Key Points
Question
Is childhood sleep apnea associated with elevated blood pressure in adolescence?
Findings
In this population-based cohort study of 421 children, persistent sleep apnea was associated with nearly 3-fold increased odds of elevated blood pressure in adolescence. The severity of adolescent sleep apnea was associated with elevated blood pressure and orthostatic hypertension in a dose-response manner.
Meaning
The results of this study suggest that sleep apnea is a risk factor for hypertension in youth and that early sleep apnea interventions may help prevent long-term cardiovascular consequences.
Abstract
Importance
Although pediatric guidelines have delineated updated thresholds for elevated blood pressure (eBP) in youth and adult guidelines have recognized obstructive sleep apnea (OSA) as an established risk factor for eBP, the relative association of pediatric OSA with adolescent eBP remains unexplored.
Objective
To assess the association of pediatric OSA with eBP and its orthostatic reactivity in adolescence.
Design, Setting, and Participants
At baseline of this population-based cohort study (Penn State Child Cohort) in 2000-2005, a random sample of 700 children aged 5 to 12 years from the general population was studied. A total of 421 participants (60.1%) were followed up in 2010-2013 after 7.4 years as adolescents (ages, 12-23 years). Data analyses were conducted from July 6 to October 29, 2020.
Main Outcomes and Measures
Outcomes were the apnea-hypopnea index (AHI) score, ascertained via polysomnography conducted in a laboratory; eBP measured in the seated position identified using guideline-recommended pediatric criteria; orthostatic hyperreactivity identified with BP assessed in the supine and standing positions; and visceral adipose tissue assessed via dual-energy x-ray absorptiometry.
Results
Among the 421 participants (mean [SD] age at follow-up, 16.5 [2.3] years), 227 (53.9%) were male and 92 (21.9%) were racial/ethnic minorities. A persistent AHI of 2 or more since childhood was longitudinally associated with adolescent eBP (odds ratio [OR], 2.9; 95% CI 1.1-7.5), while a remitted AHI of 2 or more was not (OR, 0.9; 95% CI 0.3-2.6). Adolescent OSA was associated with eBP in a dose-response manner; however, the association of an AHI of 2 to less than 5 among adolescents was nonsignificant (OR, 1.5; 95% CI, 0.9-2.6) and that of an AHI of 5 or more was approximately 2-fold (OR, 2.3; 95% CI, 1.1-4.9) after adjusting for visceral adipose tissue. An AHI of 5 or more (OR, 3.1; 95% CI, 1.2-8.5), but not between 2 and less than 5 (OR, 1.3; 95% CI, 0.6-3.0), was associated with orthostatic hyperreactivity among adolescents even after adjusting for visceral adipose tissue. Childhood OSA was not associated with adolescent eBP in female participants, while the risk of OSA and eBP was greater in male participants.
Conclusions and Relevance
The results of this cohort study suggest that childhood OSA is associated with adolescent hypertension only if it persists during this developmental period. Visceral adiposity explains a large extent of, but not all, the risk of hypertension associated with adolescent OSA, which is greater in male individuals.
This cohort study tests the association of pediatric obstructive sleep apnea with elevated blood pressure and its orthostatic reactivity in adolescence.
Introduction
Pediatric hypertension clinical practice guidelines from 2017 have provided updated thresholds to ascertain the presence of elevated blood pressure (eBP) and hypertension in youth.1 Furthermore, 2018 adult guidelines have recognized obstructive sleep apnea (OSA) as an established, fixed risk factor for eBP and cardiovascular disease in middle age.2 Despite the association observed in cross-sectional studies between pediatric OSA and eBP,3,4 its longitudinal association with eBP at later developmental stages is not well established, likely owing to either short follow-up times, relying on treatment studies, or the developmental transition examined.5,6,7,8,9 It is necessary to understand this association to establish early treatment strategies for OSA during critical developmental periods.
Evidence indicates that OSA in prepubertal children tends to remit as children develop into adolescence, with remission rates ranging from 40% to 70%.10,11,12,13,14,15 This natural developmental course may explain why the available longitudinal data assessing the association between childhood OSA and eBP in adolescence have reported mixed findings.6,7 In addition, obesity, particularly increased visceral adipose tissue (VAT), appears to play a role in the persistence and development of OSA in adolescence. Visceral adipose tissue is a stronger correlate of obstructive apneas and hypopneas than body mass index (BMI) percentile and is a key factor associated with pediatric OSA even in normal-weight youth.13,14,16,17,18,19,20 It remains unknown whether the association between OSA and adolescent eBP is independent of VAT, a known risk factor for cardiovascular disease. In addition, previous studies have indicated that vascular and cardiac-autonomic reactivity during wake and sleep are important outcomes of pediatric OSA.3 Thus, orthostatic measures of BP reactivity21,22,23,24 may be particularly able to identify cardiovascular risk in youth with OSA.
The aim of this study was to assess the association of pediatric OSA with adolescent eBP and its reactivity, and the interaction of sex and pediatric OSA’s natural course with this association, while accounting, for the first time to date, for VAT and metabolic and sleep factors in a child cohort followed up during adolescence.
Methods
Sample
The baseline portion of the Penn State Child Cohort has been previously described in detail.25,26 In brief, it was designed as a 2-phase study in which 5740 questionnaires27 were returned (79% response rate) and 700 randomly selected participants (70% response rate) underwent an in-laboratory sleep study at baseline, from 2000 to 2005. At follow-up, conducted from 2010 to 2013, 421 participants (60.1% response rate) returned 6 to 13 years later (median, 7.4 years) for a reexamination; no differences in baseline demographic characteristics were observed in the 279 participants lost to follow-up.13 The Penn State College of Medicine institutional review board approved the study. Written informed consent from the parent or legal guardian and from participants 18 years or older, and assent from those younger than 18 years, were all obtained. To compensate for their time to complete the study, participants received $100 at baseline and $200 at follow-up. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Sleep Laboratory
At both time points, participants’ sleep was monitored for 9 hours with polysomnography, including electroencephalography, electrooculography, and electromyography. Respiration was monitored with nasal pressure, thermocouple, and thoracic and abdominal strain gauges. Hemoglobin oxygen saturation was obtained from the finger. Sleep records were subsequently scored independently according to standardized criteria.28,29 As previously reported,13,18,19,25,26 apneas and hypopneas were scored using pediatric criteria for participants younger than 16 years, while adult criteria were used for participants 16 years or older, including associated decreases in oxygen saturation of 3% or more or an electroencephalographic arousal for hypopneas.29 The apnea-hypopnea index (AHI) was calculated as the number of obstructive apneas and hypopneas summed per hour of sleep. The presence of OSA in childhood was categorized based on pediatric criteria as AHI less than 2 and AHI of 2 or more.11,13 The presence of OSA in adolescence was categorized based on pediatric and adult criteria as AHI of less than 2, AHI of 2 to less than 5, and AHI of 5 or more.13,19
Key Measurements
Participants underwent a clinical history and physical examination prior to the polysomnography and included recording demographic characteristics and measurements of height, weight, and waist circumference.13,18,19,25,26 Race and ethnicity were recorded using National Institutes of Health categories and reported by the parent and/or participant. Body mass index percentile was calculated based on the Centers for Disease Control and Prevention’s formula.30 Waist circumference was measured at the top of the iliac crest. At baseline, an otolaryngology examination assessed the presence of tonsillar hypertrophy, while lifetime histories of adenotonsillectomy and antihypertensive and cardiac medication use were based on baseline and follow-up clinical histories and physical examinations.18,25,26 At follow-up, participants underwent a dual-energy x-ray absorptiometry scan to assess VAT.13,18,19 Morning fasting blood samples were collected after the polysomnography and assayed for glucose, insulin, triglyceride, and high-density lipoprotein cholesterol levels. Insulin resistance was assessed by the homeostatic model assessment method. A metabolic syndrome (MetS) risk score, without including BP, was based on the sum of the standardized scores for waist circumference, inverse high-density lipoprotein cholesterol, triglycerides, and homeostatic model assessment method.31 Finally, participants were sent home with an actigraphy device to assess sleep over 7 days.32,33
Blood Pressure and Its Orthostatic Reactivity
Blood pressure was measured in the evening during the clinical history and physical examination, using an automated system (Phillips SureSigns VM4; Koninklijke Philips NV), in 3 different positions: seated (baseline and follow-up), supine (follow-up only), and standing (follow-up only). In the seated and supine positions, 3 assessments were made after 5 minutes of rest and a mean of the second 2 assessments was used in the analysis. Blood pressure was next assessed twice immediately after standing; these 2 values were averaged for the analysis. American Academy of Pediatrics guidelines are shown in eTable 1 in the Supplement and were used to establish BP status using seated systolic BP (SBP) and diastolic BP (DBP) levels.1 Given the low prevalence of stage 1 and stage 2 hypertension in this cohort, our outcome variable analyzed was binary and identified participants with normal BP vs those with eBP (ie, all 3 categories). Orthostatic SBP was calculated as the difference between supine and standing SBP and categorized as hyperreactive, hyporeactive, or normotensive based on the 10th and 90th percentiles of orthostatic SBP, which resulted in a definition of hyporeactive as 3.0 mm Hg or less and hyperreactive as more than 15.0 mm Hg.
Statistical Analysis
Statistical analysis was conducted from July 6 to October 29, 2020. The demographic and clinical characteristics of the sample are presented as means for continuous variables and proportions for categorical variables. Analysis of variance and Cochran-Mantel-Haenszel tests were used, as appropriate, to compare these variables across groups.
We first evaluated the longitudinal association between childhood OSA (baseline) and the odds of adolescent eBP (follow-up) with a multivariable-adjusted binary logistic regression model controlling for sex, race/ethnicity, adenotonsillectomy, and antihypertensive and cardiac medication use, as well as childhood (baseline) age, tonsillar hypertrophy, BMI percentile, and SBP percentile. Multivariable-adjusted odds ratios (ORs) and their corresponding 95% CIs were computed for childhood AHI of 2 or more (vs children with an AHI less than 2). The dependent variable was the presence of adolescent eBP vs normal BP levels. Thereafter, a multivariable-adjusted binary logistic regression model examined the association between the natural course of childhood OSA from baseline to follow-up13,18 with adolescent eBP while controlling for the same covariates mentioned above. In these models, the independent variable was the natural course groups and multivariable-adjusted ORs and 95% CIs were computed for remitted, incident, and persistent AHI of 2 or more (vs children with an AHI less than 2 at both baseline and follow-up [ie, none]). In addition, incident AHI of 5 or more was also examined.
Second, analysis of covariance was used to estimate the multivariable-adjusted means (SEs) of cross-sectional SBP and DBP levels while controlling for demographic characteristics and a regression model, in which the 3 levels of adolescent OSA severity were treated as a linear component, was applied to test for a potential dose-response association. Thereafter, we evaluated the association between 3 adolescent OSA groups and the odds of eBP with multivariable-adjusted binary logistic regression models and multivariable-adjusted ORs and 95% CIs were computed for adolescent AHI between 2 and less than 5 and AHI of 5 or more (vs adolescents with an AHI less than 2). Sex, age, race/ethnicity, adenotonsillectomy, and antihypertensive and cardiac medication use as well as childhood tonsillar hypertrophy were controlled for in these logistic regression models. Visceral adipose tissue and MetS were independently controlled for in these models to assess their impact on the association between adolescent OSA and eBP; only 17 male participants and 14 female participants did not have VAT or MetS data, which were considered missing at random, and their values were imputed with their sex-specific sample mean. Finally, the association between adolescent OSA and odds of orthostatic hyporeactivity and hyperreactivity, compared with normal orthostatic BP reactivity, was examined using multivariable-adjusted polytomous logistic regression models adjusted for the same covariates mentioned above, including VAT and MetS.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc) and SPSS, version 26 (IBM Corp). All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
Characteristics of the Sample
As shown in Table 1 and eTable 2 in the Supplement, among the 421 participants, who were 5 to 12 years at baseline (median, 9 years) and 12 to 23 years at follow-up (median, 16 years), 227 (53.9%) were male and 92 (21.9%) were racial/ethnic minorities. A total of 64 participants (15.2%) had obesity (BMI ≥95th percentile).
Table 1. Descriptive Characteristics of the Overall Sample as Well as Stratified by the Presence of Adolescent OSA.
| Characteristic | All (N = 421) | AHI <2 (n = 262) | AHI 2 to <5 (n = 115) | AHI ≥5 (n = 44) | P value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 16.5 (2.3) | 16.0 (2.2) | 16.9 (2.2) | 17.8 (2.1) | <.001 |
| Male, No. (%) | 227 (53.9) | 119 (45.4) | 77 (67.0) | 31 (70.5) | <.001 |
| Racial/ethnic minority, No. (%) | 92 (21.9) | 45 (17.2) | 36 (31.3) | 11 (25.0) | .008 |
| Tonsillar hypertrophy, No. (%) | 153 (36.3) | 90 (34.3) | 43 (37.4) | 20 (45.5) | .17 |
| Adenotonsillectomy, No. (%) | 48 (11.4) | 24 (9.2) | 16 (13.9) | 8 (18.2) | .05 |
| Medication use, No. (%) | 9 (2.1) | 7 (2.7) | 2 (1.7) | 0 | .25 |
| BMI (percentile), mean (SD) | 65.3 (28.4) | 61.0 (28.6) | 70.1 (26.6) | 79.0 (25.6) | <.001 |
| MetS (z score), mean (SD) | 0.02 (2.5) | –0.2 (2.4) | 0.3 (2.4) | 0.7 (3.0) | .04 |
| Waist, mean (SD), cm | 80.3 (13.4) | 77.4 (11.4) | 83.0 (12.9) | 90.8 (18.3) | <.001 |
| Triglycerides, mean (SD), mg/dL | 94.7 (46.9) | 90.5 (43.3) | 99.4 (45.6) | 105.9 (64.7) | .07 |
| HDL cholesterol, mean (SD), mg/dL | 49.8 (12.5) | 51.2 (12.7) | 47.7 (11.0) | 47.7 (14.0) | .03 |
| Glucose, mean (SD), mg/dL | 88.7 (14.9) | 88.6 (18.0) | 89.3 (7.8) | 87.6 (8.8) | .81 |
| Insulin, mean (SD), μIU/mL | 6.4 (21.0) | 5.5 (17.2) | 8.8 (30.1) | 5.4 (7.8) | .39 |
| HOMA-IR, mean (SD) | 1.5 (5.8) | 1.2 (3.0) | 2.3 (9.9) | 1.2 (1.8) | .23 |
| VAT, mean (SD), cm2 | 59.8 (39.2) | 52.0 (32.3) | 67.3 (41.3) | 87.2 (53.9) | <.001 |
| Polysomnography | |||||
| TST, mean (SD), min | 446.8 (55.0) | 449.2 (49.2) | 444.5 (65.5) | 438.3 (57.8) | .42 |
| WASO, mean (SD), min | 69.5 (43.2) | 67.4 (40.5) | 70.7 (46.5) | 78.6 (49.2) | .33 |
| Awakenings, mean (SD), No. | 36.4 (12.1) | 34.8 (11.2) | 37.2 (11.8) | 44.0 (14.7) | <.001 |
| Arousals, mean (SD), No. | 4.1 (5.4) | 3.7 (5.2) | 4.5 (5.6) | 5.1 (6.0) | .21 |
| Stage 1, mean (SD), % | 1.0 (1.5) | 0.9 (1.2) | 1.2 (2.2) | 1.1 (1.3) | .34 |
| Sleep SpO2, mean (SD), % | 96.4 (1.2) | 96.5 (1.2) | 96.2 (1.1) | 96.2 (1.0) | .03 |
| Minimum SpO2, mean (SD), % | 91.5 (5.2) | 91.9 (5.0) | 91.1 (5.0) | 90.0 (6.6) | .06 |
| Actigraphy, mean (SD), min | |||||
| Weekdays TST | 428.1 (60.4) | 432.1 (57.4) | 418.8 (66.4) | 427.7 (60.6) | .16 |
| Weekends TST | 452.0 (90.0) | 455.1 (88.6) | 439.3 (92.3) | 467.6 (90.3) | .16 |
| Blood pressure, No. (%) | |||||
| Normal BP | 300 (71.3) | 206 (78.6) | 73 (63.5) | 21 (47.7) | <.001 |
| Elevated BP | 66 (15.7) | 33 (12.6) | 21 (18.3) | 12 (27.3) | |
| Stage 1 hypertension | 41 (9.7) | 18 (6.9) | 15 (13.0) | 8 (18.2) | |
| Stage 2 hypertension | 14 (3.3) | 5 (1.9) | 6 (5.2) | 3 (6.8) |
Abbreviations: AHI, apnea-hypopnea index; Arousals, number of arousals excluding breathing-related arousals; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment method; MetS, metabolic syndrome risk score (sum of z scores for waist, triglyceride level, inverse HDL cholesterol, and HOMA-IR); OSA, obstructive sleep apnea; SpO2, oxygen saturation; TST, total sleep time; VAT, visceral adipose tissue; WASO, wake after sleep onset.
SI conversion factors: To convert triglycerides to millimoles per liter, multiply by 0.0113; HDL cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; and insulin to picomoles per liter, multiply by 6.945.
Association of Childhood OSA With Adolescent eBP
Childhood AHI of 2 or more was not significantly associated with adolescent eBP (OR, 1.2; 95% CI, 0.6-2.5; P = .57). Male sex was a significant risk factor (OR, 3.7; 95% CI, 2.3-6.0; P < .001) that modified the association between childhood AHI of 2 or more and adolescent eBP (P = .03 for interaction). Childhood AHI of 2 or more was associated with increased odds of adolescent eBP in male participants (OR, 2.5; 95% CI, 1.0-6.6), but not in female participants (OR, 0.4; 95% CI, 0.1-1.5).
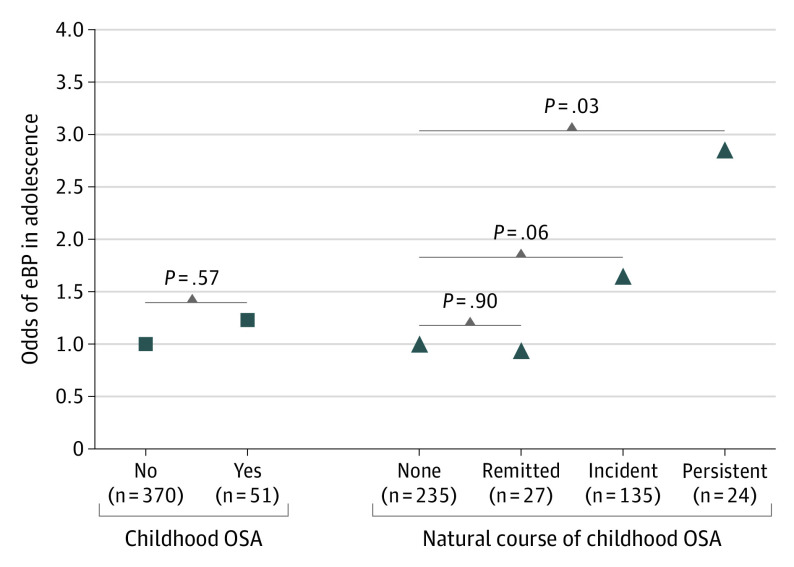
As shown in the Figure, while remitted AHI of 2 or more was not significantly associated with increased odds of adolescent eBP (OR, 0.9; 95% CI, 0.3-2.6) and incident AHI of 2 or more was only marginally associated with adolescent eBP (OR, 1.7; 95% CI, 1.0-2.8), persistent AHI of 2 or more was associated with nearly 3-fold significantly increased odds of adolescent eBP (OR, 2.9; 95% CI, 1.1-7.5). When incident OSA was defined as adolescent AHI of 5 or more (n = 38), the odds of eBP were significantly increased (OR, 2.3; 95% CI, 1.0-5.0; P = .04). Male sex remained a significant risk factor (OR, 3.4; 95% CI, 2.0-5.6; P < .001).
Figure. Association Between the Natural Course of Childhood Obstructive Sleep Apnea (OSA) and Adolescent Elevated Blood Pressure (eBP).
Data are odds ratios adjusted for race/ethnicity, sex, adenotonsillectomy, and antihypertensive and cardiac medication use, as well as childhood (baseline) age, body mass index percentile, systolic blood pressure percentile, and tonsillar hypertrophy. The 4 natural course groups were defined as none (reference), remitted, incident, and persistent OSA using an apnea-hypopnea index (AHI) of 2 or more as the pediatric threshold at baseline (childhood) and at follow-up (adolescence). When an AHI of 5 or more was used as the threshold at follow-up to define incident OSA (n = 38), the odds of eBP were 2.3-fold and significant for this more severe incident group.
Association of Adolescent OSA With Adolescent eBP and Orthostatic BP Reactivity
As shown in Table 2, there was a significant linear association between adolescent OSA and BP levels, primarily for SBP in the seated and standing positions and for its orthostatic reactivity. Univariate analyses (model 1 in Table 3) showed a significant association between adolescent OSA severity and increased odds of adolescent eBP. After multivariable adjustment, including VAT or MetS, the odds of adolescent eBP for an AHI between 2 and less than 5 were reduced from 2.1 (95% CI, 1.3-3.4) in model 1 to a nonsignificant 1.5 (95% CI, 0.9-2.6) in model 2, while the odds of adolescent eBP for an AHI of 5 or more remained significant despite being reduced from 4.0 (95% CI, 2.1-7.8) in model 1 to 2.3 (95% CI, 1.1-4.9) in model 2 and 2.6 (95% CI, 1.2-5.3) in model 3. There was no significant change in the association between adolescent OSA and eBP when controlling for any of the polysomnography or actigraphy variables (eg, OR, 2.4; 95% CI, 1.2-5.1; P = .02 for AHI≥5 after adjusting for total sleep time). Similarly, there was no significant change when controlling for gluteofemoral adiposity (eg, OR, 2.5; 95% CI, 1.2-5.3; P = .02 for AHI≥5 after adjusting for gynoid fat mass).
Table 2. Blood Pressure Levels Across Adolescent OSA Groups.
| Variable | Mean (SE), mm Hga | R | P linear | ||
|---|---|---|---|---|---|
| AHI <2 (n = 262) | AHI 2 to <5 (n = 115) | AHI ≥5 (n = 44) | |||
| Seated | |||||
| SBP | 112.6 (0.7) | 114.0 (1.1) | 117.6 (1.8) | 0.368 | .009 |
| DBP | 66.0 (0.6) | 67.1 (0.8) | 69.0 (1.4) | 0.267 | .04 |
| Supine | |||||
| SBP | 111.9 (0.6) | 112.2 (0.9) | 114.8 (1.5) | 0.356 | .14 |
| DBP | 63.8 (0.5) | 63.3 (0.7) | 65.6 (1.2) | 0.246 | .41 |
| Standing | |||||
| SBP | 117.8 (0.7) | 119.0 (1.1) | 123.9 (1.7) | 0.341 | .008 |
| DBP | 72.1 (0.5) | 72.0 (0.8) | 74.4 (1.4) | 0.212 | .22 |
| Orthostatic | |||||
| SBP | 5.9 (0.5) | 6.8 (0.7) | 9.2 (1.2) | 0.156 | .02 |
| DBP | 8.3 (0.4) | 8.7 (0.6) | 8.8 (0.9) | 0.135 | .49 |
Abbreviations: AHI, apnea-hypopnea index; DBP, diastolic blood pressure; OSA, obstructive sleep apnea; SBP, systolic blood pressure.
Adjusted for sex, race/ethnicity, and age.
Table 3. Association Between Adolescent OSA and eBP.
| Variable | Model 1a | Model 2a | Model 3a | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| AHI <2 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| AHI 2 to <5 | 2.1 (1.3-3.4) | .002 | 1.5 (0.9-2.6) | .13 | 1.6 (0.9-2.6) | .10 |
| AHI ≥5 | 4.0 (2.1-7.8) | <.001 | 2.3 (1.1-4.9) | .03 | 2.6 (1.2-5.3) | .01 |
Abbreviations: AHI, apnea-hypopnea index; eBP, elevated blood pressure; NA, not applicable; OR, odds ratio; OSA, obstructive sleep apnea.
Model 1 = univariate association of AHI with eBP. Model 2 = multivariable association of AHI with eBP, adjusting for sex, race/ethnicity, age, tonsillar hypertrophy, adenotonsillectomy, antihypertensive and cardiac medication use, and visceral adipose tissue (z score). Model 3 = multivariable association of AHI with eBP, adjusting for sex, race/ethnicity, age, tonsillar hypertrophy, adenotonsillectomy, antihypertensive and cardiac medication use, and metabolic syndrome risk score (z score).
As shown in model 1 of Table 4, the odds of orthostatic hyperreactivity associated with adolescent AHI between 2 and less than 5 were nonsignificant (OR, 1.3; 95% CI, 0.6-2.7), even after controlling for all covariates (model 2: OR, 1.3; 95% CI, 0.6-3.0; and model 3: OR, 1.5; 95% CI, 0.7-3.2). In contrast, the odds of orthostatic hyperreactivity associated with adolescent AHI of 5 or more were significant (model 1: OR, 3.3; 95% CI, 1.4-7.5) and not significantly reduced after controlling for VAT (model 2: OR, 3.1; 95% CI, 1.2-8.5) or MetS (model 3: OR, 3.9; 95% CI, 1.5-10.2).
Table 4. Association Between Adolescent OSA and Orthostatic BP Reactivity.
| Variable | Model 1a | Model 2a | Model 3a | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Hyporeactivity | ||||||
| AHI <2 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| AHI 2 to <5 | 1.1 (0.5-2.2) | .81 | 1.0 (0.5-2.2) | .96 | 1.0 (0.5-2.0) | .92 |
| AHI ≥5 | 1.6 (0.6-4.2) | .34 | 1.5 (0.5-4.3) | .45 | 1.3 (0.5-3.8) | .61 |
| Hyperreactivity | ||||||
| AHI <2 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| AHI 2 to <5 | 1.3 (0.6-2.7) | .44 | 1.3 (0.6-3.0) | .46 | 1.5 (0.7-3.2) | .32 |
| AHI ≥5 | 3.3 (1.4-7.5) | .006 | 3.1 (1.2-8.5) | .02 | 3.9 (1.5-10.2) | .005 |
Abbreviations: AHI, apnea-hypopnea index; BP, blood pressure; NA, not applicable; OSA, obstructive sleep apnea; VAT, visceral adipose tissue.
Model 1 = univariate association of AHI with orthostatic BP reactivity. Model 2 = multivariable association of AHI with orthostatic BP reactivity after adjusting for sex, race/ethnicity, age, tonsillar hypertrophy, adenotonsillectomy, antihypertensive and cardiac medication use, and VAT (z score). Model 3 = multivariable association of AHI with orthostatic BP reactivity after adjusting for sex, race/ethnicity, age, tonsillar hypertrophy, adenotonsillectomy, antihypertensive and cardiac medication use, and metabolic syndrome risk score (z score).
Sex Differences
We found evidence that male sex is associated with or modifies the association between OSA and adolescent eBP. As shown in eTable 2 in the Supplement, there were no significant sex differences in the prevalence of childhood OSA or childhood eBP; however, male participants were significantly more likely than female participants to have OSA and eBP in adolescence (OSA, 108 [47.6%] vs 51 [26.3%]; P < .001; and eBP, 90 [39.6%] vs 31 [16.0%]; P < .001). Male participants were significantly less likely than female participants to remit from childhood OSA (8 [3.5%] vs 19 [9.8%]; P < .001) and more likely to develop OSA in adolescence (93 [41.0%] vs 42 [21.6%]; P < .001). The eFigure in the Supplement plots the percentage at each level of adolescent AHI (from 1 to 5 events per hour of sleep) by sex and shows that the presence of OSA is significantly higher in male participants, starting at the threshold of AHI of 2 or more and peaking at AHI of 5 or more. In the models in Table 3, the odds of eBP for male participants were about 3-fold after controlling for VAT (model 2: OR, 3.2; 95% CI 2.0-5.3; P < .001) or MetS (model 3: OR, 3.3; 95% CI 2.0-5.3; P < .001). In contrast, when assessing orthostatic hyperreactivity (Table 4), the OR for sex was nonsignificant tending toward females (OR, 0.5; 95% CI 0.3-1.0; P = .06).
Discussion
This study reports multiple notable findings. First, we found an association between childhood OSA and adolescent eBP if OSA persisted during this developmental period or if the child was male. Second, we found that the severity of adolescent OSA was significantly associated with eBP and orthostatic hypertension in a dose-response manner. Third, there was a significant involvement of VAT and MetS in the association between adolescent OSA and eBP that, however, did not completely explain the observed risk in more severe adolescent OSA. Finally, adolescent male participants were 2 times more likely to have OSA, a ratio that was not present in childhood and is closer to that observed in adults, which suggests that sex-related adipose distribution and metabolic factors may play an etiopathogenic role in OSA and its associated cardiovascular risk as early as adolescence.
Two previous population-based studies6,7 examined whether childhood OSA is independently associated with adolescent BP levels. A cohort study found that a higher respiratory disturbance index was associated with seated SBP in the evening (P = .04)6; however, after controlling for sex and ethnicity, the association became nonsignificant (P = .08) and SBP was associated with greater BMI. In a case-control subset (N = 185) of another cohort study, baseline OSA was independently associated with 24-hour ambulatory SBP and DBP at follow-up7; in addition, baseline BMI and change in BMI were associated with follow-up SBP, leading the authors to conclude that the association between childhood OSA and increased BP was likely affected by obesity.7 These 2 previous studies differ from the present study in terms of length of follow-up (5 years and 4 years vs 7.4 years) and follow-up age of the participants (13.7 years and 14.3 years vs 16.5 years at follow-up), suggesting that the current study is able to assess the association of pediatric OSA with eBP in the transition to adolescence without overlapping developmental periods.
Childhood OSA was not independently associated with adolescent hypertension; rather, this association was modified by its persistent course and male sex. Previous reports indicate that childhood obesity is associated with the persistence of childhood OSA and that boys are more likely to persist with OSA and develop childhood OSA in the transition to adolescence.13,18 Here, we found that children in whom OSA persisted had nearly 3-fold increased odds of hypertension in adolescence. In contrast, children in whom OSA remitted were not at risk of adolescent hypertension, a finding consistent with a previous observation that weight loss (ie, a change of −4.7 BMI percentiles) was associated with the remission of childhood OSA with about 2-fold odds during this developmental period18 as well as with female participants being more likely to remit from childhood OSA (eTable 2 in the Supplement). Together with a previously reported cross-sectional association between childhood OSA and subclinical BP levels,26 the present data using a longitudinal design and guideline-recommended definitions for adolescent hypertension indicate that childhood OSA should be monitored and targeted to prevent future adverse cardiovascular outcomes. Given the high remission rate of childhood OSA and the significant association of obesity with its persistence and incidence13,18 and with inflammation20,34,35 in the transition to adolescence, future studies should examine the inflammatory and vascular mechanisms of the longitudinal association of persistent childhood OSA with hypertension.
Another notable finding of this study supported an involvement of VAT and MetS underlying the association of adolescent OSA with hypertension, similar to adults,36 but that does not completely account for the observed increased risk. Three previous studies in adolescents have examined the association between OSA and MetS, albeit with inconsistent results.37,38,39 Two of these studies included only participants with obesity38,39; one (N = 104) did not find an association between MetS and OSA, defined as AHI of 1 or more,38 while the other study (N = 42) reported a significant association between metabolic variables and OSA, defined as 4 AHI severity levels.39 In addition, a cohort study (N = 270) also found an association between metabolic factors and OSA, defined as AHI of 5 or more.37 In the current study, the association of AHI between 2 and less than 5 with hypertension was explained by VAT and MetS, while the association of AHI of 5 or more remained 2-fold and significant, supporting its independent association with increased cardiovascular risk in adolescence. Furthermore, we observed that this level of severity of adolescent OSA was also independently associated with nearly 3-fold increased odds of orthostatic hypertension, as assessed by BP hyperreactivity.
Orthostatic hypertension is an understudied phenomenon considered to be a risk factor for cardiovascular disease.21,22,23,24 As opposed to orthostatic hypotension, which was not associated with adolescent OSA in this study, the pathophysiology of orthostatic hypertension remains poorly understood but is thought to be associated with sympathetic hyperactivity, arising from hypersensitivity of the cardiopulmonary and arterial baroreceptor reflex, and α-adrenergic hyperactivation.21,22 In youth with orthostatic hypertension, a decrease in sequential baroreflex sensitivity and increase in the low-frequency to high-frequency ratio of the R-R interval has been observed when shifting from supine to standing.24 Higher antidiuretic hormone levels, lower nitric oxide and nitric oxide synthetase activity, and elevated norepinephrine levels have also been observed in individuals with orthostatic hypertension, while a vasodilation dysfunction remains unclear in youth.24 Considering prior evidence for sympathetic overflow and weaker parasympathetic modulation in pediatric OSA,3,9,20,40,41,42,43 our orthostatic BP findings support that markers of vascular reactivity, cardiac-autonomic modulation, or endothelial function may better identify OSA-associated cardiovascular risk earlier in the life span.
There was a shift in the sex distribution of OSA from childhood to adolescence. Although the prevalence of childhood OSA in male and female participants was approximately equal in childhood, the ratio shifted to 2:1 toward male participants in the adolescence follow-up. Childhood OSA was not associated with increased odds of adolescent eBP in female participants, in whom the remission of childhood OSA was higher. In contrast, the incidence and persistence of childhood OSA were higher in male participants and associated with adolescent eBP. In adults, the overall ratio of OSA between men and women is 2:1 or 4:1 depending on the AHI threshold, age, and menopausal status of women, in whom hormonal factors increase its prevalence.44,45,46 Evidence indicates that sexual maturation is associated with the trajectory of adipose tissue.47 Although females accumulate more subcutaneous adiposity during and after puberty, depositing fat preferentially in the gynoid and extremity (gluteofemoral) regions, pubertal and postpubertal males deposit more fat in the abdominal region, particularly in the visceral depot, which has been associated with the observed sex dimorphism in cardiovascular risk, greater in young males.47 An area that remains underexplored during early developmental stages is the relative association of lower gluteofemoral fat with cardiovascular risk,48 although it does not appear to show an association with OSA in youth.13,18,19 Altogether, these data suggest that VAT and/or MetS in males and hormonal changes in females may partially explain the sex-related disparities in OSA that appear to begin in the transition to adolescence and its cardiovascular sequelae persist into young adulthood.8
Limitations
This study has limitations. A total of 60.1% of the Penn State Child Cohort participated in this follow-up examination, which may introduce a slight selection bias. Nevertheless, no significant differences in demographic characteristics were found between the participants studied and the 279 lost to follow-up.13 Although data analyses included longitudinal associations between childhood OSA and adolescent eBP, some others were based on cross-sectional analyses, which limit causal inference. Finally, eBP was not based on 3 or more visits separated over time or on 24-hour ambulatory BP monitoring,1 which may lead to slight misclassification.
Conclusions
In this cohort study, children in whom pediatric OSA (AHI≥2) persisted in the developmental transition to adolescence had a nearly 3-fold increased odds of future hypertension. Adolescent OSA was associated with hypertension in a dose-response manner and VAT and MetS were involved with this association, except in adolescents with more severe OSA (AHI≥5). Sex was also involved with hypertension risk, which suggests that, at least for adolescent males, metabolic abnormalities are in the mechanistic pathway between OSA and hypertension. Obstructive sleep apnea should be screened, monitored, and targeted early in life to prevent future cardiovascular disease.
eTable 1. American Academy of Pediatrics Blood Pressure Pediatric Guidelines Endorsed by the American Heart Association
eTable 2. Childhood (Baseline) and Adolescence (Follow-up) Characteristics of the Sample Stratified by Males and Females
eFigure. Distribution of Apnea/Hypopnea Index at Each Individual Threshold of One Through Five for Males and Females
References
- 1.Flynn JT, Kaelber DC, Baker-Smith CM, et al. ; Subcommittee on Screening and Management of High Blood Pressure in Children . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e484-e594. [DOI] [PubMed] [Google Scholar]
- 3.Smith DF, Amin RS. OSA and cardiovascular risk in pediatrics. Chest. 2019;156(2):402-413. doi: 10.1016/j.chest.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DelRosso LM, Mogavero MP, Ferri R. Effect of sleep disorders on blood pressure and hypertension in children. Curr Hypertens Rep. 2020;22(11):88. doi: 10.1007/s11906-020-01100-x [DOI] [PubMed] [Google Scholar]
- 5.Ehsan Z, Ishman SL, Kimball TR, Zhang N, Zou Y, Amin RS. Longitudinal cardiovascular outcomes of sleep disordered breathing in children: a meta-analysis and systematic review. Sleep. 2017;40(3):zsx015. doi: 10.1093/sleep/zsx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161(1):26-30. doi: 10.1016/j.jpeds.2011.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li AM, Au CT, Ng C, Lam HS, Ho CKW, Wing YK. A 4-year prospective follow-up study of childhood OSA and its association with BP. Chest. 2014;145(6):1255-1263. doi: 10.1378/chest.13-1333 [DOI] [PubMed] [Google Scholar]
- 8.Chan KC, Au CT, Hui LL, Wing YK, Li AM. Childhood OSA is an independent determinant of blood pressure in adulthood: longitudinal follow-up study. Thorax. 2020;75(5):422-431. doi: 10.1136/thoraxjnl-2019-213692 [DOI] [PubMed] [Google Scholar]
- 9.Quante M, Wang R, Weng J, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. Sleep. 2015;38(9):1395-1403. doi: 10.5665/sleep.4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6- to 17-year old children—the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2010;157(1):57-61. doi: 10.1016/j.jpeds.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376. doi: 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spilsbury JC, Storfer-Isser A, Rosen CL, Redline S. Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence. Sleep. 2015;38(1):23-29. doi: 10.5665/sleep.4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bixler EO, Fernandez-Mendoza J, Liao D, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47(5):1402-1409. doi: 10.1183/13993003.01771-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan KC, Au CT, Hui LL, Ng S-K, Wing YK, Li AM. How OSA evolves from childhood to young adulthood: natural history from a 10-year follow-up study. Chest. 2019;156(1):120-130. doi: 10.1016/j.chest.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Ellenberg SS, Hou X, et al. ; Childhood Adenotonsillectomy Trial . Prognosis for spontaneous resolution of OSA in children. Chest. 2015;148(5):1204-1213. doi: 10.1378/chest.14-2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannon TS, Lee S, Chakravorty S, Lin Y, Arslanian SA. Sleep-disordered breathing in obese adolescents is associated with visceral adiposity and markers of insulin resistance. Int J Pediatr Obes. 2011;6(2):157-160. doi: 10.3109/17477166.2010.482156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7(3):268-273. doi: 10.5664/JCSM.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye SS, Fernandez-Mendoza J, Calhoun SL, et al. Childhood obesity, weight loss and developmental trajectories predict the persistence and remission of childhood sleep-disordered breathing. Pediatr Obes. 2019;14(1). doi: 10.1111/ijpo.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danisi JM, Fernandez-Mendoza J, Vgontzas AN, et al. Association of visceral adiposity and systemic inflammation with sleep disordered breathing in normal weight, never obese adolescents. Sleep Med. 2020;69:103-108. doi: 10.1016/j.sleep.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kheirandish-Gozal L, Gozal D. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci. 2019;20(3):459. doi: 10.3390/ijms20030459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fessel J, Robertson D. Orthostatic hypertension: when pressor reflexes overcompensate. Nat Clin Pract Nephrol. 2006;2(8):424-431. doi: 10.1038/ncpneph0228 [DOI] [PubMed] [Google Scholar]
- 22.Kario K. Orthostatic hypertension—a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9(12):726-738. doi: 10.1038/nrneph.2013.224 [DOI] [PubMed] [Google Scholar]
- 23.Thomas RJ, Liu K, Jacobs DR Jr, Bild DE, Kiefe CI, Hulley SB. Positional change in blood pressure and 8-year risk of hypertension: the CARDIA Study. Mayo Clin Proc. 2003;78(8):951-958. doi: 10.1016/S0025-6196(11)63142-X [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Jin H, Du J. Orthostatic hypertension in children: an update. Front Pediatr. 2020;8:425. doi: 10.3389/fped.2020.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731-736. doi: 10.1093/sleep/32.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bixler EO, Vgontzas AN, Lin H-M, et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52(5):841-846. doi: 10.1161/HYPERTENSIONAHA.108.116756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68(3):360-366. doi:10.1136/adc.68.3.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A.. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. NIMH Publication 204. US Government Printing Office; 1968.
- 29.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 31.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetol Metab Syndr. 2010;2:8. doi: 10.1186/1758-5996-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He F, Bixler EO, Berg A, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015;16(7):856-861. doi: 10.1016/j.sleep.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16(12):1489-1494. doi: 10.1016/j.sleep.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016;311(5):E851-E858. doi: 10.1152/ajpendo.00249.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Increased inflammation from childhood to adolescence predicts sleep apnea in boys: a preliminary study. Brain Behav Immun. 2017;64:259-265. doi: 10.1016/j.bbi.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: the road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. 2018;42:211-219. doi: 10.1016/j.smrv.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401-408. doi: 10.1164/rccm.200703-375OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erdim I, Akcay T, Yilmazer R, Erdur O, Kayhan FT. Is metabolic syndrome associated with obstructive sleep apnea in obese adolescents? J Clin Sleep Med. 2015;11(12):1371-1376. doi: 10.5664/jcsm.5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhushan B, Ayub B, Loghmanee DA, Billings KR. Metabolic alterations in adolescents with obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2015;79(12):2368-2373. doi: 10.1016/j.ijporl.2015.10.046 [DOI] [PubMed] [Google Scholar]
- 40.Liao D, Li X, Rodriguez-Colon SM, et al. Sleep-disordered breathing and cardiac autonomic modulation in children. Sleep Med. 2010;11(5):484-488. doi: 10.1016/j.sleep.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao D, Li X, Vgontzas AN, et al. Sleep-disordered breathing in children is associated with impairment of sleep stage–specific shift of cardiac autonomic modulation. J Sleep Res. 2010;19(2):358-365. doi: 10.1111/j.1365-2869.2009.00807.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao D, Rodríguez-Colón SM, He F, Bixler EO. Childhood obesity and autonomic dysfunction: risk for cardiac morbidity and mortality. Curr Treat Options Cardiovasc Med. 2014;16(10):342. doi: 10.1007/s11936-014-0342-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179-189. doi: 10.1016/j.smrv.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 44.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3, pt 1):608-613. doi: 10.1164/ajrccm.163.3.9911064 [DOI] [PubMed] [Google Scholar]
- 45.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181-1185. doi: 10.1164/rccm.200209-1055OC [DOI] [PubMed] [Google Scholar]
- 46.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167(9):1186-1192. doi: 10.1164/rccm.200210-1238OC [DOI] [PubMed] [Google Scholar]
- 47.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes (Lond). 2012;36(10):1261-1269. doi: 10.1038/ijo.2012.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lotta LA, Wittemans LBL, Zuber V, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. 2018;320(24):2553-2563. doi: 10.1001/jama.2018.19329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. American Academy of Pediatrics Blood Pressure Pediatric Guidelines Endorsed by the American Heart Association
eTable 2. Childhood (Baseline) and Adolescence (Follow-up) Characteristics of the Sample Stratified by Males and Females
eFigure. Distribution of Apnea/Hypopnea Index at Each Individual Threshold of One Through Five for Males and Females