Abstract
This cohort study examines cutaneous reactions in hospital employees who received messenger RNA COVID-19 vaccination.
Mucocutaneous reactions, such as pruritus, urticaria, and angioedema, may occur after COVID-19 messenger RNA (mRNA) vaccination. To our knowledge, the incidence of these reactions and recurrence with subsequent vaccination has not been described. Cutaneous reactions may contribute to unnecessary avoidance of future vaccination doses.
Methods
We prospectively studied Mass General Brigham employees who received an mRNA COVID-19 vaccine (first dose: December 16, 2020, to January 20, 2021; follow-up through February 26, 2021; eMethods in the Supplement). Institutional review board approval was provided by the Mass General Brigham human research committee with a waiver of informed consent. For 3 days after vaccination, employees completed daily symptom surveys through a multipronged approach, including email, text message, phone, and smartphone application links. Cutaneous reactions included rash or itching (other than the injection site), hives, and/or swelling of the lips, tongue, eyes, or face (eAppendix in the Supplement).
We calculated the number and frequency of self-reported cutaneous reactions with 95% confidence intervals using symptom survey respondents by dose as the denominator. We compared frequencies using χ2 tests. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute), and statistical significance was set at P < .05.
Results
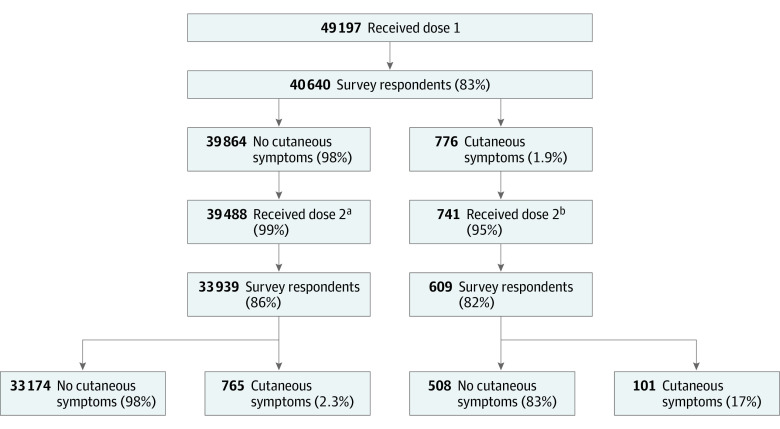
Of 49 197 employees who received the first dose of a COVID-19 vaccine, 12 464 (25%) received the Pfizer-BioNtech vaccine and 36 733 (75%) received the Moderna vaccine. At least 1 symptom survey was completed by 40 640 (83%) after the first dose of the vaccine.
Cutaneous reactions were reported by 776 respondents after dose 1 (1.9%; 95% CI, 1.8%-2.1%; Table and Figure). Rash and itching (other than at the injection site) was the most common cutaneous reaction, which was reported by 559 (1%; 95% CI, 1.8%-2.1%). The mean (SD) age of those reporting cutaneous reactions was 41 (14) years. Cutaneous reactions were more common in women (656 [85%]) than men (120 [15%]; P < .001) and differed by race (62% White individuals, 7% Black individuals, and 12% Asian individuals; P < .001]). More than one-third of employees who reported cutaneous reactions were physicians or nurses (285 [37%]).
Table. Self-reported Cutaneous Reactions After mRNA COVID-19 Vaccination.
| Reaction | No. (%) [95% CI] | ||
|---|---|---|---|
| Both mRNA vaccines | Pfizer | Moderna | |
| Dose 1 reaction | |||
| No. | 40 640 | 10 445 | 30 195 |
| Cutaneous reactiona | 776 (1.9) [1.78-2.05] | 150 (1.4) [1.22-1.68] | 626 (2.1) [1.92-2.24] |
| Itching or rashb | 599 (1.5) [1.36-1.60] | 128 (1.2) [1.02-1.46] | 471 (1.6) [1.42-1.71] |
| Hives/urticaria | 162 (0.4) [0.34-0.46] | 25 (0.2) [0.15-0.35] | 137 (0.5) [0.38-0.54] |
| Swelling/angioedema | 120 (0.3) [0.24-0.35] | 16 (0.2) [0.09-0.25] | 104 (0.3) [0.28-0.42] |
| Recurrent dose 2 reaction | |||
| No. | 609 | 124 | 485 |
| Cutaneous reactiona | 101 (17) [13.7-19.8] | 20 (16) [10.14-23.81] | 81 (17) [13.49-20.32] |
| Itching or rashb | 81 (13) [10.7-16.3] | 17 (14) [8.19-21.04] | 64 (13) [10.31-16.54] |
| Hives/urticaria | 20 (3.3) [2.02-5.03] | 3 (2.4) [0.50-6.91] | 17 (3.5) [2.05-5.55] |
| Swelling/angioedema | 16 (2.6) [1.51-4.23] | 3 (2.4) [0.50-6.91] | 13 (2.7) [1.43-4.54] |
| New dose 2 reaction | |||
| No. | 33 939 | 9055 | 24 884 |
| Cutaneous reactiona | 765 (2.3) [2.10-2.42] | 128 (1.4) [1.18-1.68] | 637 (2.6) [2.37-2.76] |
| Itching or rashb | 546 (1.6) [1.48-1.75] | 100 (1.1) [0.90-1.34] | 446 (1.8) [1.63-1.96] |
| Hives/urticaria | 194 (0.6) [0.49-0.66] | 27 (0.3) [0.20-0.43] | 167 (0.7) [0.57-0.78] |
| Swelling/angioedema | 125 (0.4) [0.31-0.44] | 15 (0.2) [0.09-0.27] | 110 (0.4) [0.36-0.53] |
Abbreviation: mRNA, messenger RNA.
Numbers do not sum to the total, as individual employees can report multiple cutaneous reactions.
Other than at injection site.
Figure. Self-reported Cutaneous Reactions After Messenger RNA COVID-19 Vaccination.
aA total of 117 employees scheduled for dose 2 and 259 not scheduled/unknown.
bA total of 5 employees scheduled for dose 2 and 30 not scheduled/unknown.
Of those with self-reported cutaneous reaction to the first dose, 741 (95%) received their second dose. Among the 609 who completed a symptom survey after the second dose, 508 (83%) reported no recurrent cutaneous reactions.
Among those with no cutaneous reaction to the first dose, 765 (2.3%) reported cutaneous reactions after the second dose. Rash and itching (other than the injection site) was the most common (546 [1.6%]; 95% CI, 1.5%-1.8%).
Discussion
In this prospective cohort of almost 50 000 health care employees, 1.9% self-reported cutaneous reactions after receiving the first dose of an mRNA COVID-19 vaccine. With more than 600 employees with first-dose cutaneous reactions included, 83% did not have recurrent cutaneous reactions. An additional 2.3% reported cutaneous reactions only after the second dose of the vaccine.
Cutaneous reactions were more commonly reported among women, which was similar to reported local injection site reactions and anaphylaxis after mRNA COVID vaccinations.1,2 To our knowledge, the underlying cause for this difference is not yet known, although drug allergies are more common in women.3
The limitations of this study include the use of self-reported data. However, many employees were clinicians; thus, the data may be more reliable. Self-reported reactions may contribute to vaccine hesitancy. This study captures cutaneous reactions that occurred within 3 days after vaccination and thus cannot assess for delayed cutaneous reactions.2
Cutaneous reactions, including itching, rash, hives and swelling, occurred in more than 4% of those who received the 2-dose mRNA COVID-19 vaccines. The spectrum of reported cutaneous reactions after mRNA vaccination include large local reactions, urticaria, and morbilliform reactions.2,4 Unlike anaphylaxis, cutaneous reactions alone are not a contraindication to revaccination.5 In this cohort, most individuals received the second dose without recurrent reactions. Referral to an allergist or dermatologist is not necessary for most reactions but should be considered for patients who experience immediate or severe reactions.6 These data are reassuring for the millions of Americans who may develop cutaneous reactions after vaccination in the coming year.
eMethods.
eAppendix. Employee Symptom Survey–Allergy Question
eReferences
References
- 1.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101-1102. doi: 10.1001/jama.2021.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273-1277. doi: 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal KG, Li Y, Acker WW, et al. Multiple drug intolerance syndrome and multiple drug allergy syndrome: Epidemiology and associations with anxiety and depression. Allergy. 2018;73(10):2012-2023. doi: 10.1111/all.13440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol.S0190-9622(21)00658-7. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25-43. doi: 10.1016/j.jaci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention . What to do if you have an allergic reaction after getting a COVID-19 vaccine. Accessed March 5, 2021. https://www.cdc.gov/coronavirus/2019ncov/vaccines/safety/allergic-reaction.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eAppendix. Employee Symptom Survey–Allergy Question
eReferences