperini C., Solaro C., Fioretti C., Bezzini D., Marastoni D., Paolicelli D., Vecchio D., Landi D., Bucciantini E., Pedrazzoli E., Signoriello E., Sbragia E., Susani E.L., Curti E., Milano E., Marinelli F., Camilli F., Boneschi F.M., Govone F., Bovis F., Calabria F., Caleri F., Rinaldi F., Vitetta F., Corea F., Crescenzo F., Patti F., Teatini F., Tabiadon G., Granella F., Boffa G., Lus G., Brichetto G., Comi G., Tedeschi G., Maniscalco G.T., Borriello G., de Luca G., Konrad G., Vaula G., Marfia G.A., Mallucci G., Liberatore G., Salemi G., Miele G., Sibilia G., Pesci I., Schiavetti I., Brambilla L., Lopiano L., Sinisi L., Pasquali L., Saraceno L., Carmisciano L., Chiveri L., Mancinelli L., Moiola L., Grimaldi L.M.E., Caniatti L.M., Capobianco M., Cava M.della, Onofrj M., Rovaris M., Salvetti M., Vercellino M., Bragadin M.M., Buccafusca M., Buscarinu M.C., Celani M.G., Grasso M.G., Stromillo M.L., Petracca M., Amato M.P., Sormani Maria Pia, L’Episcopo M.R., Sessa M., Ferrò M.T., Trojano M., Ercolani M.V., Bianco M., Re M.lo, Vianello M., Clerico M., Battaglia, Mario Alberto, Napoli M., Ponzano M., Radaelli M., Conti M.Z., Calabrese M., Mirabella M., Filippi M., Inglese M., Lucchini M., Pozzato M., Danni M.C., Zaffaroni M., Zampolini M., Ponzio M., de Riz M., de Rossi N., de Stefano N., Cavalla P., de Mitri P., Grossi P., Zaratin P., Confalonieri P., Gallo P., Immovilli P., Ragonese P., Sola P., Annovazzi P., Iaffaldano P., Nardone R., Cerqua R., Clerici R., Lanzillo R., Motta R., Balgera R., Bergamaschi R., Totaro R., Iodice R., Capra R., Marangoni S., Realmuto S., Cottone S., Montepietra S., Rasia S., Arena S., Bucello S., Banfi S., Bonavita S., Malucchi S., Tonietti S., Vollaro S., Cordera S., Aguglia U., Clerici V.T., Barcella V., Bergamaschi V., Morra V.B., Dattola V., Mantero V. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89 doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Y.Y. Ocrelizumab: a review in multiple sclerosis. CNS Drugs. 2018 doi: 10.1007/s40263-018-0568-7. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . Clinical Management of COVID-19. 2020. https://apps.who.int/iris/rest/bitstreams/1328457/retrieve [WWW Document], URL. (accessed 4.18.21) [Google Scholar]
- Yam C., Jokubaitis V., Hellwig K., Dobson R. MS, pregnancy and COVID-19. Mult. Scler. J. 2020 doi: 10.1177/1352458520949152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan S., Maillart E., Louapre C., Roux T., Lubetzki C., Papeix C. COVID-19 infection in NMO/SD patients: a French survey. J. Neurol. 2020 doi: 10.1007/s00415-020-10112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.