Abstract
We intensively studied faba bean (Vicia faba L.) and wheat (Triticum aestivum L.) intercropping and found that this type of intercropping can effectively control the occurrence of faba bean wilt under field conditions. We conducted hydroponic experiments to explore the role of plant extracts in the process of soil-borne diseases and the mechanism of disease control of faba bean and wheat intercropping. In this experiment, three concentration gradients of faba bean and wheat stem, leaf, and root extracts were added to study the effects of faba bean and wheat extracts on faba bean growth, the physiological resistance of roots, and the growth of Fusarium oxysporum f. sp. fabae (FOF). Faba bean extracts significantly inhibited the growth of faba bean seedlings and the activity of root defense enzymes and significantly stimulated the growth of FOF at high concentrations. Compared with the treatment with faba bean extracts, wheat extracts significantly enhanced the growth of faba bean seedlings, increased the activity of defense enzymes, and inhibited the growth of FOF. Based on these results, we believe that wheat extracts can effectively alleviate the autotoxicity of faba beans and also control the occurrence of faba bean wilt in the field. This provides a theoretical basis for practical intercropping to reduce the damage caused by faba bean wilt.
Introduction
The continuous planting and harvesting of single crops is a common practice in modern agriculture, and that has resulted in serious obstacles. The hazards of continuous cropping obstacles primarily include soil compaction, the frequent occurrence of soil-borne diseases, a reduction in crop yields, or even a total lack of germination of the seeds. Among them, the frequent occurrence of soil-borne diseases has always been a very difficult problem during actual production.1,2 Thus far, soil-borne diseases have seriously threatened the production of various cash crops, such as watermelon, peanut, and cotton, which has a substantial impact on agricultural production around the world.3−5 The accumulation of autotoxic substances has always been a central area of research in the study of the causes of the frequent occurrence of soil-borne diseases. Many studies have shown that the accumulation of autotoxic substances strongly promotes the occurrence of soil-borne diseases.6 For instance, the secretion of phenolic acids, such as cinnamic acid, coumaric acid, and ferulic acid, from cucumber roots, and the products of decomposition of cucumber increase the risk of Fusarium wilt.7−9 The accumulation of autotoxic substances in the rhizosphere during peanut monocropping aggravates the occurrence of soil-borne diseases of peanut.5 Other studies have shown that the main reason that autotoxic substances can promote the occurrence of diseases is that they can have a strong destructive effect on plant physiological and biochemical resistance. For example, Ye et al. found that cinnamic acid in cucumber autotoxic substances destroyed the plant antioxidant system, increased the content of active oxygen free radicals in the root, and accelerated the degree of membrane lipid peroxidation.10 Wang et al. found that exogenous syringic acid and phthalic acid significantly reduced the activity of antioxidant enzymes, such as guaiacol peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT), by inhibiting their levels of gene expression in strawberry roots.11 These effects render plants more susceptible to infection and increase the incidence of diseases. Several chemical and biological methods have been developed to control plant diseases.12 However, these methods are not environmentally friendly or sufficiently efficient.13,14
Intercropping is a planting method in which two or more crops are planted in close proximity.14 In actual production, it is used as a green and efficient planting method to control soil-borne diseases and increase the yields of crops.3,15 Allelopathy is an indispensable part of the study of the disease control mechanism of intercropping. For example, in the wheat/watermelon intercropping system, wheat allelopathic substances secreted by the root system increase the expression of watermelon defense genes, improve the ability of watermelon to resist the invasion of pathogens, and control the occurrence of wilt.16 In the intercropping system of cumin (Cuminum cyminum L.) and watermelon, the cuminic acid secreted by the root system of C. cyminum significantly increased the activity of antioxidant enzymes and defensive enzymes in the watermelon roots and improved the ability of watermelon to resist pathogens.17 Allelopathic chemicals can enter the environment in different manners to play a role in the direct or indirect effects on growth of plants. The primary manners in which allelopathic chemicals are released include following their release by aboveground volatilization and leaching and secretion by the roots.18 Root secretion is the main source of allelochemicals belowground; these substances enter the soil directly through secretions from the plant roots and play a role in the interaction of the plant with other organisms.5,6,15 The study of other allelopathic substances in plants is usually conducted with plant extracts. Leaching easily occurs in rainy and humid periods, and the allelochemicals contained in the crop surface are released into the surrounding environment from leaching by rain and fog to inhibit the growth of itself or other crops.47 The extraction method is usually used to obtain allelochemicals that enter the environment through leaching, and this extraction method has been used in many studies.40,41 Examples include extracts of rock rose (Cistus ladanifer), Arugula (Eruca Sativa), sunflower (Helianthus annuus Linn.), and alfalfa (Medicago sativa Linn.) plants that have a strong allelopathic effect on their own physiology or that of other plants.19−24 However, most of the research on the mechanism of disease control by intercropping focuses on plant root exudates, and there are few studies on the extracts of plant stems, leaves, and roots that also have allelopathic effects.
Faba beans are widely cultivated worldwide as an important legume crop.25 However, because of the continuous single planting, the yield of faba beans is greatly reduced owing to Fusarium wilt.26 In Yunnan and southwestern China, faba beans are usually planted with wheat to control faba bean wilt. We intensively studied the mechanism of control of faba bean and wheat intercropping to control the wilt disease of faba bean. Our previous research focused on the effects of allelochemicals secreted by roots on plants and microbes in the faba bean–wheat intercropping system.27,28 However, to fully demonstrate the mechanism of disease control of faba bean–wheat intercropping, data on the allelopathy of plant extracts are lacking. We conducted a preliminary experiment on the allelopathy of extract of faba bean stems and leaves from the perspective of physiological resistance based on a field experiment but using hydroponics. In this study, we aimed to (i) reveal the allelopathic capability of extracts from faba beans and wheat and (ii) explore the possible causes of effective control of faba bean Fusarium wilt in faba bean–wheat intercropping.
Results
Effect of Intercropping Wheat and Faba Bean on Fusarium Wilt of Faba Bean
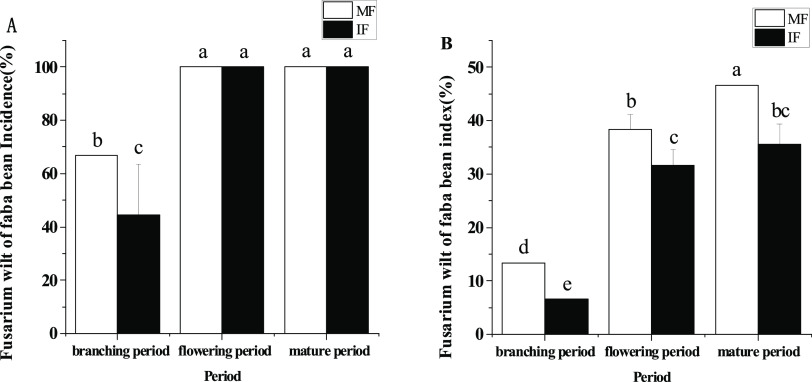
Figure 1A shows that the incidence of faba bean wilt during the mature and flowering periods was significantly higher than that during the branching period in the monocropping and intercropping models. Compared with monocropping, intercropping wheat and faba bean significantly reduced the incidence of faba bean wilt in the branching stages by 33.44%.
Figure 1.
Effect of wheat and faba bean intercropping on faba bean wilt: (A) incidence of faba bean wilt and (B) faba bean wilt disease index. MF: monocropped and IF: intercropped with wheat. The data is an average, and the standard error of three biological replicates is represented by a bar. Different letters for each index indicate significant differences at p < 0.05.
In Figure 1B, the disease index of faba bean wilt during the flowering stage was significantly higher than that during the branching stage, and the disease index in the mature stage of faba bean wilt was significantly higher than that in the flowering stage. The disease index gradually increased with time. Compared with monocropping, intercropping wheat and faba bean significantly reduced the disease index of faba bean wilt by 50, 17.39, and 23.81% during the branching, flowering, and mature stages, respectively. Intercropping with faba bean and wheat can effectively control the faba bean wilt compared with the faba bean monocropping (Figure 1A,B), and the effect is particularly significant in the suppression of the faba bean wilt disease index. Among these three periods, the branching period is when the faba bean and wheat intercropping is the most effective at controlling the disease. The incidence of faba bean wilt and the disease index decreased by 33.44 and 50%, respectively.
Effects of Wheat and Faba Bean Stem, Leaf, and Root Extracts on Faba Bean Growth
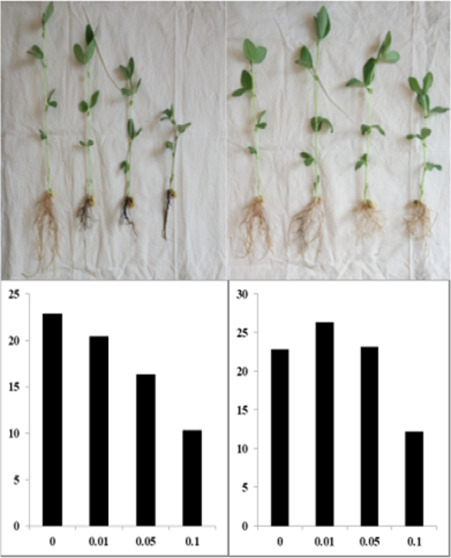
Compared with the control, the addition of three concentrations of faba bean stem and leaf extracts significantly inhibited the growth index of faba beans, which was concentration dependent (Table 1). Compared with the control, the exogenous addition of 0.01 g·mL–1 wheat stem and leaf extracts significantly increased the plant height, dry weight, and root length of faba bean. Exogenously added 0.05 g·mL–1 wheat stem and leaf extracts slightly increased these parameters compared with the control. However, when the concentration of the wheat stem and the leaf extract reached 0.1 g·mL–1, it significantly inhibited all of the growth indices of faba bean (Figure 2).
Table 1. Effect of the Faba Bean and Wheat Stem and Leaf Extracts on the Growth of Faba Bean.
| aqueous extract from leaves and stem | concentration (g·mL–1) | number of leaves per plant | max leaf length (cm) | plant height (cm) | main root length (cm) | shoot dry weight (g) | root dry weight (g) | root length (cm) |
|---|---|---|---|---|---|---|---|---|
| faba bean extract | CK | 10.00 ± 0.00a | 5.70 ± 0.60a | 22.87 ± 1.32b | 15.80 ± 0.78ab | 0.25 ± 0.06b | 0.17 ± 0.03b | 2.86 ± 0.14b |
| 0.01 | 8.00 ± 0.00bc | 4.9 ± 0.1b | 20.43 ± 1.40c | 10.60 ± 1.45c | 0.23 ± 0.03bc | 0.15 ± 0.01b | 2.41 ± 0.02c | |
| 0.05 | 7.33 ± 1.15cd | 3.87 ± 0.06c | 16.3 ± 1.39d | 8.40 ± 0.66d | 0.15 ± 0.03d | 0.09 ± 0.01c | 1.45 ± 0.16d | |
| 0.1 | 6.00 ± 0.00d | 3.20 ± 0.56d | 10.3 ± 0.95e | 6.13 ± 0.60e | 0.07 ± 0.02e | 0.04 ± 0.02d | 0.39 ± 0.09e | |
| wheat extract | 0.01 | 10.67 ± 1.15a | 6.13 ± 0.31a | 26.33 ± 0.15a | 16.50 ± 0.92a | 0.36 ± 0.07a | 0.21 ± 0.04a | 3.51 ± 0.13a |
| 0.05 | 9.33 ± 1.15ab | 5.80 ± 0.26a | 23.17 ± 1.29b | 13.70 ± 2.10b | 0.28 ± 0.01b | 0.14 ± 0.01b | 2.85 ± 0.16b | |
| 0.1 | 7.33 ± 1.15cd | 4.63 ± 0.21b | 12.20 ± 0.53e | 6.90 ± 1.21de | 0.16 ± 0.05cd | 0.11 ± 0.01c | 1.61 ± 0.12d |
CK: blank control. The data is an average, and the standard error of three biological replicates is represented by a number. Different letters for the same growth parameters among treatments with different concentrations of the extract indicate significant differences at p < 0.05.
Figure 2.
Growth of the faba beans under different treatments. BR: treatment with exogenously added faba bean root extract, BSY: treatment with exogenously added faba bean stem and leaf extracts, WR: treatment with the exogenously added wheat root extract, and WSY: treatment with exogenously added wheat stem and leaf extracts. These four photos were taken by Jiaxing Lv.
Compared with the control, the addition of 0.01 g·mL–1 faba bean root extract significantly inhibited the main root length and root length of faba bean but had no significant effect on the other indicators (Table 2). Compared with the control, the faba bean root extract with a concentration greater than or equal to 0.05 g·mL–1 significantly inhibited all of the growth indices of faba bean. In contrast, the wheat root extract had an opposite effect. Compared with the control, the addition of the 0.01 g·mL–1 wheat extract significantly increased all of the growth indices of faba bean with the exception of number of leaves. The addition of the 0.05 g·mL–1 wheat root extract significantly increased the main root length, stem dry weight, root dry weight, and root length of faba bean. However, when the concentration of the wheat root extract reached 0.1 g·mL–1, it significantly inhibited the plant height, main root length, stem weight, and root length of faba bean compared with the control and had no significant effect on the other indicators (Figure 2).
Table 2. Effect of the Faba Bean and Wheat Root Extracts on the Growth of Faba Bean.
| aqueous extract from roots | concentration (g·mL–1) | number of leaves per plant | max leaf length (cm) | plant height (cm) | main root length (cm) | shoot dry weight (g) | root dry weight (g) | root length (cm) |
|---|---|---|---|---|---|---|---|---|
| faba bean extract | CK | 10.00 ± 0.00ab | 5.70 ± 0.60bc | 22.87 ± 1.32b | 15.80 ± 0.78b | 0.25 ± 0.06c | 0.17 ± 0.03cd | 2.86 ± 0.14c |
| 0.01 | 9.33 ± 1.15bc | 5.57 ± 0.21bc | 22.60 ± 1.01b | 11.87 ± 1.67c | 0.23 ± 0.03cd | 0.17 ± 0.02c | 2.54 ± 0.11d | |
| 0.05 | 8.00 ± 0.00cd | 4.80 ± 0.10d | 17.83 ± 0.58c | 9.17 ± 0.49d | 0.16 ± 0.02e | 0.14 ± 0.01cd | 1.72 ± 0.11e | |
| 0.1 | 6.67 ± 1.15d | 3.83 ± 0.35e | 10.50 ± 1.32e | 7.17 ± 0.25e | 0.07 ± 0.01f | 0.09 ± 0.03e | 0.97 ± 0.17f | |
| wheat extract | 0.01 | 11.33 ± 1.15a | 6.43 ± 0.35a | 26.90 ± 0.53a | 18.97 ± 0.59a | 0.39 ± 0.01a | 0.27 ± 0.01a | 3.69 ± 0.14a |
| extract | 0.05 | 10.00 ± 0.00ab | 5.93 ± 0.12ab | 23.53 ± 0.96b | 17.87 ± 0.31a | 0.32 ± 0.02b | 0.22 ± 0.04b | 3.19 ± 0.20b |
| 0.1 | 8.67 ± 1.15bc | 5.33 ± 0.15cd | 13.03 ± 0.59d | 11.87 ± 1.01c | 0.18 ± 0.06de | 0.12 ± 0.02de | 2.34 ± 0.13d |
CK: blank control. The data is an average, and the standard error of three biological replicates is represented by a number. Different letters for the same growth parameters among treatments with different concentrations of the extract indicate significant differences at p < 0.05.
The most notable effect was that the wheat extracts significantly increased the growth index of faba beans at three concentrations compared with the faba bean extracts.
Effects of Extracts from Faba Bean Stems, Leaves, and Roots on the Physiological Resistance of Faba Bean Roots
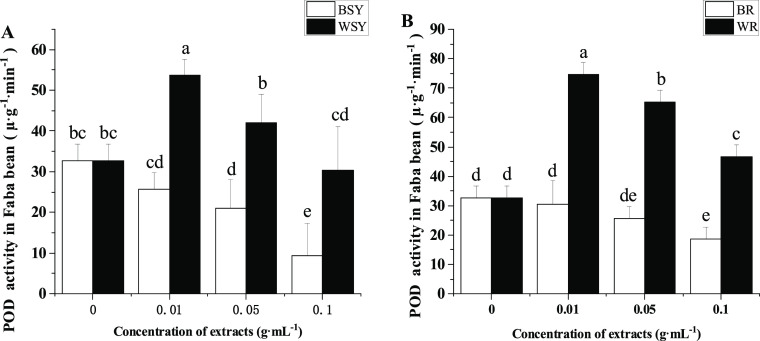
As shown in Figure 3A, compared with the control, the addition of 0.05 and 0.1 g·mL–1 faba bean stem and leaf extracts significantly reduced the POD activity of the faba bean root system. Compared with the faba bean stem and leaf extracts, the wheat stem and leaf extracts significantly increased the POD activity of the faba bean root at all concentrations tested. The faba bean root extracts significantly reduced the POD activity of faba bean root in the 0.1 g·mL–1 treatment compared with that of the control (Figure 3B). Compared with the faba bean root extracts, the wheat root extracts can significantly increase the POD activity of the faba bean root at all concentrations tested.
Figure 3.
Effects of extracts from faba bean and wheat stems, leaves, and roots on the POD activity of faba bean roots. (A) Effect of extracts from faba bean and wheat stems and leaves on the POD activity of faba bean roots and (B) effect of extracts from faba bean and wheat roots on the POD activity of faba bean roots. BR: treatment with the exogenously added faba bean root extract, BSY: treatment with exogenously added faba bean stem and leaf extracts, POD: peroxidase, WR: treatment with the exogenously added wheat root extract, and WSY: treatment with exogenously added wheat stem and leaf extracts. The data is an average, and the standard error of three biological replicates is represented by a bar. Different letters for each index indicate significant differences at the p < 0.05 level.
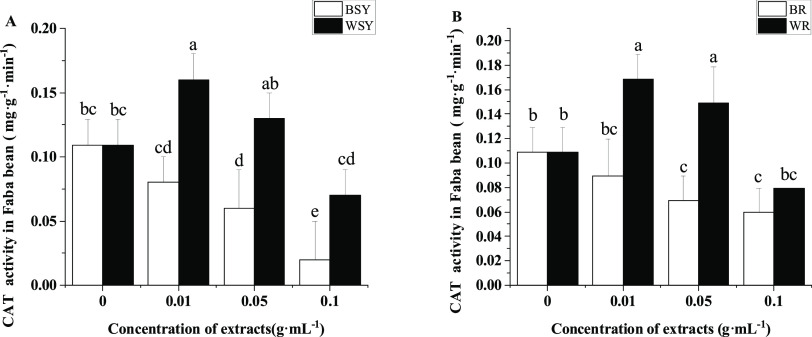
Compared with the control, the faba bean stem and leaf extracts significantly inhibited the activity of CAT in the faba bean root system at concentrations of 0.05 and 0.1 g·mL–1 (Figure 4A). Compared with the faba bean stem and leaf extracts, the wheat stem and leaf extracts can significantly increase the activity of CAT in the faba bean root system at all concentrations tested. The faba bean root extract significantly inhibited the activity of CAT in the faba bean root system compared with that of the control at concentrations of 0.05 and 0.1 g·mL–1 (Figure 4B). Compared with the faba bean root extract, the wheat root extract significantly increased the activity of CAT in the faba bean root at concentrations of 0.01 and 0.05 g·mL–1.
Figure 4.
Effects of extracts from faba bean stems, leaves, and roots on the CAT activity of faba bean roots. (A) Effect of extracts from faba bean and wheat stems and leaves on the CAT activity of faba bean roots and (B) effect of extracts from faba bean and wheat roots on the CAT activity of faba bean roots. BR: treatment with the exogenously added faba bean root extract, BSY: treatment with exogenously added faba bean stem and leaf extracts, CAT: catalase, WR: treatment with the exogenously added wheat root extract, and WSY: treatment with exogenously added wheat stem and leaf extract. The data is an average, and the standard error of three repetitions is represented by a bar. Different letters for each index indicate significant differences at the p < 0.05 level.
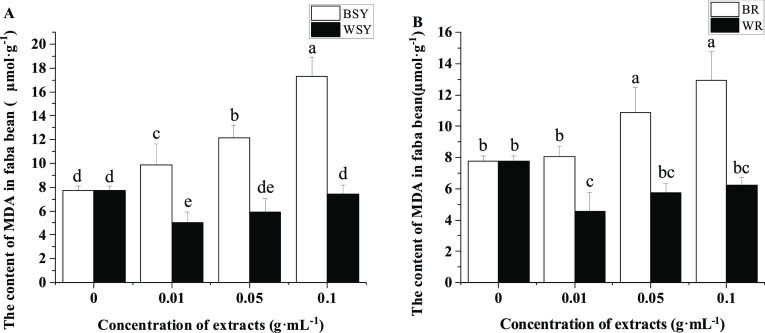
The effect of extracts from faba bean stems, leaves, and roots on the MDA content of faba bean roots is shown in Figure 5. The extracts of faba bean stems and leaves at all three concentrations significantly increased the content of MDA of faba bean roots compared with the control. This effect increases with the concentration. Compared with the faba bean stem and leaf extracts, the wheat stem and leaf extracts in the three concentrations of treatment significantly reduced the content of MDA in the faba bean root system, and the effect was most significant in the 0.1 g·mL–1 treatment. In contrast to the control, 0.05 and 0.1 g·mL–1 faba bean root extracts significantly increased the content of MDA in the faba bean root system (Figure 5B). Compared with the faba bean root extract, the wheat root extract at the three concentrations tested significantly reduced the MDA content in the faba bean root system, with the most significant effect visible at 0.1 g·mL–1.
Figure 5.
Effects of extracts from faba bean stems, leaves, and roots on the MDA content of faba bean roots. (A) Effect of extracts from faba bean and wheat stems and leaves on the MDA content of faba bean roots and (B) effect of extracts from faba bean and wheat roots on the MDA content of faba bean roots. BR: treatment with the exogenously added faba bean root extract, BSY: treatment with exogenously added faba bean stem and leaf extracts, MDA: malondialdehyde, WR: treatment with the exogenously added wheat root extract, and WSY: treatment with exogenously added wheat stem and leaf extracts. The data is an average, and the standard error of three repetitions is represented by a bar. Different letters for each index indicate significant differences at the p < 0.05 level.
Effects of Extracts from the Leaves, Stems, and Roots of Faba Bean and Wheat on FOF Spore Germination and Mycelial Growth
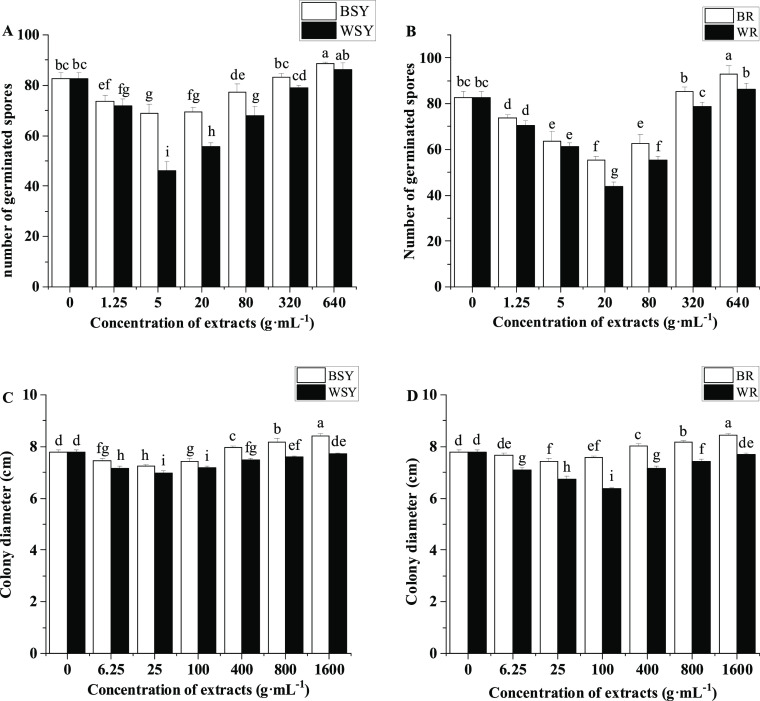
As Figure 6A indicates, compared with the control, the addition of 1.25, 5, 20, and 80 mg·L–1 faba bean stem and leaf extracts significantly inhibited the germination of FOF spores, but 640 mg·L–1 faba bean stem and leaf extracts significantly increased the germination of FOF spores. Compared with the treatment of faba bean stem and leaf extracts, the wheat stem and leaf extracts at concentrations of 1.25, 5, 20, and 80 mg·L–1 significantly inhibited the germination of FOF spores, with the strongest inhibitory effect at 5 mg·L–1 (Figure 6A). The faba bean root extracts significantly inhibited the germination of FOF spores at concentrations of 1.25, 5, 20, and 80 mg·L–1, but when the concentration reached 640 mg·L–1, the faba bean root extracts significantly promoted the germination of FOF spores (Figure 6B). The wheat root extracts significantly inhibited the spore germination of FOF compared with faba bean root extracts when tested at 20, 80, 320, and 640 mg·L–1.
Figure 6.
(A) Effects of faba bean and wheat stem and leaf extracts on the germination of Fusarium oxysporum f. sp. fabae spores, (B) faba bean and wheat root extracts on the germination of FOF spores, (C) faba bean and wheat stem and leaf extracts on FOF mycelial growth, and (D) faba bean and wheat root extracts on FOF mycelial growth. BR: treatment with the exogenously added faba bean root extract, BSY: treatment with exogenously added faba bean stem and leaf extracts, FOF: F. oxysporum f. sp. fabae, WR: treatment with the exogenously added wheat root extract, and WSY: treatment with exogenously added wheat stem and leaf extracts. The data is an average, and the standard error of three repetitions is represented by a bar. Different letters for each index indicate significant differences at the p <0.05 level.
The extracts of faba bean stems and leaves significantly inhibited the mycelial growth of FOF at 6.25, 25, and 100 mg·L–1 concentrations compared with the control, and concentrations of 400, 800, and 1,600 mg·L–1 significantly stimulated the mycelial growth of FOF (Figure 6C). Compared with the faba bean stem and leaf extracts, the wheat stem and leaf extracts significantly inhibited the mycelial growth of FOF in all concentrations. The faba bean root extracts significantly inhibited the mycelial growth of FOF at concentrations of 25 and 100 mg·L–1, but they significantly stimulated the mycelial growth of FOF at concentrations of 400, 800, and 1600 mg·L–1 (Figure 6D). Compared with the faba bean root extracts, the wheat root extracts significantly inhibited the mycelial growth of FOF at all concentrations tested.
Discussion
Autotoxicity refers to the process by which plants or their residues release toxic chemicals into the environment during decomposition, thereby inhibiting the germination and growth of the same plant and serving as a common cause of plant continuous cropping obstacles.18,19,29 This experiment showed that all concentrations tested of the extracts of faba bean stems, leaves, and roots significantly inhibited the growth of faba bean seedlings compared with the control, and the pronounced inhibition of root growth was particularly significant. Plant extracts from melon significantly inhibited the germination of its own seeds and the growth of its cotyledons, which is similar to the results that we obtained.19 Plant cells accumulate free radicals owing to reduced antioxidant capacity during adverse conditions, leading to the oxidative damage of cellular macromolecules and membranes.30,31 Furthermore, autotoxic metabolites produced by the stressed plants accelerate free radical-induced membrane peroxidation and breakdown, thereby providing nutrients to the pathogens and enhancing their ability to invade plant roots. In fact, the activity of the antioxidant enzymes POD and CAT are reliable indicators of disease resistance in plants.11 Wang et al. showed that exogenous syringic acid and phthalic acid significantly reduced the activities of POD and CAT in strawberry roots and increased the content of MDA.11 This is identical to the results obtained in this experiment. However, this experiment explores the effect of allelopathy of plant extracts on the plant defense system. Compared with the control, medium and low concentrations of faba bean stem, leaf, and root extracts significantly inhibited the activities of the antioxidant enzymes POD and CAT of the faba bean root system, while significantly enhancing the accumulation of MDA in faba bean roots. This could be because the extract of faba bean stems, leaves, and roots contains a substantial amount of phenolic acids.18 They destroy the functional pathways of antioxidant enzymes and cause enormous damage to the defense system of faba bean roots, which, in turn, clears obstacles for the pathogens to invade faba bean roots. The accumulation of pathogens is the primary cause of soil-borne diseases, and these microorganisms are difficult to remove from the soil. Experiments have proven that the three biological forms of F. oxysporum can survive for more than 11 years without changing their morphology.17 Long-term continuous crops have formed a stable and suitable environment with increased temperature and humidity, sufficient nutrients, and host conditions that are more conducive to the propagation and growth of pathogens, resulting in the aggravation of disease.33,34 In this experiment, the extracts of faba bean stems, leaves, and roots at low concentrations inhibited the spore germination and mycelial growth of FOF. However, with the increase in concentration, the inhibitory effect gradually disappeared, and at high concentration, the extracts significantly promoted germination and growth of the fungus. This may be related to the allelochemicals in the faba bean extract.7 Our previous studies have shown that the addition of cinnamic acid significantly promotes the germination of FOF spores, which is similar to the results of this study.28 Compared with previous studies, this experiment supplemented the allelopathic effects of faba bean plants from the perspective of plant extracts and demonstrated the allelopathy of faba bean plants from another perspective. A large number of studies have proven that allelochemicals produced by plants can accumulate in the soil after continuous cropping.11 Therefore, we hypothesized that in actual agricultural production, owing to years of continuous cropping, the allelochemicals in faba bean extracts accumulate to a large amount in the soil, and the concentration of these allelochemicals in the soil becomes increasingly higher, which affects the germination and growth of FOF in the soil. Based on these results, we concluded that the autotoxicity of faba bean may promote the growth of pathogen by destroying the defense system of the faba bean root system and enhancing the invasion of pathogens to the root system of faba bean, finally resulting in strong inhibition of the growth of faba beans. Intercropping is a green and efficient planting model, particularly in terms of increasing the growth index and controlling diseases. Now this advantage has been verified in many intercropping systems, such as corn and soybean intercropping, that effectively control corn crown rot and garlic/tobacco intercropping that effectively controls tobacco black shank disease.32,35 Similarly, we found that in field experiments, intercropping faba bean and wheat significantly inhibited the incidence of faba bean wilt in the faba bean branching stages, and the disease index of faba bean wilt was significantly inhibited during the branching, flowering, and podding stages of faba bean. Most research on the mechanism of intercropping disease control focuses on allelopathic substances secreted into the soil through the root system, but in actual production, these compounds can also enter the soil through the leaching and evaporation of plant roots, stems, and leaves. These allelopathic substances are easily overlooked.7 For the research on the mechanism of control by faba bean and wheat intercropping, we added different concentrations of wheat stem, leaf, and root extracts in the faba bean hydroponic experiment. Compared with the faba bean extracts, we found that the wheat extracts significantly promoted the growth of faba bean seedlings at all treatment concentrations. Studies have shown that spraying extracts of the moringa plant (Moringa oleifera) on wheat leaves can promote the growth of wheat, which is consistent with our results.36 The difference is that this study is an investigation of biological agents under a monocropping system. However, our experiment focused more on the allelopathy of plants when grown naturally. We also simultaneously found that, compared with the treatment of faba bean extracts, wheat extracts significantly enhanced the activities of faba bean root POD and CAT and effectively reduced the accumulation of faba bean root MDA. The ability of the faba bean root system to resist the invasion of pathogens had improved. A series of results on plant growth and physiological resistance show that wheat extracts can effectively alleviate the autotoxicity of faba beans. We hypothesize that this may be one of the important mechanisms of wheat and faba bean intercropping for disease control. This result is consistent with previous studies on rice/watermelon and corn/sunflower intercropping systems.11,37 However, these studies did not explore the allelopathy between host and nonhost crops in the intercropping system from the perspective of plant extracts, which is the largest innovation of this experiment. On the basis of the significant improvement of the faba bean root defense system by wheat extracts, compared with the faba bean extracts, the wheat extracts could significantly inhibit the mycelial growth and germination of spores, thereby fundamentally reducing the possibility of pathogen infection of faba beans. This is consistent with the results of studies on the wheat/watermelon and rice-water chestnut intercropping system.38,39 We have found in actual production that the faba bean–wheat intercropping can effectively reduce the amount of FOF in the faba bean rhizosphere (Figure 7). Therefore, we hypothesize that in the wheat/faba bean intercropping system, the extracts of wheat can effectively relieve the stimulatory effects of the faba bean extracts on the occurrence of faba bean wilt, thereby, further reducing the occurrence of faba bean wilt. Unexpectedly, compared with the control, the wheat extracts were effective at a low concentration, but they enhanced the inhibition of the growth of faba beans at high concentration. However, in actual production, unlike the large accumulation of the faba bean extract, wheat has no continuous cropping history. The concentration of allelochemicals in the wheat extract in the field is very low and they are easily degraded by microorganisms in the soil.34 Therefore, in the actual field intercropping mode, the concentration of allelochemicals in the wheat extract is not very high. This experiment was a hydroponic one, and our aim was to explore the allelopathy of wheat extracts of different concentrations. This does not examine the decomposition of allelochemicals by soil rhizosphere microorganisms. However, it also indicates that in actual agricultural production, we should focus on controlling the ratio of faba bean and wheat and avoiding an excessive planting density of wheat that leads to an excessive concentration of the rhizosphere wheat extract that could inhibit the growth of faba bean.
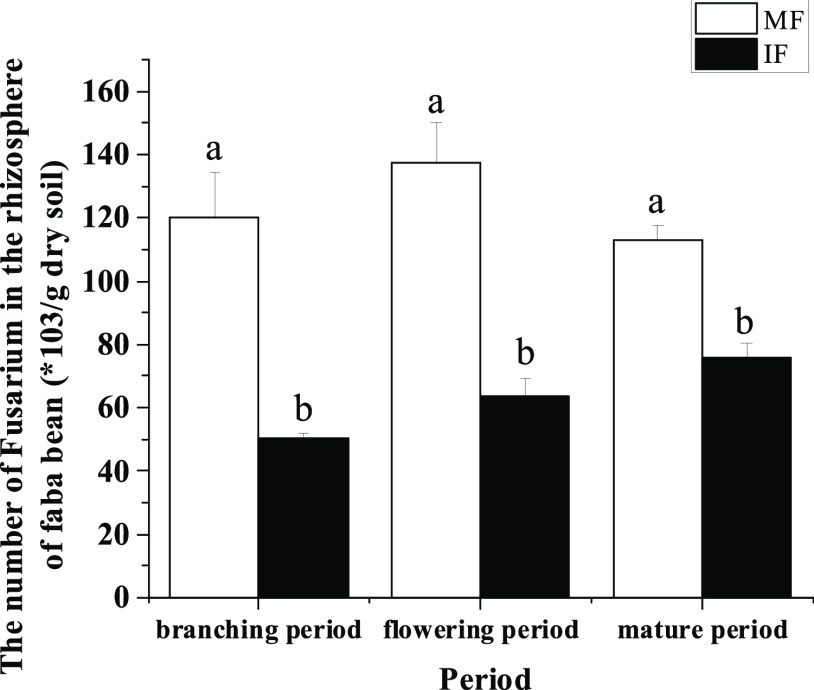
Figure 7.
Number of F. oxysporum propagules in the faba bean rhizosphere under different treatments in different periods. The data is an average, and the standard error of three repetitions is represented by a bar. Different letters for each index indicate significant differences at the p < 0.05 level.
In summary, wheat/faba bean intercropping can effectively control the occurrence of faba bean wilt. Studies on the extracts of faba beans and wheat found that the extracts of wheat improved the condition of faba bean seedlings, enhanced the physiological resistance of faba beans, eased the autotoxicity of faba beans, and suppressed pathogenic fungal growth. This experiment supplemented the mechanism of the faba bean–wheat intercropping system to control faba bean fusarium wilt. We strove to more comprehensively demonstrate the mechanism of faba bean–wheat intercropping to control faba bean wilt. Although this is only preliminary research, it provides encouraging results and a basis for future research.
Materials and Methods
Test Materials
The faba bean varieties (Vicia faba L.) used in this study, 89–147, and wheat (Triticum aestivum L.) Yunmai 53, were purchased from the Yunnan Academy of Agricultural Sciences (Kunming, China).
FOF was isolated from continuously cropped faba beans fields by the Plant-Microbe Laboratory at Yunnan Agricultural University, China. The fungus was transferred to potato dextrose agar (PDA) media, incubated at 28 °C for 7 days, and then stored at 4 °C.
Field Trials
The field test was conducted in the experimental field of Changtian, Chuxiong, Yunnan Province, China, from October 2011 to May 2012. The field had been planted with faba beans for three consecutive years. There was moderate rainfall during the planting. The field lies in the humid subtropical zone and has a paddy soil type with topsoil (0–20 cm) that contained organic matter 14.5 g·kg–1, total nitrogen 1.21 g·kg–1, alkali nitrogen 59.8 mg·kg–1, available phosphorus 29.9 mg·kg–1, available potassium 52.1 mg·kg–1, and had a pH of 6.5. We applied N, P, and K fertilizers to the soil before sowing. The nitrogen fertilizer application rate for faba bean was 90 kg·hm–2, the phosphorus fertilizer application rate was 90 kg·hm–2 (calculated as P2O5), and the potassium fertilizer application rate was 90 kg·hm–2 (calculated as K2O).
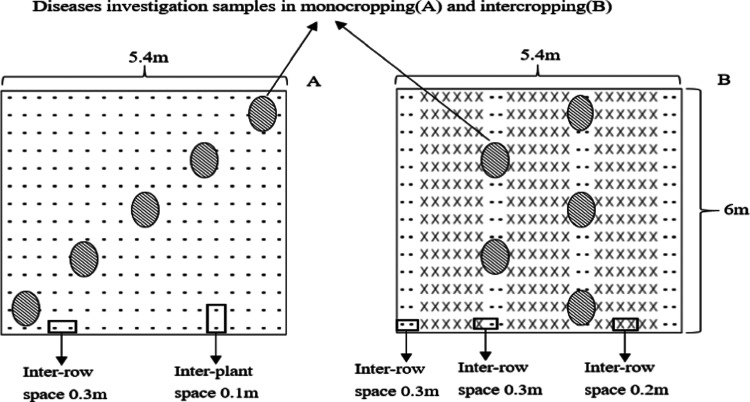
The faba beans were monocropped (MF) or intercropped with wheat (IF) in plots that measured 5.4 m × 6 m with a total area of 32.4 m2. As shown in Figure 8, the MF faba bean plants were sown at 0.1 m intervals, and the rows were spaced 0.3 m apart. Six rows of wheat and two rows of faba beans were planted alternately in the IF plot for a total of three and four strips, respectively. The faba bean rows and intercropping faba bean and wheat rows were each spaced 0.3 m, whereas the wheat rows were spaced 0.2 m. The faba bean plants from the outermost rows of the 1st and 4th strips were not sampled. In addition, a 1 m wide faba bean strip was planted around the entire test field as a protection line. Each treatment was repeated three times in six random blocks. No pesticides, fungicides, or herbicides were applied throughout the growth period. Other management was conducted according to the local agronomic customs.
Figure 8.
Diagram of the planting patterns in the field experiments: (A) monocropping faba bean plot and (B) intercropping plot of faba bean with wheat; -, faba bean and ×, wheat; shaded ovals represent sampling locations.
Measurement of the Incidence of Fusarium Wilt
We also evaluated the faba beans in field 60 days after sowing. In the MF plot, five diagonal points were randomly selected, and three plants from each point were analyzed (15 plants for each plot). In the IF plot, five points were selected on the two faba bean belts (two points in the first belt and three points in the second belt), and three plants were surveyed at each point (15 plants per plot) (Figure 1). The severity of disease was scored at different stages as follows: 0: no symptoms of infection, 1: slight plaques or discoloration at the base of the stem or peripheral roots, 2: uneven lesions at the base of the root or the stem, 3: uniform lesions, discoloration, or wilting in 1/3 to 1/2 of the stem base or root and a reduction in lateral roots, 4: completely discolored or withered roots or stem base, and 5: complete wilting of the plant and death. The disease incidence refers to the proportion of diseased plants in all plants, and the disease index refers to the severity of plant diseases. The disease index and wilt incidence were calculated as
Preparation of Aqueous Extracts
The extraction method is usually used to obtain allelochemicals that enter the environment through leaching, and this extraction method has been used in many studies.40,41 At maturity, all of the faba bean and wheat plants were collected from the experimental field, and the dust that adhered to the plant and root systems was rinsed with tap water and then deionized water. The plants were divided into two parts: roots and a combination of stems and leaves, which were desiccated in an oven at 105 °C for 30 min, dried at 65 °C to a constant weight, and cut into 1 cm long small pieces. A total of 20 g of dry samples of roots, stems, and leaves were weighed, and 200 mL of deionized water was added to each sample.40 The samples were shaken at a frequency of 100 times per minute at 40°C for 2 h. After that, the dry samples were soaked in distilled water for 48 h at 24°C in the light, and the extracts were filtered through three layers of gauze and centrifuged at 4000 rpm for 4 h.41 The supernatant was considered to be 0.1 g·mL–1 plant water infusion mother liquor and stored at −20°C for use.
Greenhouse Cultivation
Faba bean seeds were soaked for 24 h at room temperature, germinated at 25 °C, and sown in sterile quartz sand that had been soaked in deionized water. Once the faba bean seedlings had grown four to six leaves, six faba bean seedlings were transplanted into 2 L of the Hoagland nutrient solution that contained various concentrations of aqueous extracts. The nutrient solution formulation used was (mmol·L–1): K2SO4 0.75, MgSO4 0.65, KCl 0.1, KH2PO4 0.25, H3BO4 0.001, MnSO4 0.001, CuSO4 0.0001, ZnSO4 0.001, (NH4)6Mo7O24 0.000005, and Fe-EDTA 0.2. The treatments included 0 (control), 0.01, 0.05, and 0.1 g·mL–1 aqueous extracts. The controls were treated with deionized water. There were three biological replicates for these treatments that resulted in 72 plants (three replicate pots × two types of extract × three seedlings × four concentrations). The experiments were conducted under 24 h pump ventilation.
Measurements of Seedling Growth
The number of leaves per plant, maximum leaf length, height, main root length, shoot dry weight, and root dry weight were measured 30 days after transplantation.
Evaluation of Oxidative Stress Levels
POD activity was measured as previously described.42,43 Briefly, 1 g of root samples was ground, and the homogenate was mixed with 5 mL of phosphate buffer. After centrifugation at 3000 rpm for 10 min, the supernatant was aspirated. A volume of 0.1 mL of the enzyme was mixed with 1 mL of 2% H2O2, 2.9 mL of 0.05 M phosphate buffer, and 1 mL of 0.05 M guaiacol in a 25 mL volumetric flask and incubated in 34 °C water for 3 min. The absorbance at 470 nm was measured every 30 s for 5 min.
The activity of CAT was also measured as previously described.44,45 The root homogenate obtained as above was centrifuged for 15 min at 4000 rpm, and 2.5 mL of the supernatant and 0.1 M H2O2 were mixed and incubated for 10 min in a 30 °C water bath. After the addition of 2.5 mL of 10% H2SO4, the solution was titrated with 0.1 M KMnO4 until the solution turned pink. One unit of CAT is expressed as the number of milligrams of H2O2 decomposed in 1 min·g–1 of the fresh weight sample (mg·g–1·min–1).
To measure the content of malondialdehyde (MDA), the end product of membrane lipid peroxidation,46 0.5 g of the plant sample was homogenized in 5 mL of 5% trichloroacetic acid and centrifuged at 3000 rpm for 10 min. The supernatant was aspirated, and 2 mL was boiled with the same volume of 0.67% thiobarbituric acid for 30 min, cooled, and centrifuged. The absorbance was measured at 450, 532, and 600 nm.
Evaluation of FOF Growth and Conidial Germination
The pathogens used in the experiment were isolated from the field and cultured on PDA media. Mycelial discs that were 9 mm in diameter were placed on PDA and cultivated at 28 °C for 7 days. The colony diameter was measured radially in three directions on days 3 and 7. A 9 mm agar plug was cut from the 7-day-old culture, inoculated into 15 mL PD media containing 0, 0.01, 0.05, or 0.1 g·mL–1 faba bean or wheat aqueous extracts, and incubated for 7 days at 28 °C with constant shaking at 170 rpm. The culture broth was filtered, dried at 80 °C for 12 h, and weighed to determine the fungal biomass. The germination of spores was determined by washing the 7-day-old mycelia on PDA with sterile water and collecting the spores by filtration through four layers of gauze. The spore suspension obtained after washing the PDA was diluted to ≤1 × 103 CFU·mL–1, and 0.1 mL of spores was plated on each 2% (w/v) water agar plate containing 0, 0.01, 0.05, or 0.1 g·mL–1 faba bean or wheat aqueous extracts, and each extract treatment was repeated three times. The plates were incubated at 28°C for 3 days, and the number of colonies was counted.
Statistical Analysis
All of the data were analyzed using Origin 2018 (OriginLab, Northampton, MA) and SPSS v. 20.0 software (IBM, Inc., Armonk, NY). Significant differences between treatments were evaluated using a two-factor ANOVA, followed by a Tukey’s test at the 5% probability level.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31860596 and 31560586).
Glossary
Abbreviations Used
- FOF
Fusarium oxysporum f. sp. fabae
- MF
faba bean monocropped
- IF
faba bean intercropped with wheat
- POD
peroxidase
- CAT
catalase
- SOD
superoxide dismutase
- MDA
malondialdehyde
Author Contributions
† Y.G. and J.V. contributed equally.
The authors declare no competing financial interest.
References
- Zeng J.; Liu J.; Lu C.; Ou X.; Yan H.; et al. Intercropping with turmeric or ginger reduce the continuous cropping obstacles that affect pogostemon cablin (patchouli). Front. Microbiol. 2020, 11, 579719 10.3389/fmicb.2020.579719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopi R.; Singh S.; Raj C. Status of Fusarium diseases of crop plants in North East India. Indian Phytopathol 2019, 72, 637–646. 10.1007/s42360-019-00148-3. [DOI] [Google Scholar]
- Li X. G.; Wang X. X.; Dai C. C.; Zhang T. L.; Xie X. G.; Ding C. F.; Wang H. W. Effects of intercropping with atractylodes lancea and application of bio-organic fertiliser on soil invertebrates, disease control and peanut productivity in continuous peanut cropping field in subtropical china. Agroforestry Syst. 2014, 88, 41–52. 10.1007/s10457-013-9653-6. [DOI] [Google Scholar]
- Li X.; Zhang Y.; Ding C.; Xu W.; Wang X. Temporal patterns of cotton fusarium and verticillium wilt in jiangsu coastal areas of China. Sci. Rep. 2017, 7, 12581 10.1038/s41598-017-12985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. G.; Zhang T. L.; Wang X. X.; Hua K.; Zhao L.; Han Z. M. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int. J. Biol. Sci. 2013, 9, 164–173. 10.7150/ijbs.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaduzzaman M.; Asao T. Autotoxicity in beans and their allelochemicals. Sci. Hortic. 2012, 134, 26–31. 10.1016/j.scienta.2011.11.035. [DOI] [Google Scholar]
- Hao Z. P.; Wang Q.; Christie P.; Li X. Allelopathic potential of watermelon tissues and root exudates. Sci. Hortic. 2007, 112, 315–320. 10.1016/j.scienta.2006.12.030. [DOI] [Google Scholar]
- Yan Z.; He X.; Guo K.; et al. Allelochemicals from the rhizosphere of Lanzhou lily: Discovery of the autotoxic compounds of a bulb crop. Sci. Hortic. 2019, 250, 121–126. 10.1016/j.scienta.2019.02.038. [DOI] [Google Scholar]
- Yu J. Q.; Ye S. F.; Zhang M. F.; Hu W. H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. 10.1016/S0305-1978(02)00150-3. [DOI] [Google Scholar]
- Ye S. F.; Zhou Y. H.; Sun Y.; Zou L. Y.; Yu J. Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. 10.1016/j.envexpbot.2005.02.010. [DOI] [Google Scholar]
- Wang X. Q.; Du G. D.; Lu X. F.; Ma H. Y.; Lyu D. G.; Zhang H.; Song J. L. Characteristics of mitochondrial membrane functions and antioxidant enzyme activities in strawberry roots under exogenous phenolic acid stress. Sci. Hortic. 2019, 248, 89–97. 10.1016/j.scienta.2018.12.051. [DOI] [Google Scholar]
- Arie T. Microorganisms and plant activators as alternatives to chemical fumigants to control soilborne diseases in Japan. Phytopathology 2011, 101, S233. [Google Scholar]
- De Medeiros E. V.; Notaro K. D. A.; De Barros J. A.; et al. Soils from intercropped fields have a higher capacity to suppress black root rot in cassava, caused by Scytalidium lignicola. J. Phytopathol. 2019, 209–217. 10.1111/jph.12788. [DOI] [Google Scholar]
- Li X.; de Boer W.; Zhang Y. n.; Ding C.; Zhang T.; Wang X. Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol. Biochem. 2018, 116, 120–130. 10.1016/j.soilbio.2017.09.029. [DOI] [Google Scholar]
- Ren L.; Huo H.; Zhang F.; Hao W.; Xiao L.; Dong C.; Xu G. The components of rice and watermelon root exudates and their effects on pathogenic fungus and watermelon defense. Plant Signal. Behav. 2016, 11, e1187357 10.1080/15592324.2016.1187357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H.; Cao H.; Nawaz M. A.; Sohail H.; Huang Y.; Cheng F.; Kong Q.; Bie Z. Wheat Intercropping Enhances the Resistance of Watermelon to Fusarium Wilt. Front. Plant. Sci. 2018, 9, 696 10.3389/fpls.2018.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Wang Y.; Han L. R.; Zhang X.; Feng J. T. Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon. Molecules 2017, 22, 2053 10.3390/molecules22122053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K.; He X.; Yan Z.; Li X.; Ren X.; Pan L.; Qin B. Allelochemicals from the Rhizosphere Soil of Cultivated Astragalus hoantchy. J. Agric. Food Chem. 2016, 64, 3345–52. 10.1021/acs.jafc.5b06093. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Wu J.; Xi Y.; Zhang L.; Wang-Pruski G. Effects of Autotoxicity on Seed Germination, Gas Exchange Attributes and Chlorophyll Fluorescence in Melon Seedlings. J. Plant Growth Regul. 2021, 1–11. 10.1007/s00344-021-10355-w.33649694 [DOI] [Google Scholar]
- Li X.; Zhang Y.; Ding C.; Xu W.; Wang X. Temporal patterns of cotton Fusarium and Verticillium wilt in Jiangsu coastal areas of China. Sci. Rep. 2017, 7, 12581 10.1038/s41598-017-12985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mohamadawy A. J.; Al-Haidery A. A.; Aljawasim B. D. Efficiency of bio-evaporation of Arugula (Eruca sativa) leaves to control root rot disease on cucumber caused by Pythium intermedium in Greenhouse. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 928, 062016 10.1088/1757-899X/928/6/062016. [DOI] [Google Scholar]
- Hao Z. P.; Wang Q.; Christie P.; Li X. L. Allelopathic potential of watermelon tissues and root exudates. Sci. Hortic. 2007, 112, 315–320. 10.1016/j.scienta.2006.12.030. [DOI] [Google Scholar]
- Tesio F.; Vidotto F.; Ferrero A. Allelopathic persistence of Helianthus tuberosus L. residues in the soil. Sci. Hortic. 2012, 135, 98–105. 10.1016/j.scienta.2011.12.008. [DOI] [Google Scholar]
- El-Darier S. M.; El-Dien M. H. Z. Biological activity of Medicago sativa L. (alfalfa) residues on germination efficiency, growth and nutrient uptake of Lycopersicon esculentum L. (tomato) seedlings. J. Taibah Univ. Sci. 2011, 5, 7–13. 10.1016/S1658-3655(12)60033-8. [DOI] [Google Scholar]
- Dong Y.; Dong K.; Tang L.; Zheng Y.; Yang Z.; Xiao J.; Zhao P.; Hu G. The effect of wheat and broad bean intercropping on the functional diversity of broad bean rhizosphere microbial community and its relationship with the occurrence of broad bean wilt. Acta Ecol. Sin. 2013, 33, 7445–7454. 10.5846/stxb201208281214. [DOI] [Google Scholar]
- Alghamdi S. S.; Migdadi H. M.; Ammar M. H.; Paull J. G.; Siddique K. H. M. Faba bean genomics: current status and future prospects. Euphytica 2012, 186, 609–624. 10.1007/s10681-012-0658-4. [DOI] [Google Scholar]
- Stoddard F. L.; Nicholas A. H.; Rubiales D.; Thomas J.; Villegas-Fernández A. M. Integrated pest management in faba bean. Field Crop. Res. 2010, 115, 308–318. 10.1016/j.fcr.2009.07.002. [DOI] [Google Scholar]
- Guo Y.; Lv J.; Zhao Q.; et al. Cinnamic Acid Increased the Incidence of Fusarium Wilt by Increasing the Pathogenicity of Fusarium oxysporum and Reducing the Physiological and Biochemical Resistance of Faba Bean, Which Was Alleviated by Intercropping With Wheat[J]. Front. Plant Sci. 2020, 11, 1928 10.3389/fpls.2020.608389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Bie Z.-L.; Huang Y. Identification of autotoxins in rhizosphere soils under the continuous cropping of cowpea. Allelopathy J. 2010, 25, 383–392. [Google Scholar]
- Sunaina; Singh N. B. Alleviation of allelopathic stress of benzoic acid by indole acetic acid in Solanum lycopersicum. Sci. Hortic. 2015, 192, 211–217. 10.1016/j.scienta.2015.06.013. [DOI] [Google Scholar]
- Rashid M.; Hampton J. G.; Shaw M. L.; et al. Oxidative damage in forage rape (Brassica napus L.) seeds following heat stress during seed development. J. Agron. Crop Sci. 2020, 206, 101–117. 10.1111/jac.12372. [DOI] [Google Scholar]
- Xue C.; Wenjun M. U.; Jiaqin X. I.. et al. Effect of Different Crops Intercropping with Flue-cured Tobacco on Tobacco Black Shank Disease. Chin. Tob. Sci., 2015. [Google Scholar]
- Lee S.-H.; Shin H.; et al. Effect on Colony Growth Inhibition of Soil-Borne Fungal Pathogens by Available Chlorine Content in Sodium Hypochlorite. Plant Pathol.J. 2019, 156–163. 10.5423/ppj.oa.07.2018.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-g.; Ding C.-f.; Hua K.; Zhang T.-l.; Zhang Y.-n.; Zhao L.; Yang Y.-r.; Liu J.-g.; Wang X.-x. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. 10.1016/j.soilbio.2014.07.019. [DOI] [Google Scholar]
- Gao X.; Wu M.; Xu R.; Wang X.; Pan R.; Kim H. J.; Liao H. Root interactions in a maize/soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS One 2014, 9, e95031 10.1371/journal.pone.0095031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.; Basra S. M. A.; Afzal I.; Nawaz M.; Rehman H. U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 2017, 24, 27601–27612. 10.1007/s11356-017-0336-0. [DOI] [PubMed] [Google Scholar]
- Chen Y.-q.; Sui P.; Luan C.; Shi X.-p. Xanthium Suppression Under Maize||Sunflower Intercropping System. J. Integr. Agr. 2012, 11, 1026–1037. 10.1016/S2095-3119(12)60095-1. [DOI] [Google Scholar]
- Qin J.; He H.; Luo S.; Li H. Effects of rice-water chestnut intercropping on rice sheath blight and rice blast diseases. Crop. Prot. 2013, 43, 89–93. 10.1016/j.cropro.2012.09.009. [DOI] [Google Scholar]
- Hao W.-y.; Ren L.; Ran W.; Shen Q.-r. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 2010, 336, 485–497. 10.1007/s11104-010-0505-0. [DOI] [Google Scholar]
- Han C. M.; Pan K. W.; Wu N.; et al. Allelopathic effect of ginger on seed germination and seedling growth of soybean and chive. Sci. Hortic. 2008, 116, 330–336. 10.1016/j.scienta.2008.01.005. [DOI] [Google Scholar]
- Turk M. A.; Tawaha A. M. Allelopathic effect of black mustard (Brassica nigra L.) on germination and growth of wild oat (Avena fatua L.). Crop. Prot. 2003, 22, 673–677. 10.1016/S0261-2194(02)00241-7. [DOI] [Google Scholar]
- Muñoz-Muñoz J. L.; García-Molina F.; García-Ruiz P. A.; Arribas E.; Tudela J.; García-Cánovas F.; Rodríguez-López J. N. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. 10.1016/j.foodchem.2008.07.076. [DOI] [Google Scholar]
- Quintanilla-Guerrero F.; Duarte-Vázquez M. A.; García-Almendarez B. E.; Tinoco R.; Vazquez-Duhalt R.; Regalado C. Polyethylene glycol improves phenol removal by immobilized turnip peroxidase. Bioresour. Technol. 2008, 99, 8605–8611. 10.1016/j.biortech.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Kar M.; Mishra D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–9. 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Limones C.; Hervás A.; Navas-Cortés J. A.; Jiménez-Díaz R. M.; Tena M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp.ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. 10.1006/pmpp.2003.0445. [DOI] [Google Scholar]
- Bird R. P.; Hung S. S. O.; Hadley M.; Draper H. H. Determination of malonaldehyde in biological materials by high-pressure liquid chromatography. Anal. Biochem. 1983, 128, 240–244. 10.1016/0003-2697(83)90371-8. [DOI] [PubMed] [Google Scholar]
- Li S. Ecological biochemistry (2): Biochemical relationships among higher plants. J. Ecol. 1989, 008, 66–70. [Google Scholar]