Abstract
Objective
To compare 30-day mortality in long-term care facility (LTCF) residents with and without COVID-19 and to investigate the impact of 31 potential risk factors for mortality in COVID-19 cases.
Design
Retrospective cohort study.
Setting and Participants
All residents of LTCFs registered in Senior Alert, a Swedish national database of health examinations in older adults, during 2019-2020.
Methods
We selected residents with confirmed COVID-19 until September 15, 2020, along with time-dependent propensity score–matched controls without COVID-19. Exposures were COVID-19, age, sex, comorbidities, medications, and other patient characteristics. The outcome was all-cause 30-day mortality.
Results
A total of 3731 residents (median age 87 years, 64.5% female) with COVID-19 were matched to 3731 controls without COVID-19. Thirty-day mortality was 39.9% in COVID-19 cases and 5.7% in controls [relative risk 7.05, 95% confidence interval (CI) 6.10-8.14]. In COVID-19 cases, the odds ratio (OR) for 30-day mortality was 2.44 (95% CI 1.57-3.81) in cases aged 80-84 years, 2.99 (95% CI 1.93-4.65) in cases aged 85-89 years, and 3.28 (95% CI 2.11-5.10) in cases aged ≥90 years, as compared with cases aged <70 years. Other risk factors for mortality among COVID-19 cases included male sex (OR, 2.60, 95% CI 2.22-3.05), neuropsychological conditions (OR, 2.18; 95% CI 1.76-2.71), impaired walking ability (OR, 1.45, 95% CI 1.17-1.78), urinary and bowel incontinence (OR 1.51, 95% CI 1.22-1.85), diabetes (OR 1.36, 95% CI 1.14-1.62), chronic kidney disease (OR 1.37, 95% CI 1.11-1.68) and previous pneumonia (OR 1.57, 95% CI 1.32-1.85). Nutritional factors, cardiovascular diseases, and antihypertensive medications were not significantly associated with mortality.
Conclusions and Implications
In Swedish LTCFs, COVID-19 was associated with a large excess in mortality after controlling for an extensive number of risk factors. Beyond older age and male sex, several prevalent clinical risk factors independently contributed to higher mortality. These findings suggest that reducing transmission of COVID-19 in LTCFs will likely prevent a considerable number of deaths.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, residential facilities, geriatrics, epidemiology
Early in the coronavirus disease 2019 (COVID-19) pandemic, long-term care facilities (LTCFs) were pointed out as high-risk environments, requiring high priority for prevention and precaution.1 In spite of this, many countries have reported a considerable proportion of COVID-19–related deaths in LTCFs.2, 3, 4, 5 In Sweden, 50% of all deaths have occurred in LTCFs.6 Sweden has also had a much higher rate of COVID-19 mortality in LTCFs than neighboring countries, being 5-fold higher than in Denmark and 11-fold higher than in Norway and Finland.7 In line with this observation, an independent committee of inquiry appointed by the Swedish government concluded that Sweden’s strategy to protect older adults living in LTCFs has failed.8 Thus, as the world continues to battle new waves of the pandemic, it is imperative to identify the most important risk factors for COVID-19 mortality in countries where mortality has been high in LTCFs, such as in Sweden, so that premature deaths can be prevented.
Although previous research has identified male sex, older age, and comorbidity as risk factors for severe COVID-19 in the general population,9, 10, 11, 12, 13, 14 data from LTCF residents are lacking. Other than a few small studies,15, 16, 17, 18 only 1 large study has investigated risk factors for 30-day mortality following COVID-19 in LTCF residents.19 Strong risk factors in this study, apart from older age and male sex, were diabetes, chronic kidney disease, and impaired physical and cognitive function.19 Yet, the median age was only 79 years, and the study did not include a control group, highlighting that more studies in large, representative populations of LTCF residents are warranted. The aim of the present cohort study was to compare all-cause, 30-day mortality in COVID-19 cases and matched controls living in Swedish LTCFs. An additional aim was to also investigate the impact of 31 potential risk factors for all-cause 30-day mortality in COVID-19 cases.
Methods
Study Design and Population
This retrospective cohort study was approved by the Swedish Ethical Review Authority (no. 2020-02552), who waived the informed consent requirement. We considered for inclusion all residents of LTCFs in Sweden who are registered in the Senior Alert database. Launched in 2008, Senior Alert collects data for assessment and prevention of falls, pressure ulcers, malnutrition, and oral health among adults aged ≥65 years.20 It is used in hospital wards, home care services, and LTCFs in 90% of Swedish municipalities and regions.20 An estimated 73% of all Swedish LTCF residents are registered in the database.21
In the Senior Alert cohort, we identified all COVID-19 cases confirmed in Sweden until mid-September 2020 using the Swedish Public Health Agency’s SmiNet database. Reporting confirmed COVID-19 cases to SmiNet is required by law. No information regarding the method of testing was available. COVID-19 cases were excluded from the analysis if they did not have a record in Senior Alert within a year prior to the date of COVID-19 testing or diagnosis (whichever came first or was available). Cases were also excluded if the dates of testing and diagnosis were both unavailable. Persons in the Senior Alert cohort who did not have confirmed COVID-19 (i.e., controls) were included if they had a Senior Alert record in 2019 or 2020 (they were included from the latest record during these years, if there were multiple records).
The data were linked using Personal Identification Numbers, which all residents of Sweden have. Statistics Sweden replaced these numbers with pseudo-anonymized identifiers for integrity reasons.
Risk Factors and Outcome
The study outcome was all-cause, 30-day mortality, which was obtained from the Swedish Cause of Death Register.22 Body mass index (BMI, weight in kilograms divided by height in meters squared) was obtained from Senior Alert and was used to define underweight (<18.5), normal weight 18.5-24.99), overweight (25.0-29.99), and obesity (≥30). Senior Alert also provided data from 3 validated instruments, which were incorporated into the database upon the advice of an expert panel: Mini Nutritional Assessment, Downton Fall Risk Index, and Modified Norton Scale.20 The items we selected from these instruments are neuropsychological conditions, known previous falls, walking ability, fluid intake, food intake, incontinence, and general physical condition (Table 1 ).
Table 1.
Baseline Characteristics in the Total Cohort of Residents With COVID-19 and in Matched COVID-19 Cases and Controls
| Variables | All COVID-19 Cases (n = 4177) | Matched COVID-19 Cases (n = 3731) | Matched Controls (n = 3731) |
|---|---|---|---|
| Days between Senior Alert registration and baseline∗, median (IQR) | 119 (60-187) | 120 (60-188) | 120 (60-188) |
| Male sex | 1478 (35.4) | 1325 (35.5) | 1318 (35.3) |
| Age, median (IQR), y | 87 (81-92) | 87 (81-92) | 87 (81-92) |
| Age group, y | |||
| <70 | 152 (3.6) | 140 (3.8) | 164 (4.4) |
| 70-74 | 280 (6.7) | 249 (6.7) | 238 (6.4) |
| 75-79 | 519 (12.4) | 456 (12.2) | 431 (11.6) |
| 80-84 | 792 (19.0) | 706 (18.9) | 693 (18.6) |
| 85-89 | 1045 (25.0) | 938 (25.1) | 927 (24.9) |
| ≥90 | 1389 (33.3) | 1242 (33.3) | 1278 (34.3) |
| BMI, mean (SD) | 25.5 (5.1) | 25.4 (5.0) | 25.6 (5.3) |
| BMI categories | |||
| Underweight (<18.5) | 265 (6.4) | 240 (6.4) | 258 (6.9) |
| Normal weight (18.5-24.99) | 1865 (44.7) | 1672 (44.8) | 1604 (43.0) |
| Overweight (25.0-29.99) | 1334 (32.0) | 1196 (32.1) | 1182 (31.7) |
| Obesity (≥30) | 707 (17.0) | 623 (16.7) | 687 (18.4) |
| Neuropsychological conditions | |||
| None | 938 (23.7) | 886 (23.8) | 884 (23.7) |
| Mild dementia or depression | 1931 (48.7) | 1822 (48.8) | 1805 (48.4) |
| Severe dementia or depression | 1095 (27.6) | 1023 (27.4) | 1042 (27.9) |
| Known previous falls | 2059 (52.9) | 1970 (52.8) | 1950 (52.3) |
| Walking ability | |||
| Safe with or without walking aids | 1584 (40.7) | 1513 (40.6) | 1492 (40.0) |
| Unsafe walk | 1419 (36.5) | 1367 (36.6) | 1379 (37.0) |
| Unable to walk | 889 (22.8) | 851 (22.8) | 860 (23.1) |
| Fluid intake, mL/d | |||
| >1000 | 2315 (58.7) | 2191 (58.7) | 2182 (58.5) |
| 700-1000 | 1402 (35.6) | 1327 (35.6) | 1312 (35.2) |
| 500-700 | 208 (5.3) | 196 (5.3) | 210 (5.6) |
| <500 | 17 (0.4) | 17 (0.5) | 27 (0.7) |
| Food intake | |||
| Normal serving | 2747 (69.7) | 2597 (69.6) | 2.587 (69.3) |
| ¾ serving | 720 (18.3) | 686 (18.4) | 699 (18.7) |
| ½ serving | 375 (9.5) | 350 (9.4) | 341 (9.1) |
| <½ serving | 100 (2.5) | 98 (2.6) | 104 (2.8) |
| General physical condition | |||
| Good | 2210 (56.0) | 2077 (55.7) | 2034 (54.5) |
| Fair | 1597 (40.5) | 1524 (40.9) | 1545 (41.4) |
| Poor | 126 (3.2) | 121 (3.2) | 142 (3.8) |
| Very bad | 9 (0.2) | 9 (0.2) | 10 (0.3) |
| Incontinence | |||
| No | 1041 (26.4) | 986 (26.4) | 989 (26.5) |
| Temporarily but unusual | 602 (15.3) | 565 (15.1) | 556 (14.9) |
| Urinary or bowel | 965 (24.5) | 906 (24.3) | 890 (23.9) |
| Urinary and bowel | 1334 (33.8) | 1274 (34.2) | 1296 (34.7) |
| Comorbidities | |||
| Stroke | 1048 (25.1) | 942 (25.3) | 940 (25.2) |
| Myocardial infarction | 500 (12.0) | 446 (12.0) | 431 (11.6) |
| Angina pectoris | 643 (15.4) | 576 (15.4) | 574 (14.4) |
| Heart failure | 856 (20.5) | 771 (20.7) | 776 (20.8) |
| Atrial fibrillation | 1109 (26.6) | 997 (26.7) | 971 (26.0) |
| Autoimmune disease | 534 (12.8) | 487 (13.1) | 491 (13.2) |
| Diabetes | 928 (22.2) | 825 (22.1) | 845 (22.7) |
| COPD | 539 (12.9) | 483 (13.0) | 486 (13.0) |
| Asthma | 305 (7.3) | 275 (7.4) | 242 (6.5) |
| Cancer | 1833 (43.9) | 1687 (45.2) | 1661 (44.5) |
| Renal failure or chronic kidney disease | 566 (13.6) | 521 (14.0) | 536 (14.4) |
| Liver disease | 82 (2.0) | 72 (1.9) | 75 (2.0) |
| Sepsis | 350 (8.4) | 316 (8.5) | 309 (8.3) |
| Influenza | 208 (5.0) | 184 (4.9) | 193 (5.2) |
| Pneumonia | 1013 (24.3) | 915 (24.5) | 923 (24.7) |
| Alcohol intoxication | 264 (6.3) | 233 (6.2) | 221 (5.9) |
| Medications | |||
| Antithrombotics | 2461 (58.9) | 2205 (59.1) | 2253 (60.4) |
| Antihypertensives (other than diuretics) | 2494 (59.7) | 2257 (60.5) | 2271 (60.9) |
| Diuretics | 1794 (43.0) | 1611 (43.2) | 1608 (43.1) |
| Antidepressants | 2409 (57.7) | 2178 (58.4) | 2140 (57.4) |
| Psycholeptics | 2935 (70.3) | 2649 (71.0) | 2648 (71.0) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SD, standard deviation.
Data are displayed as number (percentage) unless stated otherwise.
Baseline was the date of COVID-19 test/diagnosis in cases and a corresponding date in controls.
Data on comorbidities were obtained from the Swedish National Patient Register (NPR), which includes all diagnoses made in Swedish inpatient care since 1987 and all secondary care since 2001. The NPR has been validated, and most diagnoses have a positive predictive value of at least 90% but lower sensitivity.23 Data on cancer were obtained from both the NPR and, for the years 1964-2018, the Swedish Cancer Registry. This registry includes all cancer diagnoses made in Sweden since 1958. Data on medication use were collected both from Senior Alert and the Swedish Prescribed Drug Register, which records all prescription medications dispensed at pharmacies in Sweden since July 2005. For this study, we included only recent medication use, defined as prescriptions collected during 2019-2020. Definitions of diagnoses and medications are available in Supplementary Table 1.
Statistical Analysis
COVID-19 cases and controls were 1:1 matched using time-dependent propensity scores. This method enables matching when the exposure (here, COVID-19) does not coincide with the time of cohort entry (here, the date of the Senior Alert record).24 , 25 This was done by running a Cox regression on all potential risk factors, with COVID-19 as the outcome variable and the date of the Senior Alert record as the time origin. This model was used to calculate a propensity score (the linear predictor), reflecting each individual’s probability of contracting COVID-19. Next, each COVID-19 case was matched to the control with the closest propensity score among those who were still alive at the time when the COVID-19 case occurred (time was counted as days since the Senior Alert date). Matching was done sequentially, starting with the first COVID-19 case (in terms of days since cohort entry), then the second, and so on. Controls could only be matched to 1 case, and ties in propensity scores were resolved using random selection. Owing to the relatively small number of COVID-19 cases, we did not match later-diagnosed cases as controls to earlier-diagnosed cases, which is commonly done.24 , 25 To ensure close matches, a caliper of 1/10th of the standard deviation of the propensity score was used.26 The Cox regression model included time-varying covariates for diagnoses and medications, meaning that a new propensity-score was calculated for controls at the time each case occurred. After matching, the baseline date was set to the COVID-19 date in cases and the corresponding date (in days since cohort entry) in controls.
In the matched cohort, we calculated the relative risk of all-cause 30-day mortality, with a 95% confidence interval (CI) calculated using the Mantel-Haenszel approach.27 (pp284-286) In COVID-19 cases, we used logistic regression to calculate unadjusted and fully adjusted odds ratios for mortality and 95% CIs. Furthermore, the fully adjusted model was rerun with age as a continuous variable to estimate the absolute risk of death by age and other characteristics. We used fractional polynomials to accommodate potential nonlinearity for the age variable.
In the regression models, extreme values for height (<130 cm and >200 cm) and weight (<30 kg and >200 kg) were excluded. For the variables of fluid intake, food intake, and general physical condition, the 2 upper categories were collapsed to 1 category, because the number of participants in the highest category was small (Table 1).
In a sensitivity analysis, we included only COVID-19 cases with a record in Senior Alert within 3 months prior to the date of COVID-19. This restriction was done to examine whether results were affected by the delay between measurements in Senior Alert and the baseline date (COVID-19 test or diagnosis). All analyses were performed using Stata MP version 16.1 for Mac (StataCorp, College Station, TX). Statistical significance was determined as odds ratios with 95% CIs that did not cross 1.
Results
There were 216,085 residents in LTCFs registered in Senior Alert (83,519 with a record in 2019 or 2020). Of these 216,085 residents, 5409 were confirmed with COVID-19 from 22 February 2020 to 15 September 2020. Four individuals were excluded because of missing dates of diagnosis and testing. Another 1225 residents were excluded because they did not have a record in Senior Alert within 1 year before the COVID-19 date. Three additional cases were excluded because their death date preceded their date of confirmed COVID-19. Thus, the study cohort comprised 4177 residents with COVID-19 [64.6% female, median age 87 years (interquartile range 81-92)]. Of these individuals, 3732 had complete data and 3731 could be matched to 3731 controls. Amount of missing data in residents with COVID-19 is presented in Supplementary Table 3.
Thirty-Day Mortality and Associated Risk Factors
Baseline characteristics were similar in both unmatched and matched COVID-19 cases and controls (Table 1). Thirty-day mortality was 39.9% (n = 1487) in COVID-19 cases and 5.7% (n = 211) in controls (relative risk 7.05, 95% CI 6.10-8.14). The association of risk factors with 30-day mortality is presented in Table 2 . The absolute risks by age and other risk factors are presented in Supplementary Fig. 1, Supplementary Fig. 10, Supplementary Fig. 11, Supplementary Fig. 12, Supplementary Fig. 2, Supplementary Fig. 3, Supplementary Fig. 4, Supplementary Fig. 5, Supplementary Fig. 6, Supplementary Fig. 7, Supplementary Fig. 8, Supplementary Fig. 9.
Table 2.
Risk Factors for All-Cause 30-Day Mortality in Residents With COVID-19
| Variables | n | Number of Deaths (%) | Unadjusted OR (95% CI) | Adjusted∗ OR (95% CI) |
|---|---|---|---|---|
| Male sex | 1478 | 747 (50.5) | 2.04 (1.79, 2.33) | 2.60 (2.22, 3.05) |
| Age group, y | ||||
| <70 | 152 | 34 (22.4) | 1 (ref) | 1 (ref) |
| 70-74 | 280 | 88 (31.4) | 1.59 (1.01, 2.51) | 1.45 (0.89, 2.39) |
| 75-79 | 519 | 167 (32.2) | 1.65 (1.08, 2.52) | 1.50 (0.95, 2.38) |
| 80-84 | 792 | 321 (40.5) | 2.37 (1.57, 3.55) | 2.44 (1.57, 3.81) |
| 85-89 | 1045 | 449 (43.0) | 2.62 (1.75, 3.90) | 2.99 (1.93, 4.65) |
| ≥90 | 1389 | 588 (42.3) | 2.55 (1.72, 3.79) | 3.28 (2.11, 5.10) |
| BMI categories | ||||
| Underweight (<18.5) | 265 | 120 (45.3) | 1.28 (0.99, 1.66) | 1.31 (0.98, 1.77) |
| Normal weight (18.5-24.99) | 1865 | 733 (39.3) | 1 (ref) | 1 (ref) |
| Overweight (25.0-29.99) | 1334 | 520 (39.0) | 0.99 (0.85, 1.14) | 1.03 (0.87, 1.22) |
| Obesity (≥30) | 707 | 272 (38.5) | 0.97 (0.81, 1,15) | 1.16 (0.94, 1.44) |
| Neuropsychological conditions | ||||
| None | 938 | 305 (32.5) | 1 (ref) | 1 (ref) |
| Mild dementia or depression | 1931 | 744 (38.5) | 1.30 (1.10, 1.53) | 1.47 (1.22, 1.77) |
| Severe dementia or depression | 1095 | 525 (48.0) | 1.91 (1.60, 2.29) | 2.18 (1.76, 2.71) |
| Known previous falls | 2059 | 868 (42.2) | 1.24 (1.09, 1.41) | 1.07 (0.92, 1.24) |
| Walking ability | ||||
| Safe with or without walking aids | 1584 | 498 (31.4) | 1 (ref) | 1 (ref) |
| Unsafe walk | 1419 | 631 (44.5) | 1.75 (1.50, 2.03) | 1.30 (1.09, 1.55) |
| Unable to walk | 889 | 417 (46.9) | 1.93 (1.63, 2.28) | 1.45 (1.17, 1.78) |
| Fluid intake, mL/d | ||||
| >1000 | 2315 | 900 (38.9) | 1 (ref) | 1 (ref) |
| 700-1000 | 1402 | 557 (39.7) | 1.04 (0.91, 1.19) | 0.94 (0.80, 1.09) |
| <700 | 225 | 105 (46.7) | 1.38 (1.05, 1.81) | 1.09 (0.79, 1.51) |
| Food intake | ||||
| Normal serving | 2747 | 1049 (38.2) | 1 (ref) | 1 (ref) |
| ¾ serving | 720 | 307 (42.6) | 1.20 (1.02, 1.42) | 1.15 (0.95, 1.39) |
| ≤½ serving | 475 | 206 (43.4) | 1.24 (1.02, 1.51) | 1.13 (0.89, 1.44) |
| General physical condition | ||||
| Good | 2210 | 788 (35.7) | 1 (ref) | 1 (ref) |
| Fair | 1597 | 703 (44.0) | 1.42 (1.24, 1.62) | 1.20 (1.04, 1.40) |
| Poor or very bad | 135 | 71 (52.6) | 2.00 (1.41, 2.84) | 1.41 (0.95, 2.08) |
| Incontinence | ||||
| No | 1041 | 319 (30.6) | 1 (ref) | 1 (ref) |
| Temporarily but unusual | 602 | 207 (34.4) | 1.19 (0.96, 1.47) | 1.00 (0.79, 1.27) |
| Urinary or bowel | 965 | 401 (41.6) | 1.61 (1.34, 1.94) | 1.23 (1.00, 1.52) |
| Urinary and bowel | 1334 | 635 (47.6) | 2.06 (1.74, 2.44) | 1.51 (1.22, 1.85) |
| Comorbidities | ||||
| Stroke | 1048 | 441 (42.1) | 1.16 (1.01, 1.34) | 1.02 (0.86, 1.21) |
| Myocardial infarction | 500 | 220 (44.0) | 1.24 (1.03, 1.50) | 0.93 (0.74, 1.18) |
| Angina pectoris | 643 | 278 (43.2) | 1.21 (1.02, 1.43) | 1.12 (0.91, 1.38) |
| Heart failure | 856 | 366 (42.8) | 1.19 (1.02, 1.39) | 0.95 (0.78, 1.17) |
| Atrial fibrillation | 1109 | 468 (42.2) | 1.17 (1.02, 1.35) | 0.94 (0.78, 1.13) |
| Autoimmune disease | 534 | 231 (43.3) | 1.20 (1.00, 1.44) | 1.19 (0.97, 1.47) |
| Diabetes | 928 | 428 (46.1) | 1.43 (1.23, 1.65) | 1.36 (1.14, 1.62) |
| COPD | 539 | 221 (41.0) | 1.08 (0.90, 1.30) | 1.01 (0.81, 1.27) |
| Asthma | 305 | 109 (35.7) | 0.84 (0.66, 1.08) | 0.79 (0.59, 1.05) |
| Cancer | 1833 | 736 (40.2) | 1.06 (0.93, 1.20) | 0.91 (0.78, 1.05) |
| Renal failure or chronic kidney disease | 566 | 273 (48.2) | 1.52 (1.27, 1.81) | 1.37 (1.11, 1.68) |
| Liver disease | 82 | 30 (36.6) | 0.88 (0.56, 1.39) | 0.88 (0.52, 1.50) |
| Sepsis | 350 | 147 (42.0) | 1.12 (0.90, 1.40) | 0.80 (0.62, 1.04) |
| Influenza | 208 | 90 (43.3) | 1.18 (0.89, 1.57) | 0.98 (0.71, 1.36) |
| Pneumonia | 1013 | 493 (48.7) | 1.65 (1.43, 1.91) | 1.57 (1.32, 1.85) |
| Alcohol intoxication | 264 | 86 (32.6) | 0.73 (0.56, 0.95) | 0.73 (0.53, 1.00) |
| Medications | ||||
| Antithrombotics | 2461 | 1013 (41.2) | 1.19 (1.05, 1.36) | 1.04 (0.88, 1.23) |
| Antihypertensives (other than diuretics) | 2494 | 1003 (40.2) | 1.09 (0.96, 1.23) | 1.01 (0.87, 1.18) |
| Diuretics | 1794 | 761 (42.4) | 1.25 (1.10, 1.41) | 1.14 (0.97, 1.34) |
| Antidepressants | 2409 | 979 (40.6) | 1.13 (0.99, 1.28) | 1.10 (0.95, 1.28) |
| Psycholeptics | 2935 | 1172 (39.9) | 1.07 (0.94, 1.23) | 1.03 (0.88, 1.21) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Adjusted for all variables in column 1 and days since Senior Alert registration. The analysis included n = 3732 participants and 1488 cases of death.
Supplementary Fig. 1.
Neuropsychological conditions and absolute risk of 30-day mortality in men without other risk factors.
Supplementary Fig. 10.
Diabetes and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 11.
Age and absolute risk of 30-day mortality in men without other risk factors.
Supplementary Fig. 12.
Age and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 2.
Neuropsychological conditions and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 3.
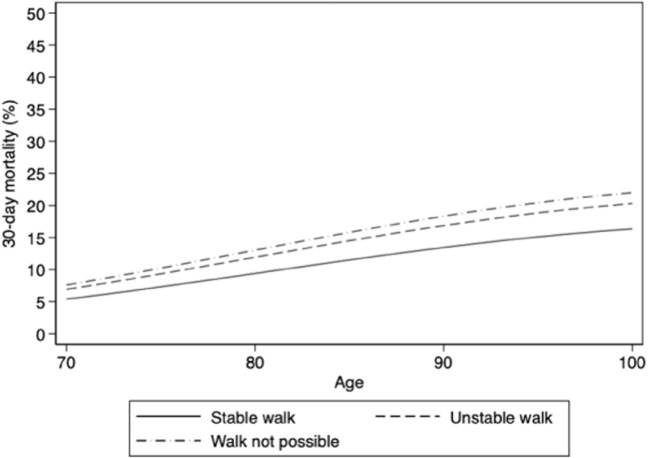
Walking ability and absolute risk of 30-day mortality in men without other risk factors.
Supplementary Fig. 4.
Walking ability and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 5.
Incontinence and absolute risk of 30-day mortality in men without other risk factors.
Supplementary Fig. 6.
Incontinence and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 7.
Pneumonia and absolute risk of 30-day mortality in men without other risk factors.
Supplementary Fig. 8.
Pneumonia and absolute risk of 30-day mortality in women without other risk factors.
Supplementary Fig. 9.
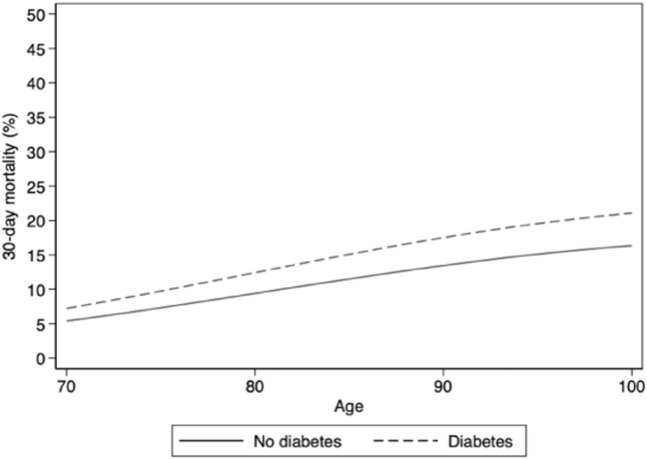
Diabetes and absolute risk of 30-day mortality in men without other risk factors.
Men had 2.6-fold higher odds of 30-day mortality than women after adjustment for other risk factors. The absolute risk of death increased with increasing age; for example, 30-day mortality was approximately 13% in 70-year-old men and 30% in 90-year-old men without other risk factors (Supplementary Figure 11). Factors related to nutrition (BMI, fluid intake, and food intake) were not associated with 30-day mortality after adjustment, although there was a trend toward increased risk from underweight. Mild and severe neuropsychological conditions (dementia or depression) were both highly prevalent and associated with higher 30-day mortality after adjustment. Compared to those with no conditions, residents with severe conditions had more than twice the odds of 30-day mortality. Neuropsychological conditions were also strongly associated with absolute risk of mortality. For example, a 90-year-old male resident with severe neuropsychological conditions had a 30-day mortality risk of almost 50%, which would have been almost 30% if he had not had neuropsychological conditions (Supplementary Figure 1). With respect to walking ability, most residents walked unsafely or were unable to walk, which was associated with higher 30-day mortality after adjustment. Compared to those in good general physical condition, residents in poorer condition had higher odds of 30-day mortality after adjustment. A third of residents had urinary and bowel incontinence, which was associated with 1.5-fold higher odds of 30-day mortality after adjustment.
Comorbidities associated with higher adjusted odds of 30-day mortality included diabetes, renal failure or chronic kidney disease, and pneumonia. Importantly, diabetes and previous pneumonia were also highly prevalent. Cancer, chronic obstructive pulmonary diseases, and antihypertensives (other than diuretics) were not associated with 30-day mortality before or after adjustment. Cardiovascular diseases, antithrombotic medication, and diuretics were only associated with higher 30-day mortality before adjustment.
Sensitivity Analysis
The sensitivity analysis comprised 1421 residents registered in Senior Alert within 3 months of confirmed COVID-19 (median 47 days, interquartile range 27-68). Of these, 566 died within 30 days (39.9%). Overall, this analysis confirmed the results of the main analysis (Supplementary Table 2).
Discussion
This study showed that 30-day mortality was 40% in Swedish LTCF residents with COVID-19, which was 7 times higher than in matched controls without COVID-19. Beyond older age and male sex, independent risk factors for higher mortality were neuropsychological conditions, impaired walking ability, incontinence, diabetes, chronic kidney disease, and previous pneumonia. These risk factors, most of which are not modifiable, were highly prevalent, and associated with a high absolute risk of death, altogether emphasizing the importance of preventing COVID-19 transmission to LTCFs.
The 40% mortality rate in our study is almost twice as high as in a US study of more than 5000 nursing home residents.19 This difference likely reflects that our study cohort was older. Smaller studies, conducted in age groups similar to ours, showed more comparable mortality rates.15 , 28 , 29 A limitation of all these studies is that they lacked a control group, impeding assessment of excess mortality. In this sense, our results add important evidence regarding the profound dangers of COVID-19 in LTCFs, as illustrated by the 7-fold higher mortality. Although the reason for the high mortality is likely multifactorial and complex, the disease indisputably has a tremendous significance from a public health perspective, affecting older adults, especially those living in LTCFs, disproportionally. In support, a recent study showed that LTCF residents had 4 times higher risk of COVID-19 mortality, compared with community-dwelling older adults.30 Further, another study showed that COVID-19 is more dangerous for older adults compared to seasonal influenza, especially for older adults with certain comorbidities, being associated with a 5-fold higher risk of death.31 Our study provides additional evidence that COVID-19 mortality is high also in older adults without other risk factors. Altogether, the findings from our study suggest that COVID-19 has caused a large number of premature deaths in Swedish LTCFs.
In our study, older age, male sex, and neuropsychological conditions were among the most important risk factors for 30-day mortality in LTCF residents with COVID-19. Although these risk factors are known from previous studies,15 , 16 , 19 , 29 less is known about their additive effects. Therefore, we also examined how the absolute risk of death varied depending on these 3 risk factors. For example, a 90-year-old male resident with severe neuropsychological conditions had a 30-day mortality risk of around 50%, which would have been 30% if he had not had neuropsychological conditions. In women, the corresponding difference was around 25% vs 15%. These large absolute differences strengthen the clinical importance of these 3 risk factors, and pinpoints groups that are especially critical to protect against being infected in LTCFs.
Two other common patient characteristics that were associated with higher mortality were impaired walking ability and urine and bowel incontinence. Previous studies found physical function and frailty to be risk factors for 30-day mortality after COVID-19 in LTCFs15 , 19 and in-hospital mortality in older adults.32 In one study, bowel incontinence was a risk factor for COVID-19 diagnosis.17 Thus, our study shows that in a large cohort of LTCF residents, easy-to-assess characteristics such as walking ability and incontinence are prevalent and independent risk factors for mortality after COVID-19.
In contrast, no association was found between obesity and mortality. Although obesity is a well-known risk factor for developing severe COVID-19 in the general population,14 studies in older people have shown conflicting results.33 , 34 The lack of association in our study may be related to the well-known obesity paradox in very old people,35 for whom body-mass-index is a poor indicator of body composition and body fat distribution.36 It has also been hypothesized that malnutrition could be an important risk factor,37 , 38 but we did not find an association between food intake and mortality after adjustment for other risk factors, as in a previous study.18 However, there was a trend toward an increased risk of mortality in those with the lowest BMI. Although this did not reach statistical significance, likely because of the small number of people in that BMI category, it cannot be ruled out that underweight is a risk factor for COVID-19 mortality in LTCF residents.
Having diabetes or renal failure or chronic kidney disease was both common and associated with increased risk of 30-day mortality. Both these conditions have previously been identified as risk factors for mortality following COVID-19 in LTCF residents.19 Also, history of pneumonia was common and a strong risk factor for 30-day mortality. Although we are not aware of any other studies that have investigated pneumonia as a risk factor in LTCF residents, it was recently shown that previous pneumonia is a risk factor for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in the general Swedish population.39 Hypothetically, previous pneumonia could be a marker of impaired immune function that increases one’s susceptibility for severe COVID-19 infection.
In our study, antihypertensives were not associated with mortality after COVID-19. This extends the results of observational studies showing that hypertension is not a risk factor in LTCF residents19 , 29 and is supported by randomized studies showing that continuation of antihypertensive treatment did not increase the risk of severe outcomes, as compared to discontinuation, in patients hospitalized with COVID-19.40 , 41 Similarly, many other common diseases or medications were not associated with mortality in this study, including cardiovascular disease, antithrombotics, pulmonary disease, and cancer. An explanation for these findings could be that many prevalent diagnoses have little impact on mortality risk in very frail older people who have lived to an old age. It should also be noted that because different conditions are likely less often diagnosed in LTCFs, and primary care diagnoses care are not captured in the NPR, the sensitivity to capture different diagnoses is likely lower than in community-dwelling individuals. Regardless, our results are similar to previous studies of nursing home residents,15 , 19 , 29 geriatric patients,32 and veterans.42
This study has several important strengths. To our knowledge, this is the first study to evaluate 30-day mortality following COVID-19 in LTCFs using a control group. The study cohort included a large representative population of LTCF residents from the whole of Sweden, who are of particular importance to study given that they have experienced the highest mortality rates from COVID-19. Moreover, more than 30 potential risk factors and clinical patient characteristics were available, and these were investigated through a comprehensive set of analyses, increasing the credibility of our findings. Some limitations of this study should also be considered. First, the data obtained from Senior Alert may not be completely accurate for the time of COVID-19 infection owing to the lag time between assessment in Senior Alert and baseline (COVID-19), although a sensitivity analysis suggested that this did not bias associations. Second, because the study cohort was restricted to residents in LTCFs with a record in Senior Alert in the past year, generalizability to all LTCF residents in Sweden may in theory be limited. However, our data captured 5409 cases compared to the 7143 cases confirmed in LTCFs in Sweden until mid-September according to official data,43 meaning that the coverage in our study was high. Third, the accuracy regarding identification of certain risk factors may be limited. For example, neuropsychological conditions comprised both dementia and depression, although these are clearly different conditions. Yet, using this item, we observed an odds ratio of similar magnitude to that shown in a previous study where cognitive function was assessed using the Minimum Data Set.19 Moreover, although we had access to a wide array of potential risk factors, we lacked data on symptoms at COVID-19 presentation, which have previously been associated with mortality following COVID-19 in LTCF residents.19 Fifth, data on LTCF characteristics were lacking, for example data on staffing and structure, which could have influenced the transmission and mortality of COVID-19. Sixth, the results are not necessarily generalizable to other countries. Finally, although COVID-19 cases and controls were matched, there may be unmeasured differences between the groups that may partly have contributed to the higher mortality in cases.
Conclusions and Implications
In summary, 30-day mortality was 7 times higher in Swedish LTCF residents with COVID-19 than in matched controls, suggesting that the excess mortality is due to the COVID-19 infection itself and not older age or poorer health status. In addition, beyond older age and male sex, some diagnoses and simple measures of health status predict short-term mortality. Because large-scale community transmission has been deemed one of the primary explanations for the high COVID-19 mortality rates in Swedish LTCFs,8 our findings emphasize that reducing transmission of COVID-19 to LTCFs, would likely prevent a considerable number of deaths in this frail group of older individuals.
Footnotes
The authors received funding used for salaries from Foundation Stockholms Sjukhem (MK), Academy of Finland (MK), Läkarsällskapet (MK), and the Swedish Research Council (MK, AN, PN). The funders had no role in any part of this manuscript or the decision to publish.
The authors declare no conflicts of interest.
Supplementary Material 1
Measures taken by Swedish authorities during the first wave of the COVID-19 pandemic.
An in-depth review of Sweden’s COVID-19 strategy, including a detailed timeline of key events and adopted measures, during the first wave of the pandemic is available from Ludvigsson JF. The first eight months of Sweden’s COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr 2020; 109:2459–2471. Available at: https://onlinelibrary.wiley.com/doi/10.1111/apa.15582.
Supplementary Table 1.
Definitions of Comorbidities and Prescription Medications
| Variable | Definition | Code Type | Codes |
|---|---|---|---|
| Comorbidities | |||
| Myocardial infarction | Myocardial infarction | ICD-9/10-SE | I21, I22, 410 |
| Stroke | Stroke | ICD-9/10-SE | I60-I64, 431-434 |
| Angina pectoris | Angina pectoris | ICD-9/10-SE | I20, 413 |
| Heart failure | Heart failure | ICD-9/10-SE | I50, 428 |
| Atrial fibrillation | Atrial fibrillation | ICD-9/10-SE | I48, 427D |
| Autoimmune disease | Rheumatoid arthritis | ICD-9/10-SE | M05, M06, 714 |
| Inflammatory bowel disease | ICD-9/10-SE | K50-K52, 555 | |
| Multiple sclerosis | ICD-10-SE | G35 | |
| Autoimmune hepatitis | ICD-10-SE | K754 | |
| Systemic lupus erythematosus | ICD-9/10-SE | M32, 710A | |
| Psoriatic arthritis | ICD-10-SE | L405, M073 | |
| Ankylosing spondylitis | ICD-9/10-SE | M45, 720A | |
| Giant cell arteritis | ICD-9/10-SE | M315, M316, 446F | |
| Polymyalgia rheumatica | ICD-9/10-SE | M353, 725 | |
| Sjögren/sicca syndrome | ICD-9/10-SE | M350, 710C | |
| Systemic sclerosis | ICD-9/10-SE | M34, 710B | |
| Diabetes | Diabetes | ICD-9/10-SE | E10, E11, 250 |
| Antidiabetic agent | ATC | A10 | |
| Cancer | Malignant neoplasms | ICD-10-SE | C |
| Malignant neoplasms | ICD-7-SE∗ | 140-209∗ | |
| Chronic obstructive pulmonary disease | Chronic obstructive pulmonary disease | ICD-9/10-SE | J20, J40-J44, 491, 492 |
| Asthma | Asthma | ICD-9/10-SE | J45, J46, 493 |
| Renal failure or chronic kidney disease | Renal failure or chronic kidney disease | ICD-9/10-SE | N17-N19, 584-586 |
| Liver disease | Liver disease | ICD-10-SE | K70-K77 |
| Sepsis | Sepsis | ICD-10-SE | A40, A41 |
| Influenza | Influenza | ICD-10-SE | J09-J11 |
| Pneumonia | Pneumonia | ICD-10-SE | J12-J18 |
| Alcohol intoxication | Alcohol intoxication | ICD-9/10-SE | F10, 291, 303 |
| Prescription medications | |||
| Antithrombotics | Antithrombotics | ATC | B01A |
| Antihypertensives† (other than diuretics) | Angiotensin-converting enzyme inhibitors/angiotensin II receptor blocker | ATC | C09 |
| Calcium-receptor blocker | ATC | C08 | |
| Diuretic† | Diuretic | ATC | C03 |
| Antidepressants† | Antidepressants | ATC | N06A |
| Psycholeptics† | Psycholeptics | ATC | N05 |
ATC, anatomical therapeutic chemical; ICD-9/10-SE, International Classification of Diseases, 9th/10th Revision, Swedish Version.
Obtained from the Swedish Cancer Registry from 1964-2018.
Information regarding use of these medications were additionally obtained from the Senior Alert assessment. Thus, use of these medications were defined as either a prescription in the Prescribed Drug Register or as use reported in Senior Alert.
Supplementary Table 2.
Risk Factors for All-Cause 30-Day Mortality in the Sensitivity Analysis Including Residents With COVID-19 Who Were Registered in Senior Alert Within 3 Months of COVID-19 Diagnosis
| Variables | Adjusted∗ OR (95% CI) |
|---|---|
| Male sex | 2.67 (2.06, 3.46) |
| Age group | |
| <70 | 1 (ref) |
| 70-74 | 1.45 (0.68, 3.11) |
| 75-79 | 1.11 (0.55, 2.27) |
| 80-84 | 1.53 (0.77, 3.06) |
| 85-89 | 1.99 (1.00, 3.93) |
| ≥90 | 2.51 (1.27, 4.98) |
| BMI categories | |
| Underweight (<18.5) | 1.11 (0.67, 1.83) |
| Normal weight (18.5-24.99) | 1 (ref) |
| Overweight (25.0-29.99) | 1.41 (1.08, 1.84) |
| Obesity (≥30) | 1.21 (0.83, 1.77) |
| Neuropsychological conditions | |
| None | 1 (ref) |
| Mild dementia or depression | 1.12 (0.83, 1.52) |
| Severe dementia or depression | 1.84 (1.28, 2.64) |
| Known previous falls | 1.27 (0.99, 1.62) |
| Walking ability | |
| Safe with or without walking aids | 1 (ref) |
| Unsafe walk | 1.45 (1.08, 1.95) |
| Unable to walk | 1.78 (1.26, 2.49) |
| Fluid intake, mL/d | |
| >1000 | 1 (ref) |
| 700-1000 | 0.96 (0.74, 1.24) |
| <700 | 0.74 (0.43, 1.28) |
| Food intake | |
| Normal serving | 1 (ref) |
| ¾ serving | 0.94 (0.70, 1.28) |
| ≤½ serving | 1.12 (0.76, 1.64) |
| General physical condition | |
| Good | 1 (ref) |
| Fair | 1.44 (1.12, 1.85) |
| Poor or very bad | 1.48 (0.74, 2.96) |
| Incontinence | |
| No | 1 (ref) |
| Temporarily but unusual | 0.93 (0.62, 1.40) |
| Urinary or bowel | 1.18 (0.84, 1.66) |
| Urinary and bowel | 1.47 (1.04, 2.07) |
| Comorbidities | |
| Stroke | 0.95 (0.71, 1.25) |
| Myocardial infarction | 0.87 (0.60, 1.27) |
| Angina pectoris | 1.00 (0.70, 1.42) |
| Heart failure | 1.02 (0.73, 1.42) |
| Atrial fibrillation | 0.88 (0.65, 1.19) |
| Autoimmune disease | 1.12 (0.79, 1.58) |
| Diabetes | 1.31 (0.98, 1.74) |
| COPD | 0.73 (0.50, 1.06) |
| Asthma | 0.80 (0.50, 1.29) |
| Cancer | 0.87 (0.69, 1.11) |
| Renal failure or chronic kidney disease | 1.12 (0.79, 1.60) |
| Liver disease | 1.08 (0.44, 2.66) |
| Sepsis | 0.66 (0.42, 1.03) |
| Influenza | 0.70 (0.39, 1.23) |
| Pneumonia | 1.79 (1.35, 2.38) |
| Alcohol intoxication | 0.58 (0.35, 0.98) |
| Medications | |
| Antithrombotics | 1.21 (0.92, 1.58) |
| Antihypertensives (other than diuretics) | 1.10 (0.85, 1.43) |
| Diuretics | 0.84 (0.64, 1.09) |
| Antidepressants | 1.16 (0.90, 1.48) |
| Psycholeptics | 1.15 (0.87, 1.51) |
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Adjusted for all variables in column 1. The analysis included N = 1421 participants and 566 cases of death.
Supplementary Table 3.
Amount of Missing Data in Residents With COVID-19
| Variables | Missing Data in the Total COVID-19 Cohort (n = 4177) | Missing Data Among Those Who Died Within 30 d (n = 1647) | Missing Data Among Those Alive at 30 d (n = 2530) |
|---|---|---|---|
| Sex | 0 | 0 | 0 |
| Age | 0 | 0 | 0 |
| BMI | 6 | 2 | 4 |
| Neuropsychological conditions | 213 | 73 | 140 |
| Known previous falls | 285 | 101 | 184 |
| Walking ability | 285 | 101 | 184 |
| Fluid intake, mL/d | 235 | 85 | 150 |
| Food intake | 235 | 85 | 150 |
| General physical condition | 235 | 85 | 150 |
| Incontinence | 235 | 85 | 150 |
| Comorbidities | |||
| Stroke | 0 | 0 | 0 |
| Myocardial infarction | 0 | 0 | 0 |
| Angina pectoris | 0 | 0 | 0 |
| Heart failure | 0 | 0 | 0 |
| Atrial fibrillation | 0 | 0 | 0 |
| Autoimmune disease | 0 | 0 | 0 |
| Diabetes | 0 | 0 | 0 |
| COPD | 0 | 0 | 0 |
| Asthma | 0 | 0 | 0 |
| Cancer | 0 | 0 | 0 |
| Renal failure or chronic kidney disease | 0 | 0 | 0 |
| Liver disease | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 0 |
| Influenza | 0 | 0 | 0 |
| Pneumonia | 0 | 0 | 0 |
| Alcohol intoxication | 0 | 0 | 0 |
| Medications | |||
| Antithrombotics | 0 | 0 | 0 |
| Antihypertensives (other than diuretics) | 0 | 0 | 0 |
| Diuretics | 0 | 0 | 0 |
| Antidepressants | 0 | 0 | 0 |
| Psycholeptics | 0 | 0 | 0 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
References
- 1.World Health Organization Infection prevention and control guidance for long-term care facilities in the context of COVID-19: Interim guidance, 21 March 2020. https://apps.who.int/iris/handle/10665/331508 Available at:
- 2.ECDC Public Health Emergency Team. Danis K., Fonteneau L., Georges S. High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Euro Surveill. 2020;25:2000956. doi: 10.2807/1560-7917.ES.2020.25.22.2000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson D.C., Barbu M.G., Beiu C. The impact of COVID-19 pandemic on long-term care facilities worldwide: An overview on international issues. Biomed Res Int. 2020;2020:8870249. doi: 10.1155/2020/8870249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepulveda E.R., Stall N.M., Sinha S.K. A comparison of COVID-19 mortality rates among long-term care residents in 12 OECD countries. J Am Med Dir Assoc. 2020;21:1572–1574.e1573. doi: 10.1016/j.jamda.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau-Ng R., Caruso L.B., Perls T.T. COVID-19 deaths in long-term care facilities: A critical piece of the pandemic puzzle. J Am Geriatr Soc. 2020;68:1895–1898. doi: 10.1111/jgs.16669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socialstyrelsen Statistik om smittade och avlidna med covid-19 bland äldre efter boendeform. https://www.socialstyrelsen.se/globalassets/1-globalt/covid-19-statistik/statistik-om-covid-19-bland-aldre-efter-boendeform/faktablad-statistik-om-smittade-och-avlidna-med-covid-19-bland-aldre-efter-boendeform.pdf Available at: Published May 6, 2020.
- 7.The Corona Commission International experiences of covid-19 in nursing homes. 2020. https://www.regeringen.se/4af363/contentassets/a8e708fff5e84279bf11adbd0f78fcc1/internationella-erfarenheter-av-covid-19-i-aldreboenden_webb.pdf Available at: Published 2020.
- 8.The Corona Commission Elderly care during the pandemic. 2020. https://www.regeringen.se/4af379/contentassets/a8e708fff5e84279bf11adbd0f78fcc1/sou_2020_80_aldreomsorgen-under-pandemin.pdf Available at: Published 2020.
- 9.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim L., Garg S., O'Halloran A. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) Clin Infect Dis. 2021;72:e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reilev M., Kristensen K.B., Pottegard A. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: A nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Lu Y., Huang Y.M. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielza R., Sanz J., Zambrana F. Clinical characteristics, frailty, and mortality of residents with COVID-19 in nursing homes of a region of Madrid. J Am Med Dir Assoc. 2021;22:245–252.e2. doi: 10.1016/j.jamda.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham N.S.N., Junghans C., Downes R. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81:411–419. doi: 10.1016/j.jinf.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S.M., Bakaev I., Chen H. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21:1378–1383.e1371. doi: 10.1016/j.jamda.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarteret P., Strazzulla A., Rouyer M. Clinical features and medical care factors associated with mortality in French nursing homes during the COVID-19 outbreak. Int J Infect Dis. 2020;104:125–131. doi: 10.1016/j.ijid.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panagiotou O.A., Kosar C.M., White E.M. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181:439–448. doi: 10.1001/jamainternmed.2020.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edvinsson J., Rahm M., Trinks A., Hoglund P.J. Senior alert: A quality registry to support a standardized, structured, and systematic preventive care process for older adults. Qual Manag Health Care. 2015;24:96–101. doi: 10.1097/QMH.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 21.Senior Alert Annual report 2019. https://plus.rjl.se/info_files/infosida40605/118_Senior_alert_Arsrapport_2019.pdf Available at:
- 22.Brooke H.L., Talbäck M., Hörnblad J. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61:721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y.P., Propert K.J., Rosenbaum P.R. Balanced risk set matching. J Am Stat Assoc. 2001;96:870–882. [Google Scholar]
- 26.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman K.J., Greenland S., Lash T.L. Lippincott Williams & Wilkins; Baltimore, MD: 2008. Modern Epidemiology. [Google Scholar]
- 28.Atalla E., Zhang R., Shehadeh F. Clinical presentation, course, and risk factors associated with mortality in a severe outbreak of COVID-19 in Rhode Island, USA, April-June 2020. Pathogens. 2020;10:8. doi: 10.3390/pathogens10010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouns S.H., Bruggemann R., Linkens A. Mortality and the use of antithrombotic therapies among nursing home residents with COVID-19. J Am Geriatr Soc. 2020;68:1647–1652. doi: 10.1111/jgs.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandén M., Aradhya S., Kolk M. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: A population-based, observational study using individual-level data. Lancet Healthy Longev. 2020;1:e80–e88. doi: 10.1016/S2666-7568(20)30016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y., Bowe B., Maddukuri G., Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with COVID-19 and seasonal influenza: Cohort study. BMJ. 2020;371:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagg S., Jylhava J., Wang Y. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559.e1552. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattar N., Ho F.K., Gill J.M. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes Metab Syndr. 2020;14:1149–1151. doi: 10.1016/j.dsx.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobolowsky F.A., Bardossy A.C., Currie D.W. Signs, Symptoms, and Comorbidities Associated With Onset and Prognosis of COVID-19 in a Nursing Home. J Am Med Dir Assoc. 2021;22(3):498–503. doi: 10.1016/j.jamda.2021.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S., Ren J. Obesity paradox in aging: From prevalence to pathophysiology. Prog Cardiovasc Dis. 2018;61:182–189. doi: 10.1016/j.pcad.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Kuk J.L., Saunders T.J., Davidson L.E., Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Lidoriki I., Frountzas M., Schizas D. Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly? Med Hypotheses. 2020;144:109946. doi: 10.1016/j.mehy.2020.109946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li T., Zhang Y., Gong C. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergman J., Ballin M., Nordström A., Nordström P. Risk factors for COVID-19 diagnosis, hospitalization and subsequent all-cause mortality in Sweden: A nationwide study. Eur J Epidemiol. 2021;36:287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopes R.D., Macedo A.V.S., de Barros E. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: A randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J.B., Hanff T.C., William P. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: A prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou G.N., Locke E., Green P. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US Veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Public Health Agency of Sweden Weekly report on covid-19, week 37. 2020. https://www.folkhalsomyndigheten.se/globalassets/statistik-uppfoljning/smittsamma-sjukdomar/veckorapporter-covid-19/2020/covid-19-veckorapport-vecka-37-final.pdf Available at: Published September 18, 2020.