This genetic association study investigates the extent of shared genetic architecture between schizophrenia and brain cortical surface area and thickness.
Key Points
Question
To what extent is the genetic architecture of schizophrenia shared with cortical brain surface area and thickness?
Findings
In this genetic association study, data sets revealed that 94% of the genetic variants associated with total cortical surface area and all variants associated with average cortical thickness were also associated with the genetic risk of schizophrenia, despite nonsignificant genetic correlations.
Meaning
The amount of shared genetic variants between schizophrenia and cortical brain structure suggests overlapping molecular genetic mechanisms between cortical development and schizophrenia.
Abstract
Importance
Schizophrenia is a complex heritable disorder associated with many genetic variants, each with a small effect. While cortical differences between patients with schizophrenia and healthy controls are consistently reported, the underlying molecular mechanisms remain elusive.
Objective
To investigate the extent of shared genetic architecture between schizophrenia and brain cortical surface area (SA) and thickness (TH) and to identify shared genomic loci.
Design, Setting, and Participants
Independent genome-wide association study data on schizophrenia (Psychiatric Genomics Consortium and CLOZUK: n = 105 318) and SA and TH (UK Biobank: n = 33 735) were obtained. The extent of polygenic overlap was investigated using MiXeR. The specific shared genomic loci were identified by conditional/conjunctional false discovery rate analysis and were further examined in 3 independent cohorts. Data were collected from December 2019 to February 2021, and data analysis was performed from May 2020 to February 2021.
Main Outcomes and Measures
The primary outcomes were estimated fractions of polygenic overlap between schizophrenia, total SA, and average TH and a list of functionally characterized shared genomic loci.
Results
Based on genome-wide association study data from 139 053 participants, MiXeR estimated schizophrenia to be more polygenic (9703 single-nucleotide variants [SNVs]) than total SA (2101 SNVs) and average TH (1363 SNVs). Most SNVs associated with total SA (1966 of 2101 [93.6%]) and average TH (1322 of 1363 [97.0%]) may be associated with the development of schizophrenia. Subsequent conjunctional false discovery rate analysis identified 44 and 23 schizophrenia risk loci shared with total SA and average TH, respectively. The SNV associations of shared loci between schizophrenia and total SA revealed en masse concordant association between the discovery and independent cohorts. After removing high linkage disequilibrium regions, such as the major histocompatibility complex region, the shared loci were enriched in immunologic signature gene sets. Polygenic overlap and shared loci between schizophrenia and schizophrenia-associated regions of interest for SA (superior frontal and middle temporal gyri) and for TH (superior temporal, inferior temporal, and superior frontal gyri) were also identified.
Conclusions and Relevance
This study demonstrated shared genetic loci between cortical morphometry and schizophrenia, among which a subset are associated with immunity. These findings provide an insight into the complex genetic architecture and associated with schizophrenia.
Introduction
Schizophrenia is a severe brain disorder with an estimated heritability up to 80%1 in twin studies, indicating a prominent genetic component in its cause. Genetic risk of schizophrenia has been suggested to influence early brain development,2 and patients with schizophrenia exhibit many brain structural abnormalities.3,4 Recent structural magnetic resonance imaging studies have reported a smaller total cortical surface area (SA) and thinner average cortical thickness (TH) in individuals with schizophrenia, with the largest effect sizes found in the frontal and temporal lobes.3,5 Similar frontotemporal patterns in SA and TH were also reported in nonaffected relatives of patients with schizophrenia6 and in comparison with polygenic risk for schizophrenia in healthy population.7 Because both SA and TH are highly heritable with common single-nucleotide variants (SNVs) explaining 26% to 34% of the phenotypic variation,8 the above findings proposed that the genetic mechanisms contributing to the risk of schizophrenia may also influence brain anatomy. This hypothesis is further supported by evidence that polygenic risk scores of schizophrenia is associated with global TH in healthy individuals9,10 and that genetic variance of schizophrenia is shared with SA (0.5%) and TH (6.3%) in twins.11
While previous studies have indicated an overlapping genetic basis between schizophrenia and brain anatomy,12,13 the polygenic overlap of schizophrenia and cortical brain structure remains elusive. A recent genetic-wide association study (GWAS) of SA and TH found no significant genetic correlation with schizophrenia using linkage disequilibrium (LD) score regression.8 However, LD score regression14 is not able to capture genetic overlap with mixed-effect directions, which is often the case between complex phenotypes.15,16 To address that, Frei et al16 developed MiXeR, which evaluates genetic overlap regardless of effect directions and estimates the overall shared polygenic architecture. Moreover, identifying specific shared genetic loci offers biological insights into the polygenic overlap between 2 traits. Because both schizophrenia and brain anatomy are associated with many small-effect genetic variants, applying statistical tools, such as conditional/conjunctional false discovery rate (cFDR),15,17,18 to improve the yield of existing GWAS provides a cost-efficient approach to boost power for discovery. The cFDR was built on an empirical bayesian statistical framework, and it improves power of SNV detection by leveraging the combined power of 2 GWASs.19,20,21,22,23,24,25 For example, while conventional statistical tools reported no evidence of genetic overlap between schizophrenia and subcortical volumes,26 cFDR identified shared genomic loci between schizophrenia and subcortical volumes using cross-trait SNV enrichment.27
Here we applied MiXeR16 to investigate whether schizophrenia shares a genetic basis with cortical SA and TH and the cFDR approach15,17,18 to identify specific shared loci using GWAS summary statistics. By exploring the shared genetic architecture of schizophrenia and brain structure, we ultimately aim to provide key insights into the pathophysiology underlying schizophrenia.
Methods
GWAS Data Sets
Details of data sets and methods are provided in the eMethods in Supplement 1 and corresponding publications.8,16,17,18,28,29 The GWAS data sets were collected from December 2019 to February 2021, and data analysis was performed from May 2020 to February 2021.
All GWAS data sets included in this study were approved by the relevant ethics committees, and informed consent was obtained from all participants. The Regional Committee for Medical and Health Research Ethics of South-East Norway approved the inclusion of data from UK Biobank.
For the discovery analyses, the GWAS summary statistics for schizophrenia (40 675 cases and 64 643 controls) were generated by meta-analyzing the schizophrenia cases from UK (CLOZUK) and an independent Psychiatric Genomics Consortium data sets.28,29 The summary statistics of total SA and average TH were generated from UK Biobank (n = 33 735).30 For validation of findings, we included summary statistics of independent cohorts, including schizophrenia from a Norwegian cohort (961 cases and 5000 controls) and total SA and average TH from the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) cohort of European ancestry (n = 23 909).7 We also included summary statistics for schizophrenia in East Asian cohorts (22 778 cases and 35 362 healthy controls).31
Summary statistics for regional SA and TH were acquired from the UK Biobank with 33 735 volunteers.30 We selected regions that have been reported as significantly different with the largest effect sizes between schizophrenia cases and healthy controls in a previous large-scale study,3 including fusiform, inferior temporal gyrus, middle temporal gyrus, precentral, and superior frontal gyrus for SA and fusiform, inferior temporal gyrus, middle temporal gyrus, superior frontal gyrus, and superior temporal gyrus, insula and pars opercularis for TH.
As height showed phenotypic association,32 limited shared genomic regions,33 and nonsignificant genetic correlation34 with schizophrenia, we included a height GWAS data set as a heritable, polygenic, non–brain-related comparator.35
Statistical Analysis
We constructed conditional quantile-quantile (QQ) plots to visualize polygenic enrichment, after excluding SNVs within 4 regions with complex LD pattern (major histocompatibility complex [MHC] region: chr6:25119106–33854733; 8p23.1: chr8:7200000–12500000; MAPT region: chr17:40000000–47000000; apolipoprotein E region: chr19:44909039–45912650).36 Each QQ plot reveals the distribution of P values for the primary phenotype conditioning on the significance of association with the secondary trait at the level of P < .10, P < .010, and P < .001.
Polygenic overlap irrespective of genetic correlation between selected phenotypes was evaluated by MiXeR.16,37 Univariate analysis estimated polygenicity (estimated number of variants) and discoverability (the average magnitude of additive genetic associations across variants) of each phenotype. Bivariate analysis modeled additive genetic associations with 2 traits as a mixture of 4 bivariate Gaussian components, representing variants influencing only 1 trait, variants affecting both traits, and variants have no association with either trait. The number of variants associated with the unique polygenic components and of the shared polygenic component were estimated. MiXeR calculated a Dice coefficient, a ratio of shared variants to the total number of variants, to evaluate the polygenic overlap. Based on Akaike information criterion, MiXeR evaluated model fitting based on current power of input summary statistics. A comparison between MiXeR and LD score regression is provided in the eMethods in Supplement 1.
The cFDR method,38 including conditional FDR (condFDR) and conjunctional FDR (conjFDR) analysis, was applied to identify specific shared loci.15,17,18 The condFDR analysis, as an extension of standard FDR, uses the associations between variants and the secondary phenotype to rerank test statistics and to recalculate the associations between these variants and the primary phenotype. Thus, we applied condFDR to boost the power of SNV discovery for each individual trait. The conjFDR analysis is a conservative estimate of the posterior probability that an SNV has no association with either trait, given that the P values for that SNV in both the primary and secondary traits are lower than the observed P values. After repeating condFDR for both traits, we applied conjFDR analysis to identify shared genetic loci. The FDR significance cutoffs were 0.05 for conjFDR and 0.01 for condFDR in line with prior studies.15,17 We also included shared loci with conjFDR less than 0.01 in the eResults in Supplement 1.
For identified loci, we examined significance and directionality of allelic association in independent cohorts using lead SNVs. For genomic loci with a primary lead SNV missing in an independent data set with European ancestry, we assigned a secondary lead SNV if available. SNV sign test26,29 verified directionality of allelic association between the discovery and independent cohorts with the null hypothesis of randomly oriented associations. Sign concordancy for shared lead SNVs was defined only when SNV associations of both phenotypes showed congruent associations in independent cohorts.
Identify Genomic Loci, Functional Annotation, and Novelty
Independent genomic loci were defined using FUMA protocol.39 A locus that was not physically overlapping with findings from the original GWASs, National Human Genome Research Institute–European Bioinformatics Institute GWAS Catalog,40 or previous cFDR studies16,17,18 was considered as novel. The z scores from original GWASs reflected the directionality of allelic association. FUMA39 annotated Combined Annotation Dependent Depletion,41 RegulomeDB scores,42 and chromatin states43,44 for candidate SNVs with FDR of 0.1 or less. We performed 3 gene mapping strategies to candidate SNVs, including (1) positional mapping by physical position using a 10-kb window; (2) expression quantitative trait locus [eQTL] association; and (3) chromatin interaction mapping.39 Genes mapped by all 3 mapping strategies were considered as credible genes. After excluding mapped genes in the regions with complex LD pattern (MHC, 8p23.1, MAPT, apolipoprotein E regions), we applied the rest in gene set analysis.39 The Molecular Signatures Database evaluated enrichment in immunological signature gene sets.45 GTEx portal (version 8) obtained eQTL associations in brain tissues for lead SNVs and expression trajectories for genes.46
Results
Genetic Overlap Between Schizophrenia and Global Cortical Structural Measures
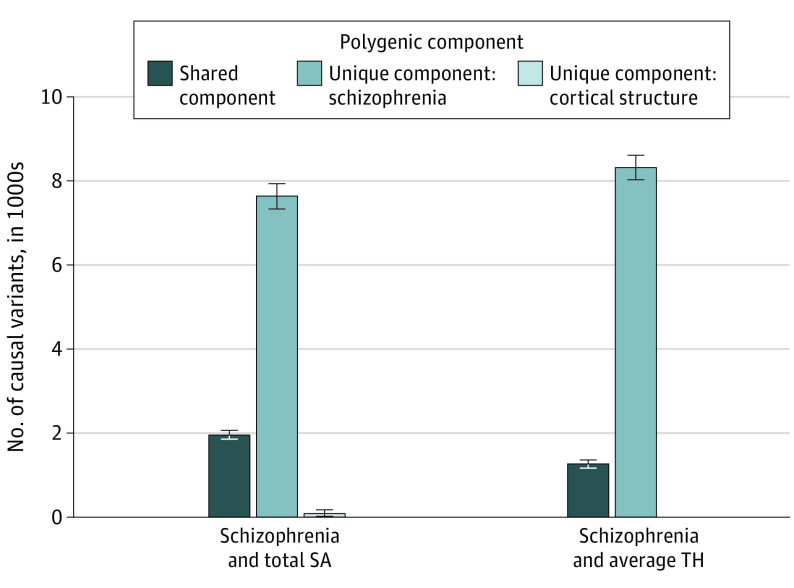
The stratified conditional QQ plots showed SNV enrichment for schizophrenia as a function of their associations with total SA and average TH and vice versa (eFigure 1 in Supplement 1), suggesting the existence of polygenic overlap. While the corresponding genetic correlations were not significant (schizophrenia and total SA: genetic r = −0.01; schizophrenia and average TH: genetic r = 0.01), 94% of variants associated with total SA (1966 of 2101; Dice coefficient = 0.33) and 97% of variants associated with average TH (1322 of 1363; Dice coefficient = 0.24) may contribute to the risk of schizophrenia (Figure 1; eTables 1 and 2 and eFigure 2 in Supplement 1). Specifically, 51% of shared variants between schizophrenia and total SA and 50% shared variants between schizophrenia and average TH showed concordant associations with both traits. MiXeR also revealed a higher polygenicity in schizophrenia than in total SA and average TH with 7737 and 8380 variants associated with schizophrenia but not associated with SA and TH, respectively. As a somatic control, height shared a smaller proportion of variants (1049 of 4026 [26%]; Dice coefficient = 0.15; eTables 1 and 2 and eFigure 2 in Supplement 1) with schizophrenia, with a nonsignificant genetic correlation.
Figure 1. Polygenic Comparison of Schizophrenia and Global Cortical Measures in Discovery Study.
Bar diagram visualizing the estimated unique and shared variants, shown in thousands. Error bars indicate standard errors. SA indicates surface area; TH, thickness.
Shared and Novel Loci Between Schizophrenia and Global Cortical Structural Measures
The conjFDR analysis identified 44 genomic loci shared between schizophrenia and total SA (Figure 2), with 19 and 37 novel loci for schizophrenia and total SA, respectively. Schizophrenia and average TH shared 23 genomic loci (Figure 2), with 8 and 19 loci novel for schizophrenia and average TH, respectively. Four loci were jointly associated with schizophrenia, total SA, and average TH. The shared loci showed mixed directions of allelic associations, with 17 of 44 loci (39%) showing concordant associations with schizophrenia and total SA, and 8 of 23 loci (35%) had concordant associations with schizophrenia and average TH. The shared genomic loci, candidate SNVs, allelic associations, and novelty for each trait are summarized in Supplement 2, eTable 3 in Supplement 1, Supplement 3, and Supplement 4.
Figure 2. Shared Genetic Loci Between Schizophrenia and Global Cortical Measures in Discovery Study.
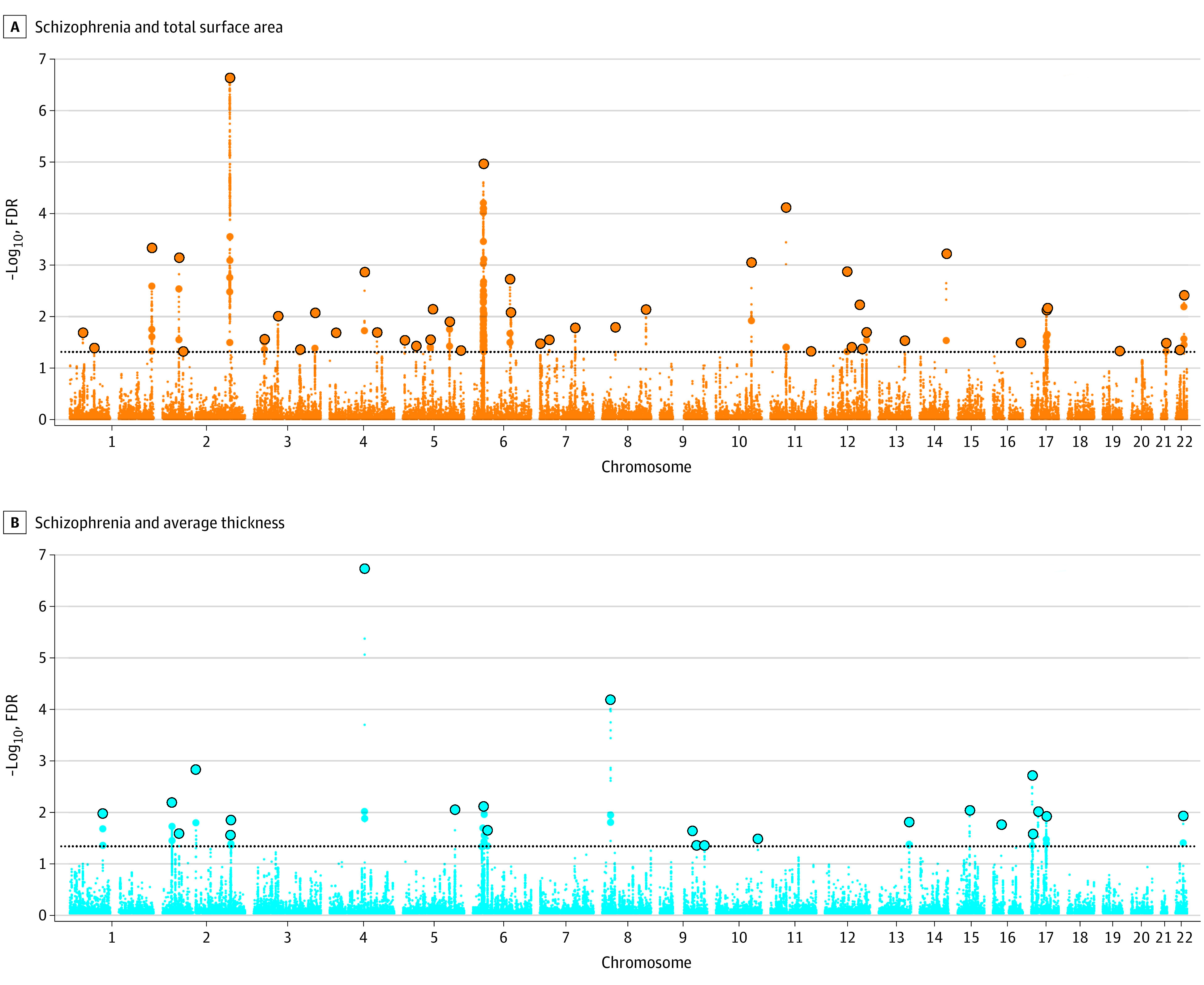
The y-axis represents –log10–transformed conjunctional false discovery rate (FDR) values for each single-nucleotide variant, and the x-axis reflects the chromosomal position. The dotted horizontal line is the cutoff for significance. Details for genomic loci and candidate single-nucleotide variants are provided in eTable 2 in Supplement 1, Supplement 3, and Supplement 4.
Among those shared loci, Combined Annotation Dependent Depletion scores indicated 3 lead SNVs (schizophrenia and SA: rs7146019; schizophrenia and TH: rs13107325 and rs11975) as pathogenic (Combined Annotation Dependent Depletion score >12.37; Supplement 2). A subset of lead SNVs had a minimum chromatin state of less than 8, indicating a location within regulatory regions (Supplement 2). We found 92 significant eQTLs for lead SNVs shared between schizophrenia and total SA and 61 significant eQTLs for lead SNVs shared between schizophrenia and average TH, implying shared loci alter gene expressions in brain tissues (eTable 4 in Supplement 1). After excluding high-LD regions, mapped genes for schizophrenia and total SA were enriched in 26 GO terms, including “GO_COMMITMENT_OF_NEURONAL_CELL_TO_SPECIFIC_NEURON_TYPE_IN_FOREBRAIN” and “GO_POSITIVE_REGULATION_OF_TYPE_I_INTERFERON_PRODUCTION” and 27 immunologic signature gene sets. Mapped genes for schizophrenia and average TH showed significant enrichments in 2 GO terms and 10 immunologic signature gene sets. Mapped genes and gene sets are listed in eTables 5 and 6 in Supplement 1, Supplement 5, and Supplement 6.
In addition, condFDR analysis identified 214 schizophrenia-associated genomic loci, in which 33 were novel for schizophrenia, conditional on their associations with SA (Supplement 7). Conditioning on SNV associations with TH, we found 201 genomic loci associated with schizophrenia, including 28 novel loci. Conditioning on SNV associations with schizophrenia, condFDR analysis reported 76 genomic loci for total SA and 57 loci for average TH, with 52 and 35 novel for total SA and average TH, respectively (Supplement 8).
Concordancy and Significance of Identified Genomic Loci in Independent Samples
Of 43 lead SNVs that were shared between schizophrenia and total SA and available in the independent Norwegian and ENIGMA data sets, 67% (29 of 43) showed a consistent direction of association between the discovery and independent cohorts (binomial P = .02), suggesting corresponding SNV associations replicated en masse in independent cohorts. As for schizophrenia and average TH, 13 of 23 shared lead SNVs showed the same direction of association (binomial P = .34) in independent cohorts. Examining the association of those 23 loci on each phenotype separately showed significant sign concordancy in average TH (concordant sign: 19 of 23 lead SNVs [83%]; binomial P = .001) but not in schizophrenia (concordant sign: 15 of 23 lead SNVs [65%]; binomial P = .11). Further, the genetic association of shared lead SNVs with schizophrenia replicated en masse in East Asian samples31 (schizophrenia and total SA: binomial P = 1.56 × 10−4; schizophrenia and average TH: binomial P = 3.64 × 10−4). Lead SNVs identified in condFDR analysis revealed en masse concordant association in independent cohorts as well as in East Asian samples (eResults in Supplement 1).
A group of shared loci (schizophrenia and total SA: 23 of 43 [54%]; schizophrenia and average TH: 12 of 23 [52%]) showed nominal significance between lead SNVs with at least 1 phenotype (P < .05; Table 1 and Table 2).47,48 For those shared loci, 5 credible mapped genes, including INA, ATP5G1, SREBF2, MAPT, and DNPH1, showed a high expression in the brain cortex (transcripts per million >4049; Figure 3). More details are included in the eResults in Supplement 1, Supplement 9, Supplement 10, Supplement 11, and Supplement 12.
Table 1. Twenty-Three Shared Genomic Loci Between Schizophrenia and Total Surface Area That Showed conjFDR Less Than 0.05 and Significance in Independent Cohortsa.
| Genomic loci | Discovery study | Independent GWAS data setsc | Noveltye | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CHR | Minimum BP | Maximum BP | Primary lead SNV | conjFDR | A1 | A2 | Credible mapped gene | Cor_Effb | Lead SNV | P value | Rep_Dird | ||
| SCZ GWAS | Total SA GWAS | |||||||||||||
| 3 | 1 | 243639859 | 244014490 | rs1058304 | 4.74 × 10−4 | T | C | NA | − | rs1058304 | .675 | 2.35 × 10−4 | 0 | Total SA |
| 4 | 2 | 48177487 | 48707841 | rs72872724 | 7.34 × 10−4 | G | A | FOXN2 | + | rs72872724 | .972 | 1.17 × 10−4 | + | SCZ, total SA |
| 8 | 3 | 71481192 | 71611630 | rs7610856 | 0.010 | A | C | NA | − | rs7610856 | .058 | .005 | + | Total SA |
| 12 | 4 | 103112470 | 103387161 | rs34316881 | 0.001 | T | C | NA | + | rs34316881 | .407 | .001 | + | Total SA |
| 13 | 4 | 139927828 | 140130524 | rs28565133 | 0.021 | T | C | NA | − | rs28565133 | .069 | 5.01 × 10−5 | + | SCZ, total SA |
| 14 | 5 | 5426431 | 5533414 | rs3111175 | 0.030 | G | T | NA | − | rs2578558 | .532 | .030 | + | SCZ, total SA |
| 15 | 5 | 38922826 | 39094257 | rs12233987 | 0.038 | C | T | NA | − | rs12233987 | .220 | .002 | + | SCZ, total SA |
| 19 | 5 | 170776747 | 170848124 | rs7715167 | 0.047 | T | C | NA | − | rs7715167 | .031 | 1.37 × 10−5 | + | SCZ, total SA |
| 21 | 6 | 108868422 | 109035704 | rs9398172 | 0.002 | G | A | FOXO3 | − | rs9398172 | .272 | 4.69 × 10−4 | + | |
| 23 | 7 | 1860728 | 2167939 | rs11764960 | 0.035 | A | G | NA | − | rs11764960 | .472 | .013 | + | Total SA |
| 24 | 7 | 28454580 | 28482115 | rs2391665 | 0.029 | G | A | NA | − | rs2391665 | .383 | .008 | 0 | SCZ, total SA |
| 25 | 7 | 104587323 | 105063372 | rs3779210 | 0.017 | T | C | NA | + | rs3779210 | .101 | 8.44 × 10−6 | + | Total SA |
| 26 | 8 | 40712159 | 40771375 | rs1004412 | 0.017 | T | C | NA | − | rs1004412 | .036 | .664 | 0 | SCZ, total SA |
| 28 | 10 | 104965551 | 105176914 | rs7082288 | 9.11 × 10−4 | T | C | INA; PDCD11 | − | rs7082288 | .013 | 3.35 × 10−8 | + | |
| 29 | 11 | 46257757 | 47242761 | rs6485671 | 7.75 × 10−5 | A | G | NA | + | rs6485671 | .106 | .030 | + | Total SA |
| 31 | 12 | 66277698 | 66384362 | rs61921611 | 0.001 | C | T | NA | − | rs61921611 | .632 | 6.94 × 10−8 | + | SCZ |
| 32 | 12 | 79760388 | 79891269 | rs2895728 | 0.041 | C | T | NA | − | rs2895728 | .327 | 4.01 × 10−4 | 0 | SCZ, total SA |
| 35 | 12 | 123447928 | 123891209 | rs61955214 | 0.021 | T | C | OGFOD2; PITPNM2; MPHOSPH9; RP11-282O18.3; hsa-mir-8072; SETD8 | + | rs61955214 | .665 | .018 | 0 | Total SA |
| 37 | 14 | 99733954 | 99751588 | rs7146019 | 6.16 × 10−4 | A | G | NA | + | rs7146019 | .700 | .004 | 0 | Total SA |
| 39 | 17 | 43463493 | 44865603 | rs2532392 | 0.008 | A | G | MAPT-AS1; MAPT; STH; KANSL1-AS1; RP11-259G18.1; WNT3 | + | rs199504 | .301 | 1.99 × 10−22 | + | SCZ |
| 40 | 17 | 46835629 | 47047868 | rs35929648 | 0.007 | A | G | TTLL6; SUMO2P17; ATP5G1; UBE2Z | − | rs35929648 | .774 | .001 | 0 | Total SA |
| 43 | 22 | 29360297 | 29399244 | rs2213645 | 0.046 | A | G | NA | − | rs2213645 | .976 | 5.50 × 10−5 | + | SCZ, total SA |
| 44 | 22 | 41437526 | 42308739 | rs12170228 | 0.004 | T | C | POLR3H; CSDC2; DESI1; MEI1; SREBF2 | − | rs12170228 | .023 | .899 | + | Total SA |
Abbreviations: A1, allele 1 (effect allele); A2, allele 2 (noneffect allele); BP, genomic position (hg19); conjFDR, conjunctional false discovery rate; CHR, chromosome; Cor_Eff, concordancy of association directions between 2 traits; GWAS, genome-wide association study; NA, not applicable; Rep_Dir, concordancy of association directions between the discovery and independent cohorts; SA, surface area; SCZ, schizophrenia; SNV, single-nucleotide variant.
This table provides 23 genomic loci that identified as shared between SCZ and total SA in a discovery study and showed a significant association with at least 1 phenotype in the independent Norwegian and Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) cohorts.
The directionality of allelic associations with both phenotypes in discovery data sets using z scores is shown here. + Indicates the same direction and −, the opposite direction.
For genomic loci of which the primary lead SNV is not available in independent data sets, we assigned a secondary lead SNV.
The “Rep_Dir” shows the concordancy of association directions of lead SNVs between the discovery and independent samples using z scores. + For both phenotypes show the same direction between the discovery and independent cohorts and 0, for at least 1 phenotype shows opposite direction.
Whether a locus was novel for 2 traits, according to the original GWAS, National Human Genome Research Institute–European Bioinformatics Institute GWAS Catalog, and previous conditional FDR/conjFDR publications is indicated. More information about all identified genomic loci are listed in Supplement 2 and Supplement 9, with details of defining genomic loci, lead SNV, credible mapped genes, and novelty in the eMethods in Supplement 1.
Table 2. Twelve Shared Genomic Loci Between Schizophrenia and Average Thickness That Showed conjFDR Less Than 0.05 and Significance in Independent Cohortsa.
| Genomic loci | Discovery study | Independent GWAS data setsc | Noveltye | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CHR | Minimum BP | Maximum BP | Primary lead SNV | conjFDR | A1 | A2 | Credible mapped gene | Cor_Effb | LEAD_SNV | P value | Rep_Dird | ||
| SCZ GWAS | Average TH GWAS | |||||||||||||
| 1 | 1 | 98336823 | 98664991 | rs10875133 | 0.012 | A | G | NA | + | rs10875133 | .632 | .002 | + | Average TH |
| 2 | 2 | 26961166 | 27351279 | rs34472003 | 0.007 | C | T | SLC35F6; DPYSL5; KHK | − | rs7602823 | .430 | 6.90 × 10−4 | 0 | Average TH |
| 4 | 2 | 97349315 | 98599499 | rs78381888 | .002 | G | A | NA | + | rs78381888 | .636 | .025 | + | Average TH |
| 7 | 4 | 102702364 | 103387161 | rs13107325 | 1.97 × 10−7 | T | C | NA | − | rs13107325 | .209 | 9.97 × 10−5 | + | Average TH |
| 9 | 6 | 26410800 | 33548090 | rs2523548 | 0.008 | A | G | NA | − | rs3130100 | .225 | .006 | + | Average TH |
| 10 | 6 | 43160375 | 43212493 | rs9462876 | 0.024 | G | A | CUL9; DNPH1 | + | rs9462876 | .026 | .848 | 0 | Average TH |
| 11 | 8 | 26119170 | 26279173 | rs147506820 | 7.01 × 10−5 | A | G | SDAD1P1 | + | rs147506820 | .725 | .012 | 0 | Average TH |
| 15 | 10 | 123902771 | 123912298 | rs7902292 | 0.036 | T | C | NA | − | rs7902292 | .681 | .023 | 0 | SCZ, average TH |
| 17 | 15 | 58873555 | 59115995 | rs4774310 | 0.010 | G | T | NA | + | rs4774310 | .354 | .003 | + | Average TH |
| 19 | 17 | 2532052 | 2586229 | rs6502460 | 0.002 | G | T | NA | + | rs6502460 | .775 | .022 | + | SCZ, average TH |
| 21 | 17 | 19897445 | 20384834 | rs7209734 | 0.011 | C | A | NA | − | rs7209734 | .544 | .004 | + | Average TH |
| 22 | 17 | 43463493 | 44865603 | rs2532392 | 0.013 | A | G | MAPT-AS1; MAPT; STH; KANSL1-AS1; RP11-259G18.1; WNT3 | − | rs199504 | .301 | 1.47 × 10−4 | + | SCZ, average TH |
Abbreviations: A1, allele 1 (effect allele); A2, allele 2 (noneffect allele); BP, genomic position (hg19); conjFDR, conjunctional false discovery rate; CHR, chromosome; Cor_Eff, concordancy of association directions between 2 traits; GWAS, genome-wide association study; NA, not applicable; Rep_Dir, concordancy of association directions between the discovery and independent cohorts; SCZ, schizophrenia; SNV, single-nucleotide variant; TH, thickness.
This table provides 12 genomic loci that identified as shared between SCZ and average TH in a discovery study and showed significant associations with at least 1 phenotype in the independent Norwegian and Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) cohorts.
The directionality of allelic associations with both phenotypes in discovery data sets using z scores is shown here. + Indicates the same direction and −, the opposite direction.
For genomic loci of which the primary lead SNV is not available in independent data sets, we assigned a secondary lead SNV.
The “Rep_Dir” shows the concordancy of association directions of lead SNVs between the discovery and independent samples using z scores. + For both phenotypes show the same direction between the discovery and independent cohorts and 0, for at least 1 phenotype shows opposite direction.
Whether a locus was novel for 2 traits, according to the original GWAS, National Human Genome Research Institute–European Bioinformatics Institute GWAS Catalog, and previous conditional FDR/conjFDR publications is indicated. More information about all identified genomic loci are listed in Supplement 2 and Supplement 9, with details of defining genomic loci, lead SNV, credible mapped genes and novelty in the eMethods in Supplement 1.
Figure 3. Expression of Credible Genes of Shared Loci in Brain Tissue.
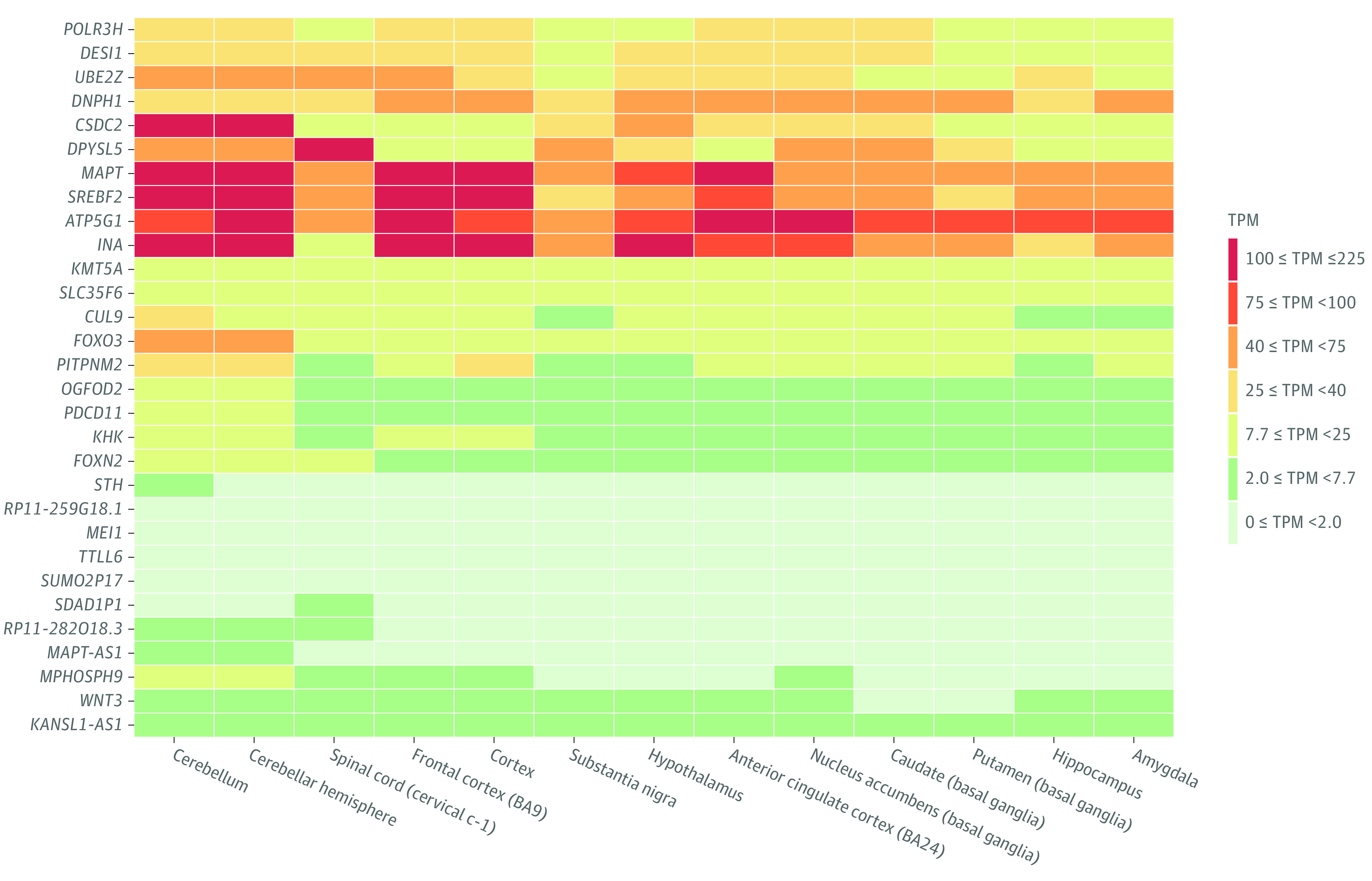
Among the shared loci that were significantly associated with at least 1 phenotype in the independent cohorts, 31 credible genes were mapped by all 3 strategies: positional mapping, expression quantitative trait locus mapping, and chromatin interaction mapping. Corresponding expression patterns in brain tissue were extracted from the Genotype-Tissue Expression Portal. The expression pattern for has-mir-8072 was not found. The KMT5A is also named as SETD8. Transcripts per million (TPM) is used to measure gene expression levels. BA indicates Brodmann area.
Shared Genetic Mechanisms Between Schizophrenia and Schizophrenia-Associated Regional Cortical Measurements
We observed SNV enrichment in QQ plots for 2 regions of interest for SA (superior frontal gyrus and middle temporal gyrus) and 3 for TH (superior temporal gyrus, inferior temporal gyrus, and superior frontal gyrus) with schizophrenia and vice versa (eFigures 3-6 in Supplement 1). The largest overlap as measured with Dice coefficient was observed in middle temporal gyrus among SA and schizophrenia (Dice coefficient = 0.32) and in superior temporal gyrus among TH and schizophrenia (Dice coefficient = 0.40; eTable 1 in Supplement 1). For the conjFDR analysis, schizophrenia shared 9, 16, 9, 11, and 10 genomic loci with regional SA (superior frontal gyrus and middle temporal gyrus) and regional TH (superior temporal gyrus, inferior temporal gyrus, and superior frontal gyrus), respectively (Supplement 13).
Discussion
In the present study, we identified a shared genetic basis between schizophrenia and SA and TH of cerebral cortex using MiXeR and cFDR. Specifically, we (1) showed that a substantial proportion of SNVs associated with total SA and average TH were associated with the risk of schizophrenia; (2) identified shared genomic loci between schizophrenia and total SA and average TH; (3) revealed polygenic overlap between schizophrenia and relevant regions of interest for SA (superior frontal gyrus and middle temporal gyrus) and for TH (superior temporal gyrus, inferior temporal gyrus, and superior frontal gyrus); and (4) suggested immune functions may be associated with cortical development and the pathology of schizophrenia.
Individual differences in cortical brain structure have been linked to cognition, mood, and behavior.50,51 Previous studies have suggested cortical brain structure as an endophenotype of schizophrenia,52 mainly supported by phenotypic associations.3,4 Here we identified genetic overlap between schizophrenia and cortical structure, suggesting that previously reported group differences in these brain magnetic resonance imaging features between patients with schizophrenia and healthy controls are partly genetically determined, in line with the reported association between polygenic risk score of schizophrenia and cortical TH.7,9,10 Specifically, MiXeR revealed many variants shared between schizophrenia and cortical morphology with a distinct pattern of association distributions that approximately 50% of shared variants demonstrated concordant associations with schizophrenia and cortical structure. The mixed directions or associations across the shared genetic variants15,16 help explain the polygenic overlap despite nonsignificant genetic correlation.8 Meanwhile, MiXeR reported a higher polygenicity for schizophrenia compared with cortical SA and TH, which is likely associated with the biological complexity and clinical heterogeneity of schizophrenia.16,53,54 These findings indicate a polygenic pleiotropy15 model for the brain in which polygenic phenotypes related to brain may have overlapping genetic determinants along with comparatively distinct genetic characteristics, including different polygenicities and phenotype-specific association distributions. Regional TH measures showed a bigger genetic overlap with schizophrenia than global measures, while SA did not show this pattern. While the brain magnetic resonance imaging associations with schizophrenia show substantial between-individual heterogeneity,7,10,55 this pattern generally complies with the anatomical distribution of associations observed for TH in schizophrenia while the association with SA appears to be global.3
Notably, after excluding the regions with complex LD patterns, genes implicated by the shared loci were significantly enriched in biological processes related to inflammation. Previous studies have suggested that the activity of neuroinflammatory receptors affects cortical development,56 along with significant associations between inflammation makers with cortical SA57 and TH.58 Immune dysfunction has been proposed as an important factor in the pathogenesis of schizophrenia59 as evidenced by the involvement of immune-related genes29 and altered various inflammatory makers in schizophrenia.60 Immune-related synapse pruning, which decreases cortical TH,61 has been reported in schizophrenia.62 Our results suggest immune processes may be involved in the shared genetic component between schizophrenia and cortical structure, providing an insight into the above observations. Although most prior reports highlighted the MHC region, the enrichment in inflammation-related gene sets without MHC implies that genetic factors linking to the immune system to cerebral cortex and schizophrenia are not only driven by the strong and complex association at the MHC region. Further experimental studies are needed to understand the roles of immunity in cortical structure and in schizophrenia.
Our study found 5 credible mapped genes, including INA, ATP5G1, SREBF2, MAPT, and DNPH1, have a high expression in the brain cortex. INA encodes neurofilaments, which are involved in the morphogenesis of neurons and work as biomarkers in neurological disorders.63 Previous studies have suggested ATP5G1, encoding a subunit of mitochondrial ATP synthase, is associated with major depression disorder and Alzheimer disease.64,65 SREBP2 has been indicated as a genetic susceptibility factor in schizophrenia and an antipsychotic-activated transcription factor. MAPT encodes microtubule-associated protein tau, which participates in axonal transport and neurite outgrowth.66 Variants within MAPT are associated with neurodegenerative disorders,23 learning disability,67 and alterations in cortical TH, gray matter volume, and white matter integrity.68,69 DNPH1 encodes a hydrolase, and a GWAS has reported DNPH1 is associated with risk-taking behavior.70
Limitations
However, some limitations should be noted. First, the sample size of the independent Norwegian GWAS data set for schizophrenia was small with a limited power. Given the shared loci between schizophrenia and average TH failed to show concordancy in the Norwegian cohort, a future examination with a large cohort is necessary. Second, although gene set analysis provided potential insights, whether the overlapping genetic components reflect shared biological pathways or more unspecific associations of brain-expressed genes warrants further research.
Conclusions
In summary, our study uncovers shared polygenicity between schizophrenia and cortical brain structure. Mapped genes of shared loci along with previous reports suggest potential connections of immunity to the development of cortical brain structure and to the pathology of schizophrenia. These findings provide molecular genetic insights into the phenotypic correlations between schizophrenia and brain structure.
eMethods.
eResults.
eReferences.
eFigure 1. Conditional QQ plots for schizophrenia conditioned on global cortical measures and vice versa in discovery study
eFigure 2. Shared polygenicity underlying schizophrenia and global cortical measures in discovery study
eFigure 3. Conditional QQ plots for schizophrenia conditioned on regions of interest for surface area and vice versa
eFigure 4. Shared polygenicity underlying schizophrenia and regional surface area
eFigure 5. Conditional QQ plots for schizophrenia conditioned on regions of interest for thickness and vice versa
eFigure 6. Shared polygenicity underlying schizophrenia and regional thickness
eTable 1. Univariate analysis using MiXeR
eTable 2. Bivariate analysis using MiXeR
eTable 3. Previously reported significant associations of shared genomic loci based on the NHGRI-EBI GWAS Catalog using candidate SNPs as index
eTable 4. Significant eQTLs of lead SNPs of shared genomic loci in discovery study in the GTEx database (v8)
eTable 5. Gene-sets significantly enriched by mapped genes of shared loci between schizophrenia (SCZ) and total surface area (SA)
eTable 6. Gene-sets significantly enriched by mapped genes of shared loci between schizophrenia (SCZ) and average thickness (TH)
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and brain cortical structural measures (total surface area, SA; average thickness, TH) at conjFDR<0.05 in discovery study
eTable. Candidate SNPs shared between schizophrenia (SCZ) and total surface area (SA) in discovery study
eTable. Candidate SNPs shared between schizophrenia (SCZ) and average thickness (TH) in discovery study
eTable. Genes mapped to candidate SNPs shared between schizophrenia (SCZ) and total surface area (SA) in discovery study
eTable. Genes mapped to candidate SNPs shared between schizophrenia (SCZ) and average thickness (TH) in discovery study
eTable. Distinct genomic loci associated with schizophrenia (SCZ) at condFDR<0.01 given association with total surface area(SA) or average thickness (TH) in discovery study
eTable. Distinct genomic loci associated with total surface area (SA) or average thickness (TH) at condFDR<0.01 given association with schizophrenia (SCZ) in discovery study
eTable. Lead SNPs for conjFDR genomic loci in independent datasets
eTable. Lead SNPs for conjFDR genomic loci in an East Asian GWAS dataset
eTable. Lead SNPs for condFDR genomic loci in independent datasets
eTable. Lead SNP for condFDR genomic loci in an East Asian GWAS dataset
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and regional cortical structural measures(regional surface area, SA; regional thickness, TH) at conjFDR<0.05
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and brain cortical structural measures (total surface area, SA; average thickness, TH) at conjFDR<0.01 in discovery study
eTable. Lead SNPs for conjFDR genomic loci at conjFDR<0.01 in independent datasets
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187-1192. doi: 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18(12):727-740. doi: 10.1038/nrn.2017.125 [DOI] [PubMed] [Google Scholar]
- 3.van Erp TGM, Walton E, Hibar DP, et al. ; Karolinska Schizophrenia Project . Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644-654. doi: 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547-553. doi: 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moberget T, Doan NT, Alnæs D, et al. ; KaSP . Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 2018;23(6):1512-1520. doi: 10.1038/mp.2017.106 [DOI] [PubMed] [Google Scholar]
- 6.de Zwarte SMC, Brouwer RM, Tsouli A, et al. Running in the family? structural brain abnormalities and IQ in offspring, siblings, parents, and co-twins of patients with schizophrenia. Schizophr Bull. 2019;45(6):1209-1217. doi: 10.1093/schbul/sby182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alnæs D, Kaufmann T, van der Meer D, et al. ; Karolinska Schizophrenia Project Consortium . Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry. 2019;76(7):739-748. doi: 10.1001/jamapsychiatry.2019.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasby KL, Jahanshad N, Painter JN, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE Consortium; EPIGEN Consortium; IMAGEN Consortium; SYS Consortium; Parkinson’s Progression Markers Initiative; Enhancing NeuroImaging Genetics through Meta-Analysis Consortium (ENIGMA)—Genetics working group . The genetic architecture of the human cerebral cortex. Science. 2020;367(6484):eaay6690. doi: 10.1126/science.aay6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neilson E, Shen X, Cox SR, et al. Impact of polygenic risk for schizophrenia on cortical structure in UK Biobank. Biol Psychiatry. 2019;86(7):536-544. doi: 10.1016/j.biopsych.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 10.Westlye LT, Alnæs D, van der Meer D, Kaufmann T, Andreassen OA. Population-based mapping of polygenic risk for schizophrenia on the human brain: new opportunities to capture the dimensional aspects of severe mental disorders. Biol Psychiatry. 2019;86(7):499-501. doi: 10.1016/j.biopsych.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Bohlken MM, Brouwer RM, Mandl RC, Kahn RS, Hulshoff Pol HE. Genetic variation in schizophrenia liability is shared with intellectual ability and brain structure. Schizophr Bull. 2016;42(5):1167-1175. doi: 10.1093/schbul/sbw034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee PH, Baker JT, Holmes AJ, et al. Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol Psychiatry. 2017;22(8):1224. doi: 10.1038/mp.2017.42 [DOI] [PubMed] [Google Scholar]
- 13.Ohi K, Shimada T, Kataoka Y, et al. Genetic correlations between subcortical brain volumes and psychiatric disorders. Br J Psychiatry. 2020;216(5):280-283. doi: 10.1192/bjp.2019.277 [DOI] [PubMed] [Google Scholar]
- 14.Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeland OB, Frei O, Dale AM, Andreassen OA. The polygenic architecture of schizophrenia: rethinking pathogenesis and nosology. Nat Rev Neurol. 2020;16(7):366-379. doi: 10.1038/s41582-020-0364-0 [DOI] [PubMed] [Google Scholar]
- 16.Frei O, Holland D, Smeland OB, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019;10(1):2417. doi: 10.1038/s41467-019-10310-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreassen OA, Djurovic S, Thompson WK, et al. ; International Consortium for Blood Pressure GWAS; Diabetes Genetics Replication and Meta-analysis Consortium; Psychiatric Genomics Consortium Schizophrenia Working Group . Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92(2):197-209. doi: 10.1016/j.ajhg.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr Bull. 2014;40(1):13-17. doi: 10.1093/schbul/sbt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann T, van der Meer D, Doan NT, et al. ; Karolinska Schizophrenia Project (KaSP) . Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019;22(10):1617-1623. doi: 10.1038/s41593-019-0471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeland OB, Frei O, Kauppi K, et al. ; NeuroCHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) Cognitive Working Group . Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry. 2017;74(10):1065-1075. doi: 10.1001/jamapsychiatry.2017.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahrami S, Steen NE, Shadrin A, et al. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry. 2020;77(5):503-512. doi: 10.1001/jamapsychiatry.2019.4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreassen OA, Harbo HF, Wang Y, et al. ; Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; International Multiple Sclerosis Genetics Consortium (IMSGC) . Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20(2):207-214. doi: 10.1038/mp.2013.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan RS, Schork AJ, Wang Y, et al. ; ADNI, ADGC, GERAD, CHARGE and IPDGC Investigators . Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry. 2015;20(12):1588-1595. doi: 10.1038/mp.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meer D, Rokicki J, Kaufmann T, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Pediatric Imaging, Neurocognition and Genetics Study . Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatry. 2020;25(11):3053-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell KS, Shadrin A, Bahrami S, et al. Identification of genetic overlap and novel risk loci for attention-deficit/hyperactivity disorder and bipolar disorder. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0613-z [DOI] [PubMed] [Google Scholar]
- 26.Franke B, Stein JL, Ripke S, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium; ENIGMA Consortium . Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19(3):420-431. doi: 10.1038/nn.4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeland OB, Wang Y, Frei O, et al. Genetic overlap between schizophrenia and volumes of hippocampus, putamen, and intracranial volume indicates shared molecular genetic mechanisms. Schizophr Bull. 2018;44(4):854-864. doi: 10.1093/schbul/sbx148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardiñas AF, Holmans P, Pocklington AJ, et al. ; GERAD1 Consortium; CRESTAR Consortium . Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381-389. doi: 10.1038/s41588-018-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripke S, Neale BM, Corvin A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Meer D, Shadrin AA, Connell K, et al. Improved prediction of schizophrenia by leveraging genetic overlap with brain morphology. medRxiv. Preprint posted online August 4, 2020. doi: 10.1101/2020.08.03.20167510 [DOI]
- 31.Lam M, Chen C-Y, Li Z, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Indonesia Schizophrenia Consortium; Genetic REsearch on schizophreniA neTwork-China and the Netherlands (GREAT-CN) . Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51(12):1670-1678. doi: 10.1038/s41588-019-0512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nopoulos P, Flaum M, Arndt S, Andreasen N. Morphometry in schizophrenia revisited: height and its relationship to pre-morbid function. Psychol Med. 1998;28(3):655-663. doi: 10.1017/S0033291797006417 [DOI] [PubMed] [Google Scholar]
- 33.Bacanu SA, Chen X, Kendler KS. The genetic overlap between schizophrenia and height. Schizophr Res. 2013;151(1-3):226-228. doi: 10.1016/j.schres.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anttila V, Bulik-Sullivan B, Finucane HK, et al. ; Brainstorm Consortium . Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yengo L, Sidorenko J, Kemper KE, et al. ; GIANT Consortium . Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641-3649. doi: 10.1093/hmg/ddy271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartzman A, Lin X. The effect of correlation in false discovery rate estimation. Biometrika. 2011;98(1):199-214. doi: 10.1093/biomet/asq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GitHub. precimed/mixer. Accessed May 13, 2021. https://github.com/precimed/mixer
- 38.GitHub. precimed/pleiofdr. Accessed May 13, 2021. https://github.com/precimed/pleiofdr
- 39.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buniello A, MacArthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005-D1012. doi: 10.1093/nar/gky1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310-315. doi: 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyle AP, Hong EL, Hariharan M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790-1797. doi: 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Zhang F, Hu H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481-487. doi: 10.1038/ng.3538 [DOI] [PubMed] [Google Scholar]
- 44.Kundaje A, Meuleman W, Ernst J, et al. ; Roadmap Epigenomics Consortium . Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317-330. doi: 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739-1740. doi: 10.1093/bioinformatics/btr260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.GTEx Consortium . Human genomics: the Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648-660. doi: 10.1126/science.1262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912-919. doi: 10.1038/s41588-018-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JJ, Wedow R, Okbay A, et al. ; 23andMe Research Team; COGENT (Cognitive Genomics Consortium); Social Science Genetic Association Consortium . Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50(8):1112-1121. doi: 10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomaznoy M, Sethi A, Greenbaum J, Peters B. Identifying inaccuracies in gene expression estimates from unstranded RNA-seq data. Sci Rep. 2019;9(1):16342. doi: 10.1038/s41598-019-52584-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser DA, Doucet GE, Ing A, et al. An integrated brain-behavior model for working memory. Mol Psychiatry. 2018;23(10):1974-1980. doi: 10.1038/mp.2017.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility: linking memory and mood. Nat Rev Neurosci. 2017;18(6):335-346. doi: 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636-645. doi: 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- 53.Holland D, Frei O, Desikan R, et al. Beyond SNP heritability: polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model. PLoS Genet. 2020;16(5):e1008612. doi: 10.1371/journal.pgen.1008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor LJ, Schoech AP, Hormozdiari F, Gazal S, Patterson N, Price AL. Extreme polygenicity of complex traits is explained by negative selection. Am J Hum Genet. 2019;105(3):456-476. doi: 10.1016/j.ajhg.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolfers T, Rokicki J, Alnæs D, et al. Replicating extensive brain structural heterogeneity in individuals with schizophrenia and bipolar disorder. medRxiv. 2021:2020.2005.2008.20095091. [DOI] [PMC free article] [PubMed]
- 56.Choi GB, Yim YS, Wong H, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933-939. doi: 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsland AL, Gianaros PJ, Kuan DCH, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195-204. doi: 10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prats-Soteras X, Jurado MA, Ottino-González J, et al. Inflammatory agents partially explain associations between cortical thickness, surface area, and body mass in adolescents and young adulthood. Int J Obes (Lond). 2020;44(7):1487-1496. doi: 10.1038/s41366-020-0582-y [DOI] [PubMed] [Google Scholar]
- 59.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258-270. doi: 10.1016/S2215-0366(14)00122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696-1709. doi: 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schüz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol. 1989;286(4):442-455. doi: 10.1002/cne.902860404 [DOI] [PubMed] [Google Scholar]
- 62.Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22(3):374-385. doi: 10.1038/s41593-018-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 64.Liang WS, Reiman EM, Valla J, et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105(11):4441-4446. doi: 10.1073/pnas.0709259105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng D, He S, Ma C, et al. Co-expression network analysis revealed that the ATP5G1 gene is associated with major depressive disorder. Front Genet. 2019;10:703-703. doi: 10.3389/fgene.2019.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343(6257):461-463. doi: 10.1038/343461a0 [DOI] [PubMed] [Google Scholar]
- 67.Shaw-Smith C, Pittman AM, Willatt L, et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet. 2006;38(9):1032-1037. doi: 10.1038/ng1858 [DOI] [PubMed] [Google Scholar]
- 68.Panman JL, Jiskoot LC, Bouts MJRJ, et al. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging. 2019;76:115-124. doi: 10.1016/j.neurobiolaging.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 69.Canu E, Boccardi M, Ghidoni R, et al. H1 haplotype of the MAPT gene is associated with lower regional gray matter volume in healthy carriers. Eur J Hum Genet. 2009;17(3):287-294. doi: 10.1038/ejhg.2008.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karlsson Linnér R, Biroli P, Kong E, et al. ; 23and Me Research Team; eQTLgen Consortium; International Cannabis Consortium; Social Science Genetic Association Consortium . Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51(2):245-257. doi: 10.1038/s41588-018-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eReferences.
eFigure 1. Conditional QQ plots for schizophrenia conditioned on global cortical measures and vice versa in discovery study
eFigure 2. Shared polygenicity underlying schizophrenia and global cortical measures in discovery study
eFigure 3. Conditional QQ plots for schizophrenia conditioned on regions of interest for surface area and vice versa
eFigure 4. Shared polygenicity underlying schizophrenia and regional surface area
eFigure 5. Conditional QQ plots for schizophrenia conditioned on regions of interest for thickness and vice versa
eFigure 6. Shared polygenicity underlying schizophrenia and regional thickness
eTable 1. Univariate analysis using MiXeR
eTable 2. Bivariate analysis using MiXeR
eTable 3. Previously reported significant associations of shared genomic loci based on the NHGRI-EBI GWAS Catalog using candidate SNPs as index
eTable 4. Significant eQTLs of lead SNPs of shared genomic loci in discovery study in the GTEx database (v8)
eTable 5. Gene-sets significantly enriched by mapped genes of shared loci between schizophrenia (SCZ) and total surface area (SA)
eTable 6. Gene-sets significantly enriched by mapped genes of shared loci between schizophrenia (SCZ) and average thickness (TH)
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and brain cortical structural measures (total surface area, SA; average thickness, TH) at conjFDR<0.05 in discovery study
eTable. Candidate SNPs shared between schizophrenia (SCZ) and total surface area (SA) in discovery study
eTable. Candidate SNPs shared between schizophrenia (SCZ) and average thickness (TH) in discovery study
eTable. Genes mapped to candidate SNPs shared between schizophrenia (SCZ) and total surface area (SA) in discovery study
eTable. Genes mapped to candidate SNPs shared between schizophrenia (SCZ) and average thickness (TH) in discovery study
eTable. Distinct genomic loci associated with schizophrenia (SCZ) at condFDR<0.01 given association with total surface area(SA) or average thickness (TH) in discovery study
eTable. Distinct genomic loci associated with total surface area (SA) or average thickness (TH) at condFDR<0.01 given association with schizophrenia (SCZ) in discovery study
eTable. Lead SNPs for conjFDR genomic loci in independent datasets
eTable. Lead SNPs for conjFDR genomic loci in an East Asian GWAS dataset
eTable. Lead SNPs for condFDR genomic loci in independent datasets
eTable. Lead SNP for condFDR genomic loci in an East Asian GWAS dataset
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and regional cortical structural measures(regional surface area, SA; regional thickness, TH) at conjFDR<0.05
eTable. Distinct genomic loci shared between schizophrenia (SCZ) and brain cortical structural measures (total surface area, SA; average thickness, TH) at conjFDR<0.01 in discovery study
eTable. Lead SNPs for conjFDR genomic loci at conjFDR<0.01 in independent datasets