Key Points
Question
Can death within 90 days be accurately predicted before esophagectomy for cancer?
Findings
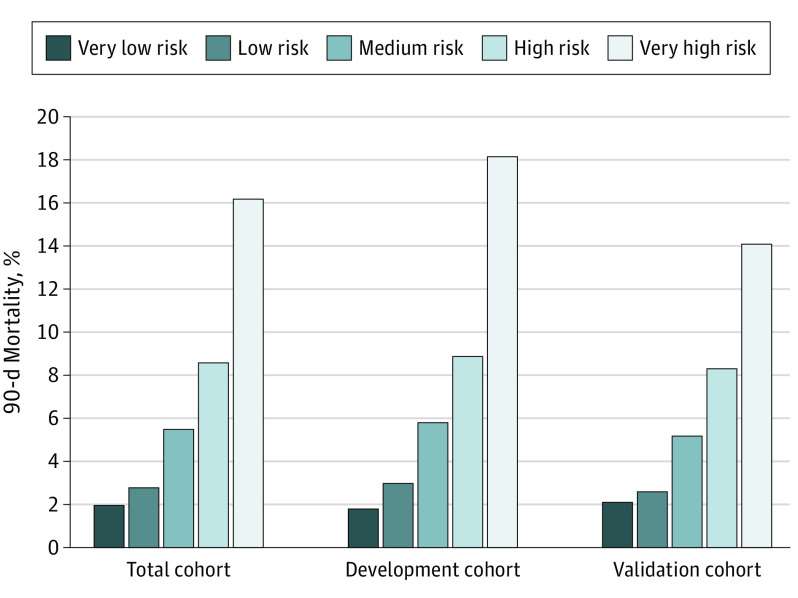
In this diagnostic/prognostic study, a scoring system that predicted death within 90 days based on logistic regression β coefficients was developed for 8403 patients randomly assigned to development (n = 4172) and validation (n = 4231) cohorts. On the basis of 10 preoperative variables, the final score allowed stratification into 5 risk groups: very low risk (1.8%), low risk (3.0%), medium risk (5.8%), high risk (8.9%), and very high risk (18.2%) of death within 90 days.
Meaning
The International Esodata Study Group risk prediction model allowed for stratification of an individual patient’s risk of death within 90 days after esophagectomy and may aid decision-making and the consent process between patients and surgeons.
Abstract
Importance
Ninety-day mortality rates after esophagectomy are an indicator of the quality of surgical oncologic management. Accurate risk prediction based on large data sets may aid patients and surgeons in making informed decisions.
Objective
To develop and validate a risk prediction model of death within 90 days after esophagectomy for cancer using the International Esodata Study Group (IESG) database, the largest existing prospective, multicenter cohort reporting standardized postoperative outcomes.
Design, Setting, and Participants
In this diagnostic/prognostic study, we performed a retrospective analysis of patients from 39 institutions in 19 countries between January 1, 2015, and December 31, 2019. Patients with esophageal cancer were randomly assigned to development and validation cohorts. A scoring system that predicted death within 90 days based on logistic regression β coefficients was conducted. A final prognostic score was determined and categorized into homogeneous risk groups that predicted death within 90 days. Calibration and discrimination tests were assessed between cohorts.
Exposures
Esophageal resection for cancer of the esophagus and gastroesophageal junction.
Main Outcomes and Measures
All-cause postoperative 90-day mortality.
Results
A total of 8403 patients (mean [SD] age, 63.6 [9.0] years; 6641 [79.0%] male) were included. The 30-day mortality rate was 2.0% (n = 164), and the 90-day mortality rate was 4.2% (n = 353). Development (n = 4172) and validation (n = 4231) cohorts were randomly assigned. The multiple logistic regression model identified 10 weighted point variables factored into the prognostic score: age, sex, body mass index, performance status, myocardial infarction, connective tissue disease, peripheral vascular disease, liver disease, neoadjuvant treatment, and hospital volume. The prognostic scores were categorized into 5 risk groups: very low risk (score, ≥1; 90-day mortality, 1.8%), low risk (score, 0; 90-day mortality, 3.0%), medium risk (score, –1 to –2; 90-day mortality, 5.8%), high risk (score, −3 to −4: 90-day mortality, 8.9%), and very high risk (score, ≤−5; 90-day mortality, 18.2%). The model was supported by nonsignificance in the Hosmer-Lemeshow test. The discrimination (area under the receiver operating characteristic curve) was 0.68 (95% CI, 0.64-0.72) in the development cohort and 0.64 (95% CI, 0.60-0.69) in the validation cohort.
Conclusions and Relevance
In this study, on the basis of preoperative variables, the IESG risk prediction model allowed stratification of an individual patient’s risk of death within 90 days after esophagectomy. These data suggest that this model can help in the decision-making process when esophageal cancer surgery is being considered and in informed consent.
This diagnostic/prognostic study develops and validates a risk prediction model for 90-day mortality after esophagectomy for cancer.
Introduction
Esophageal cancer resections, notwithstanding significant improvements in surgical technique and perioperative care in the modern era, still carry a risk of death greater than most common oncologic resections, with 5% or less a modern benchmark. These cancers also carry a high risk of major morbidity that ranges from 35% to 60%, depending on definitions used and irrespective of whether the operation is performed by open, minimally invasive, hybrid, or robotic-assisted technique.1,2,3,4,5,6,7,8
In the reporting of operative mortality, it is clear that the traditional practice of reporting only death within the first 30 days masks a considerable percentage of postoperative deaths, up to double, when compared with 90-day mortality.2,6,7,8 Seen as a new indicator of quality of care, 90-day mortality is a more reliable metric to examine contemporary cancer management. Factors associated with 90-day mortality include tumor-related variables, patient comorbidities and characteristics, operative interventions, and perioperative management.9,10
Considering 90-day mortality as an emerging standard to evaluate outcomes that reflect all patients whose deaths were surgery related, prediction of mortality should be adapted to this new benchmark. Risk prediction is essential to determine eligibility for surgery, promote a care-related quality improvement process, and assist patients and practitioners in the decision-making process that guides treatment. Several risk prediction models for mortality after esophagectomy have been developed; however, few models were designed to predict death within 90 days.11,12,13,14,15 Moreover, clinical application of these preexisting models remains limited because of low performance, poor discrimination, absence of some pivotal variables, and lack of proper validation studies.11,12,13,14,15 A key limitation is the power of the studies because of small patient cohorts within these models. The focus of this study was to develop a novel risk prediction model using a large contemporaneous cohort. The premise is that this would be a reliable metric that would add granularity to risk assessment of surgical management in a period during which the comprehensive approach to the overall treatment of esophageal cancer is currently being scrutinized and reassessed.
The International Esodata Study Group (IESG) is currently the most powerful and largest prospective, multicenter collection of standardized complications after esophagectomy.2,16,17,18,19 We hypothesized that the IESG database, having achieved consensus on predictor definition and on standardized methods of reporting, would allow the development and the validation of a risk prediction model for 90-day mortality after esophagectomy for cancer.
Methods
Setting and Study Population
Patient data were prospectively acquired between January 1, 2015, and December 31, 2019, on the online platform of the IESG data set (Esodata) for standardized reporting of complications after esophagectomy2,16,19 from 39 institutions in 19 countries worldwide. All participating centers are tertiary centers, mainly academic medical centers (eAppendix in Supplement 1). All contributors approved collecting all esophagectomy procedures for benign and malignant diseases and were committed to providing 90-day follow-up for each included patient. Patient data were retrospectively extracted, encrypted, and deidentified to comply with international data privacy agreements in May 2020. Because of the retrospective design of this diagnostic/prognostic study, the Assistance Publique–Hôpitaux de Marseille Institutional Review Board approved the study and waived the need for patient consent. This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.
Patient Eligibility and Primary Outcome
The study population comprised patients who had been operated on for cancer of the esophagus and gastroesophageal junction. We excluded patients operated on for benign diseases and perforations. The primary end point was 90-day mortality, defined as all-cause death within 90 days, including deaths within the initial hospitalization, after discharge, after transfer to other acute care facilities, and requiring readmission.
Development and Validation Cohorts
The whole cohort was randomly assigned (Stata random generation) to define development and validation cohorts. Patients with perforations and benign disease were excluded afterward. A predictive model was developed using the development cohort data set and its performance tested in the predictive model from the validation cohort. We identified the following nonprespecified pretreatment- and treatment-related predictive variables present in Esodata: age; body mass index (BMI, calculated as weight in kilograms divided by height in meters squared); sex; World Health Organization/Eastern Cooperative Oncology Group performance status; American Society of Anesthesiologists score; Charlson Comorbidity Index with each comorbidity; hospital volume; tumor location; cTNM stage; elective or emergency procedure; preoperative treatment; tumor histologic subtype; surgical approaches (minimally invasive or open); level of anastomosis; cervical lymphadenectomy; and digestive conduit. Hospital volume was calculated on the basis of the mean of the number of esophagectomies per center per year and categorized in quartiles.
Statistical Analysis
A univariate logistic regression model was used to test the ability of potential baseline risk factors to predict the risk of death within 90 days. Variables with P < .10 in the univariate model were considered to be incorporated in a multiple logistic regression model.
After exclusion of variables exhibiting very low prevalence, variables with clinical relevance and identified bivariately as statistically significant were included in a multiple regression model to identify multivariably significant risk factors for death within 90 days. Continuous variables were presented as median (interquartile range) and as number (percentage) for categorical variables. The Fisher exact test and χ2 test were used as appropriate.
After a multiple logistic regression model was established, significant predictive variables were allocated weighted points that were proportional to their regression β coefficient values as already reported in previous risk prediction models.20,21 The final score for each patient was the result of the addition of all weighted points in the developmental and validation cohorts. Patients were categorized according to the integer of the final score and then collapsed into homogeneous 90-day mortality risk groups for calculation of 90-day mortality rates. The predicted probability of surgical 90-day mortality (P for mortality), expressed in percentage, was calculated with the following formula:
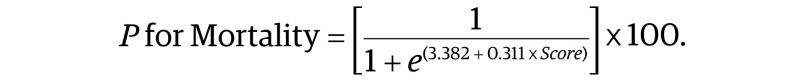 |
The reliability of the predictive model was assessed with respect to discrimination and calibration. Predictive model discrimination was analyzed by the area under the receiver operating characteristic curve. The model’s calibration was tested with a Hosmer-Lemeshow goodness-of-fit test. Comparison of the 90-day mortality rates across risk groups in both cohorts was assessed using the calculation of odds of death within 90 days. All analyses were performed with Stata/SE, version 16.1 (StataCorp LLC). A 2-sided P < .05 was considered statistically significant.
Results
During a 5-year period, the IESG data set included 8640 patients. After Strata random generation and after excluding 237 patients (193 benign cases and 44 perforations), the study cohort was represented by 8403 patients with cancer (mean [SD] age, 63.6 [9.0] years; 6641 [79.0%] male) (eFigure 1). The key patient characteristics of the development and validation groups are given in Table 1. eTable 1 in Supplement 1 details further comparative data on comorbidities, clinical and pathologic tumor staging, performance status, and surgical approach.
Table 1. Demographic and Clinical Characteristics of the Development and Validation Cohortsa.
| Characteristic | Total (N = 8403) | Development cohort (n = 4172) | Validation cohort (n = 4231) | P valueb |
|---|---|---|---|---|
| Sex | ||||
| Male | 6641 (79.0) | 3288 (78.8) | 3353 (79.2) | .62 |
| Female | 1762 (21.0) | 884 (21.2) | 878 (20.8) | |
| Age, mean (SD), y | 63.6 (9.0) | 63.8 (9.0) | 63.4 (10.0) | .73 |
| Age group, y | ||||
| ≤40 | 170 (2.0) | 75 (1.8) | 95 (2.2) | .24 |
| 41-50 | 658 (7.8) | 321 (7.7) | 337 (8.0) | |
| 51-60 | 2076 (24.7) | 1038 (24.9) | 1038 (24.5) | |
| 61-70 | 3357 (40.0) | 1641 (39.3) | 1716 (40.6) | |
| 71-80 | 1920 (22.8) | 975 (23.4) | 945 (22.3) | |
| >80 | 222 (2.5) | 122 (2.9) | 100 (2.4) | |
| BMI, mean (SD) | 26.04 (5.0) | 26.07 (5.0) | 26.0 (5.0) | .57 |
| BMI group | ||||
| <18.5 | 445 (5.3) | 227 (5.4) | 218 (5.2) | .90 |
| 18.5-24.9 | 3386 (40.3) | 1668 (40.0) | 1718 (40.6) | |
| 25-29.9 | 2966 (35.3) | 1478 (35.4) | 1488 (35.2) | |
| ≥30 | 1606 (19.1) | 799 (19.2) | 807 (19.1) | |
| WHO/ECOG performance status | ||||
| 0 | 4205 (50.0) | 2073 (49.7) | 2132 (50.4) | .85 |
| 1 | 3753 (44.7) | 1878 (45.0) | 1875 (44.3) | |
| 2 | 379 (4.5) | 186 (4.5) | 193 (4.6) | |
| 3 | 66 (0.8) | 35 (0.8) | 31 (0.7) | |
| Myocardial infarction | 442 (5.3) | 215 (5.2) | 227 (5.4) | .66 |
| Connective tissue disease | 71 (0.8) | 36 (0.9) | 35 (0.8) | .85 |
| Chronic pulmonary disease | 845 (10.1) | 417 (10.0) | 428 (10.1) | .85 |
| Moderate to severe renal disease | 136 (1.6) | 66 (1.6) | 70 (1.7) | .79 |
| Peripheral vascular disease | 433 (5.2) | 220 (5.3) | 213 (5.0) | .62 |
| Diabetes (uncomplicated) | 1058 (12.6) | 550 (13.2) | 508 (12.0) | .10 |
| Diabetes (end-organ damage) | 62 (0.7) | 33 (0.8) | 29 (.7) | .57 |
| Liver disease (mild) | 167 (2.0) | 84 (2.0) | 83 (2.0) | .86 |
| Liver disease (moderate to severe) | 35 (0.4) | 20 (0.5) | 15 (0.4) | .37 |
| Solid tumor present | 8228 (97.9) | 4086 (97.9) | 4142 (97.9) | .89 |
| Neoadjuvant therapy | ||||
| Chemoradiotherapy | 3916 (46.6) | 1969 (47.2) | 1947 (46.0) | .49 |
| Chemotherapy | 2393 (28.5) | 1199 (28.7) | 1194 (28.2) | |
| Definitive chemoradiotherapy | 154 (1.7) | 76 (1.8) | 78 (1.8) | |
| Radiotherapy alone | 13 (0.1) | 6 (0.1) | 7 (0.1) | |
| None | 1927 (23.0) | 922 (22.0) | 1005 (23.8) | |
| Volume activity, mean per year per center | ||||
| 0-45.9 | 2169 (25.8) | 1102 (26.4) | 1067 (25.2) | .40 |
| 46-71.6 | 1975 (23.5) | 980 (23.5) | 995 (23.5) | |
| 71.7-108.6 | 2355 (28.0) | 1138 (27.3) | 1217 (28.8) | |
| >108.6 | 1904 (22.7) | 952 (22.8) | 952 (22.5) | |
| Deaths within 30 postoperative days | 164 (2.0) | 89 (2.1) | 75 (1.8) | .23 |
| Grade V (Clavien-Dindo) | 196 (2.3) | 100 (2.3) | 96 (2.4) | .69 |
| Deaths within 90 postoperative days | 353 (4.2) | 184 (4.4) | 169 (4.0) | |
| In-hospital before discharge | 191/353 (54.1) | 100/184 (54.3) | 91/169 (53.8) | .34 |
| No readmission within 30 d of discharge | 116/353 (32.9) | 59/184 (32.1) | 57/169 (33.7) | |
| Readmission related within 30 d of discharge | 34/353 (9.6) | 18/184 (9.7) | 16/169 (9.4) | |
| Readmission status within 30 d not known | 6/353 (1.7) | 3/184 (1.6) | 3/169 (1.7) | |
| Unrelated readmission within 30 d of discharge | 6/353 (1.7) | 4/184 (2.1) | 2/169 (1.1) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Data are presented as number/total number (percentage) of patients unless otherwise indicated.
Fisher exact test and χ2 test were used as appropriate.
Among the 8403 patients, 164 patients (2.0%) died within 30 days and 353 patients (4.2%) within 90 days, corresponding to a 2-fold increase in mortality rate. The different mortality patterns are given in Table 1. A total of 191 of the 353 patients (54.1%) who died within 90 days died in the hospital before discharge, 34 (9.6%) after readmission related to esophagectomy and 6 (1.7%) after unrelated readmission within 30 days of discharge, whereas 116 patients (32.9%) died within 90 days after hospital discharge without readmission.
The development cohort (n = 4172) and the validation cohort (n = 4231) were not significantly different in key domains (Table 1; eTable 1 in Supplement 1). The rate of congestive heart failure was the sole difference and was significantly higher in the validation cohort. The rates of 30-day mortality (2.1% in the development cohort and 1.8% in the validation cohort; P = .23) and 90-day mortality (4.4% in the development cohort and 4.0% in the validation cohort; P = .34) were similar between the 2 cohorts.
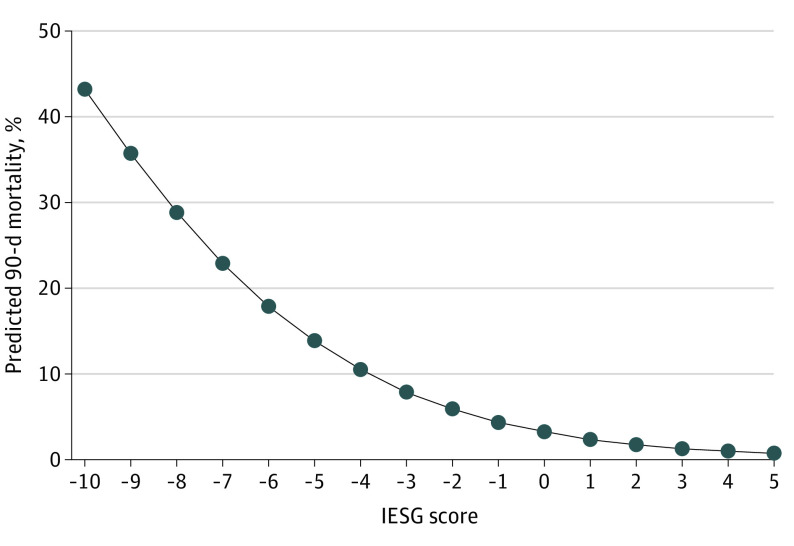
eTable 2 in Supplement 1 gives the results of a univariate logistic regression model of the development cohort that compares patients alive after 90 postoperative days (n = 3988) with those who died within 90 postoperative days (n = 184). The univariate regression model revealed 10 variables with P < .10 that were thus considered as potential factors to predict the risk of death within 90 days: age, BMI, sex, World Health Organization/Eastern Cooperative Oncology Group classification, history of myocardial infarction, connective tissue disease, peripheral vascular disease, liver disease (moderate to severe), neoadjuvant treatment, and hospital volume (number of admissions per year per center) (eFigure 2 in Supplement 1). The 10 variables were included in a multiple logistic regression analysis using the stepwise proportional hazards regression model to identify significant risk factors for death within 90 days. Patients treated with radiotherapy alone (n = 13) were not included in the multiple logistic regression. Weighted points were assigned to each variable using a linear transformation of the regression β coefficients (Table 2).20,21 At the last step, a final risk score was obtained by addition of all 10 weighted points for each patient. A negative score was associated with a higher risk of death within 90 days. A positive score was associated with a lower risk of death within 90 days. The final risk score ranged from −10 to +5, determining 16 risk groups of patients. Figure 1 depicted the predicted 90-day mortality according to the risk score. The distribution of patients according to their score is reported in eFigure 3 in Supplement 1. The best cutoff was applied to categorize the development cohort into 5 different homogeneous groups of risk according to their score: very low risk (score, ≥1), low risk (score, 0), medium risk (score, −1 to −2), high risk (score, −3 to −4), and very high risk (score, ≤−5); details of categorization of the patients into the 5 risk groups are provided in eTables 3, 4, and 5 in Supplement 1.
Table 2. Multiple Logistic Regression Model and Weighted Point Assignment in the Development Cohorta.
| Variable | OR (95% CI) | β | P value | Weighted points |
|---|---|---|---|---|
| Age, y | ||||
| ≤40 | 1.17 (0.35-3.87) | 0.157 | .80 | 0 |
| 41-50 | 0.81 (0.41-1.61) | −0.207 | .55 | 1 |
| 51-60 | 0.79 (0.52-1.20) | −0.241 | .26 | 1 |
| 61-70 | NA | 1 [Reference] | 0 | |
| 71-80 | 1.06 (0.72-1.60) | 0.058 | .77 | 0 |
| >80 | 2.98 (1.54-5.75) | 1.092 | .001 | −3 |
| BMI | ||||
| <18.5 | 2.57 (1.48-4.48) | 0.944 | .001 | −3 |
| 18.5-24.9 | NA | 1 [Reference] | 0 | |
| 25-29.9 | 0.60 (0.42-0.86) | −0.516 | .005 | 1 |
| ≥30 | 0.62 (0.40-0.97) | −0.476 | .04 | 1 |
| Sex | ||||
| Male | NA | 1 [Reference] | 0 | |
| Female | 0.50 (0.32-0.78) | −0.696 | .002 | 2 |
| WHO/ECOG performance statusb | ||||
| 0 | NA | 1 [Reference] | 0 | |
| 1 | 1.26 (0.91-1.75) | 0.231 | .17 | −1 |
| 2 | 1.83 (0.98-3.42) | 0.606 | .06 | −2 |
| 3 | 3.69 (1.18-11.56) | 1.305 | .02 | −4 |
| Myocardial infarctionc | ||||
| No | 1 [Reference] | 0 | ||
| Yes | 2.18 (1.31-3.60) | 0.777 | .002 | −2 |
| Connective tissue diseased | ||||
| No | 1 [Reference] | 0 | ||
| Yes | 3.07 (1.02-9.25) | 1.123 | .046 | −3 |
| Peripheral vascular diseasee | ||||
| No | 1 [Reference] | 0 | ||
| Yes | 1.83 (1.10-3.03) | 0.604 | .02 | −2 |
| Liver disease (moderate to severe)f | ||||
| No | 1 [Reference] | 0 | ||
| Yes | 5.06 (1.63-15.72) | 1.622 | .005 | −5 |
| Neoadjuvant therapy | ||||
| None | 1 [Reference] | 0 | ||
| Definitive chemoradiotherapy | 2.10 (0.76-5.77) | 0.739 | .15 | −2 |
| Chemotherapy only | 1.23 (0.75-2.04) | 0.215 | .40 | 0 |
| Chemoradiotherapy | 1.68 (1.09-2.57) | 0.516 | .02 | −1 |
| Hospital volume, mean per year per center | ||||
| 0-45.9 | 1 [Reference] | 0 | ||
| 46-71.6 | 1.00 (0.66-1.50) | −0.003 | .99 | 0 |
| 71.7-108.6 | 0.96 (0.64-1.45) | −0.036 | .86 | 0 |
| >108.6 | 1.60 (0.37-0.98) | −0.512 | .04 | 1 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; OR, odds ratio; WHO/ECOG, World Health Organization/Eastern Cooperative Oncology Group.
The method ENTER was used. The 10 variables were significant, and none were removed. Negative and positive scores are associated with higher and lower risk of death, respectively. The linear transformation was obtained by dividing the corresponding β coefficient by −0.696 (the lowest β value of individual variable [female sex]) multiplied by a constant (2) and rounded to the nearest integer.19,20
The WHO/ECOG performance statuses: 0, fully active, able to perform all predisease performance without restriction; 1, restricted in physically strenuous activity but ambulatory and able to perform work of a light or sedentary nature; 2, ambulatory and capable of all self-care but unable to perform any work activities; up and about more than 50% of waking hours; 3, capable of only limited self-care; confined to bed or chair more than 50% of waking hours; 4, completely disabled; cannot carry on any self-care; totally confined to bed or chair; and 5, dead.
History of definite or probable myocardial infarction (electrocardiogram changes and/or enzyme changes).
Systemic lupus erythematosus, polymyositis, mixed connective tissue disease, polymyalgia rheumatica, and moderate to severe rheumatoid arthritis.
Intermittent claudication or past bypass for chronic arterial insufficiency, history of gangrene or acute arterial insufficiency, or untreated thoracic or abdominal aneurysm (≥6 cm).
Cirrhosis and portal hypertension but no variceal bleeding history (moderate) or cirrhosis and portal hypertension with variceal bleeding history (severe). Hospital volume was based on the sum of esophagectomies per hospital during the study period and was expressed in mean per year per center.
Figure 1. Predicted Surgical 90-Day Mortality According to International Esodata Study Group (IESG) Risk Score Associated With the Sum Score.
See the Methods section for the formula used to calculate the predicted probability of surgical 90-day mortality (P for mortality).
The distribution of deaths within 90 days was similar according to risk score stratification between the validation and development cohorts (Figure 2 and Table 3). In terms of discrimination, the areas under the curve were 0.68 (95% CI, 0.64-0.72) in the development cohort and 0.64 (95% CI, 0.60-0.69) in the validation cohort (eFigure 4 in Supplement 1). In terms of calibration, the model was supported by a nonsignificant Hosmer-Lemeshow χ2 test in both cohorts (eFigure 5 in Supplement 1). The odds ratio was calculated for each risk group, with the very low–risk group considered the reference (Table 3). A simple comparison between predictive scores of patients with a very low risk (score, ≥1) and those of patients with a very high risk (score, ≤−5) demonstrated an 11-fold increase in 90-day mortality in the development cohort (1.8% vs 18.2%) and a 7-fold increase in 90-day mortality in the validation cohort (2.1% vs 14.1%). eTables 6, 7, and 8 in Supplement 1 provide an algorithm for calculation of the final risk score with a range for prediction of death within 90 days and show repartition of mortality and complications in each risk group.
Figure 2. Surgical 90-Day Mortality According to Risk Group Score in the Development and Validation Cohorts.
The best cutoff was reached to collapse the cohort into 5 different homogeneous groups of risk according to their score: very low risk (score, ≥1), low risk (score, 0), medium risk (score, −1 to −2), high risk (score, −3 to −4), and very high risk (score, ≤−5).
Table 3. Ninety-Day Mortality, ORs for Death, and Risk Performance by Groupa.
| Risk group (score) | Development cohort (n = 4172) | Validation cohort (n = 4231) | ||||
|---|---|---|---|---|---|---|
| 90-d Mortality, No./total No. (%) | OR (95% CI) | P value | 90-d Mortality, No./total No. (%) | OR (95% CI) | P value | |
| Very low risk (≥1) | 25/1335 (1.8) | 1 [Reference] | 29/1350 (2.1) | 1 [Reference] | ||
| Low risk (0) | 32/1058 (3.0) | 1.63 (1.00-2.77) | <.001 | 31/1173 (2.6) | 1.23 (1.0-2.0) | .004 |
| Medium risk (−1 to −2) | 76/1308 (5.8) | 3.23 (2.04-5.11) | <.001 | 65/1246 (5.2) | 2.5 (1.6-3.9) | <.001 |
| High risk (−3 to −4) | 34/378 (8.9) | 5.17 (3.04-8.79) | <.001 | 31/370 (8.3) | 4.16 (2.4-7.0) | <.001 |
| Very high risk (≤−5) | 17/93 (18.2) | 11.7 (6.07-22.6) | <.001 | 13/92 (14.1) | 7.5 (3.7-14.9) | <.001 |
Abbreviation: OR, odds ratio.
Discrimination refers to the ability to distinguish patients who will die from those who will survive. The model’s good calibration refers to the concordance between observed outcomes and predicted probabilities of death within 90 days. The area under the receiver operating characteristic curve was 0.68 (95% CI, 0.64-0.72) for the development cohort and 0.64 (95% CI, 0.60-0.69) for the validation cohort. Calibration (Hosmer-Lemeshow goodness-of-fit test) was χ28 = 8.38 (P = .39) for the development cohort and χ28 = 9.46 (P = .30) for the validation cohort. A nonsignificant test indicated that the model was calibrated. Akaike information criteria were 1429.45 for the development cohort and 1378.24 for the validation cohort.
Discussion
This diagnostic/prognostic study provided a unique opportunity to analyze a data set of more than 8000 patients and enabled development of a model that allowed stratification of individual risk of death within 90 days in 5 distinctive groups. For everyday practice, the data sets that are incorporated are readily accessible before surgery. The application of the IESG score at the first patient visit or at the multidisciplinary tumor board would add granularity to patient selection and would aid practitioners and patients in the decision-making process and in providing informed consent. Moreover, the IESG score would also provide a specific method for flagging patients who are identified as being at higher risk for postsurgical death. A further potential application of the IESG score may be in clinical trials, including ongoing clinical trials such as SANO (Surgery as Needed in Esophageal Cancer), which examines the concept of a surgery-as-needed approach to esophageal resection.22,23 In a broader context, patients with a complete clinical response may be more appropriately selected for a conservative surveillance approach, particularly if the IESG risk assessment is very high.
Age is a significant factor in decision-making and risk assessment, and this study confirms that an age of 80 years or older is an important component of the surgical risk of death within more than 90 days, being ascribed −3 points. Although appropriately selected older patients can undergo surgical treatment without demonstrating elevated mortality rates,24,25,26 older patients represent a high-risk population requiring careful oncogeriatric assessment and optimized treatment pathways.27,28,29 In this study, the data suggest that in patients 80 years or older, the target IESG risk score should ideally be between −3 and −4 to keep the patient in an acceptable risk range (predicted mortality, 8.9%). This selection of older candidates implies the necessary exclusion or correction of other negative prognosticators that compound the final score, including additive comorbidities, body weight loss, or poor performance status. This approach emphasizes the potentially beneficial impact of a preoperative target intervention to optimize correctable factors. Combining risks, the data also suggest that neoadjuvant treatment (−1 point) can be considered in selected candidates 80 years or older without additive negative prognosticators.
The nutritional spectrum of esophageal cancer ranges from underweight and malnourishment to the common phenotype of a male with obesity, in particular visceral obesity, with esophageal adenocarcinoma. In this study, when BMI was less than 18.5, the weight on the final score was strongly negative at −3 points. Patients who are underweight should be seen as a target subgroup for optimal preoperative nutritional intervention.30,31 However, the study design does not allow exploration of the impact of malnutrition because BMI reported in Esodata was probably the measure of BMI at the time of the surgery. The weight loss before surgery and potential preoperative nutritional intervention were not available. Paradoxically, a BMI of 25 or greater appeared to be a protective factor for death within 90 days, which is consistent with other reports.30,31,32,33 Although patients with overweight and type 1 obesity are considered to be at lower risk for complications, extremes of BMI (underweight and type 3 obesity) are associated with high risks of major morbidity and death.33 Interestingly, the large data set included few patients with a BMI greater than 35, limiting subgroup analysis across the obesity categories.
Male patients carried a higher risk than female patients, with 2 points, although the mechanism is unclear. Women are reported to have a better prognosis in squamous cell carcinoma but not in adenocarcinoma.34,35,36,37 In this study, squamous cell carcinoma was more common in women (634/1762 [36.0%]) compared with men (1118/6641 [16.8%]). Differences between sexes can possibly be explained by different distribution of tumor histologic subtype, ethnic origin, socioeconomic factors, risk factor exposures, and comorbidities. However, these factors were nonadjusted in this study. Moreover, because of the anonymization of the data, distinction between Western and Eastern contributing centers was not possible.
Among comorbidities, a history of myocardial infarction (−2 points) and peripheral vascular disease (−2 points) were 2 important negative prognosticators. These 2 comorbidities may be linked; however, the multiple logistic regression model did not find any collinearity effect. For instance, only 12% of patients presented simultaneously with the 2 comorbidities. These data support the proposal that coronary heart disease and peripheral arterial disease involve nonidentical drivers and pathogenesis.38 Despite a shared mixture of determinants, the 2 diseases are different and require a careful preoperative cardiovascular workup. Connective tissue disease (−3 points) is also a strongly negative prognosticator that must be seen as a multifaceted illness regarding related organ end-stage diseases and the related toxic effects of long-term treatment inherent to these diseases.36,39
Liver disease (moderate and severe) provided the strongest negative impact on 90-day mortality (−5 points). This finding supports the advice from the existing literature that extreme caution should be taken in the treatment of patients with moderate and severe liver disease and suggests that this is a relative contraindication for curative esophagectomy. However, surgery can be undertaken for milder disease, such as Child-Pugh A disease with normal liver function test results and without any edematoascitic decompensation.40,41,42,43,44 The Model for End-stage Liver Disease compared with Child-Pugh classification has been suggested to supply a more appropriate risk assessment in patients with cirrhosis, deserving further investigation.42
With respect to neoadjuvant therapy, neoadjuvant chemoradiotherapy was a negative prognosticator of death within 90 days (−1 point), whereas chemotherapy alone had no weight on the final score. Whether neoadjuvant chemoradiotherapy might increase postoperative complications is a matter of discussion.45,46,47,48,49,50,51,52 On the one hand, the neoadjuvant treatment provides significant oncologic advantages, but this may be at the cost of increased respiratory, cardiovascular, and anastomotic leak rates.52 Although controversial, neoadjuvant chemoradiotherapy has been suggested to be associated with higher 30-day mortality and in-hospital mortality on network meta-analysis, when timing of surgery was too delayed, or when chemoradiotherapy was indicated for early stages of the disease.14,49,50,51,52,53 Regarding definitive chemoradiotherapy (−2 points), salvage esophagectomy increases postoperative mortality, especially when the cumulative dose of radiotherapy exceeded 50 Gy and when patients exhibited persistence of tumor rather than recurrence of the disease.54,55,56 However, even though there is a small group of patients undergoing salvage esophagectomy, the current study does not allow a comprehensive assessment on how this risk score would predict the outcomes of definitive chemoradiotherapy.
A further important dimension, and critical in the context of centralization of esophageal cancer surgery, is whether hospital volume is associated with mortality; this study provides supportive data. In this study, this dimension was scored at +1 point for the highest quartile (ie, volume >108.6 procedures per year per center), whereas it had no weight in the 3 other quartiles. The explanation for the lack of an association of hospital volume with mortality is not clear, but arguably the nature of the participating centers, all tertiary with optimal structures and processes, enables equivalent outcomes.57,58,59,60 Thus, in these institutions, the hospital volume is less likely to demonstrate a significant effect compared with lower-operative-volume institutions without centralization.
Limitations
This study has several limitations. The results were restricted to the strict framework of the Esodata without any possibility to undertake an in-depth analysis. Moreover, some variable definitions need to be refined and clarified, such as for BMI and connective tissue diseases. Although the model was supported by a specific calibration test, the discrimination remains moderate. This limitation is common to every reported prediction model for mortality when reporting on rare clinical events and reflecting the great complexity of causes underlying 90-day mortality.12,13,14,15 Nonetheless, the data analysis has significant relevance, being abstracted from the most powerful and largest prospective collection of standardized complications worldwide, with more than 4000 patients each in the development and validation cohorts. In addition, all of the patient data were extracted from a contemporary database during a recent 5-year period, reflecting modern oncologic patient management and including relevant modern variables known to affect early outcomes.
Conclusions
On the basis of a prospectively collected, large, contemporary data set and on preoperative variables combining clinical, demographic, and hospital volume data, the proposed IESG risk prediction model allows for stratification of individual patient risk of death within 90 days after esophagectomy for cancer. The IESG risk score is easily accessible and provides an evidence-based schema for assigning risk to 1 component of the multimodality treatment of patients with esophageal cancer and for allocation of the most appropriate treatment. Moreover, the IESG provides an adjustment-scoring system that permits comparison between practitioners and institutions. Independent validation of the score is required.
eAppendix. List of Contributing Centers (10 America, 23 Europe, 5 Asia, 1 Australia)
eTable 1. Additional Demographic and Clinical Comparison Between Developmental and Validation Cohort
eTable 2. Association Between Risk Factors With 90-Day Mortality in the Development Cohort
eTable 3. Repartition of Patients According to the Score and Collapsing in Homogeneous Risk Group
eTable 4. Repartition of Patients According to the Final Score and According to Risk Groups
eTable 5. Risk Group With Respective Mortality and Number of Patients
eTable 6. Predicted Mortality in Relation to the Sum Score
eTable 7. Calculation of the Score
eTable 8. Repartition of Death and Complications Among the Whole Cohort and Among Each Risk Group
eFigure 1. Flowchart of the Study
eFigure 2. Funnel Plot Showing the 90-Day Deaths According to the Hospital Volume Represented by the Mean Number of Procedures of Esophagectomy/Year/Center
eFigure 3. Distribution of Patients in the Validation and in the Development Cohort According to Final Score
eFigure 4. ROC Curve of Prediction 90-Day Mortality in Validation, in Development Cohort, and in Total Cohort of the Final Score
eFigure 5. Calibration of 90-Day Mortality After Esophagectomy (n = 8403)
Nonauthor Collaborators. International Esodata Study Group members.
References
- 1.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887-1892. doi: 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 2.Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg. 2019;269(2):291-298. doi: 10.1097/SLA.0000000000002611 [DOI] [PubMed] [Google Scholar]
- 3.In H, Palis BE, Merkow RP, et al. Doubling of 30-day mortality by 90 days after esophagectomy: a critical measure of outcomes for quality improvement. Ann Surg. 2016;263(2):286-291. doi: 10.1097/SLA.0000000000001215 [DOI] [PubMed] [Google Scholar]
- 4.Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. ; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group . Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380(2):152-162. doi: 10.1056/NEJMoa1805101 [DOI] [PubMed] [Google Scholar]
- 5.van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg. 2019;269(4):621-630. doi: 10.1097/SLA.0000000000003031 [DOI] [PubMed] [Google Scholar]
- 6.D’Journo XB, Berbis J, Jougon J, et al. External validation of a risk score in the prediction of the mortality after esophagectomy for cancer. Dis Esophagus. 2017;30(1):1-8. doi: 10.1111/dote.12447 [DOI] [PubMed] [Google Scholar]
- 7.Talsma AK, Lingsma HF, Steyerberg EW, Wijnhoven BP, Van Lanschot JJ. The 30-day versus in-hospital and 90-day mortality after esophagectomy as indicators for quality of care. Ann Surg. 2014;260(2):267-273. doi: 10.1097/SLA.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 8.Walters DM, McMurry TL, Isbell JM, Stukenborg GJ, Kozower BD. Understanding mortality as a quality indicator after esophagectomy. Ann Thorac Surg. 2014;98(2):506-511. doi: 10.1016/j.athoracsur.2014.03.041 [DOI] [PubMed] [Google Scholar]
- 9.Horne ZD, Wegner RE, Colonias A, et al. Drivers of 30- and 90-day postoperative death after neoadjuvant chemoradiation for esophageal cancer. Ann Thorac Surg. 2020;109(3):921-926. doi: 10.1016/j.athoracsur.2019.10.057 [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, McMurry TL, Stukenborg GJ, Kozower BD. Readmission predicts 90-day mortality after esophagectomy: analysis of Surveillance, Epidemiology, and End Results Registry linked to Medicare outcomes. J Thorac Cardiovasc Surg. 2015;150(5):1254-1260. doi: 10.1016/j.jtcvs.2015.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs HF, Harnsberger CR, Broderick RC, et al. Simple preoperative risk scale accurately predicts perioperative mortality following esophagectomy for malignancy. Dis Esophagus. 2017;30(1):1-6. doi: 10.1093/dote/dox022 [DOI] [PubMed] [Google Scholar]
- 12.Bosch DJ, Pultrum BB, de Bock GH, Oosterhuis JK, Rodgers MG, Plukker JT. Comparison of different risk-adjustment models in assessing short-term surgical outcome after transthoracic esophagectomy in patients with esophageal cancer. Am J Surg. 2011;202(3):303-309. doi: 10.1016/j.amjsurg.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 13.van den Boorn HG, Engelhardt EG, van Kleef J, et al. Prediction models for patients with esophageal or gastric cancer: a systematic review and meta-analysis. PLoS One. 2018;13(2):e0192310. doi: 10.1371/journal.pone.0192310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Neville BA, Koppert LB, et al. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006;24(26):4277-4284. doi: 10.1200/JCO.2005.05.0658 [DOI] [PubMed] [Google Scholar]
- 15.Warnell I, Chincholkar M, Eccles M. Predicting perioperative mortality after oesophagectomy: a systematic review of performance and methods of multivariate models. Br J Anaesth. 2015;114(1):32-43. doi: 10.1093/bja/aeu294 [DOI] [PubMed] [Google Scholar]
- 16.Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262(2):286-294. doi: 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 17.Reynolds JV, Donlon N, Elliott JA, et al. Comparison of esophagectomy outcomes between a national center, a national audit collaborative, and an international database using the Esophageal Complications Consensus Group (ECCG) standardized definitions. Dis Esophagus. 2021;34(1):doaa060. doi: 10.1093/dote/doaa060 [DOI] [PubMed] [Google Scholar]
- 18.van der Werf LR, Busweiler LAD, van Sandick JW, van Berge Henegouwen MI, Wijnhoven BPL; Dutch Upper GI Cancer Audit (DUCA) Study Group. Reporting national outcomes after esophagectomy and gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann Surg. 2020;271(6):1095-1101. doi: 10.1097/SLA.0000000000003210 [DOI] [PubMed] [Google Scholar]
- 19.Kuppusamy MK, Low DE; International Esodata Study Group (IESG) . Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of >6000 patients using ECCG definitions and the online Esodata database. Ann Surg. Published online October 14, 2020. doi: 10.1097/SLA.0000000000004309 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DT, Graviss EA. Development and validation of a prognostic score to predict tuberculosis mortality. J Infect. 2018;77(4):283-290. doi: 10.1016/j.jinf.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Rassi A Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355(8):799-808. doi: 10.1056/NEJMoa053241 [DOI] [PubMed] [Google Scholar]
- 22.Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. ; SANO-Study Group . Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18(1):142. doi: 10.1186/s12885-018-4034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (Esostrate). ClinicalTrials.gov identifier: NCT02551458. September 16, 2015. Accessed May 10, 2021. https://clinicaltrials.gov/ct2/show/NCT02551458
- 24.Baranov NS, van Workum F, van der Maas J, et al. The influence of age on complications and overall survival after Ivor Lewis totally minimally invasive esophagectomy. J Gastrointest Surg. 2019;23(7):1293-1300. doi: 10.1007/s11605-018-4062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133(5):1186-1192. doi: 10.1016/j.jtcvs.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 26.Internullo E, Moons J, Nafteux P, et al. Outcome after esophagectomy for cancer of the esophagus and GEJ in patients aged over 75 years. Eur J Cardiothorac Surg. 2008;33(6):1096-1104. doi: 10.1016/j.ejcts.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 27.Schlottmann F, Strassle PD, Nayyar A, Herbella FAM, Cairns BA, Patti MG. Postoperative outcomes of esophagectomy for cancer in elderly patients. J Surg Res. 2018;229:9-14. doi: 10.1016/j.jss.2018.03.050 [DOI] [PubMed] [Google Scholar]
- 28.Giannotti C, Sambuceti S, Signori A, et al. Frailty assessment in elective gastrointestinal oncogeriatric surgery: predictors of one-year mortality and functional status. J Geriatr Oncol. 2019;10(5):716-723. doi: 10.1016/j.jgo.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 29.Markar SR, Karthikesalingam A, Thrumurthy S, Ho A, Muallem G, Low DE. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus. 2013;26(3):250-262. doi: 10.1111/j.1442-2050.2012.01353.x [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Li Y, Sun H, et al. Predictive value of body mass index for short-term outcomes of patients with esophageal cancer after esophagectomy: a meta-analysis. Ann Surg Oncol. 2019;26(7):2090-2103. doi: 10.1245/s10434-019-07331-w [DOI] [PubMed] [Google Scholar]
- 31.Scarpa M, Cagol M, Bettini S, et al. Overweight patients operated on for cancer of the esophagus survive longer than normal-weight patients. J Gastrointest Surg. 2013;17(2):218-227. doi: 10.1007/s11605-012-2023-2 [DOI] [PubMed] [Google Scholar]
- 32.Mitzman B, Schipper PH, Edwards MA, Kim S, Ferguson MK. Complications after esophagectomy are associated with extremes of body mass index. Ann Thorac Surg. 2018;106(4):973-980. doi: 10.1016/j.athoracsur.2018.05.056 [DOI] [PubMed] [Google Scholar]
- 33.Wightman SC, Posner MC, Patti MG, et al. Extremes of body mass index and postoperative complications after esophagectomy. Dis Esophagus. 2017;30(5):1-6. doi: 10.1093/dote/dow006 [DOI] [PubMed] [Google Scholar]
- 34.Geller AD, Zheng H, Gaissert H, et al. Relative incremental cost of postoperative complications of esophagectomy. Semin Thorac Cardiovasc Surg. 2019;31(2):290-299. doi: 10.1053/j.semtcvs.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 35.Kauppila JH, Wahlin K, Lagergren P, Lagergren J. Sex differences in the prognosis after surgery for esophageal squamous cell carcinoma and adenocarcinoma. Int J Cancer. 2019;144(6):1284-1291. doi: 10.1002/ijc.31840 [DOI] [PubMed] [Google Scholar]
- 36.Backemar L, Lagergren P, Johar A, Lagergren J. Impact of co-morbidity on mortality after oesophageal cancer surgery. Br J Surg. 2015;102(9):1097-1105. doi: 10.1002/bjs.9854 [DOI] [PubMed] [Google Scholar]
- 37.Sundelöf M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44(11):1566-1571. doi: 10.1016/j.ejca.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 38.Tunstall-Pedoe H, Peters SAE, Woodward M, Struthers AD, Belch JJF. Twenty-year predictors of peripheral arterial disease compared with coronary heart disease in the Scottish Heart Health Extended Cohort (SHHEC). J Am Heart Assoc. 2017;6(9):e005967. doi: 10.1161/JAHA.117.005967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filip B, Scarpa M, Cavallin F, et al. Postoperative outcome after oesophagectomy for cancer: nutritional status is the missing ring in the current prognostic scores. Eur J Surg Oncol. 2015;41(6):787-794. doi: 10.1016/j.ejso.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 40.Deng HY, Zheng X, Zha P, Liang H, Huang KL, Peng L. Can we perform esophagectomy for esophageal cancer patients with concomitant liver cirrhosis? a comprehensive systematic review and meta-analysis. Dis Esophagus. 2019;32(6):doz003. doi: 10.1093/dote/doz003 [DOI] [PubMed] [Google Scholar]
- 41.Asti E, Sozzi M, Bonitta G, Bernardi D, Bonavina L. Esophagectomy in patients with liver cirrhosis: a systematic review and Bayesian meta-analysis. J Visc Surg. 2018;155(6):453-464. doi: 10.1016/j.jviscsurg.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 42.Valmasoni M, Pierobon ES, De Pasqual CA, et al. Esophageal cancer surgery for patients with concomitant liver cirrhosis: a single-center matched-cohort study. Ann Surg Oncol. 2017;24(3):763-769. doi: 10.1245/s10434-016-5610-8 [DOI] [PubMed] [Google Scholar]
- 43.Schizas D, Giannopoulos S, Vailas M, et al. The impact of cirrhosis on esophageal cancer surgery: an up-to-date meta-analysis. Am J Surg. 2020;220(4):865-872. doi: 10.1016/j.amjsurg.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 44.Mariette C. Is there a place for esogastric cancer surgery in cirrhotic patients? Ann Surg Oncol. 2008;15(3):680-682. doi: 10.1245/s10434-007-9765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mungo B, Molena D, Stem M, et al. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus. 2015;28(7):644-651. doi: 10.1111/dote.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markar SR, Johar A, Maisey N, Lagergren P, Lagergren J. Complications during neoadjuvant therapy and prognosis following surgery for esophageal cancer. Dis Esophagus. 2018;31(5). doi: 10.1093/dote/dox151 [DOI] [PubMed] [Google Scholar]
- 47.Sabra MJ, Smotherman C, Kraemer DF, Nussbaum MS, Tepas JJ, Awad ZT. The effects of neoadjuvant therapy on morbidity and mortality of esophagectomy for esophageal cancer: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) 2005-2012. J Surg Oncol. 2017;115(3):296-300. doi: 10.1002/jso.24493 [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: a meta-analysis based on clinical trials. PLoS One. 2018;13(8):e0202185. doi: 10.1371/journal.pone.0202185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin Q, Xu H, Liu J, et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? a meta-analysis. Int J Surg. 2018;59:11-18. doi: 10.1016/j.ijsu.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 50.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416-2422. doi: 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 51.Zhou HY, Zheng SP, Li AL, et al. Clinical evidence for association of neoadjuvant chemotherapy or chemoradiotherapy with efficacy and safety in patients with resectable esophageal carcinoma (NewEC study). EClinicalMedicine. 2020;24:100422. doi: 10.1016/j.eclinm.2020.100422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjoquist KM, Burmeister BH, Smithers BM, et al. ; Australasian Gastro-Intestinal Trials Group . Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681-692. doi: 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 53.Chan KKW, Saluja R, Delos Santos K, et al. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta-analysis. Int J Cancer. 2018;143(2):430-437. doi: 10.1002/ijc.31312 [DOI] [PubMed] [Google Scholar]
- 54.Markar S, Gronnier C, Duhamel A, et al. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol. 2015;33(33):3866-3873. doi: 10.1200/JCO.2014.59.9092 [DOI] [PubMed] [Google Scholar]
- 55.Markar SR, Karthikesalingam A, Penna M, Low DE. Assessment of short-term clinical outcomes following salvage esophagectomy for the treatment of esophageal malignancy: systematic review and pooled analysis. Ann Surg Oncol. 2014;21(3):922-931. doi: 10.1245/s10434-013-3364-0 [DOI] [PubMed] [Google Scholar]
- 56.Mitchell KG, Nelson DB, Corsini EM, et al. Morbidity following salvage esophagectomy for squamous cell carcinoma: the MD Anderson experience. Dis Esophagus. 2020;33(3):doz067. doi: 10.1093/dote/doz067 [DOI] [PubMed] [Google Scholar]
- 57.Chang AC. Centralizing esophagectomy to improve outcomes and enhance clinical research: invited expert review. Ann Thorac Surg. 2018;106(3):916-923. doi: 10.1016/j.athoracsur.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 58.Speicher PJ, Englum BR, Ganapathi AM, et al. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer. Ann Surg. 2017;265(4):743-749. doi: 10.1097/SLA.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg. 2012;16(5):1055-1063. doi: 10.1007/s11605-011-1731-3 [DOI] [PubMed] [Google Scholar]
- 60.Munasinghe A, Markar SR, Mamidanna R, et al. Is it time to centralize high-risk cancer care in the United States? comparison of outcomes of esophagectomy between England and the United States. Ann Surg. 2015;262(1):79-85. doi: 10.1097/SLA.0000000000000805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. List of Contributing Centers (10 America, 23 Europe, 5 Asia, 1 Australia)
eTable 1. Additional Demographic and Clinical Comparison Between Developmental and Validation Cohort
eTable 2. Association Between Risk Factors With 90-Day Mortality in the Development Cohort
eTable 3. Repartition of Patients According to the Score and Collapsing in Homogeneous Risk Group
eTable 4. Repartition of Patients According to the Final Score and According to Risk Groups
eTable 5. Risk Group With Respective Mortality and Number of Patients
eTable 6. Predicted Mortality in Relation to the Sum Score
eTable 7. Calculation of the Score
eTable 8. Repartition of Death and Complications Among the Whole Cohort and Among Each Risk Group
eFigure 1. Flowchart of the Study
eFigure 2. Funnel Plot Showing the 90-Day Deaths According to the Hospital Volume Represented by the Mean Number of Procedures of Esophagectomy/Year/Center
eFigure 3. Distribution of Patients in the Validation and in the Development Cohort According to Final Score
eFigure 4. ROC Curve of Prediction 90-Day Mortality in Validation, in Development Cohort, and in Total Cohort of the Final Score
eFigure 5. Calibration of 90-Day Mortality After Esophagectomy (n = 8403)
Nonauthor Collaborators. International Esodata Study Group members.