Abstract
Background:
Bridge to transplant (BTT) with left ventricular assist devices (LVAD) is a mainstay of therapy for heart failure in patients awaiting heart transplantation (HT). Criteria for HT listing does not differ between patients medically managed versus mechanically bridged to HT. The objectives of the current study were to evaluate the impact of BTT with LVAD on post-transplant survival, to describe differences in causes of 1-year mortality in medically and mechanically bridged patients, and to evaluate differences in risk factors for 1-year mortality between those with and without LVAD at the time of HT.
Methods:
Using the United Network of Organ Sharing (UNOS) database, we identified 5486 adult, single-organ HT recipients transplanted between 2008 and 2015. Patients were propensity matched for likelihood of LVAD at the time of HT. Kaplan-Meier survival estimates were used to assess the impact of BTT on 1-year and 5-year mortality. Logistic regression analysis was used to evaluate the odds ratio of 1-year mortality for patients BTT with LVAD as compared to medical management across clinically significant variables at various thresholds.
Results:
Early mortality was higher in mechanically bridged patients: 9.5% vs. 7.2% mortality at 1-year (p-value < 0.001). BTT patients incurred an increased risk of 1-year mortality with an eGFR of 40-60 mL/min/1.73m2 (OR: 1.69, p=0.003) and with an eGFR of < 40mL/min (OR: 2.16, p=0.005). A similar trend was seen in patients with a BMI of 25-30 kg/m2 (OR:1.88, p=0.024) and a BMI of >30 kg/m2 (OR: 2.11, p<0.001). When patients were stratified by BTT status and the presence of risk factors including Age > 60, eGFR < 40 mL/min/1.73m2, and BMI > 30kg/m2 there were significant differences in 1-year mortality between medium- and high-risk medically and mechanically bridged patients, with 1-year mortality in high-risk BTT patients at 17.6% compared to high risk medically managed patients at 10.4%.
Conclusion:
Bridge to HT with LVAD, while necessary due to organ scarcity and capable of improving waitlist survival, confers a significantly higher risk of early post-transplant mortality. Patients bridged with mechanical support may require more careful consideration for transplant eligibility after LVAD placement.
Keywords: heart transplant, left ventricular assist device, bridge to transplant, primary graft dysfunction
Introduction
Bridge to transplantation (BTT) with durable, continuous flow left ventricular assist devices (LVAD) has become a widely adopted strategy for managing patients awaiting heart transplantation (HT). Recent reports from the International Society of Heart and Lung Transplant Registry suggests that in 2017, over 50% of HT recipients were bridged with mechanical circulatory support (MCS) – the majority with durable LVADs. While multiple prior studies have shown that LVADs decrease death or delisting for worsening status in patients awaiting HT, there have been conflicting reports of their impact on post-transplant outcomes1, 2. Older, single-center studies have been unable to demonstrate an adverse impact of BTT on post-HT survival3–6. Newer studies, however, have suggested a negative impact on outcomes, particularly in those patients with longer duration of LVAD support prior to HT7–9. Recent data also suggest that LVAD support may increase the risk of primary graft dysfunction (PGD) and perioperative vasoplegia: both of which may affect early post-transplant mortality10–12. Lastly, it is important to note that despite the marked variation in practice patterns across transplant centers, current listing criteria do not distinguish between medically managed and mechanically bridged patients1.
The objectives of the current study were to 1) evaluate the impact of BTT with contemporary LVAD on post-transplant survival, 2) describe differences in causes of 1-year mortality in medically and mechanically bridged patients, 3) to evaluate differences in risk factors for 1-year mortality between those with and without LVAD at the time of HT, and 4) to explore the potential impact of these differences on the development of future heart allocation scoring system.
Methods
Study Design, Variables, and Definitions
The United Network for Organ Sharing (UNOS) database was queried to identify adult patients (≥18 years old) who underwent single-organ HT between 2008 and 2015. For the purposes of the current study, patients with a durable, continuous flow LVAD at the time of HT were considered BTT. Patients with durable, pulsatile flow left ventricular assist devices, extracorporeal devices (including venoarterial extracorporeal membranous oxygenation), and total artificial hearts were excluded from the current study. We first compared baseline characteristics including demographics, etiology of heart failure, comorbid conditions, functional status, UNOS status at listing, and pre-transplant hemodynamics between patients who were BTT with LVAD and those who were medically managed. Based on these variables, a propensity score was generated for each patient assessing the likelihood of having a durable LVAD in place at the time of HT. Variables included in the propensity score were gender, ethnicity, smoking history, blood type O, body mass index (BMI), etiology of heart failure, pulmonary vascular resistance (PVR), estimated glomerular filtration rate (eGFR), UNOS status at listing, UNOS status at transplant, functional status, wait list time, and UNOS region. eGFR was calculated using the Modification of Diet in Renal Disease equation13. A propensity matched cohort was utilized for the remainder of the analysis. Logistic regression analysis and Kaplan-Meier survival estimates were used to quantify the impact of BTT with LVAD on post-HT outcomes. Cause of death data was then analyzed for patients who experienced 1-year mortality. All causes of death coded as “Other” were analyzed and recoded when appropriate as detailed in Supplemental Table 1. The distribution of various causes of death was then compared between patients BTT with LVAD and those who were medically managed. Risk factors for 1-year mortality were separately assessed in medically and mechanically bridged patients. The risk of 1-year mortality in mechanically versus medically bridged patients was quantified at various strata of clinically significant variables. Lastly, patients in both groups were then classified as low, medium, or high risk based upon the presence or absence of age > 60 years, eGFR < 40 mL/min/1.73m2, and BMI > 30 kg/m2, in order to evaluate the impact of bridging strategy and risk strata on post-HT outcomes. This study was approved by the Columbia University Institutional Review Board. The analytic methods will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The authors are not permitted to share data from the UNOS/SRTR database, but data can be requested by researchers for the purpose of reproducing the analysis.
Statistical Analysis
Descriptive analyses were conducted for all baseline variables and are presented as means and standard deviations for continuous variables and numbers and percentages for categorical variables. Differences between baseline characteristics of LVAD patients and those medically managed were assessed using independent Student’s t-test and chi-square test where appropriate. The propensity score for LVAD support at the time of transplant was generated based upon multivariate logistic regression analysis. Propensity matching was performed using one to one nearest neighbor matching with specified caliper distance of 0.25. Absolute standard differences were assessed before and after matching to ensure acceptable balance. Kaplan-Meier survival estimates were used to assess early and late post-transplant survival, with log-rank testing used to compare those with and without LVAD at the time of transplant. Univariate and multivariate logistic regression analysis were used to identify risk factors for 1-year mortality in the mechanically and medically bridged population, respectively. Odds ratios for 1-year mortality in mechanically bridged as compared to medically managed patients were analyzed for each clinically significant variable at multiple thresholds. Those patients without 1-year of follow up data were excluded from all logistic regression analysis. All patients alive at 1-year were included in a sensitivity analysis to assess long-term survival conditional upon survival to 1-year. All tests were two-sided and p-values <0.05 were considered statistically significant. STATA version 13.1 (Stata corp., College Station, TX) was used to perform statistical analysis.
Results
Patient Population and Overall Trends in Use of LVAD as BTT
A total of 14357 patients were included in the current study (Figure 1). Among these, 5359 (37.3%) were BTT with LVAD and 8998 (62.6%) were medically managed. The proportion of patients BTT with LVAD increased during the study period such that by 2013, almost 50% of patients were mechanically supported prior to HT (Figure 2A). This trend existed in parallel with increasing wait list time, driven primarily by an increase in wait list time for BTT patients (Figure 2B). Among patients with LVAD at the time of HT, there was also a trend in increased time on support prior to HT (Figure 2C). Importantly, the number of BTT patients transitioned from a destination therapy (DT) strategy to HT – assessed based upon the presence of absence of LVAD support at the time of listing - also increased substantially during the study period (Figure 2D).
Figure 1:
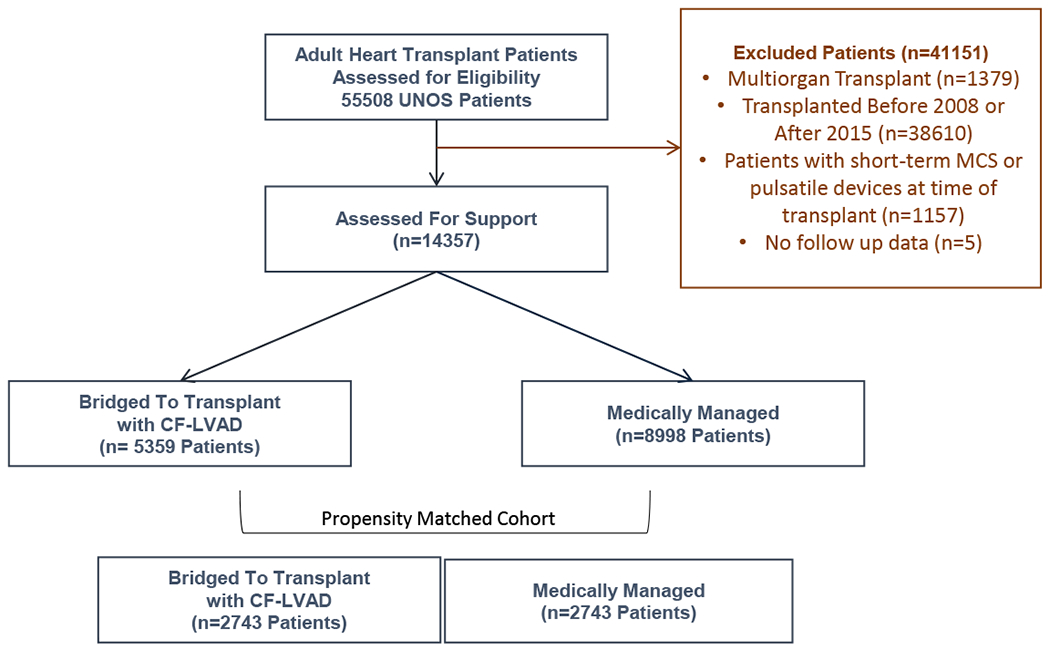
Study Design and Patient Population
Figure 2:
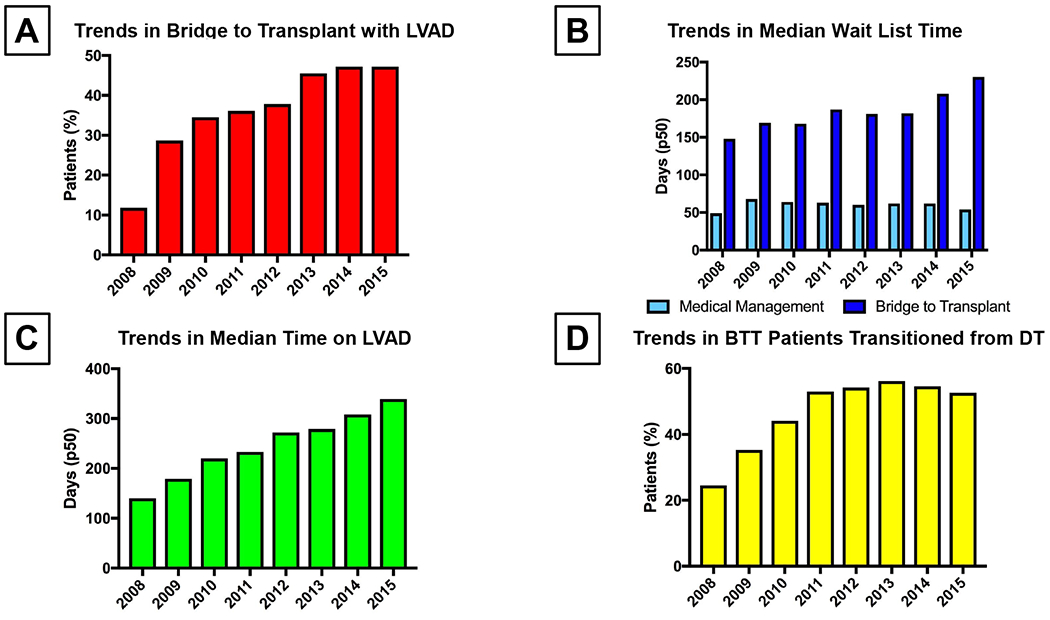
(A) Trends in Use of LVAD (Left Ventricular Assist Device) as BTT (Bridge to Transplant) During the Study Period
(B) Trends in Wait List Time Among Patients Bridged to Transplant Versus Medically Managed
(C) Trends in Time on LVAD (Left Ventricular Assist Device) Support Prior to Transplant
(D) Trends in Percent of BTT (Bridge to Transplant) Patients Who Were Initially Implanted as Destination Therapy
Pre-transplant characteristics of the overall study population prior to propensity score matching are summarized in Table 1. LVAD patients were more likely to be male, had a higher prevalence of comorbidities including diabetes and smoking history, and were more likely to have a high BMI and blood type O. Non-dilated cardiomyopathies, inclusive of restrictive cardiomyopathy, hypertrophic cardiomyopathy, and congenital heart disease, comprised 15% of medically managed patients, but only 2% of patients BTT with LVAD. There was no clinically significant difference in eGFR, donor age, or ischemic time. Importantly, patients with LVAD at HT spent a median of 191 days on the wait list as compared to 60 days for medically managed patients.
Table 1:
Pre-Transplant Characteristics of Study Population Prior to Propensity Matching
| Variable |
All Patients (n=14357) |
Bridge to Transplant (n=5359) |
Medical Management (n=8998) |
p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 56 (47 – 63) | 56 (47 – 63) | 56 (47 – 63) | 0.523 |
| Male Gender, n(%) | 10556 (73.4) | 4306 (80.4) | 6250 (69.5) | <0.001 |
| Ethnicity | <0.001 | |||
| White | 9730 (67.8) | 3586 (66.9) | 6144 (68.3) | |
| African American | 2901 (20.2) | 1223 (22.8) | 1678 (18.7) | |
| Hispanic | 1097 (7.6) | 349 (6.5) | 748 (8.3) | |
| Other | 629 (4.4) | 201 (3.8) | 428 (4.8) | |
| Diabetes, n(%) | 3939 (27.5) | 1641 (30.7) | 2298 (25.6) | <0.001 |
| CVA, n(%) | 768 (5.4) | 300 (5.6) | 468 (5.2) | 0.293 |
| Smoking, n(%) | 6803 (47.5) | 2860 (53.5) | 3943 (44.0) | <0.001 |
| AICD, n(%) | 11350 (79.5) | 4287 (80.5) | 7063 (79.0) | 0.024 |
| Blood Type O, n(%) | 5500 (38.3) | 2422 (45.2) | 3078 (34.2) | <0.001 |
| BMI (mg/kg2) | 27.1 ± 4.8 | 28.1 ± 4.9 | 26.5 ± 4.7 | <0.001 |
| Non-Dilated Myopathy, n(%) | 1510 (10.5) | 107 (2.0) | 1403 (15.6) | <0.001 |
| Status @ Listing | <0.001 | |||
| Status 1A | 3212 (22.8) | 1493 (28.9) | 1719 (19.3) | |
| Status 1B | 6389 (45.4) | 2675 (51.8) | 3714 (41.7) | |
| Status 2 | 4461 (31.7) | 992 (19.2) | 3469 (40.0) | |
| Status 1A @ Transplant | 8230 (57.3) | 3820 (71.3) | 4410 (49.0) | <0.001 |
| Functional Status | <0.001 | |||
| Poor | 4041 (28.8) | 1451 (27.7) | 2563 (29.4) | |
| Moderate | 7747 (55.5) | 2871 (54.7) | 4876 (56.0) | |
| Excellent | 2196 (15.7) | 926 (17.6) | 1270 (14.6) | |
| eGFR (mL/min/1.73m2) | 72.5 ± 33.6 | 73.8 ± 29.9 | 71.7 ± 35.6 | <0.001 |
| Days on WL, median(IQR) | 98 (29 – 264) | 191 (77 – 404) | 60 (20 – 176) | <0.001 |
| Mean PA Pressures (mmHg) | 27.7 ± 10.1 | 26.3 ± 10.1 | 28.6 ± 10.0 | <0.001 |
| PVR (Woods Units) | 2.46 ± 1.75 | 2.29 ± 1.72 | 2.57 ± 1.76 | <0.001 |
CVA, cerebrovascular accident; AICD, automated internal cardioverter defibrillator; BMI, body mass index; eGFR, estimated glomerular filtration rate; WL, waiting list; PA, pulmonary artery; PVR, pulmonary vascular resistance; IQR, interquartile range
Predictors of Bridge to Transplant with LVAD and Generation of Propensity Score
In order to identify clinical characteristics associated with the presence of LVAD support at the time of transplant, univariable and multivariable regression analyses were performed utilizing basic patient demographics, past medical history, hemodynamics, laboratory values, listing status and location. Male gender, ethnicity, smoking history, blood type, BMI, etiology of heart failure, UNOS status at listing, UNOS status at transplant, functional status, wait list time, eGFR, PVR, and UNOS region were strongly associated with LVAD utilization as BTT (Table 2). Using these variables, a propensity score was generated and used to identify a propensity matched cohort of 5486 patients (2743 mechanically supported, 2743 medically bridged). Propensity scores ranged from 0.004 to 0.963 and showed a relatively normal distribution within the study population (Supplemental Figure 1). Baseline characteristics between patients who received mechanical versus medical bridging strategies were well balanced after propensity score matching (Table 3). Only a history of stroke, the presence of an AICD, and the number of patients with a wait time more than 6 months remained significantly different between groups after propensity matching.
Table 2:
Variables Associated With LVAD Support at time of Transplant
| Variable | OR (95% CI) | p-value | OR (95% CI) | p-value |
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||
| Age (Years) | 1.00 (1.00 – 1.00) | 0.557 | ||
| Male Gender, n(%) | 1.80 (1.66 – 1.95) | <0.001 | 1.42 (1.27 – 1.58) | <0.001 |
| Ethnicity | <0.001 | <0.001 | ||
| White | (Reference) | (Reference) | ||
| African American | 1.25 (1.15 – 1.36) | 1.21 (1.08- 1.36) | ||
| Hispanic | 0.80 (0.70 – 0.91) | 0.85 (0.70 – 1.02) | ||
| Other | 0.80 (0.68 – 0.96) | 0.97 (0.77 – 1.23) | ||
| Diabetes, n(%) | 1.29 (1.19 – 1.39) | <0.001 | 1.06 (0.96 – 1.18) | 0.184 |
| CVA, n(%) | 1.08 (0.93 – 1.26) | 0.293 | ||
| Smoking, n(%) | 1.46 (1.37 – 1.57) | <0.001 | 1.26 (1.15 – 1.38) | <0.001 |
| AICD, n(%) | 1.10 (1.01 – 1.20) | 0.025 | 0.87 (0.76 – 0.98) | 0.070 |
| Blood Type O, n(%) | 1.59 (1.48 – 1.70) | <0.001 | 1.28 (1.16 – 1.40) | <0.001 |
| BMI (kg/m2) | 1.08 (1.07 – 1.09) | <0.001 | 1.05 (1.04 – 1.06) | <0.001 |
| Non-Dilated Myopathy | 0.11 (0.09 – 0.13) | <0.001 | 0.12 (0.09 – 0.15) | <0.001 |
| Status @ Listing | <0.001 | <0.001 | ||
| Status 1A | (Reference) | (Reference) | ||
| Status 1B | 0.83 (0.76 – 0.90) | 0.71 (0.63 – 0.80) | ||
| Status 2 | 0.87 (0.81 – 0.93) | 0.16 (0.14 – 0.19) | ||
| Status 1A @ Transplant | 2.58 (2.40 – 2.78) | <0.001 | 2.94 (2.65 – 3.26) | <0.001 |
| Functional Status | <0.001 | <0.001 | ||
| Poor | (Reference) | (Reference) | ||
| Moderate | 1.04 (0.96 – 1.13) | 1.69 (1.51 – 1.89) | ||
| Excellent | 1.29 (1.16 – 1.43) | 2.29 (1.98 – 2.66) | ||
| eGFR (mL/min/1.73m2) | 1.00 (1.00 – 1.00) | <0.001 | 1.01 (1.00 – 1.01) | <0.001 |
| PVR (Woods Units) | 0.89 (0.87 – 0.91) | <0.001 | 0.93 (0.91 – 0.96) | <0.001 |
| Wait List Time > 6mo. | 3.33 (3.10 – 3.58) | <0.001 | 5.90 (5.28 – 6.59) | <0.001 |
| UNOS Region | <0.001 | <0.001 | ||
| 1 | (Reference) | (Reference) | ||
| 2 | 0.61 (0.50 – 0.74) | 0.63 (0.49 – 0.82) | ||
| 3 | 0.38 (0.31 – 0.47) | 0.33 (0.25 – 0.43) | ||
| 4 | 0.51 (0.42 – 0.62) | 0.57 (0.44 – 0.75) | ||
| 5 | 0.40 (0.33 – 0.49) | 0.57 (0.44 – 0.74) | ||
| 6 | 1.75 (1.35 – 2.27) | 3.28 (2.33 – 4.61) | ||
| 7 | 1.20 (0.98 – 1.47) | 1.35 (1.03 – 1.76) | ||
| 8 | 0.69 (0.56 – 0.85) | 0.88 (0.66 – 1.16) | ||
| 9 | 1.11 (0.90 – 1.37) | 0.92 (0.70 – 1.22) | ||
| 10 | 1.06 (0.87 – 1.30) | 1.21 (0.93 – 1.58) | ||
| 11 | 0.79 (0.65 – 0.96) | 0.86 (0.67 – 1.12) | ||
CVA, cerebrovascular accident; AICD, automated internal cardioverter defibrillator; BMI, body mass index; eGFR, estimated glomerular filtration rate; WL, waiting list; PA, pulmonary artery; PVR, pulmonary vascular resistance; UNOS, United Network of Organ Sharing
Table 3:
Pre-Transplant Characteristics of the Propensity Matched Cohort.
| Variable |
Overall (n=5486) |
Bridge to Transplant (n=2743) |
Medical Management (n=2743) |
p-value | ASD Prior to PSM (%) | ASD After PSM (%) |
|---|---|---|---|---|---|---|
| Age (years) | 54.0 ± 12.1 | 54.0 ± 12.1 | 54.1 ± 12.1 | 0.628 | 1.0 | 1.3 |
| Male Gender, n(%) | 4239 (77.3) | 2105 (76.7) | 2134 (77.8) | 0.350 | 25.3 | 2.5 |
| Ethnicity | 0.999 | 4.8 | 0.2 | |||
| White | 3754 (68.4) | 1877 (68.4) | 1877 (68.4) | |||
| African American | 1146 (20.9) | 572 (20.9) | 574 (20.9) | |||
| Hispanic | 365 (6.7) | 183 (6.7) | 182 (6.6) | |||
| Other | 221 (4.0) | 111 (4.1) | 110 (4.0) | |||
| Diabetes, n(%) | 1613 (29.5) | 820 (30.0) | 793 (29.0) | 0.423 | 11.4 | 2.2 |
| CVA, n(%) | 304 (5.6) | 175 (6.4) | 129 (4.7) | 0.006 | 1.8 | 7.4 |
| Smoking, n(%) | 2834 (51.7) | 1421 (51.8) | 1413 (51.2) | 0.829 | 19.1 | 0.6 |
| AICD, n(%) | 4508 (82.7) | 2221 (81.6) | 2287 (83.8) | 0.039 | 3.9 | 5.7 |
| Blood Type O, n(%) | 2137 (39.0) | 1064 (38.8) | 1073 (39.1) | 0.803 | 22.6 | 0.7 |
| BMI (kg/m2) | 27.4 ± 4.7 | 27.4 ± 4.7 | 27.4 ± 4.7 | 0.654 | 36.3 | 1.2 |
| Non-Dilated Myopathy | 173 (3.2) | 81 (3.0) | 92 (3.4) | 0.395 | 49.4 | 2.3 |
| Status @ Listing | 0.400 | 15.5 | 1.9 | |||
| Status 1A | 1443 (26.3) | 723 (26.4) | 720 (26.3) | |||
| Status 1B | 2611 (47.6) | 1284 (46.8) | 1327 (48.4) | |||
| Status 2 | 1432 (26.1) | 736 (26.8) | 696 (25.4) | |||
| Status 1A @ Transplant | 3469 (63.2) | 1736 (63.3) | 1733 (63.2) | 0.933 | 46.7 | 0.2 |
| Functional Status | 0.654 | 7.4 | 1.3 | |||
| Poor | 1598 (29.1) | 785 (28.6) | 813 (29.6) | |||
| Moderate | 3012 (54.9) | 1522 (55.5) | 1490 (54.3) | |||
| Excellent | 876 (15.9) | 436 (15.9) | 440 (16.0) | |||
| eGFR (mL/min/1.73m2) | 72.7 ± 27.8 | 72.6 ± 27.2 | 72.9 ± 28.4 | 0.701 | 9.5 | 1.0 |
| PVR (Woods Units) | 2.41 ± 1.49 | 2.39 ± 1.51 | 2.37 ± 1.50 | 0.940 | 17.2 | 1.3 |
| Wait Time > 6 Months | 1938 (35.3) | 932 (34.0) | 1006 (36.7) | 0.037 | 58.9 | 5.6 |
CVA, cerebrovascular accident; AICD, automated internal cardioverter defibrillator; BMI, body mass index; eGFR, estimated glomerular filtration rate; WL, waiting list; PA, pulmonary artery; PVR, pulmonary vascular resistance.
Impact of BTT with LVAD on Post-Transplant Survival
A total of 441 patients (8.0%) died during the first post-transplant year. In multivariate modeling including BTT with LVAD and the additional covariates that remained significantly different between groups after propensity matching, only BTT with LVAD (OR: 1.42, CI(1.17 – 1.73, p=0.001) and a wait time of more than 6 months (OR: 1.31, CI (1.07 – 1.61), p=0.009) were associated with 1-year mortality. Adjusted Kaplan-Meier survival estimates were then used to assess the short- and long- term impact of BTT with LVAD on post-transplant survival in the propensity matched cohort (Figure 3A). At 1 year, 92.8 % of medically bridged patients were alive as compared to 90.5% of LVAD patients (log rank p<0.001). This difference was diminished at 5-years post-HT where 78.9% of medically patients were alive as compared to 78.0% of BTT patients (Figure 3B). Kaplan-Meier survival estimates conditional upon survival to 1-year confirmed that there was no difference in risk after the first post-transplant year (Figure 4). When cumulative incidence of mortality was assessed at various time points, the majority of events occurred in the early post-transplant period (Table 4).
Figure 3:
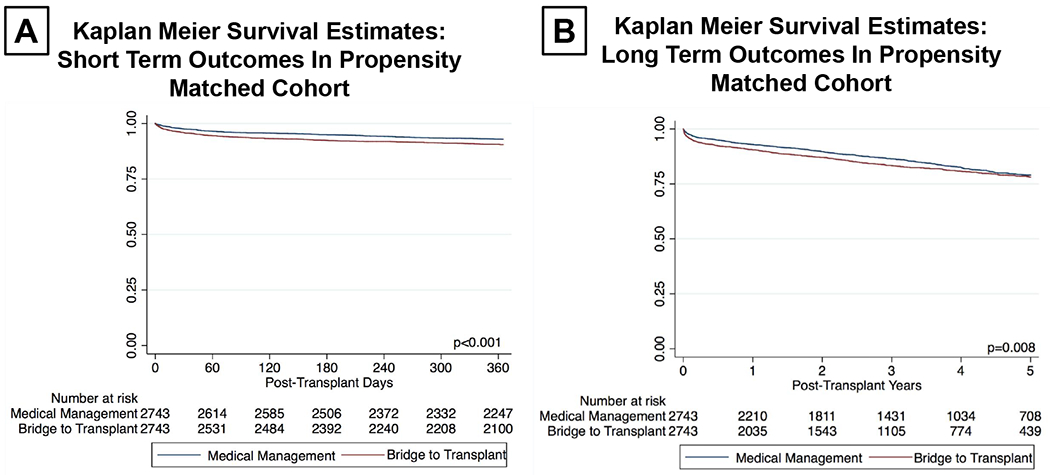
(A) Kaplan-Meier Survival Estimates for 1-Year Mortality in Propensity Matched Cohort Adjusted for Wait List Time > 6 Months
(B) Kaplan-Meier Survival Estimates for 5-Year Mortality in Propensity Matched Cohort Adjusted for Wait List Time > 6 Months
Figure 4:
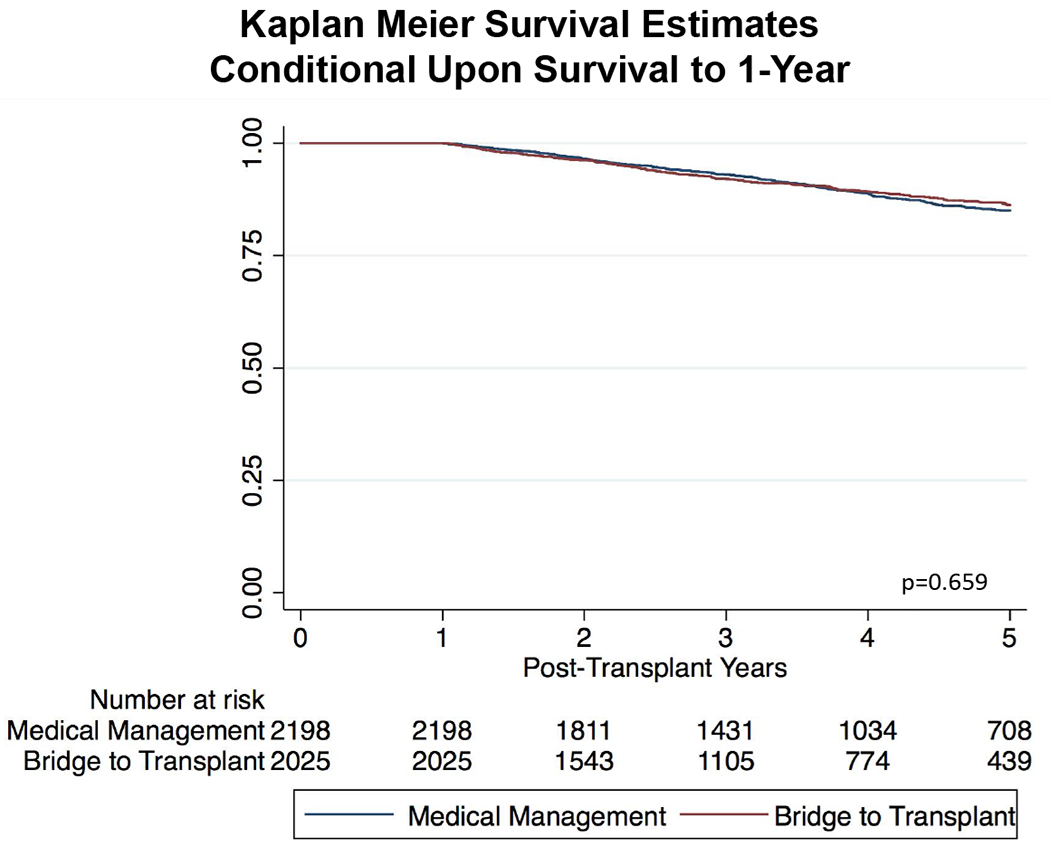
Kaplan-Meier Survival Estimates of 5-Year Survival Conditional Upon Survival to 1-Year
Table 4:
Predictors of 1-Year Post-Transplant Mortality in Medically versus Mechanically Bridged Patients
|
Variable |
OR (95% CI) | p-value | OR (95% CI) | p-value |
|---|---|---|---|---|
| Univariable Analysis | ||||
| Medically Bridged | BTT w/ CF-LVAD | |||
| Age (years) | 1.02 (1.00 – 1.03) | 0.017 | 1.03 (1.01 – 1.04) | <0.001 |
| eGFR (mL/min/1.73m2) | 0.99 (0.99 – 1.00) | 0.008 | 0.98 (0.98 – 0.99) | <0.001 |
| PVR (Woods Units) | 1.06 (0.97 – 1.16) | 0.225 | 1.08 (1.01 –1.17) | 0.063 |
| BMI (kg/m2) | 1.00 (0.97 – 1.03) | 0.953 | 1.07 (1.04 – 1.09) | <0.001 |
| Ethnicity | 0.628 | 0.104 | ||
| White | (Reference) | (Reference) | ||
| African American | 0.85 (0.58 – 1.25) | 1.45 (1.06 – 1.97) | ||
| Hispanic | 0.93 (0.50 – 1.72) | 1.04 (0.61 – 1.79) | ||
| Other | 0.66 (0.26 – 1.66) | 1.44 (0.78 – 2.64) | ||
| Diabetes | 1.05 (0.76 – 1.45) | 0.785 | 1.42 (1.08 – 1.86) | 0.011 |
| History of Stroke | 1.12 (0.59 – 2.19) | 0.727 | 1.28 (0.81 – 2.01) | 0.297 |
| Smoking History | 0.89 (0.67 – 1.19) | 0.440 | 1.07 (0.83 – 1.40) | 0.596 |
| Implantable Defibrillator | 0.73 (0.50 – 1.06) | 0.099 | 1.01 (0.71 – 1.42) | 0.975 |
| Blood Type O | 1.14 (0.85 – 1.55) | 0.379 | 0.87 (0.66 – 1.14) | 0.317 |
| UNOS Status @ Listing | 0.264 | 0.030 | ||
| Status 1A | (Reference) | (Reference) | ||
| Status 1B | 0.74 (0.52 – 1.06) | 0.75 (0.55 – 1.01) | ||
| Status 2 | 0.86 (0.57 – 1.27) | 1.12 (0.80 – 1.57) | ||
| UNOS Status 1A @ Transplant | 0.99 (0.73 – 1.35) | 0.749 | 0.93 (0.71 – 1.22) | 0.612 |
| Functional Status @ Transplant | 0.847 | 0.265 | ||
| Poor | (Reference) | (Reference) | ||
| Moderate | 1.09 (0.77 – 1.53) | 0.93 (0.69 – 1.25) | ||
| Excellent | 1.09 (0.70 – 1.72) | 0.71 (0.46 – 1.09) | ||
| Wait Time > 6 Months | 1.09 (0.80 – 1.48) | 0.581 | 1.47 (1.12 – 1.92) | 0.005 |
| Ischemic Time (hours) | 1.09 (0.94 – 1.26) | 0.249 | 1.14 (1.01 – 1.28) | 0.041 |
| Donor Age (years) | 1.02 (1.01 – 1.03) | 0.001 | 1.00 (0.99 – 1.01) | 0.723 |
| Time on LVAD > 6 Months | --- | --- | 1.38 (1.05 – 1.82) | 0.020 |
| Multivariable Analysis | ||||
| Medically Bridged | BTT w/ CF-LVAD | |||
| Age (years) | 1.01 (0.99 – 1.02) | 0.212 | 1.02 (1.00 – 1.03) | 0.010 |
| eGFR (mL/min/1.73m2) | 0.99 (0.99 – 1.00) | 0.041 | 0.99 (0.98 – 0.99) | <0.001 |
| PVR (Woods Units) | 1.09 (1.00 – 1.17) | 0.044 | ||
| BMI (kg/m2) | 1.06 (1.03 – 1.09) | <0.001 | ||
| Diabetes | 1.06 (0.79 – 1.42) | 0.717 | ||
| Wait Time > 6 Months | 1.36 (1.03 – 1.80) | 0.031 | ||
| Ischemic Time (hours) | 1.13 (1.00 – 1.28) | 0.054 | ||
| Donor Age (years) | 1.02 (1.01 – 1.03) | 0.002 | ||
CVA, cerebrovascular accident; AICD, automated internal cardioverter defibrillator; BMI, body mass index; eGFR, estimated glomerular filtration rate; WL, waiting list; PA, pulmonary artery; PVR, pulmonary vascular resistance; UNOS, United Network for Organ Sharing; LVAD, left ventricular assist device.
Device-Specific Risk Factors for Early Mortality
A total of 1736 patients BTT with LVAD were transplanted as UNOS status 1A, among whom 747 (43.3%) were upgraded as a result of device complications. These included 110 (14.7%) with device thrombus, 313 (41.9%) with device infection, 90 (12.1%) with device malfunction, 46 (6.2%) with ventricular arrhythmia, and 188 (25.2%) with other indications for 1A(b) upgrade. When post-transplant outcomes were assessed between BTT patients with device complications as indication for status 1A and those with other indications for upgrade, there was no significant difference in 1-year mortality (Supplemental Figure 2A). A total of 2629 (95.8%) of BTT patients had data available on the duration of LVAD support prior to HT. Median time of support was 213 days (IQR: 121 – 377 days). As compared to patients with less than 6 months of support, those patients with longer duration of device support experienced increased mortality in the first year post-transplant (Supplemental Figure 2B). Seventy-nine percent of BTT were supported with axial flow devices. When 1-year mortality was compared by device type (axial v. centrifugal) there was no significant differences in outcomes (Supplemental Figure 2C).
Causes of Early Post-Transplant Mortality in Medically Managed Versus Mechanically Bridged Patients
Among the 441 deaths in the first post-transplant year, median time to death was 48 days. The most common causes of deaths were cardiovascular (23.1%), infection (21.3%), and organ failure (15.9%) (Supplemental Figure 3A). Within cardiovascular death, the most common etiology was UNOS-defined PGD (51.9%), affecting twice the number of BTT patients (n=35) as compared to those medically managed (n=18) (Supplemental Figure 3B).
Comparison of Risk Factors for Early Mortality Between Medical and Mechanical Bridging Strategies
Next, we assessed risk factors for early post-transplant mortality in the propensity matched cohort, evaluating mechanically and medically bridged patients independently. While age and renal function were significant predictors of early death in both cohorts, BMI, PVR, and ischemic time were significantly associated with 1-year mortality only in BTT patients. In contrast, donor age was a significant predictor only in those patients who were medically managed. We then evaluated the odds ratios for 1-year mortality of mechanically bridged versus medically bridged patients for various thresholds of age, eGFR, BMI, PVR, ischemic time, and donor age (Figure 5A). We found a significant, dose-dependent increase in risk for BTT patients as compared to medically managed patients across strata of eGFR, in particular for those patients with an eGFR of 40-60 mL/min/1.73m2 (OR: 1.69, CI: 1.19 – 2.39, p=0.003) and eGFR of < 40 mL/min/1.73m2 (OR: 2.16, CI: 1.26 – 3.69, p=0.005). Those patients BTT with LVAD who had a BMI > 30 kg/m2 experienced a two-fold increase in risk of 1-year mortality (OR: 2.11, CI: 1.48 – 3.00, p<0.001). Patients BTT with LVAD who had a PVR of more than 2 Wood units were at higher risk than medically managed patients with similar PVRs.
Figure 5:
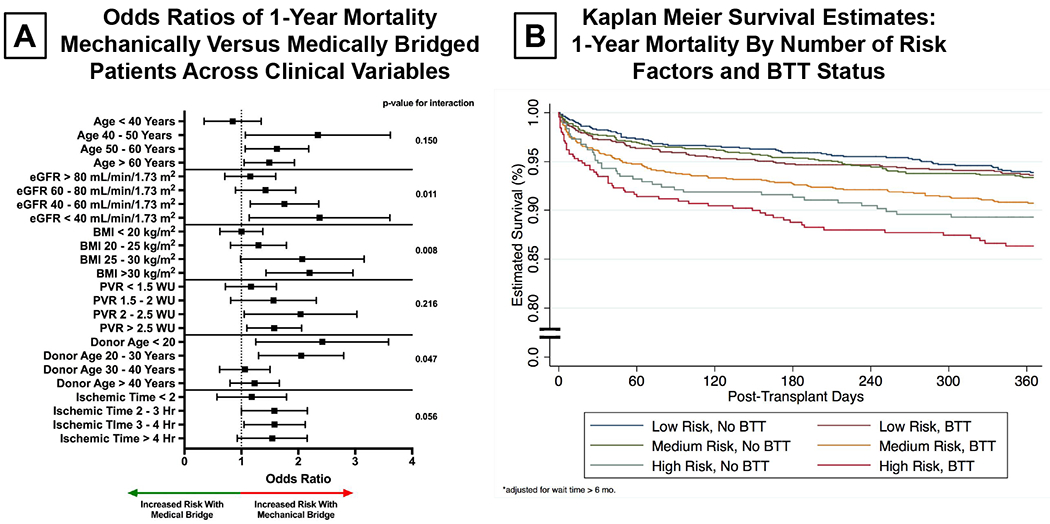
(A) Comparison of Odds Ratios of 1-Year Mortality for BTT (Bridge to Transplant) Versus Medically Managed Patients by Age, estimated glomerular filtration rate, body mass index, pulmonary vascular resistance, donor age, and ischemic time thresholds. Adjusted for Wait Time > 6 Months.
(B) Kaplan Meier Survival Estimates of 1-Year Mortality By BTT (Bridge to Transplant) Status and Risk Factors Adjusted for Wait List Time > 6 Months
In order to quantify the cumulative impact of age, eGFR, and BMI on outcomes we stratified patients as low medium and high risk based upon the presence or absence of Age > 60 years, eGFR< 40 mL/min/1.73m2, and BMI > 30 kg/m2. Patients with no risk factors were considered low risk, those with one risk factor medium risk, and those with 2-3 risk factors high risk. The Kaplan-Meier survival estimates for 1-year mortality by risk strata and BTT status are shown in Figure 5B. While low-risk BTT and non-BTT patients had similar outcomes, there was a marked difference in 1-year mortality between medium- and high-risk BTT patients as compared to those medically managed. In particular, Kaplan Meier estimates of 1-year survival in high-risk BTT patients was unacceptably low – with only 82.4% of high risk BTT patients alive at 1-year as compared to 89.6% of high risk medically managed patients.
Discussion
The current study examines the impact of LVAD as BTT on early post-transplant outcomes. The important findings include 1) in a propensity matched cohort, BTT with LVAD was associated with increased early post-transplant mortality, 2) this increase in early death was, in part, driven by cardiovascular mortality, specifically PGD, and 3) mechanically bridged patients appear to be at higher risk for post-HT mortality across thresholds of clinically significant variables suggesting that optimal risk thresholds may differ between the two groups.
With an increasing number of patients living with end-stage heart failure, LVAD utilization has rapidly increased, largely guided by INTERMACS criteria to determine which patients are eligible for this life-saving therapy14. It is important to note, however, that only a portion of these patients are considered eligible for HT by ISHLT criteria at the time of LVAD implant. In this population, the decision to BTT with LVAD is driven largely by expected wait list time as predicted by blood type, BMI, and organ scarcity in a given UNOS region1. For the remainder of patients transplanted from LVAD, the device was initially implanted as destination therapy. This means that only after optimization of eGFR, PVR, or BMI and/or the development of a device complication necessitating evaluation for HT, that many patients become candidates for transplant. Thus, for many patients in the contemporary era the decision to implant an LVAD is often separate in time and space from the decision to list a patient for HT. As such, and as shown in the current study, an increasing number of patients are being transplanted after a prolonged period of non-pulsatile, non-laminar blood flow potentially resulting in maladaptive changes in peripheral vasoreactivity, endothelial cell dysfunction, and interstitial fibrosis in end-organ vasculature15. While the effects of non-pulsatile flow on device complications including stroke and GI bleeding are beginning to be elucidated, the effect of a prolonged non-pulsatile state on post-transplant physiology and perioperative morbidity and mortality remain unknown. In this way, the physiology of LVAD patients differs markedly from those patients medically managed prior to transplant, raising the question, for the first time, of how this device-host interaction affects traditional risk factors for post-transplant mortality.
A major finding of the current study was the significant difference in short-term post-transplant survival between medically and mechanically bridged patients. Recent single-center studies have also been unable to demonstrate a significant difference between post-HT outcomes of BTT and non-BTT patients, though many have been small and inclusive of older, pulsatile flow devices4, 6, 16. Nativi et al. previously utilized the ISHLT registry database to study patients BTT with both pulsatile flow and continuous flow LVADs between 2004 and 200817. In this study, inclusive of only 417 continuous flow devices, the authors were unable to demonstrate a significant difference in post-transplant outcomes up to 4-year after HT. Of note, however, treatment effect was estimated after traditional covariate adjustment as compared to the propensity matching approach used in the current study. This approach is highly relevant, since LVAD use depends heavily on etiology of heart failure, body size, and other clinical risk factors, which may introduce significant confounding by indication when assessing its impact on post-transplant outcomes.
Our next step was to evaluate the causes of death which contributed to early mortality. We found that cardiovascular death, infection, and multisystem organ failure were the most common etiologies of death within the first year after HT. Among cardiovascular etiologies, PGD was the most common cause of death – affecting 35 mechanically supported patients and 18 medically managed patients. This confirms the findings of multiple studies suggestive of an increased risk of PGD in patients BTT with LVAD, including our own institutional experience, and could represent one of the underlying mechanism of increased post-HT mortality11, 12, 18, 19, 20. Postulated mechanisms for the link between BTT with LVAD and PGD include occult RV dysfunction during prolonged LVAD support, changes in peripheral and coronary vasoreactivity due to prolonged state of continuous flow, and upregulated inflammatory milieu to name a few. Lastly, vasoplegia – an often under recognized cause of morbidity and mortality following HT – has been identified as a predictor of poor outcomes following both transplant and LVAD implantation, and may also contribute to the increased cardiovascular death in this patient population10, 21–23.
We hypothesized that many accepted risk factors for post-transplant mortality may confer differing amounts of risk, and at different thresholds, when patients BTT with LVAD were compared to medically managed patients. In the current study, we demonstrate that odds ratios for 1-year mortality differ significantly between BTT and non-BTT patients at varying cutoffs of many clinically significant variables. Currently, ISHLT guidelines recommend using an eGFR of less than 30 mL/min as an absolute contraindication to HT24. However, it has previously been shown that despite similar pre-HT eGFR, patients supported with LVAD are at higher risk for post-transplant renal dysfunction, raising the possibility that eGFR may not be representative of the degree of underlying renal disease present in patients supported by MCS25, 26. In parallel with these observations, we found that transplant patients with eGFR <60 mL/min/1.73m2 have an increased risk of death with LVAD bridge as compared to medical therapy. The current study demonstrates similar findings for BMI. Risk of 1-year mortality appeared relatively similar across age strata with the exception of ages 40-50, in which BTT patients had a significantly higher proportion of patients with high BMI (>30 kg/m2) than medically managed patients, possibly explaining this trend. It is also worth noting that LVAD bridged patients with a PVR of > 2 Woods units experienced an increased risk of mortality as compared to medically managed patients. We believe that PVR is a poor marker of pulmonary vascular remodeling in LVAD patients, since it is a flow-dependent measure and is invariably reduced following LVAD support27. Taken together, these findings suggest that medically managed and mechanically bridged patients may exhibit different thresholds of risk which must be accounted for at the patient, center, and policy level in order to improve post-transplant outcomes.
In reality, however, the risk of LVAD prior to BTT extends beyond a cross-sectional assessment of renal function, body habitus, and or pulmonary vascular remodeling. It is, in a way, a surrogate of a prolonged, complicated course that begins, in many cases, years prior to evaluation for HT. It often represents either clinical deterioration despite continuous IV inotropes necessitating MCS, resulting in the exposure to cardiopulmonary bypass, at least one median sternotomy, and a prolonged recovery time with or without perioperative morbidity (e.g. renal failure, infection, respiratory failure, clinical or subclinical RV failure). Furthermore, in regions of particular organ scarcity, it may be only after the development of a complication while on device support that the patient is upgraded to Status 1A and may receive an organ. This is in direct contrast to the course of the medically managed patient who awaits HT at home with a single high-dose inotrope or in an ICU with invasive hemodynamic monitoring and dual inotropes, without prior sternotomy or cardiac surgery. Thus, while current transplant eligibility criteria rely on cross-sectional assessments of end-organ function to identify those at high risk for post-transplant morbidity and mortality, these metrics fail to capture the risk associated with natural history of LVAD support. As future heart allocation scores are developed, particular attention must be paid to the risks associated with MCS as BTT – both qualitative and quantitative – in order to ensure appropriate status designations based both upon risk of wait list mortality and likelihood of poor outcomes after HT.
The current study also has many inherent limitations. First, because of its retrospective nature and the pre-specified time points at which data was collected, important information about patient status and acuity at the time of LVAD implant cannot be included. Similarly, timing of right heart catheterization in relationship to LVAD implantation is often difficult to discern. Cause of death was coded for each patient by their respectively institution, but certain etiologies of death – including PGD – are not universally defined. Although propensity matching strives to eliminate bias in the comparison of medically and mechanically bridged patients, we must acknowledge the likelihood of residual confounding still present in the analysis. Additionally, because risk stratification was performed without a validation cohort, our analysis likely overestimates the differences in mortality among groups. Lastly, data was analyzed from a large registry, raising the possibility of data missingness and error in data entry.
Bridge to HT with LVAD, while both necessary in the context of organ scarcity and capable of improving waitlist survival in advanced heart failure patients, confers a significantly higher risk of early post-transplant mortality. As such, patients bridged with mechanical support may require more careful consideration for transplant eligibility after LVAD placement. These findings should be taken into consideration when developing and/or refining heart allocation systems.
Supplementary Material
Clinical Perspective.
What Is New?
In this propensity matched analysis of the United Network of Organ Sharing (UNOS) database, we demonstrate that bridge to transplant with continuous flow left ventricular assist devices (LVAD) is associated with an increased risk of early post-transplant mortality and, in particular, primary graft failure.
Increasing age, worsening renal function, and higher body mass indices all impart greater risk of mortality in patients bridged to transplant with LVAD when compared to medically managed patients
What Are The Clinical Implications?
In advanced heart failure patients, the benefits of improved survival while awaiting heart transplantation must be weighed against the risks of post-transplant mortality in heart transplant candidates.
Our findings suggest that medically managed and mechanically bridged patients may exhibit different thresholds of risk which must be accounted for at the patient, center, and policy level in order to improve post-transplant outcomes.
Sources of Funding:
This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Disclosures:
Dr. Naka received consulting fees from Abbott and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Truby LK, Garan AR, Givens RC, Takeda K, Takayama H, Trinh PN, Yuzefpolskaya M, Farr MA, Naka Y, Colombo PC and Topkara VK Ventricular Assist Device Utilization in Heart Transplant Candidates: Nationwide Variability and Impact on Waitlist Outcomes. Circ Heart Fail. 2018;11:e004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wever-Pinzon O, Drakos SG, Kfoury AG, Nativi JN, Gilbert EM, Everitt M, Alharethi R, Brunisholz K, Bader FM, Li DY, Selzman CH and Stehlik J Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United network for organ sharing thoracic organ allocation policy justified? Circulation. 2013;127:452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleveland JC Jr., Grover FL, Fullerton DA, Campbell DN, Mitchell MB, Lindenfeld J, Wolfel EE, Lowes BD, Shakar SF, Brieke A, Cannon A and Robertson AD Left ventricular assist device as bridge to transplantation does not adversely affect one-year heart transplantation survival. J Thorac Cardiovasc Surg. 2008;136:774–777. [DOI] [PubMed] [Google Scholar]
- 4.John R, Pagani FD, Naka Y, Boyle A, Conte JV, Russell SD, Klodell CT, Milano CA, Rogers J, Farrar DJ and Frazier OH Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010;140:174–181. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JA, Park Y, Kherani AR, Vigilance DW, Cheema FH, Oz MC and Naka Y Does bridging to transplantation with a left ventricular assist device adversely affect posttransplantation survival? A comparative analysis of mechanical versus inotropic support. J Thorac Cardiovasc Surg. 2003;126:1188–1190. [DOI] [PubMed] [Google Scholar]
- 6.Kamdar F, John R, Eckman P, Colvin-Adams M, Shumway SJ and Liao K Postcardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2013;145:575–581. [DOI] [PubMed] [Google Scholar]
- 7.Patlolla V, Patten RD, Denofrio D, Konstam MA and Krishnamani R The effect of ventricular assist devices on post-transplant mortality an analysis of the United network for organ sharing thoracic registry. J Am Coll Cardiol. 2009;53:264–271. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JO, Lober C, Smedira NG, Navia JL, Sopko N and Gonzalez-Stawinski GV One hundred days or more bridged on a ventricular assist device and effects on outcomes following heart transplantation. Eur J Cardiothorac Surg. 2008;34:295–300. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Takayama H, Kalesan B, Uriel N, Colombo PC, Jorde UP, Yuzefpolskaya M, Mancini DM and Naka Y Outcome of cardiac transplantation in patients requiring prolonged continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2015;34:89–99. [DOI] [PubMed] [Google Scholar]
- 10.Truby LK, Takeda K, Farr M, Beck J, Yuzefpolskaya M, Colombo PC, Topkara VK, Mancini D, Naka Y and Takayama H Incidence and Impact of On-Cardiopulmonary Bypass Vasoplegia During Heart Transplantation. ASAIO J. 2018;64:43–51. [DOI] [PubMed] [Google Scholar]
- 11.Chinnadurai Tea. Primary Graft Failure is More Common in Patients Bridged to Heart Transplant with LVAD: Role of Early Periphearl ECMO. J Heart Lung Transplant. 2018;37:S349. [Google Scholar]
- 12.Truby L Bridge to Transplant with Continuous Flow Left Ventricular Assist Devices Increases Risk for Severe Primary Graft Dysfunction. J Heart Lung Transplant. 2018;37:S328. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F and Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT and Young JB Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. [DOI] [PubMed] [Google Scholar]
- 15.Purohit SN, Cornwell WK, Pal JD, Lindenfeld J and Ambardekar AV Living Without a Pulse: The Vascular Implications of Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail. 2018;11:e004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo MJ, Hong KN, Davies RR, Chen JM, Sorabella RA, Ascheim DD, Williams MR, Gelijns AC, Stewart AS, Argenziano M and Naka Y Posttransplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;138:1425–32 e1–3. [DOI] [PubMed] [Google Scholar]
- 17.Nativi JN, Drakos SG, Kucheryavaya AY, Edwards LB, Selzman CH, Taylor DO, Hertz MI, Kfoury AG and Stehlik J Changing outcomes in patients bridged to heart transplantation with continuous- versus pulsatile-flow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2011;30:854–861. [DOI] [PubMed] [Google Scholar]
- 18.Nicoara A, Ruffin D, Cooter M, Patel CB, Thompson A, Schroder JN, Daneshmand MA, Hernandez AF, Rogers JG, Podgoreanu MV, Swaminathan M, Kretzer A, Stafford-Smith M, Milano CA and Bartz RR Primary graft dysfunction after heart transplantation: Incidence, trends, and associated risk factors. Am J Transplant. 2017. 2018;18:1461–1470. [DOI] [PubMed] [Google Scholar]
- 19.Russo MJ, Iribarne A, Hong KN, Ramlawi B, Chen JM, Takayama H, Mancini DM and Naka Y Factors associated with primary graft failure after heart transplantation. Transplantation. 2010;90:444–450. [DOI] [PubMed] [Google Scholar]
- 20.Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F, Luu M, Mancini D, Patel J, Razi R, Reichenspurner H, Russell S, Segovia J, Smedira N, Stehlik J, Wagner F and Consensus Conference p. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33:327–340. [DOI] [PubMed] [Google Scholar]
- 21.Tecson KM, Lima B, Lee AY, Raza FS, Ching G, Lee CH, Felius J, Baxter RD, Still S, Collier JDG, Hall SA and Joseph SM Determinants and Outcomes of Vasoplegia Following Left Ventricular Assist Device Implantation. J Am Heart Assoc. 2018; 7: pii: e008377. doi: 10.1161/JAHA.117.008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JL, Kobashigawa JA, Aintablian TL, Dimbil SJ, Perry PA, Patel JK, Kittleson MM, Czer LS, Zarrini P, Velleca A, Rush J, Arabia FA, Trento A and Esmailian F Characterizing Predictors and Severity of Vasoplegia Syndrome After Heart Transplantation. Ann Thorac Surg 2018;105:770–777. [DOI] [PubMed] [Google Scholar]
- 23.Chan JL, Kobashigawa JA, Aintablian TL, Li Y, Perry PA, Patel JK, Kittleson MM, Czer LS, Zarrini P, Velleca A, Rush J, Arabia FA, Trento A and Esmailian F Vasoplegia after heart transplantation: outcomes at 1 year. Interact Cardiovasc Thorac Surg. 2017;25:212–217. [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR Guidelines for Listing Candidates for Heart Transplant: A 10-Year Update. JAMA Cardiol. 2017;2:98–99. [DOI] [PubMed] [Google Scholar]
- 25.Topkara PNT VK, Masoumi A, Garan AR, Yuzefpolskaya M, Takeda K, Takayama H, Haythe J, Maurer M, Latif F, Restaino S, Farr MA, Mancini DM, Naka Y, Colombo PC. Risk of Post-Transplant Renal Dysfunction Is Increased in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. J Heart Lung Transplant. 2016;35:S287–S288. [Google Scholar]
- 26.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR and Testani JM Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014;7:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salzberg SP, Lachat ML, von Harbou K, Zund G and Turina MI Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg. 2005;27:222–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


