Key Points
Question
What is the prevalence of obstructive epicardial coronary artery disease and coronary microvascular dysfunction in hospitalized patients with heart failure with preserved ejection fraction?
Findings
In a cohort study, 106 consecutive participants with preserved ejection fraction were evaluated with coronary angiography, invasive coronary physiologic and vasoreactivity testing, and cardiac magnetic resonance imaging. A total of 51% of the study participants had obstructive epicardial coronary artery disease, 66% had endothelium-independent coronary microvascular dysfunction, and 24% had endothelium-dependent coronary microvascular dysfunction.
Meaning
The findings of this study suggest that obstructive epicardial coronary artery disease and coronary microvascular dysfunction are common and often unrecognized in hospitalized patients with heart failure with preserved ejection fraction and may be therapeutic targets.
Abstract
Importance
Coronary artery disease (CAD) and coronary microvascular dysfunction (CMD) may contribute to the pathophysiologic characteristics of heart failure with preserved ejection fraction (HFpEF). However, the prevalence of CAD and CMD have not been systematically studied.
Objective
To examine the prevalence of CAD and CMD in hospitalized patients with HFpEF.
Design, Setting, and Participants
A total of 106 consecutive patients hospitalized with HFpEF were evaluated in this prospective, multicenter, cohort study conducted between January 2, 2017, and August 1, 2018; data analysis was performed from March 4 to September 6, 2019. Participants underwent coronary angiography with guidewire-based assessment of coronary flow reserve, index of microvascular resistance, and fractional flow reserve, followed by coronary vasoreactivity testing. Cardiac magnetic resonance imaging was performed with late gadolinium enhancement and assessment of extracellular volume. Myocardial perfusion was assessed qualitatively and semiquantitatively using the myocardial-perfusion reserve index.
Main Outcomes and Measures
The prevalence of obstructive epicardial CAD, CMD, and myocardial ischemia, infarction, and fibrosis.
Results
Of 106 participants enrolled (53 [50%] women; mean [SD] age, 72 [9] years), 75 had coronary angiography, 62 had assessment of coronary microvascular function, 41 underwent coronary vasoreactivity testing, and 52 received cardiac magnetic resonance imaging. Obstructive epicardial CAD was present in 38 of 75 participants (51%, 95% CI, 39%-62%); 19 of 38 (50%; 95% CI, 34%-66%) had no history of CAD. Endothelium-independent CMD (ie, coronary flow reserve <2.0 and/or index of microvascular resistance ≥25) was identified in 41 of 62 participants (66%; 95% CI, 53%-77%). Endothelium-dependent CMD (ie, abnormal coronary vasoreactivity) was identified in 10 of 41 participants (24%; 95% CI, 13%-40%). Overall, 45 of 53 participants (85%; 95% CI, 72%-92%) had evidence of CMD and 29 of 36 (81%; 95% CI, 64%-91%) of those without obstructive epicardial CAD had CMD. Cardiac magnetic resonance imaging findings included myocardial-perfusion reserve index less than or equal to 1.84 (ie, impaired global myocardial perfusion) in 29 of 41 patients (71%; 95% CI, 54%-83%), visual perfusion defect in 14 of 46 patients (30%; 95% CI, 19%-46%), ischemic late gadolinium enhancement (ie, myocardial infarction) in 14 of 52 patients (27%; 95% CI, 16%-41%), and extracellular volume greater than 30% (ie, diffuse myocardial fibrosis) in 20 of 48 patients (42%; 95% CI, 28%-56%). Patients with obstructive CAD had more adverse events during follow-up (28 [74%]) than those without obstructive CAD (17 [46%]).
Conclusions and Relevance
In this cohort study, 91% of patients with HFpEF had evidence of epicardial CAD, CMD, or both. Of those without obstructive CAD, 81% had CMD. Obstructive epicardial CAD and CMD appear to be common and often unrecognized in hospitalized patients with HFpEF and may be therapeutic targets.
This cohort study examines the prevalence of epicardial coronary artery disease and coronary microvascular dysfunction in hospitalized patients with heart failure with preserved ejection fraction.
Introduction
Myocardial ischemia due to epicardial coronary artery disease (CAD), coronary microvascular dysfunction (CMD), or both, may represent a disease mechanism and therapeutic target in some patients with heart failure with preserved ejection fraction (HFpEF).1,2,3
Myocardial ischemia can cause left-ventricular (LV) diastolic and systolic dysfunction, both of which are common in HFpEF.4,5 Inflammation-associated CMD may also play a role in the pathophysiologic characteristics of HFpEF,6 which is a possibility supported by noninvasive studies, an autopsy series, and small invasive studies.3,7,8,9
However, to our knowledge, the prevalence of epicardial CAD, CMD, and coronary endothelial dysfunction have not been systematically studied in patients with HFpEF. We performed comprehensive invasive and noninvasive assessments of epicardial and microvascular function to evaluate the prevalence of CAD, CMD, and coronary endothelial dysfunction in prospectively recruited hospitalized patients with HFpEF.
Methods
Consecutive patients hospitalized with HFpEF were evaluated in this prospective, multicenter, cohort study conducted between January 2, 2017, and August 1, 2018; data analysis was performed from March 4 to September 6, 2019. Patients with HFpEF were prospectively recruited from 3 centers. The inclusion and exclusion criteria are described in the eMethods in the Supplement. The major exclusion criteria included an estimated glomerular filtration rate less than 30 mL/min/1.73 m2 (to allow safe administration of contrast agents during investigations) and severe frailty (ie, Clinical Frailty Scale score >6),10 because invasive coronary angiography was considered high-risk and clinically inappropriate in patients with these limitations.
Study procedures included invasive coronary angiography with guidewire-based physiologic assessment and vasoreactivity testing. In the absence of contraindications, patients also underwent multiparametric cardiac magnetic resonance imaging (CMRI). All participants provided written informed consent and the study was approved by the West of Scotland Research Ethics Committee; participants did not receive financial compensation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Testing
Invasive coronary assessment was performed by experienced operators, and computer-assisted quantitative coronary angiography analysis was performed using QAngio XA 7.3 (Medis). Coronary guidewire assessment was performed on a single major epicardial coronary artery. The left anterior descending artery was the preferred vessel, although if use of this artery was technically impossible, the left circumflex or right coronary artery was used instead; details of measurement of fractional flow reserve, coronary flow reserve (CFR), and index of microcirculatory resistance (IMR) are given in the eMethods in the Supplement and shown in Figure 1. Obstructive epicardial CAD was defined as greater than 70% stenosis of a major epicardial coronary artery (≥50% stenosis if the left main coronary artery was affected) or a 50% to 70% stenosis with a fractional flow reserve value less than or equal to 0.80.11 In patients with obstructive epicardial stenosis, CFR and IMR were measured in another (nonobstructed) coronary artery to ensure accurate assessment of microvascular function. Coronary flow reserve represents the coronary vasodilator capacity (epicardial and microvascular) and was calculated using thermodilution as the resting mean transit time divided by the hyperemic mean transit time.12 The IMR reflects the minimal resistance offered by the coronary microvasculature and was calculated as the product of the mean distal coronary artery pressure and the mean transit time measured simultaneously during hyperemia.13 Endothelium-independent CMD was defined as a CFR less than 2.0 and/or an IMR greater than or equal to 25.12,13
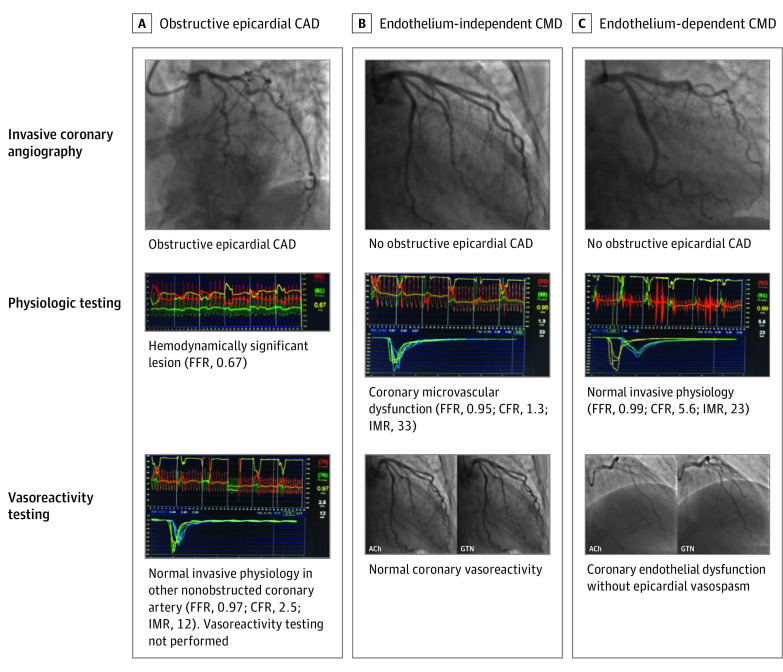
Figure 1. Overview of Clinical Diagnoses Based on Invasive Coronary Assessment Findings.
Three cases investigated with invasive coronary angiography, physiologic testing, and/or vasoreactivity testing. A, A case of obstructive epicardial coronary artery disease (CAD) with a flow-limiting lesion identified on fractional flow reserve (FFR) testing. B, A case of endothelium-independent coronary microvascular dysfunction (CMD) without obstructive epicardial CAD, with an abnormal coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) but normal coronary vasoreactivity. C, A case of endothelium-dependent CMD with no obstructive epicardial CAD and normal CFR and IMR, but abnormal coronary vasoreactivity (without epicardial vasospasm). ACh indicates acetylcholine; GTN, glyceryl trinitrate.
Coronary vasomotor function was assessed using intracoronary infusions of acetylcholine (eMethods in the Supplement; Figure 1). Coronary vasoreactivity testing was not performed in most patients with obstructive epicardial disease owing to the risk of acute myocardial ischemia from the combination of obstructive CAD and coronary spasm.14 Microvascular coronary vasospasm, reflecting endothelium-dependent CMD and vascular smooth muscle dysfunction, was defined as 20% to 90% luminal constriction and/or ischemic electrocardiographic changes in response to intracoronary acetylcholine infusions.15,16 Epicardial coronary vasospasm was defined as greater than 90% luminal constriction and ischemic electrocardiographic changes in response to intracoronary acetylcholine infusions.17 An overview of the clinical diagnoses based on the invasive coronary assessment findings is presented in Figure 1.
If no contraindications were noted, CMRI was performed with gadolinium contrast, T1 mapping, and adenosine stress perfusion imaging (MAGNETOM Prisma 3.0 T; Siemens Healthcare). The details of the CMRI protocols are given in the eMethods in the Supplement. Perfusion imaging was analyzed using QMass 8.1 (Medis), and the myocardial-perfusion reserve index (MPRI) was calculated.18,19 Impaired global myocardial perfusion was defined as an MPRI less than or equal to 1.84.20 Cardiac magnetic resonance imaging–proven myocardial infarction was defined as subendocardial or transmural late gadolinium enhancement (LGE) in the distribution of a coronary artery territory, and an extracellular volume greater than 30% was considered to represent diffuse myocardial fibrosis.21
Patients were followed up for a minimum of 12 months, using electronic medical record linkage, to document readmissions, death, and the causes of readmissions and deaths. We assessed the following composite end points: all-cause death or hospitalization for any reason, all-cause death or hospitalization for a cardiovascular cause, all-cause death or hospitalization for HF, and all-cause death or hospitalization for a non-CV cause.
Statistical Analysis
We calculated the prevalence (95% CI) of obstructive epicardial CAD, endothelium-independent and -dependent CMD, and CMRI evidence of impaired myocardial perfusion, myocardial infarction, and diffuse myocardial fibrosis in the study participants. The participants were then divided into those with and those without each condition and the clinical characteristics and investigation results were compared. We used the t test or nonparametric equivalent, when indicated, to compare continuous variables and the χ2 test or nonparametric equivalent, when indicated, to compare categorical variables. Time-to-event analysis was analyzed using the Kaplan-Meier method. With 2-sided, unpaired testing the significance threshold was P < .05. All statistical analyses were performed using Stata, version 14.2 (StataCorp LLC).
Results
Between January 2, 2017, and August 1, 2018, 2285 consecutive patients hospitalized with suspected HF were screened, of whom 628 had a diagnosis of HFpEF (eFigure 1 in the Supplement). Of these, 106 patients (17%) met the inclusion criteria and agreed to participate in the study. The most common reasons for exclusion were severe frailty (196 [38%]), severe kidney impairment (104 [20%]), and lack of capacity to consent (88 [17%]). Twenty-three enrolled patients (22%) did not undergo invasive angiography or CMRI, predominantly owing to a deterioration in clinical status. Seventy-five participants (71%) underwent invasive angiography and 52 individuals (49%) underwent CMRI. Sixty-two participants who underwent angiography (58% of all participants; 83% of those undergoing angiography) had coronary physiologic function testing and 41 (39% of all participants; 55% of those undergoing angiography) had vasoreactivity testing. Of the 52 participants undergoing CMRI, 44 (42% of all participants; 85% of those having CMRI) had both invasive coronary angiography and CMRI. The median time from presentation to CMRI was 17 (interquartile range, 10-31) days and to invasive coronary assessment was 87 (interquartile range, 56-98) days. The mean (SD) age of the participants was 72 (9) years, 53 (50%) were women, and 53 (50%) were men. On admission, 56% patients were in New York Heart Association functional class III and 42% were in class IV. Most participants had mild frailty (mean [SD] CFR, 3.4 [1.2]).10
Obstructive CAD
Obstructive epicardial CAD was present in 38 of 75 participants (51%; 95% CI, 39%-62%) who underwent angiography (Figure 2; eFigure 2 in the Supplement); 20 participants (53%) had 1-vessel, 13 (34%) had 2-vessel, and 5 (13%) had 3-vessel disease. Nineteen of 38 participants (50%; 95% CI, 34%-66%) had no history of CAD. Those with obstructive CAD were more frequently male and more likely to have a history of CAD, myocardial infarction, coronary revascularization, and chronic kidney disease than those without obstructive disease (Table 1). Patients with obstructive CAD had higher estimated LV filling pressures on echocardiography and were less likely to have mild to moderate valve disease than those without obstructive epicardial disease.
Figure 2. Prevalence of Coronary Artery Disease (CAD), Coronary Microvascular Dysfunction (CMD), and Imaging Evidence of Impaired Myocardial Perfusion, Myocardial Infarction (MI), and Diffuse Myocardial Fibrosis.
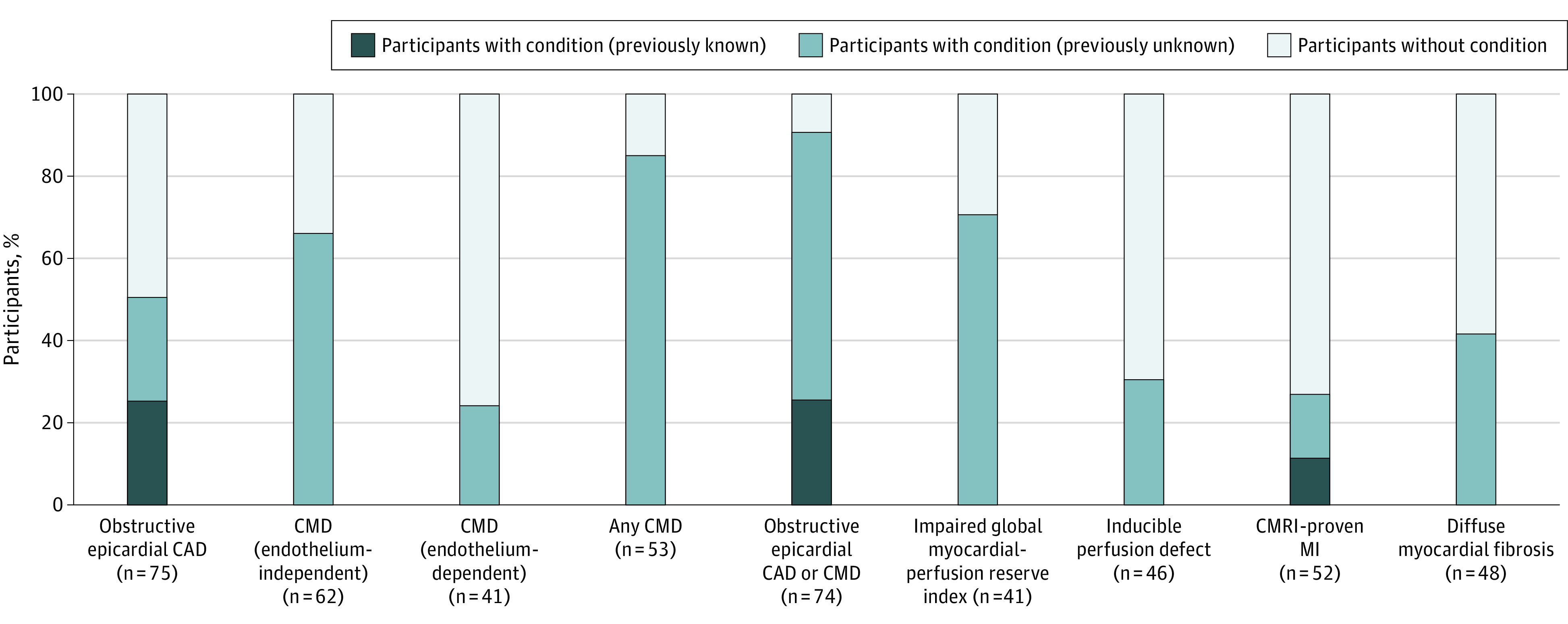
CMRI indicates cardiac magnetic resonance imaging.
Table 1. Clinical Characteristics by Presence or Absence of Obstructive Epicardial CAD.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All coronary angiography (n = 75) | No obstructive epicardial CAD (n = 37) | Obstructive epicardial CAD (n = 38) | ||
| Age, mean (SD), y | 72 (9) | 72 (9) | 73 (9) | .40 |
| Sex | ||||
| Women | 37 (49) | 23 (62) | 14 (37) | .03 |
| Men | 38 (51) | 14 (62) | 24 (63) | |
| BMI, mean (SD) | 33 (8) | 34 (38) | 31 (7) | .08 |
| Clinical frailty scale | ||||
| 1: Very fit | 1 (1) | 1 (3) | 0 | .26 |
| 2: Well | 15 (20) | 8 (22) | 7 (18) | |
| 3: Managing well | 28 (37) | 15 (41) | 13 (34) | |
| 4: Vulnerable | 15 (20) | 5 (14) | 10 (26) | |
| 5: Mildly frail | 13 (17) | 5 (14) | 8 (21) | |
| 6: Moderately frail | 3 (4) | 3 (8) | 0 | |
| NYHA functional class | ||||
| II | 2 (3) | 1 (3) | 1 (3) | .94 |
| III | 40 (53) | 19 (51) | 21 (55) | |
| IV | 33 (44) | 17 (46) | 16 (42) | |
| Vital signs, mean (SD) | ||||
| Heart rate, bpm | 83 (25) | 90 (28) | 73 (21) | .01 |
| Systolic blood pressure, mm Hg | 150 (29) | 152 (31) | 148 (29) | .58 |
| Medical history | ||||
| Previous heart failure diagnosis | 28 (37) | 13 (35) | 15 (39) | .70 |
| Any CAD | 26 (35) | 7 (19) | 19 (50) | <.01 |
| MI | 17 (23) | 4 (11) | 13 (34) | .02 |
| Angina | 11 (15) | 3 (8) | 8 (21) | .11 |
| Revascularization | 12 (16) | 2 (5) | 10 (26) | .01 |
| Percutaneous coronary intervention | 11 (15) | 2 (5) | 9 (24) | .03 |
| Coronary artery bypass grafting | 3 (4) | 0 | 3 (8) | .08 |
| Hypertension | 55 (73) | 28 (76) | 27 (71) | .65 |
| Atrial fibrillation | 49 (65) | 26 (70) | 23 (61) | .38 |
| Cerebrovascular disease | 15 (20) | 5 (14) | 10 (26) | .17 |
| Peripheral arterial disease | 8 (11) | 2 (5) | 6 (16) | .15 |
| Diabetes | 39 (52) | 15 (41) | 24 (63) | .05 |
| Chronic kidney disease | 22 (29) | 6 (16) | 16 (42) | .01 |
| Smoking history | 42 (56) | 20 (54) | 22 (58) | .74 |
| Admission medication | ||||
| Loop diuretic | 36 (48) | 18 (49) | 18 (47) | .91 |
| ACEI/ARB | 50 (67) | 22 (59) | 28 (74) | .19 |
| β-Blocker | 48 (64) | 22 (59) | 26 (68) | .42 |
| Mineralocorticoid receptor antagonist | 3 (4) | 1 (3) | 2 (5) | .57 |
| Antiplatelet | 27 (36) | 8 (22) | 19 (50) | .01 |
| Statin | 52 (69) | 23 (62) | 29 (76) | .18 |
| Laboratory test | ||||
| eGFR, mean (SD), mL/min/1.73 m2 | 64 (20) | 68 (21) | 61 (19) | .17 |
| CRP, median (IQR), mg/dL | 1.4 (0.5-2.3) | 1.2 (0.4-2.4) | 1.5 (0.5-1.8) | .80 |
| Hb, mean (SD), g/dL | 12.3 (1.9) | 12.5 (1.8) | 12.1 (2.0) | .43 |
| hsTnI, median (IQR), ng/L | 16 (9-29) | 16 (10-27) | 18 (7-34) | .89 |
| No. | 46 | 21 | 25 | |
| BNP, median (IQR), pg/mL | 319 (173-856) | 323 (185-717) | 315 (167-904) | .90 |
| No. | 38 | 17 | 21 | |
| NT-proBNP, median (IQR), pg/mL | 1376 (894-2819) | 1532 (1287-2819) | 1132 (818-2494) | .37 |
| Echocardiography, mean (SD) | ||||
| LVEF, % | 59 (6) | 60 (6) | 58 (6) | .37 |
| E/e' | 14.9 (6.3) | 12.9 (4.3) | 16.4 (7.3) | .03 |
| LA volume index, mL/m2 | 45 (16) | 47 (16) | 44 (16) | .37 |
| Estimated PASP, mm Hg | 40 (16) | 38 (13) | 41 (19) | .57 |
| Valve disease (mild or moderate) | 57 (76) | 33 (89) | 24 (63) | <.01 |
| CMRI, mean (SD) | ||||
| No. | 44 | 20 | 24 | |
| LVEF, % | 60 (7) | 61 (6) | 59 (7) | .23 |
| LVEDV index, mL/m2 | 76 (22) | 69 (21) | 82 (22) | .05 |
| LV mass index, g/m2 | 67 (16) | 65 (19) | 69 (13) | .47 |
| LA volume index, mL/m2 | 68 (22) | 70 (15) | 66 (26) | .51 |
| RVEF, % | 53 (9) | 52 (9) | 54 (8) | .36 |
| RVEDV index, mL/m2 | 80 (28) | 75 (25) | 84 (30) | .29 |
| Any LGE | 27 (61) | 9 (45) | 18 (75) | .04 |
| Ischemic LGE | 13 (30) | 2 (10) | 11 (46) | <.01 |
| Nonischemic LGE | 16 (36) | 7 (35) | 9 (38) | .86 |
| Native T1, ms | 1283 (64) | 1268 (74) | 1296 (53) | .17 |
| ECV, % | 28.4 (4.2) | 26.5 (3.3) | 29.9 (4.3) | .01 |
| MPRI, median (IQR) | 1.49 (1.33-1.85) | 1.65 (1.39-1.87) | 1.41 (1.26-1.75) | .23 |
| Inducible perfusion defect | 13 (34) | 5 (29) | 8 (38) | .57 |
| Ischemic LV segments | 2 (4) | 3 (5) | 2 (4) | .76 |
| Invasive assessment | ||||
| No. | 75 | 37 | 38 | |
| Angiographically normal | 11 (15) | 11 (30) | NA | NA |
| Coronary artery assessed with invasive physiologic testing | ||||
| No. | 62 | 36 | 26 | |
| LAD | 43 (69) | 28 (78) | 15 (58) | .10 |
| LCx | 8 (13) | 2 (6) | 6 (23) | |
| RCA | 11 (18) | 6 (17) | 5 (19) | |
| Resting Tmn, median (IQR), s | 0.71 (0.42-1.22) | 0.94 (0.56-1.35) | 0.51 (0.34-0.80) | <.01 |
| Hyperemic Tmn, median (IQR), s | 0.35 (0.21-0.51) | 0.38 (0.22-0.55) | 0.31 (0.18-0.39) | .07 |
| FFR, median (IQR)a | 0.91 (0.86-0.94) | 0.91 (0.85-0.94) | 0.91 (0.88-0.94) | .95 |
| CFR, median (IQR) | 2.1 (1.4-2.7) | 2.4 (1.5-3.1) | 2.0 (1.2-2.4) | .06 |
| CFR <2.0 | 28 (45) | 15 (42) | 15 (50) | .52 |
| IMR, median (IQR) | 23 (15-39) | 27 (19-43) | 18 (12-26) | .02 |
| IMR ≥25 | 32 (52) | 21 (58) | 11 (42) | .21 |
| Endothelium-independent CMD | 41 (66) | 25 (69) | 16 (62) | .52 |
| No. | 41 | 36 | 5 | |
| Endothelium-dependent CMD | 10 (24) | 10 (28) | 0 | .18 |
| No. | 69 | 35 | 34 | |
| LVEDP, mean (SD), mm Hg | 12 (5) | 12 (4) | 12 (7) | .90 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; CAD, coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CMRI, cardiac magnetic resonance imaging; CRP, C-reactive protein; E/e', estimated LV filling pressures on echocardiography; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; Hb, hemoglobin; hsTnI, high-sensitivity troponin I; IMR, index of microcirculatory resistance; IQR, interquartile range; LA, left atrial; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEDP, LV end-diastolic pressure; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; MI, myocardial infarction; MPRI, myocardial-perfusion reserve index; NA, not applicable; NT-proBNP, N-terminal prohormone BNP; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RCA, right coronary artery; RVEDV, right-ventricular end-diastolic volume; RVEF, right-ventricular ejection fraction; Tmn, mean transit time.
SI conversion factors: To convert BNP to nanograms per liter, multiply by 1; CRP to milligrams per liter, multiply by 10; Hb to grams per liter, multiply by 10.
In patients with obstructive epicardial stenosis, FFR value measured in another nonobstructed coronary artery.
Coronary Microvascular Dysfunction
Endothelium-independent CMD was present in 41 of the 62 participants (66%; 95% CI, 53%-77%) who underwent coronary physiologic function testing (Figure 2; eFigure 2 in the Supplement). The prevalence of CMD was similar in participants with (62%) and without (69%) obstructive CAD. The clinical characteristics of participants with and without CMD were generally similar, although patients with CMD had higher B-type natriuretic peptide levels (Table 2).
Table 2. Clinical Characteristics by Presence or Absence of Endothelium-Independent Coronary Microvascular Dysfunction.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All coronary physiologic testing (n = 62) | No endothelium-independent CMD (n = 21) | Endothelium-independent CMD (n = 41) | ||
| Age, mean (SD), y | 72 (9) | 74 (8) | 72 (9) | .41 |
| Sex | ||||
| Women | 33 (53) | 11 (52) | 22 (54) | .92 |
| Men | 29 (47) | 10 (48) | 19 (46) | |
| BMI, mean (SD) | 33 (8) | 33 (9) | 33 (7) | .80 |
| Clinical frailty scale | ||||
| 1: Very fit | 1 (2) | 1 (5) | 0 | .54 |
| 2: Well | 12 (19) | 5 (24) | 7 (17) | |
| 3: Managing well | 24 (39) | 8 (38) | 16 (39) | |
| 4: Vulnerable | 11 (18) | 4 (19) | 7 (17) | |
| 5: Mildly frail | 11 (18) | 3 (14) | 8 (20) | |
| 6: Moderately frail | 3 (5) | 0 | 3 (7) | |
| NYHA functional class | ||||
| II | 2 (3) | 1 (5) | 1 (2) | .68 |
| III | 31 (50) | 9 (43) | 22 (54) | |
| IV | 29 (47) | 11 (52) | 18 (44) | |
| Vital signs, mean (SD) | ||||
| Heart rate, bpm | 85 (26) | 89 (32) | 82 (22) | .36 |
| Systolic blood pressure, mm Hg | 151 (31) | 155 (33) | 149 (30) | .44 |
| Medical history | ||||
| Previous HF diagnosis | 23 (37) | 5 (24) | 18 (44) | .12 |
| Any CAD | 19 (31) | 7 (33) | 12 (29) | .74 |
| MI | 13 (21) | 4 (19) | 9 (22) | .79 |
| Angina | 6 (10) | 3 (14) | 3 (7) | .38 |
| Revascularization | 8 (13) | 2 (10) | 6 (15) | .57 |
| PCI | 8 (13) | 2 (10) | 6 (15) | .57 |
| CABG | 1 (2) | 0 | 1 (2) | .47 |
| Hypertension | 47 (76) | 15 (71) | 32 (78) | .56 |
| AF | 40 (65) | 11 (52) | 29 (71) | .15 |
| CVD | 13 (21) | 6 (29) | 7 (17) | .29 |
| PAD | 7 (11) | 4 (19) | 3 (7) | .17 |
| Diabetes | 33 (53) | 11 (52) | 22 (54) | .92 |
| CKD | 19 (31) | 9 (43) | 10 (24) | .14 |
| Smoking history | 34 (55) | 11 (52) | 23 (56) | .78 |
| Admission medication | ||||
| Loop diuretic | 28 (45) | 8 (38) | 20 (49) | .42 |
| ACEI/ARB | 42 (68) | 13 (62) | 29 (71) | .48 |
| β-Blocker | 42 (68) | 14 (67) | 28 (68) | .90 |
| MRA | 1 (2) | 0 | 1 (2) | .47 |
| Antiplatelet | 21 (34) | 9 (43) | 12 (29) | .28 |
| Statin | 42 (68) | 12 (57) | 30 (73) | .20 |
| Laboratory tests | ||||
| eGFR, mean (SD), mL/min/1.73 m2 | 65 (21) | 63 (15) | 66 (24) | .58 |
| CRP, median (IQR), mg/L | 13 (5-21) | 9 (4-22) | 13 (7-21) | .61 |
| Hb, mean (SD), g/L | 123 (19) | 119 (20) | 125 (19) | .32 |
| hsTnI, median (IQR), ng/L | 16 (7-29) | 20 (14-36) | 16 (5-25) | .22 |
| No. | 37 | 11 | 26 | |
| BNP, median (IQR), pg/mL | 355 (177-904) | 197 (123-623) | 569 (189-1253) | .04 |
| No. | 33 | 13 | 20 | |
| NT-proBNP, median (IQR), pg/mL | 1385 (1040-2819) | 1366 (414-2494) | 1459 (1152-2948) | .37 |
| Echocardiography, mean (SD) | ||||
| LVEF, % | 58 (6) | 60 (6) | 57 (5) | .06 |
| E/e' | 14.1 (4.9) | 13.5 (4.2) | 14.4 (5.3) | .54 |
| LA volume index, mL/m2 | 46 (15) | 43 (11) | 47 (17) | .26 |
| Estimated PASP, mm Hg | 39 (14) | 42 (16) | 36 (12) | .25 |
| Valve disease (mild or moderate) | 50 (81) | 17 (81) | 33 (80) | .97 |
| CMRI, mean (SD) | ||||
| No. | 35 | 11 | 24 | |
| LVEF, % | 59 (7) | 58 (7) | 59 (7) | .81 |
| LVEDV index, mL/m2 | 74 (22) | 75 (19) | 74 (23) | .94 |
| LV mass index, g/m2 | 67 (15) | 70 (18) | 65 (13) | .32 |
| LA volume index, mL/m2 | 68 (22) | 65 (14) | 69 (25) | .63 |
| RVEF, % | 52 (9) | 49 (8) | 54 (9) | .18 |
| RVEDV index, mL/m2 | 77 (22) | 84 (27) | 73 (18) | .19 |
| Any LGE | 22 (63) | 7 (64) | 15 (62) | .95 |
| Ischemic LGE | 10 (29) | 3 (27) | 7 (29) | .91 |
| Nonischemic LGE | 13 (37) | 4 (36) | 9 (38) | .95 |
| Native T1, ms | 1279 (67) | 1308 (70) | 1266 (63) | .10 |
| ECV, % | 28.0 (4.2) | 29.5 (3.4) | 27.4 (4.5) | .23 |
| MPRI, median (IQR) | 1.66 (1.39-1.87) | 1.55 (1.33-1.85) | 1.70 (1.39-1.97) | .37 |
| Inducible perfusion defect | 11 (38) | 4 (40) | 7 (37) | .87 |
| Ischemic LV segments | 3 (4) | 3 (5) | 3 (4) | .98 |
| Invasive assessment | ||||
| Obstructive epicardial CAD | 26 (42) | 10 (48) | 16 (39) | .52 |
| Angiographically normal | 11 (18) | 3 (14) | 8 (20) | .61 |
| Coronary artery assessed with invasive physiologic tests | ||||
| LAD | 43 (69) | 17 (81) | 26 (63) | .29 |
| LCx | 8 (13) | 1 (5) | 7 (17) | |
| RCA | 11 (18) | 3 (14) | 8 (20) | |
| Resting Tmn, median (IQR), s | 0.71 (0.42-1.22) | 0.66 (0.45-0.81) | 0.83 (0.42-1.27) | .27 |
| Hyperemic Tmn, median (IQR), s | 0.35 (0.21-0.51) | 0.23 (0.18-0.30) | 0.41 (0.33-0.65) | <.001 |
| FFR, median (IQR)a | 0.91 (0.86-0.94) | 0.90 (0.85-0.91) | 0.93 (0.89-0.95) | .03 |
| CFR, median (IQR) | 2.1 (1.4-2.7) | 2.6 (2.4-3.1) | 1.7 (1.3-2.4) | <.001 |
| IMR, median (IQR) | 23 (15-39) | 15 (12-20) | 29 (23-45) | <.001 |
| No. | 41 | 12 | 29 | |
| Endothelium-dependent CMD | 10 (24) | 4 (33) | 6 (21) | .39 |
| No. | 59 | 21 | 38 | |
| LVEDP, mean (SD), mm Hg | 12 (5) | 12 (4) | 13 (6) | .42 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CFR, coronary flow reserve; CKD, chronic kidney disease; CMD, coronary microvascular dysfunction; CMRI, cardiac magnetic resonance imaging; CRP, C-reactive protein; CVD, cerebrovascular disease; E/e', estimated LV filling pressures on echocardiography; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; Hb, hemoglobin; HF, heart failure; hsTnI, high-sensitivity troponin I; IMR, index of microcirculatory resistance; IQR, interquartile range; LA, left atrial; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEDP, LV end-diastolic pressure; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; MI, myocardial infarction; MPRI, myocardial-perfusion reserve index; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal prohormone BNP; NYHA, New York Heart Association; PAD, peripheral arterial disease; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; RCA, right coronary artery; RVEDV, right-ventricular end-diastolic volume; RVEF, right-ventricular ejection fraction; Tmn, mean transit time.
SI conversion factors: To convert BNP to nanograms per liter, multiply by 1; CRP to milligrams per liter, multiply by 10; Hb to grams per liter, multiply by 10.
In patients with obstructive epicardial stenosis, FFR value measured in another non-obstructed coronary artery.
Coronary flow reserve and IMR were both normal in 21 patients (34%); 13 patients (21%) had normal CFR but high IMR (ie, preserved flow reserve and high microvascular resistance), 9 patients (15%) had low CFR and normal IMR (ie, impaired flow reserve and normal microvascular resistance), and 19 patients (31%) had low CFR and high IMR (ie, impaired flow reserve and high microvascular resistance) (eFigure 3 in the Supplement). There were no significant differences between the characteristics of these groups (eTable 1 in the Supplement).
None of the 41 participants who underwent vasoreactivity testing demonstrated epicardial coronary artery vasoconstriction in response to acetylcholine infusion, but 10 patients (24%; 95% CI, 13%-40%) had microvascular vasospasm (Figure 2; eFigure 2 in the Supplement). These patients were more often women and had more atrial fibrillation than those without coronary microvascular endothelial or smooth muscle dysfunction, but fewer had a smoking history and they had less LGE (Table 3).
Table 3. Clinical Characteristics by Presence or Absence of Endothelium-Dependent Coronary Microvascular Dysfunction.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All coronary vasoreactivity testing (n = 41) | No endothelium-dependent CMD (n = 31) | Endothelium-dependent CMD (n = 10) | ||
| Age, mean (SD), y | 71 (9) | 71 (10) | 71 (9) | .84 |
| Sex | ||||
| Women | 25 (61) | 16 (52) | 9 (90) | .03 |
| Men | 16 (39) | 15 (48) | 1 (10) | |
| BMI, mean (SD) | 34 (8) | 34 (8) | 34 (10) | .82 |
| Clinical frailty scale | ||||
| 1: Very fit | 1 (2) | 1 (3) | 0 | .87 |
| 2: Well | 8 (20) | 6 (19) | 2 (20) | |
| 3: Managing well | 17 (41) | 13 (42) | 4 (40) | |
| 4: Vulnerable | 6 (15) | 4 (13) | 2 (20) | |
| 5: Mildly frail | 6 (15) | 4 (13) | 2 (20) | |
| 6: Moderately frail | 3 (7) | 3 (10) | 0 | |
| NYHA functional class | ||||
| II | 2 (5) | 2 (6) | 0 | .64 |
| III | 21 (51) | 15 (48) | 6 (60) | |
| IV | 18 (44) | 14 (45) | 4 (40) | |
| Vital signs, mean (SD) | ||||
| Heart rate, bpm | 90 (27) | 89 (27) | 92 (27) | .74 |
| Systolic blood pressure, mm Hg | 152 (30) | 155 (32) | 142 (18) | .22 |
| Medical history | ||||
| Previous HF diagnosis | 15 (37) | 11 (35) | 4 (40) | .80 |
| Any CAD | 9 (22) | 7 (23) | 2 (20) | .86 |
| MI | 6 (15) | 5 (16) | 1 (10) | .63 |
| Angina | 3 (7) | 2 (5) | 1 (10) | .71 |
| Revascularization | 4 (10) | 2 (5) | 2 (20) | .21 |
| PCI | 4 (10) | 2 (5) | 2 (20) | .21 |
| CABG | 0 | 0 | 0 | NA |
| Hypertension | 31 (76) | 24 (77) | 7 (70) | .63 |
| AF | 28 (68) | 18 (58) | 10 (100) | .01 |
| CVD | 7 (17) | 6 (19) | 1 (10) | .49 |
| PAD | 3 (7) | 3 (10) | 0 | .31 |
| Diabetes | 18 (44) | 15 (48) | 3 (30) | .31 |
| CKD | 8 (20) | 8 (26) | 0 | .07 |
| Smoking history | 23 (56) | 21 (68) | 2 (20) | <.01 |
| Admission medication | ||||
| Loop diuretic | 20 (49) | 14 (45) | 6 (60) | .41 |
| ACEI/ARB | 26 (63) | 21 (68) | 5 (50) | .31 |
| β-Blocker | 25 (61) | 17 (55) | 8 (80) | .16 |
| MRA | 1 (2) | 1 (3) | 0 | .57 |
| Antiplatelet | 11 (27) | 10 (32) | 1 (10) | .17 |
| Statin | 28 (68) | 22 (71) | 6 (60) | .52 |
| Laboratory tests | ||||
| eGFR, mean (SD), mL/min/1.73 m2 | 69 (22) | 67 (23) | 75 (17) | .37 |
| CRP, median (IQR), mg/L | 12 (5-24) | 14 (7-35) | 5 (4-18) | .14 |
| Hb, mean (SD), g/L | 126 (19) | 126 (21) | 124 (10) | .68 |
| hsTnI, median (IQR), ng/L | 16 (10-25) | 16 (10-29) | 16 (9-25) | .68 |
| No. | 23 | 19 | 4 | |
| BNP, median (IQR), pg/mL | 323 (177-794) | 355 (177-1017) | 254 (154-559) | .57 |
| No. | 21 | 15 | 6 | |
| NT-proBNP, median (IQR), pg/mL | 1385 (1132-2819) | 1366 (1132-3076) | 1562 (540-2108) | .97 |
| Echocardiography, mean (SD) | ||||
| LVEF, % | 59 (6) | 58 (6) | 62 (7) | .11 |
| E/e' | 12.8 (4.0) | 13.0 (4.1) | 11.9 (3.6) | .56 |
| LA volume index, mL/m2 | 47 (17) | 45 (17) | 55 (17) | .15 |
| Estimated PASP, mm Hg | 37 (13) | 36 (14) | 40 (12) | .45 |
| Valve disease (mild or moderate) | 35 (85) | 26 (84) | 9 (90) | .63 |
| CMRI, mean (SD) | ||||
| No. | 22 | 18 | 4 | |
| LVEF, % | 60 (7) | 59 (7) | 64 (5) | .21 |
| LVEDV index, mL/m2 | 71 (22) | 74 (22) | 58 (17) | .17 |
| LV mass index, g/m2 | 64 (17) | 67 (17) | 52 (9) | .12 |
| LA volume index, mL/m2 | 72 (24) | 72 (27) | 71 (9) | .91 |
| RVEF, % | 52 (9) | 52 (10) | 53 (6) | .96 |
| RVEDV index, mL/m2 | 75 (23) | 79 (22) | 61 (20) | .18 |
| Any LGE | 11 (50) | 11 (61) | 0 | .03 |
| Ischemic LGE | 4 (22) | 4 (22) | 0 | .30 |
| Nonischemic LGE | 8 (73) | 8 (44) | 0 | .10 |
| Native T1, ms | 1276 (75) | 1272 (74) | 1295 (90) | .59 |
| ECV, % | 27.4 (4.1) | 27.3 (4.1) | 27.7 (4.4) | .88 |
| MPRI, median (IQR) | 1.60 (1.39-1.87) | 1.60 (1.39-1.87) | 1.60 (1.49-1.71) | .87 |
| Inducible perfusion defect | 5 (26) | 4 (27) | 1 (25) | .95 |
| Ischemic LV segments | 2 (4) | 2 (5) | 1 (1) | .42 |
| Invasive assessment | ||||
| Obstructive epicardial CAD | 5 (12) | 5 (16) | 0 | .18 |
| Angiographically normal | 11 (27) | 7 (23) | 4 (40) | .28 |
| Coronary artery assessed with invasive physiologic tests | ||||
| LAD | 30 (73) | 21 (68) | 9 (90) | .25 |
| LCx | 4 (10) | 3 (10) | 1 (10) | |
| RCA | 7 (17) | 7 (23) | 0 | |
| Resting Tmn, median (IQR), s | 0.84 (0.49-1.28) | 0.99 (0.54-1.42) | 0.56 (0.34-1.22) | .15 |
| Hyperemic Tmn, median (IQR), s | 0.36 (0.21-0.54) | 0.42 (0.23-0.66) | 0.27 (0.20-0.38) | .10 |
| FFR, median (IQR)a | 0.92 (0.87-0.94) | 0.91 (0.85-0.94) | 0.94 (0.91-0.95) | .22 |
| CFR, median (IQR) | 2.3 (1.4-3.0) | 2.4 (1.3-3.0) | 2.0 (1.5-3.8) | .99 |
| CFR <2.0 | 19 (46) | 14 (45) | 5 (50) | .79 |
| IMR, median (IQR) | 26 (18-42) | 29 (20-50) | 21 (14-28) | .07 |
| IMR ≥25 | 23 (56) | 19 (61) | 4 (40) | .24 |
| Endothelium-independent CMD | 29 (71) | 23 (74) | 6 (60) | .39 |
| No. | 40 | 30 | 10 | |
| LVEDP, mean (SD), mm Hg | 12 (5) | 12 (5) | 12 (5) | .90 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CFR, coronary flow reserve; CKD, chronic kidney disease; CMD, coronary microvascular dysfunction; CMRI, cardiac magnetic resonance imaging; CRP, C-reactive protein; CVD, cerebrovascular disease; E/e', estimated LV filling pressures on echocardiography; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; Hb, hemoglobin; HF, heart failure; hsTnI, high-sensitivity troponin I; IMR, index of microcirculatory resistance; IQR, interquartile range; LA, left atrial; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LGE, late gadolinium enhancement; LV, left ventricular; LVEDP, LV end-diastolic pressure; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; MI, myocardial infarction; MPRI, myocardial-perfusion reserve index; MRA, mineralocorticoid receptor antagonist; NA, not applicable; NT-proBNP, N-terminal prohormone BNP; NYHA, New York Heart Association; PAD, peripheral arterial disease; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; RCA, right coronary artery; RVEDV, right-ventricular end-diastolic volume; RVEF, right-ventricular ejection fraction; Tmn, mean transit time.
SI conversion factors: To convert BNP to nanograms per liter, multiply by 1; CRP to milligrams per liter, multiply by 10; Hb to grams per liter, multiply by 10.
In patients with obstructive epicardial stenosis, FFR value was measured in another nonobstructed coronary artery.
Any CMD (ie, either endothelium independent or dependent) was present in 45 of 53 participants (85%; 95% CI, 72%-92%) (Figure 2; eFigure 2 in the Supplement); vasoreactivity testing was contraindicated in 9 participants with obstructive epicardial CAD but no endothelium-independent CMD. Twenty-nine of 36 participants (81%; 95% CI, 64%-91%) without obstructive epicardial CAD had CMD. The prevalence of CMD was not significantly different in patients with and without obstructive CAD. Participants with CMD more frequently had preexisting HF, more atrial fibrillation, lower troponin levels, and lower LV ejection fraction than those without CMD (eTable 2 in the Supplement). The overlap of the invasive coronary assessment findings is displayed in eFigure 4 in the Supplement.
Impaired Myocardial Perfusion
Forty-six participants underwent adenosine stress perfusion CMRI, of whom 41 had adequate semiquantitative assessment of MPRI. Twenty-nine of the 41 participants had an MPRI less than or equal to 1.84 (71%; 95% CI, 54%-83%), consistent with impaired global myocardial perfusion, and 14 of 46 participants had a qualitative inducible perfusion defect (30%; 95% CI, 19%-46%) (Figure 2).
Those with low MPRI had fewer myocardial infarctions, larger left atrial volumes, and a higher extracellular volume on CMRI than those with preserved global myocardial perfusion (eTable 3 in the Supplement). Participants with and without a low MPRI value had similar rates of obstructive epicardial CAD, endothelium-independent CMD, and endothelium-dependent CMD.
Patients with an inducible perfusion defect were younger and had more history of CAD, myocardial infarction, revascularization, and smoking, but had less atrial fibrillation than those with no perfusion defect (eTable 4 in the Supplement). Participants with an inducible perfusion defect had larger LV volumes and higher LV mass shown on CMRI than those without a perfusion defect. Those with a perfusion defect had a higher burden of ischemic LGE than those without an inducible defect, but a similar global MPRI. Participants with and without a perfusion defect had similar rates of epicardial CAD, endothelium-independent CMD, and endothelium-dependent CMD.
Imaging Evidence of Myocardial Infarction
Fifty-two participants underwent LGE CMRI. Fourteen individuals (27%; 95% CI, 16%-41%) had subendocardial or transmural LGE in the distribution of a coronary artery territory consistent with previous myocardial infarction (Figure 2), of whom 8 patients had no clinical history of myocardial infarction. Participants with CMRI evidence of myocardial infarction were more likely to have a clinical history of CAD, myocardial infarction, and coronary revascularization, and were less likely to have atrial fibrillation than those without CMRI evidence of myocardial infarction (eTable 5 in the Supplement). Those with CMRI-proven myocardial infarction had more obstructive CAD than those without evidence of myocardial infarction, but there were similar rates of endothelium-independent and endothelium-dependent CMD.
Diffuse Myocardial Fibrosis
Of the 48 patients who had pre- and post-contrast T1 mapping, 20 (42%; 95% CI, 28%-56%) had an extracellular volume greater than 30%, consistent with diffuse myocardial fibrosis (Figure 2). There were no major differences in the clinical characteristics of individuals with and without a high extracellular volume (eTable 6 in the Supplement). On CMRI, participants with diffuse myocardial fibrosis had larger right ventricular end-diastolic volumes and lower MPRI than participants with a normal extracellular volume. Those with an elevated extracellular volume had more obstructive CAD than those with a normal extracellular volume, but a similar rate of endothelium-independent and -dependent CMD. The overlap of the CMRI findings is displayed in eFigure 5 in the Supplement.
Clinical Outcomes
During a median follow-up of 18 (interquartile range, 14-22) months, the composite outcomes examined were more common in patients with vs without obstructive epicardial CAD, although the number of events was small (eFigure 6 in the Supplement). Patients with obstructive CAD had more adverse events during follow-up (28 [74%]) than those without obstructive CAD (17 [46%]). Eight patients (21% of those with obstructive CAD) underwent percutaneous coronary revascularization.
There were no significant differences in outcomes between patients with and without endothelium-independent CMD (eFigure 7 and eFigure 8 in the Supplement), endothelium-dependent CMD (eFigure 9 in the Supplement), or any CMD (eFigure 10 in the Supplement), but the number of events was small. The composite outcomes were more common in patients with vs without an impaired global MPRI (eFigure 11 in the Supplement). Those with an inducible perfusion defect (eFigure 12 in the Supplement), imaging evidence of myocardial infarction (eFigure 13 in the Supplement), and an elevated extracellular volume (eFigure 14 in the Supplement) had more adverse clinical outcomes than those without.
Discussion
We found that 91% of patients hospitalized with HFpEF had epicardial CAD, CMD, or both. Of those without epicardial CAD, more than 80% had CMD (endothelium-independent or -dependent). Reliable estimates of prevalence of epicardial CAD can be obtained only from invasive or noninvasive coronary imaging or autopsy studies. One autopsy study including 119 patients with HFpEF examined over a 33-year period reported that 65% of the patients had 50% or more stenosis of at least 1 epicardial coronary artery.7 Several coronary angiography studies have been conducted, reporting a CAD prevalence of 35% to 76%, although these studies have largely been convenience samples of patients undergoing clinically indicated coronary angiography, have used different anatomic criteria to define CAD, and did not include coronary physiologic function assessments.1,22,23,24,25,26,27,28,29,30 Consequently, it is unclear whether the high CAD prevalence reported represents referral bias or whether the real burden of CAD in patients with HFpEF is underrecognized. We conducted a systematic study to assess CAD prevalence in a relatively unselected HFpEF cohort using coronary angiography, although, inevitably, there was still selection bias due to the exclusion of patients with severe kidney dysfunction, frailty, or both. We also conducted invasive coronary physiologic function studies to determine whether stenoses were flow-limiting and to detect CMD, and CMRI to assess myocardial perfusion and fibrosis (ie, to ensure systematic assessment of CAD, myocardial ischemia/infarction, and myocardial fibrosis in HFpEF).
Half of the patients with invasively documented CAD in this study had no history of CAD, highlighting the high burden of unrecognized CAD in HFpEF, consistent with other studies.7,22 In addition, we found that neither semiquantitative CMR perfusion imaging (using MPRI) nor the presence of a visual perfusion defect predicted obstructive epicardial CAD on invasive investigation, suggesting that angiography may be necessary to diagnose CAD in patients with HFpEF. This finding is consistent with the results of one retrospective study that reported poor diagnostic accuracy of noninvasive ischemia testing in detecting epicardial CAD.1 The reasons why CMRI assessments of myocardial perfusion did not identify obstructive CAD are uncertain but may include the presence of impaired perfusion due to CMD, the absence of reversible ischemia in the context of nonviable myocardium, or collateral perfusion of a territory supplied by a diseased epicardial artery. Furthermore, MPRI represents global myocardial perfusion, which may not be influenced by areas of regional ischemia.
In this exploratory analysis, we found that patients with obstructive epicardial CAD had higher rates of adverse clinical outcomes than those without obstructive disease, predominantly related to hospitalizations, although there were few events overall. To our knowledge, the most appropriate medical therapy and the potential role of revascularization in HFpEF have never been investigated in randomized clinical trials.
Another novel finding was that two-thirds of patients had endothelium-independent CMD identified on invasive coronary physiologic function testing, with a similar prevalence in those with (62%) and without (69%) obstructive epicardial disease. A similar prevalence of CMD was found in a convenience sample of 30 patients with HFpEF undergoing clinically indicated angiography.9 However, in another retrospective study of 162 patients with HFpEF undergoing clinically indicated coronary microvascular function testing over a 25-year period, only 43% of the participants had endothelium-independent CMD (defined as CFR ≤2.5).8
In the PROMIS-HFpEF study, echocardiographic measurement of CFR in the left anterior descending coronary artery identified CMD in 75% of the participants, using a CFR threshold less than 2.5 (compared with 65% in our study using the same cutoff point). Epicardial CAD was not systematically excluded in PROMIS-HFpEF and clinically unrecognized obstructive CAD, which was present in 39% of our cohort, could have confounded the results. In addition to the different techniques used to measure CFR, other variations in study design may have contributed to different prevalence estimates, including the clinical status of the HFpEF population studied (ambulatory vs hospitalized) and the lower LV ejection fraction inclusion criterion (≥40%) in PROMIS-HFpEF. Earlier studies, including PROMIS-HFpEF, have found that CMD is associated with adverse outcomes in patients with HFpEF.31,32
In assessing endothelium-dependent coronary vasomotor function by administering intracoronary acetylcholine, we found that only 24% of the patients had microvascular vasospasm, reflecting coronary microvascular endothelial dysfunction and smooth muscle dysfunction. Our findings suggest that CMD in patients with HFpEF is predominantly due to endothelium-independent abnormalities, such as abnormal vascular remodeling, extrinsic vascular compression, and microvascular rarefaction, rather than endothelial and vascular smooth muscle dysfunction. This finding may explain the neutral outcomes of trials of therapies targeting nitric oxide-cyclic guanosine monophosphate protein kinase G signaling.33
We also examined the potential myocardial consequences associated with CAD and CMD in HFpEF, finding that 27% of the patients had imaging evidence of myocardial infarction on LGE. Even among those with no clinical history, 18% had evidence of clinically unrecognized myocardial infarction on CMRI. Our prevalence estimate of previous myocardial infarction is lower than the estimate in an autopsy study in patients with HF and LV ejection fraction greater than or equal to 40% (42% on gross pathologic examination),7 but higher than in a CMRI study using LGE in ambulatory patients with HFpEF (10%).34
Myocardial fibrosis may contribute to myocardial stiffness and diastolic dysfunction in HFpEF.7,35 Extracellular volume assesses myocardial fibrosis, and 42% of our patients had high extracellular volume (>30%). Although the numbers were small, patients with high extracellular volume had more adverse events than those with normal extracellular volume, consistent with previous evidence that myocardial fibrosis is associated with poor outcomes in HFpEF.36,37,38 Mineralocorticoid receptor antagonists might be most beneficial when targeted at patients with high extracellular volume.
In summary, we found 91% of participants with hospitalized HFpEF had evidence of epicardial CAD, CMD, or both (Figure 2). Of those without obstructive epicardial CAD, over 80% of patients had CMD.
Limitations
The study has limitations. Although we sought to conduct a systematic study of consecutive hospitalized patients with HFpEF at 3 centers, most of such patients did not meet inclusion criteria or did not agree to participate in the study. Furthermore, the enrolled patients did not undergo all study procedures, limiting our ability to fully compare coronary evaluation modalities across most patients. Some patients dropped out before invasive and noninvasive investigations, predominantly owing to a deterioration in their clinical status or kidney function. It was necessary to exclude patients with severe kidney dysfunction to allow safe administration of contrast agents during the imaging studies. In addition, patients with severe frailty were excluded because invasive assessment was believed to be clinically inappropriate. These factors limit the generalizability of the study results to these groups.
The delay between recruitment and performing the invasive coronary assessment may have affected the results of coronary microvascular testing. Elevated LV filling pressures can contribute to CMD as a result of extravascular compression of arterioles.39 Invasively assessed LV end-diastolic pressure was normal in more than half of the study participants, but they may not have had the assessment performed during the index hospitalization.
Our results may not be representative of ambulatory patients with HFpEF and we did not have an age- and comorbidity-matched control group, which would have been ethically difficult. The clinical outcomes were not adjudicated and are exploratory; further studies are required to assess the prognostic impact of invasively assessed epicardial CAD and CMD in HFpEF.
Conclusions
In this cohort study, epicardial CAD and CMD were common in the patients analyzed. These conditions might be unrecognized in hospitalized patients with HFpEF and may be therapeutic targets.
eMethods. Detailed Methods
eFigure 1. Study Screening and Recruitment Flow Diagram
eFigure 2. Overview of Invasive Coronary Assessment
eFigure 3. Microvascular Status Based on Coronary Flow Reserve and the Index of Microcirculatory Resistance
eFigure 4. Venn Diagram of Invasive Coronary Assessment Findings
eFigure 5. Venn Diagram of Cardiac Magnetic Resonance Imaging Findings
eFigure 6. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Obstructive Epicardial Coronary Artery Disease
eFigure 7. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Endothelium-Independent Coronary Microvascular Dysfunction
eFigure 8. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Microvascular Status Group
eFigure 9. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Endothelium-Dependent Coronary Microvascular Dysfunction
eFigure 10. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Any CMD
eFigure 11. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Impaired Global Myocardial-Perfusion Reserve Index
eFigure 12. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of A Qualitative Inducible Perfusion Defect
eFigure 13. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of CMR-Proven Myocardial Infarction
eFigure 14. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Diffuse Myocardial Fibrosis
eTable 1. Selected Clinical Characteristics by Microvascular Status Group
eTable 2. Selected Clinical Characteristics by Presence or Absence of Any Coronary Microvascular Dysfunction
eTable 3. Selected Clinical Characteristics by Presence or Absence of Impaired Global Myocardial-Perfusion Reserve Index
eTable 4. Selected Clinical Characteristics by Presence or Absence of a Qualitative Inducible Perfusion Defect
eTable 5. Selected Clinical Characteristics by Presence or Absence of CMR-Proven Myocardial Infarction
eTable 6. Clinical Characteristics by Presence or Absence of Diffuse Myocardial Fibrosis
eReferences
References
- 1.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25, pt A):2817-2827. doi: 10.1016/j.jacc.2014.03.034 [DOI] [PubMed] [Google Scholar]
- 2.Crea F, Bairey Merz CN, Beltrame JF, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS) . The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38(7):473-477. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39(37):3439-3450. doi: 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953-1959. doi: 10.1056/NEJMoa032566 [DOI] [PubMed] [Google Scholar]
- 5.Kraigher-Krainer E, Shah AM, Gupta DK, et al. ; PARAMOUNT Investigators . Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447-456. doi: 10.1016/j.jacc.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 7.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550-559. doi: 10.1161/CIRCULATIONAHA.114.009625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):432-441. doi: 10.1002/ejhf.1671 [DOI] [PubMed] [Google Scholar]
- 9.Dryer K, Gajjar M, Narang N, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;314(5):H1033-H1042. doi: 10.1152/ajpheart.00680.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489-495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bruyne B, Pijls NHJ, Kalesan B, et al. ; FAME 2 Trial Investigators . Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 12.Kern MJ, Lerman A, Bech JW, et al. ; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology . Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114(12):1321-1341. doi: 10.1161/CIRCULATIONAHA.106.177276 [DOI] [PubMed] [Google Scholar]
- 13.Melikian N, Vercauteren S, Fearon WF, et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5(8):939-945. doi: 10.4244/EIJV5I8A158 [DOI] [PubMed] [Google Scholar]
- 14.Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129(17):1723-1730. doi: 10.1161/CIRCULATIONAHA.113.004096 [DOI] [PubMed] [Google Scholar]
- 15.Ong P, Camici PG, Beltrame JF, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20. doi: 10.1016/j.ijcard.2017.08.068 [DOI] [PubMed] [Google Scholar]
- 16.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23, pt A):2841-2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Beltrame JF, Crea F, Kaski JC, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565-2568. [DOI] [PubMed] [Google Scholar]
- 18.Al-Saadi N, Nagel E, Gross M, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101(12):1379-1383. doi: 10.1161/01.CIR.101.12.1379 [DOI] [PubMed] [Google Scholar]
- 19.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108(4):432-437. doi: 10.1161/01.CIR.0000080915.35024.A9 [DOI] [PubMed] [Google Scholar]
- 20.Thomson LEJ, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction: a National Heart, Lung, and Blood Institute–sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4):8. doi: 10.1161/CIRCIMAGING.114.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trevisan L, Cautela J, Resseguier N, et al. Prevalence and characteristics of coronary artery disease in heart failure with preserved and mid-range ejection fractions: a systematic angiography approach. Arch Cardiovasc Dis. 2018;111(2):109-118. doi: 10.1016/j.acvd.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 23.Koller L, Kleber M, Goliasch G, et al. C-reactive protein predicts mortality in patients referred for coronary angiography and symptoms of heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(7):758-766. doi: 10.1002/ejhf.104 [DOI] [PubMed] [Google Scholar]
- 24.Schmaltz HN, Southern DA, Maxwell CJ, Knudtson ML, Ghali WA; APPROACH Investigators . Patient sex does not modify ejection fraction as a predictor of death in heart failure: insights from the APPROACH cohort. J Gen Intern Med. 2008;23(12):1940-1946. doi: 10.1007/s11606-008-0804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arques S, Bonello L, Roux E, et al. Angiographic coronary artery disease associated with hypertensive heart failure and normal ejection fraction: insights from a prospective monocenter study. Int J Cardiol. 2008;130(1):75-77. doi: 10.1016/j.ijcard.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Stough WG, Shaw LK, O’Connor CM. Anaemia and coronary artery disease severity in patients with heart failure. Eur J Heart Fail. 2006;8(1):54-57. doi: 10.1016/j.ejheart.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 27.East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148(1):151-156. doi: 10.1016/j.ahj.2004.01.017 [DOI] [PubMed] [Google Scholar]
- 28.Arques S, Ambrosi P, Gelisse R, Roux E, Lambert M, Habib G. Prevalence of angiographic coronary artery disease in patients hospitalized for acute diastolic heart failure without clinical and electrocardiographic evidence of myocardial ischemia on admission. Am J Cardiol. 2004;94(1):133-135. doi: 10.1016/j.amjcard.2004.03.046 [DOI] [PubMed] [Google Scholar]
- 29.Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. Am Heart J. 2000;140(3):451-455. doi: 10.1067/mhj.2000.108828 [DOI] [PubMed] [Google Scholar]
- 30.Judge KW, Pawitan Y, Caldwell J, Gersh BJ, Kennedy JW. Congestive heart failure symptoms in patients with preserved left ventricular systolic function: analysis of the CASS registry. J Am Coll Cardiol. 1991;18(2):377-382. doi: 10.1016/0735-1097(91)90589-2 [DOI] [PubMed] [Google Scholar]
- 31.Hage C, Svedlund S, Saraste A, et al. Association of coronary microvascular dysfunction with heart failure hospitalizations and mortality in heart failure with preserved ejection fraction: a follow-up in the PROMIS-HFpEF Study. J Card Fail. 2020;26(11):1016-1021. doi: 10.1016/j.cardfail.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 32.Allan T, Dryer K, Fearon WF, Shah SJ, Blair JEA. Coronary microvascular dysfunction and clinical outcomes in patients with heart failure with preserved ejection fraction. J Card Fail. 2019;25(10):843-845. doi: 10.1016/j.cardfail.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Anstrom KJ, Lewis GD, et al. ; National Heart, Lung, and Blood Institute Heart Failure Clinical Research Network . Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA. 2018;320(17):1764-1773. doi: 10.1001/jama.2018.14852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanagala P, Cheng ASH, Singh A, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction—implications for clinical trials. J Cardiovasc Magn Reson. 2018;20(1):4. doi: 10.1186/s12968-017-0424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54(5):410-418. doi: 10.1016/j.jacc.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy C, Slimani A, de Meester C, et al. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2018;20(1):55. doi: 10.1186/s12968-018-0477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duca F, Kammerlander AA, Zotter-Tufaro C, et al. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2016;9(12):9. doi: 10.1161/CIRCIMAGING.116.005277 [DOI] [PubMed] [Google Scholar]
- 38.Kanagala P, Cheng ASH, Singh A, et al. Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2019;12(11, pt 2):2291-2301. doi: 10.1016/j.jcmg.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 39.Van Herck PL, Carlier SG, Claeys MJ, et al. Coronary microvascular dysfunction after myocardial infarction: increased coronary zero flow pressure both in the infarcted and in the remote myocardium is mainly related to left ventricular filling pressure. Heart. 2007;93(10):1231-1237. doi: 10.1136/hrt.2006.100818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eFigure 1. Study Screening and Recruitment Flow Diagram
eFigure 2. Overview of Invasive Coronary Assessment
eFigure 3. Microvascular Status Based on Coronary Flow Reserve and the Index of Microcirculatory Resistance
eFigure 4. Venn Diagram of Invasive Coronary Assessment Findings
eFigure 5. Venn Diagram of Cardiac Magnetic Resonance Imaging Findings
eFigure 6. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Obstructive Epicardial Coronary Artery Disease
eFigure 7. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Endothelium-Independent Coronary Microvascular Dysfunction
eFigure 8. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Microvascular Status Group
eFigure 9. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Endothelium-Dependent Coronary Microvascular Dysfunction
eFigure 10. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Any CMD
eFigure 11. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Impaired Global Myocardial-Perfusion Reserve Index
eFigure 12. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of A Qualitative Inducible Perfusion Defect
eFigure 13. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of CMR-Proven Myocardial Infarction
eFigure 14. Kaplan-Meier Curves for Combined Endpoints of Death and Hospitalizations by Presence or Absence of Diffuse Myocardial Fibrosis
eTable 1. Selected Clinical Characteristics by Microvascular Status Group
eTable 2. Selected Clinical Characteristics by Presence or Absence of Any Coronary Microvascular Dysfunction
eTable 3. Selected Clinical Characteristics by Presence or Absence of Impaired Global Myocardial-Perfusion Reserve Index
eTable 4. Selected Clinical Characteristics by Presence or Absence of a Qualitative Inducible Perfusion Defect
eTable 5. Selected Clinical Characteristics by Presence or Absence of CMR-Proven Myocardial Infarction
eTable 6. Clinical Characteristics by Presence or Absence of Diffuse Myocardial Fibrosis
eReferences