Abstract
BACKGROUND
Robotic-assisted stereotaxy has been increasingly adopted for lead implantation in stereoelectroencephalography based on its efficiency, accuracy, and precision. Despite initially being developed for use in deep brain stimulation (DBS) surgery, adoption for this indication has not been widespread.
OBJECTIVE
To describe a recent robotic-assisted stereotaxy experience and workflow for DBS lead implantation in awake patients with and without microelectrode recording (MER), including considerations for intraoperative research using electrocorticography (ECoG).
METHODS
A retrospective review of 20 consecutive patients who underwent simultaneous bilateral DBS lead implantation using robotic-assisted stereotaxy was performed. Radial error was determined by comparing the preoperative target with the DBS lead position in the targeting plane on postoperative computed tomography. Information regarding any postoperative complications was obtained by chart review.
RESULTS
A novel method for robot coregistration was developed. We describe a standard workflow that allows for MER and/or ECoG research, and a streamlined workflow for cases in which MER is not required. The overall radial error for lead placement across all 20 patients was 1.14 ± 0.11 mm. A significant difference (P = .006) existed between the radial error of the first 10 patients (1.46 ± 0.19 mm) as compared with the second 10 patients (0.86 ± 0.09 mm). No complications were encountered.
CONCLUSION
Robotic-assisted stereotaxy has the potential to increase precision and reduce human error, compared to traditional frame-based DBS surgery, without negatively impacting patient safety or the ability to perform awake neurophysiology research.
Keywords: Robotic-assisted stereotaxy, Deep brain stimulation
ABBREVIATIONS
- AC-PC
anterior commissure-posterior commissure
- CT
computed tomography
- DBS
deep brain stimulation
- ECoG
electrocorticography
- GPi
globus pallidus interna
- MER
microelectrode recording
- MRI
magnetic resonance imaging
- RMS
root mean square
- SEM
standard error of the mean
- STN
subthalamic nucleus
- Vim
ventrointermediate nucleus of thalamus
Surgical robots aim to ensure precision and increase the accuracy of a given procedure. Robots have predetermined, reproducible, and exact paths that limit excursions, error, and the potential for injury to nearby structures if utilized properly.1 Neurosurgeons recognized the utility of robotic-assistance over 30 yr ago with the PUMA (Advance Research and Robotics) and Minerva computed tomography (CT)-guided biopsy (University of Lausanne) systems, introduced in 1985.2-4 These systems were ultimately abandoned because of high rates of malfunction and initial safety concerns related to lack of operational safeguards and clinical experience. Additional robotic systems followed suit, with the NeuroMate (Integrated Surgical Systems) in 1987,5 an magnetic resonance imaging (MRI)-compatible system in 1995,6 and the CyberKnife System (Accuray Incorporated) in 1998.7
Modern robotic systems for cranial surgery have been increasingly adopted in the United States following demonstration of their utility in Europe.8-13 Benabid5 first described a computer-driven system for stereotaxy connected to CT and MRI in 1987. The neurosurgical center in Grenoble, France, has utilized a stereotactic robot since 1989 and a microscope robot since 1995 for various surgical procedures.14
As experience mounted, this team expanded the indications for robotic-assisted stereotaxy to include deep brain stimulation (DBS), stereoelectroencephalography, and tumor biopsies or resections.8,15 The ROSA Brain system (Medtech, Zimmer Biomet) was initially released in 2007 and gained FDA approval in 2012 for cranial surgery. Despite increasing adoption of the ROSA system for stereoelectroencephalography implantation,16,17 there is only one case report of its use for DBS in the United States.18 In addition, there is minimal information in the international literature regarding the ROSA system workflow for DBS cases involving awake patients in which microelectrode recording (MER) is undertaken.
We recently converted our DBS practice to the exclusive use of robotic stereotactic assistance with the ROSA Brain system in an effort to optimize accuracy for lead placement and to standardize stereotactic workflows between epilepsy and movement disorders surgery. Here, we describe our current strategies for bilateral DBS lead implantation in awake patients using ROSA in cases with and without MER. In addition, we describe the incorporation of techniques for temporary placement of subdural electrodes for research purposes, including considerations for clinical and research behavioral testing.
DESCRIPTION OF WORKFLOW
The ROSA Brain system, Neuro Omega recording system (Alpha Omega; Medtech, Zimmer Biomet), and all clinical components utilized in this work have been approved by the FDA for intracranial surgery and DBS. Additionally, the University of Pittsburgh Institutional Review Board approved all electrocorticography (ECoG) and intraoperative research protocols prior to their implementation. Informed consent was obtained from patients for participation in intraoperative research protocols.
Frame Placement, Imaging, and Target Planning
Standard patient selection criteria and imaging protocols are used.19,20 A 3-Tesla MRI is obtained on an outpatient basis. We place a Leksell frame (Elekta) for 2 reasons: 4-point fixation is more secure than 3-point fixation, especially for awake patients, and the frame pins are used for registration of the ROSA system. For the latter reason, titanium pins are always used to reduce scatter artifact on CT imaging. A thin-sliced, volumetric CT scan is then obtained. We obtain a contrast CT angiogram if an MRI with contrast was not obtained preoperatively, eg, if contraindicated because of a cardiac pacemaker. The CT imaging includes the entire Leksell frame, including the base and its screws to provide additional registration points if necessary. The volumetric CT scan is then merged to the preoperative MRI on the ROSA software.21
Target planning can be accomplished on the robot directly or transferred preoperatively from the ROSA laptop using the same software. This system currently does not support internet-based software for remote planning.21 Newer users may avoid unnecessary complications by noting the following: first, the anterior commissure-posterior commissure (AC-PC) reference point can be adjusted with a slider on the software, and it is important to verify that the desired reference point (eg, AC-PC midpoint) is selected each time that one leaves that particular window in the software; second, there is a single-trajectory view, but one can rotate the 3-dimensional brain around the axis of the trajectory in order to view the trajectory through different oblique planes; and third, the trajectory length must be set manually and account for typical adjustments made when one uses a stereotactic frame and microdrive. It is recommended to orient the planes in one's typical targeting planes when checking trajectories, but overall, the rotational feature can be quite helpful for determining where to move the trajectory to avoid vessels, sulci, etc. We use the microdrive setup in all of our DBS cases, regardless of whether MER is performed, in order to maintain consistent workflow across all cases. When MER is not performed, the microdrive serves solely as a reliable lead holder.
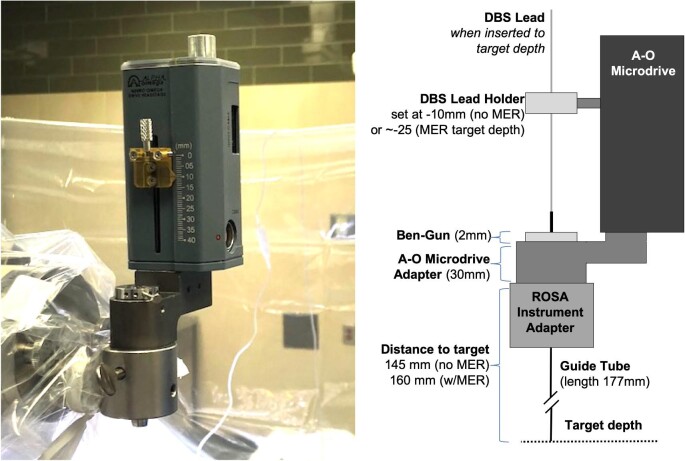
When calculating the distance to target for DBS planning on the ROSA software, we find it most useful to retain the standard Leksell distance to arc center (190 mm) as a starting point and adjust the depth according to the same distances accounted for when using the Leksell frame and Neuro Omega microdrive and guide tubes, as shown in Figure 1. For example, when using the Leksell arc in MER cases, we drop the guide slide 30 mm toward the patient in order for the microelectrode to exit a guide tube of 175-mm working length 15 mm above target. Thus, using the ROSA system, the distance to target is set at 160 mm. The starting depth on the microdrive remains that which we used for Leksell cases: –10 mm (–25 is target depth), again to exit the guide tube 15 mm above target. For cases without MER, in which we intend to insert the guide tube all the way to target, the additional 15 mm, over which the guide tube would normally be inserted, is accounted for by reducing the ROSA target distance to 145 mm. The microdrive starting distance is not adjusted from the –10 mm starting point used for MER in order for the microdrive setting to never vary between case types. Alternately, one might prefer that the ROSA target distance never varies from 160 mm, in which case the microdrive starting depth would be set to –25 for lead placement in non-MER cases, in which the microdrive serves solely as the lead holder. Obviously, the distances to target on the ROSA software must be determined in accordance with whichever microdrive system would otherwise be used at a particular institution.
FIGURE 1.
Microdrive setup and ROSA measurements. The ROSA is positioned at a distance from target based upon measurements of the ROSA adapter, Neuro Omega microdrive, and guide tube length.
Patient Positioning, Registration, and Scalp Marking
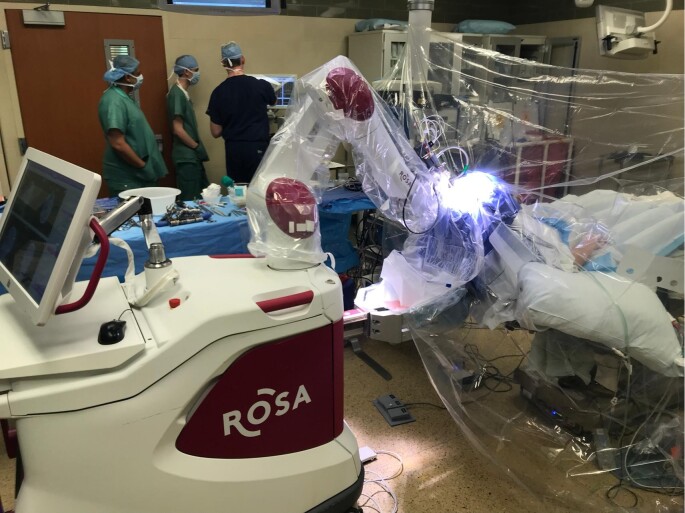
The patient is placed in a reclined beach-chair position with the neck slightly extended to facilitate airway maintenance during sedation periods. The height of the head is limited by the height of the ROSA system and the need to affix the Leksell frame into the ROSA Leksell adapter. For further safety, the ROSA system's wheels are locked, and the operative bed movement controls are disconnected to prevent accidental manipulation of either the robot or bed position while the patient's head is secured to the ROSA system. Figure 2 shows a typical setup, where the ROSA system is attached in line with the patient.
FIGURE 2.
Typical operating setup. The ROSA is positioned in line with the patient, whose head is elevated to the maximum height possible for attachment of the Leksell frame to the ROSA. A clear operative drape is used to facilitate monitoring of the patient during the “awake” portions of the case.
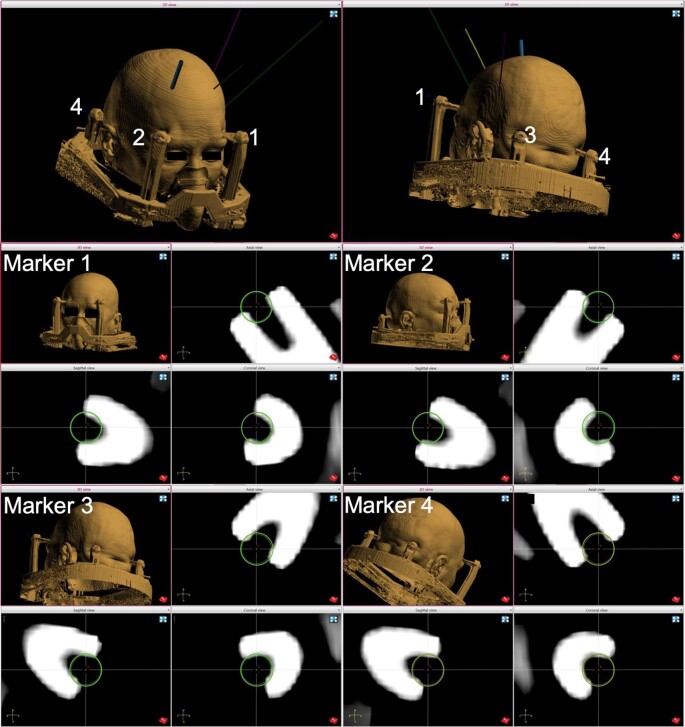
Two standard registration methods are available for the ROSA system: laser registration using facial features and registrations using skull fiducial screws.21 We adopted a third method in which titanium pins of the Leksell frame serve as bone fiducials.22 Registration points are chosen in the ROSA software, using the CT obtained with the frame placed at the center of the opening in each pin head, which can be visualized in 3 planes (Figure 3). The registration tool is then placed at the center of each pin opening (Figure 4), and this location is recorded by the robotic system. The order of pin site fiducial registration should match the numbering order for the software marker/fiducial sites. We currently proceed with the surgical case when registration root mean square (RMS) does not exceed 0.50 mm; however, RMS was tolerated up to 0.70 mm in our initial series of patients. Of note, the ROSA software tolerates up to 2.00 mm RMS error before exhibiting and error warning, when the font color changes from green to red.21 The presence of screws along the Leksell frame base provides the opportunity to add supplementary registration sites if needed to improve registration accuracy.
FIGURE 3.
Setting fiducial points on the frame CT. For each of 4 registration points, the center of the titanium pin head is identified on the CT scan and these locations are set as “marker” reference points (green circles) in the ROSA software.
FIGURE 4.
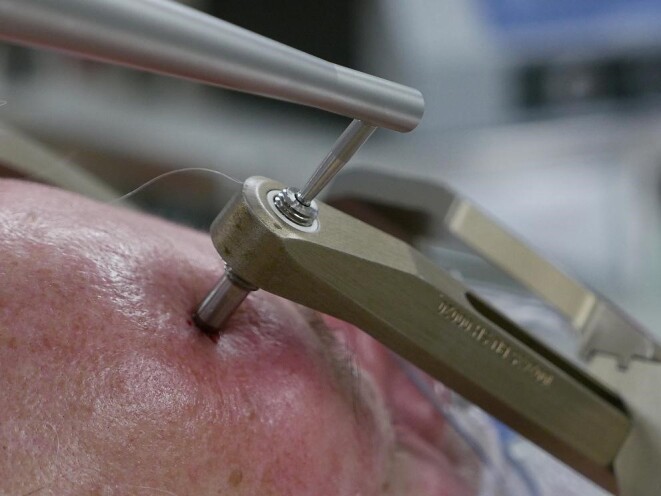
Registration of a frame pin. The ROSA registration tool is shown positioned in the center of the titanium pin opening at the site of the registration marker. The circle corresponding to the registration marker tool has been enhanced in this figure for readability.
Once the ROSA system is registered to the patient, the entry point(s) are marked on the scalp with a skin marker. The incisions are then delineated. Whereas the traditional stereotactic frame requires prepping, draping, and applying the arc prior to marking the entry points, the ROSA allows entry point determination prior to prepping and results in the need to shave less hair. In addition, if research ECoG strip electrodes are to be placed, aiming points can be planned in the software and subsequently marked on the scalp at this stage.23,24
Operative Workflow Prior to Patient Awakening
After opening both incisions, the ROSA system is brought to the entry point, and a guide tube is placed through the center of the Ben-Gun array, held by the microdrive adaptor, to mark the entry points on the skull. If MER is to be performed, the perforator drill bit is used to produce standard 14-mm burr holes. The ROSA arm is then brought to the target position to verify an unobstructed path for each guide tube position to be used in the Ben-Gun. This procedure is then completed for the second side, after which the patient is allowed to emerge from anesthesia. While the patient is waking up, the base of the lead fixation system is secured at each burr hole, and the lead locks are tested.
If MER is not performed, an electronic hand drill and ROSA-mounted drill guide are used to produce 2.4-mm burr holes in a similar fashion to the standard technique used for placing anchor bolts for stereoelectroencephalography leads. The ROSA software is used to estimate bone thickness with adjustment of the stop on the drill bit accordingly. We then affix a titanium dog bone with a single 4-mm screw, which eventually is used to secure the DBS lead in place. The trajectory is then tested for alignment by verifying a lack of bone collision when passing a guide tube to the dural surface. In cases in which the small burr hole may not be perfectly aligned, as may occur if the drill skives, the ROSA can be programmed to make micromovements pivoted around the target. If this type of correction is made, the surgeon should verify that the target and not entry point was selected as the pivot point, as both options are available on the robot. The guide tube subsequently can be tested for insertion through the burr hole without bone collision following each micromovement. Alternatively, the burr hole may be widened slightly with a drill bit to accommodate the original trajectory.
Operative Workflow During Awake Surgery
Once the patient is awake and participatory with examination, an adequate baseline has been obtained, and the blood pressure is controlled, the dura is opened. For non-MER cases, a biopsy needle is inserted through the center of the Ben-Gun array to pierce the dura and underlying pia. Once the trajectory is aligned and the dura pierced, the lead is inserted in standard fashion.
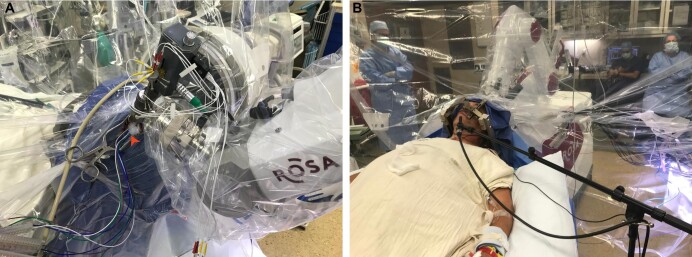
For MER cases, the dura is opened widely, which is especially important when performing research ECoG in order to accompany the insertion of subdural strip electrodes. It is recommended to bring the ROSA arm to the intermediate or home position during this step to ensure adequate visualization through the burr hole. Likewise, once the site of corticectomy is determined by lining up the guide tube in the Ben-Gun, it is recommended to again move the ROSA arm well out of the way in order to open the pia with full visualization within the burr hole. If an ECoG electrode is placed, this is done prior to making the corticectomy. Of note, the arm can be moved at this time into position to mark an aiming location on the scalp for ECoG electrode placement (alternately, these scalp markings can be made while waiting for the patient to wake up from sedation). We have found that there is adequate working room around the ROSA arm for the implantation of 2 strip electrodes and 3 microelectrode guide tubes (Figure 5A) during a speech production research task (Figure 5B).25,26
FIGURE 5.
Patient positioning for MER and ECoG. A, ROSA arm in target position, supporting the microdrive and 3 microelectrodes, with the implantation of 2 strip electrodes evidenced by their wires protruding from the burr hole (red arrowhead) seen below the ROSA arm. B, Attachment to the ROSA does not preclude the patient from participating comfortably in behavioral research tasks during intracranial recording.
The remainder of the microelectrode and lead implantation surgery is carried out in typical fashion. We have found the bed position to be adequate for comfortable patient participation in both clinical testing and intraoperative cognitive neuroscience research (Figure 5B). It is wise to double check the patient's neck positioning prior to ROSA registration, as the position cannot be changed subsequently if the patient experiences discomfort. We have found the ROSA arm to be completely stable in the setting of significant patient tremor and anxiety, as would be expected from attaching the Leksell frame to the approximately 320-kg ROSA machine.21
Once the first lead is secured, the ROSA arm is quickly moved to the second side, without the need to readjust stereotactic frame coordinates, as would be required with a frame-based system. This time-saving maneuver reduces the duration of the awake portion of the case. We do not obtain intraoperative imaging confirmation of lead placement, as intraoperative CT is not available at our institution. Without using separate stereotactic planning software and setting up the arc ring supports, the ability to confirm final 2-dimensional location with fluoroscopy is not an option. Also note that the ROSA arm becomes off-balance after attaching the Alpha Omega microdrive system during the case, such that there is an initial “plunge” or drift of the arm when the pedal is activated once the microdrive is attached. Thus, it is important to ensure that the ROSA arm is not unlocked once the guide tubes have been inserted, although this mistake would be difficult to accomplish as it requires both switching to “drive to trajectory” mode and pressing the foot petal.21
ACCURACY OF LEAD PLACEMENT AND COMPLICATIONS
We completed a retrospective review of 20 consecutive patients who underwent DBS lead placement by the senior author in the ventrointermediate nucleus of thalamus (Vim), subthalamic nucleus (STN), or globus pallidus interna (GPi) using the ROSA platform (Medtech, Zimmer Biomet; Table). Patients who underwent MER were included in analysis of placement accuracy only if the DBS lead was implanted in the central tract. Accuracy was defined as the 2-dimensional radial error obtained when comparing the target location to the location of the implanted lead in the axial targeting plane.27,28 Measurements were made by 2 blinded evaluators with expertise in stereotactic software (A.F. and V.K.), working independently, using a fusion of the postoperative volumetric CT to the original trajectory plan in the ROSA software. Information regarding any postoperative complications was obtained by chart review. Data are presented as mean ± standard error of the mean (SEM).
TABLE.
Subject List
| RMS | Radial | Radial | Intraop | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Microelectrode | error | error, left | error, right | research | |||
| number | Sex | (yr) | Pathology | Target | recording | (mm) | (mm) | (mm) | (ECoG) |
| 1 | M | 65 | PD | Bilateral STN | Noa | 0.44 | 1.85 | 3.04 | No |
| 2 | F | 77 | ET | Bilateral VIM | No | 0.63 | 1.07 | 1.08 | No |
| 3 | M | 60 | ET | Bilateral VIM | No | 0.33 | 1.16 | 0.69 | No |
| 4 | F | 64 | ET | Bilateral VIM | No | 0.24 | 1.17 | 1.75 | No |
| 5 | M | 79 | ET | Bilateral VIM | No | 0.25 | 2.51 | 2.42 | No |
| 6 | M | 59 | ET | Bilateral VIM | No | 0.59 | 2.24 | 0.51 | No |
| 7 | F | 35 | D | Bilateral GPI | Yes | 0.62 | 0.80 | b | No |
| 8 | M | 63 | PD | Bilateral STN | Yes | 0.48 | b | 1.28 | No |
| 9 | M | 67 | ET | Left VIM | No | 0.26 | 2.04 | Not applicable | No |
| 10 | M | 69 | ET | Bilateral VIM | No | 0.82 | 0.53 | 0.62 | Yes |
| 11 | M | 76 | PD | Bilateral STN | Yes | 0.4 | 0.40 | 1.25 | No |
| 12 | M | 61 | PD | Bilateral STN | Yes | 0.22 | 0.18 | 1.27 | Yes |
| 13 | F | 63 | PD | Bilateral STN | Yes | 0.67 | 0.36 | b | Yes |
| 14 | M | 69 | ET | Bilateral VIM | No | 0.28 | 1.31 | 0.69 | No |
| 15 | M | 46 | ET | Bilateral VIM | No | 0.5 | 0.66 | 1.24 | No |
| 16 | M | 73 | ET | Bilateral VIM | No | 0.32 | 0.61 | 1.20 | No |
| 17 | M | 76 | PD | Bilateral STN | Yes | 0.51 | 0.43 | 0.96 | No |
| 18 | M | 69 | PD | Bilateral GPI | Yes | 0.33 | 0.71 | 0.60 | Yes |
| 19 | M | 68 | PD | Bilateral STN | Yes | 0.38 | 1.39 | 0.59 | No |
| 20 | M | 62 | ET | Bilateral VIM | No | 0.43 | 1.22 | 1.39 | Yes |
| Average | 0.44 | 1.09 | 1.21 | ||||||
| SEM | 0.04 | 0.15 | 0.16 |
PD, Parkinson disease; ET, essential tremor; D, dystonia.
aMER was aborted in this case because of microdrive malfunction.
bLead placements occurred in tracts other than the central tract in these cases, which were excluded from error analysis.
A total of 36 DBS lead implantations (14 STN, 1 GPi, and 21 Vim) in 20 patients met inclusion criteria for DBS lead accuracy analysis. Research ECoG strip electrode placement occurred on the first (left) side in 5 patients. The overall error for lead placement across all patients was 1.14 ± 0.11 mm, as shown in Table.
Although the overall radial error in our cohort was acceptable as compared to traditional frame-based DBS surgery,29 we observed an improving trend as our experience evolved. In the first 10 patients, the overall error was 1.46 ± 0.19 mm. In the second half of patients, the average error improved to 0.86 ± 0.09 mm. A significant difference (P = .006) in radial error existed between the first and second 10 patient cohorts; however, no difference (P = .42) was noted in RMS error between the groups, suggesting that RMS registration error variance at the level we observed does not predict subsequent accuracy. Nonetheless, we believe the error difference may represent a learning curve resulting from increasing improvement of the registration technique, including the use of the CT bone window. Additionally, there was no difference between radial error for the left vs right side in either ROSA cohort (P = .59 overall, .88 first cohort, and .11 second cohort). No complications, including hemorrhage, infection, or lead misplacement, were noted.
Finally, the operative case duration for patients not undergoing interoperative research was examined (15 of 20 patients). The total case time without MER (N = 10) was 113 ± 11 min, and the total case time with MER (N = 5) was 218 ± 7 min. The process of Leksell frame (Elekta) placement, transport to CT, CT imaging, and ROSA registration typically takes approximately 30 to 45 min.
DISCUSSION AND CONCLUSIONS
Robotic-assisted DBS surgery increases precision and reduces the potential for human error associated with traditional frame-based surgery, in which several settings must be changed manually and multiple times. In this study, improvement in lead placement error corresponded with accumulated experience using the ROSA system (Medtech, Zimmer Biomet), resulting in values less than or similar to those reported by other investigators.9,12,18,30 We demonstrated good lead placement accuracy, which improved from 1.46 ± 0.19 mm in the first 10 patients to 0.86 ± 0.09 mm in the second 10 patients, consistent with a small, but significant, learning curve.
Neudorfer et al9 have previously described improved accuracy with the ROSA system in an even larger cohort of patients (N = 80), reporting an average error of 0.76 ± 0.04 mm with ROSA vs 1.11 ± 0.07 mm with the modified Riechert-Mundinger stereotactic apparatus. We note that Ho et al30 have reported their experience with a different robotic system in a series of patients undergoing awake or general anesthesia for robotic-assisted DBS using the Mazor Renaissance (Mazor Robotics) system. They noted a mean radial error of 1.40 ± 0.11 mm with a significant reduction in operative time compared to their standard frame-based approach, especially as experience with the methodology increased.
Some investigators advocate that increased accuracy with robotic assistance may obviate the need for intraoperative MER or awake clinical examination. Lefranc et al15 evaluated clinical efficacy in patients undergoing awake vs general anesthesia for robotic-assisted DBS with the ROSA system, using the mean voltage threshold for side effects in the active contact at 12 mo following surgery as the primary endpoint, and found no significant difference. Similarly, a case report from Vadera et al18 described bilateral subthalamic DBS under general anesthesia with MER, reporting left lead accuracy of 1.14 mm and right lead accuracy of 1.68 mm; however, the investigators used a frameless ROSA registration method. A series of pediatric patients underwent pallidal DBS under general anesthesia using the Neuromate (Renishaw) robotic system and noted accuracy of 1.24 ± 0.29 mm from the target; however, surgical time was noted to be approximately 8 h.13 In patients required to be under general anesthesia, we still feel that the optimal method for implanting the correct region of each target nucleus is real-time guidance with intraoperative MRI.27,31 DBS lead implantation with ROSA, however, is a valid alternative given its potential for submillimetric accuracy.
In summary, we have converted our DBS practice to the exclusive use of robotic stereotactic assistance with the ROSA system and developed an operative workflow applicable to awake surgery with MER and intraoperative research in the awake patient. We found awake DBS lead placement with the ROSA system to be safe, accurate, and efficient.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
The data from this manuscript was previously presented, in part, as an oral presentation at the Congress of Neurological Surgeons Annual Meeting on October 21, 2019, in San Francisco, California.
Contributor Information
Amir H Faraji, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Vasileios Kokkinos, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts.
James C Sweat, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Donald J Crammond, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
R Mark Richardson, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts.
Operative Neurosurgery Speaks! Audio abstracts available for this article at www.operativeneurosurgery-online.com.
COMMENT
The authors described the application of robotic assisted device for deep brain stimulation surgery. Although not completely new to the literature, the method is still considered novel and, at some degree, intriguing due to the lack of clear benefits in comparison to more conventional stereotactic methods. Nevertheless, it is clear that robotic devices have the potential to add additional accuracy and safety for deep brain stimulation surgery, as illustrated in this well documented manuscript. I commend the authors for their interesting and highly applicable work.
Jorge González-Martínez
Cleveland, Ohio
Operative Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Davies B. A review of robotics in surgery. Proc Inst Mech Eng H. 2000;214(1):129-140. [DOI] [PubMed] [Google Scholar]
- 2. Drake JM, Joy M, Goldenberg A, Kreindler D. Computer- and robot-assisted resection of thalamic astrocytomas in children. Neurosurgery. 1991;29(1):27-33. [DOI] [PubMed] [Google Scholar]
- 3. Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35(2):153-160. [DOI] [PubMed] [Google Scholar]
- 4. Glauser D, Fankhauser H, Epitaux M, Hefti JL, Jaccottet A. Neurosurgical robot Minerva: first results and current developments. J Image Guid Surg. 1995;1(5):266-272. [DOI] [PubMed] [Google Scholar]
- 5. Benabid AL, Cinquin P, Lavalle S, Le Bas JF, Demongeot J, de Rougemont J. Computer-driven robot for stereotactic surgery connected to CT scan and magnetic resonance imaging. Technological design and preliminary results. Appl Neurophysiol. 1987;50(1-6):153-154. [DOI] [PubMed] [Google Scholar]
- 6. Masamune K, Kobayashi E, Masutani Yet al. Development of an MRI-compatible needle insertion manipulator for stereotactic neurosurgery. J Image Guid Surg. 1995;1(4):242-248. [DOI] [PubMed] [Google Scholar]
- 7. Adler JR Jr, Murphy MJ, Chang SD, Hancock SL. Image-guided robotic radiosurgery. Neurosurgery. 1999;44(6):1299-1306; discussion 1306-1297. [PubMed] [Google Scholar]
- 8. Lefranc M, Le Gars D. Robotic implantation of deep brain stimulation leads, assisted by intra-operative, flat-panel CT. Acta Neurochir. 2012;154(11):2069-2074. [DOI] [PubMed] [Google Scholar]
- 9. Neudorfer C, Hunsche S, Hellmich M, El Majdoub F, Maarouf M. Comparative study of robot-assisted versus conventional frame-based deep brain stimulation stereotactic neurosurgery. Stereotact Funct Neurosurg. 2018;96(5):327-334. [DOI] [PubMed] [Google Scholar]
- 10. Eljamel MS. Robotic neurological surgery applications: accuracy and consistency or pure fantasy? Stereotact Funct Neurosurg. 2009;87(2):88-93. [DOI] [PubMed] [Google Scholar]
- 11. Eljamel MS. Validation of the PathFinder neurosurgical robot using a phantom. Int J Med Robotics Comput Assist Surg. 2007;3(4):372-377. [DOI] [PubMed] [Google Scholar]
- 12. Lefranc M, Capel C, Pruvot ASet al. The impact of the reference imaging modality, registration method and intraoperative flat-panel computed tomography on the accuracy of the ROSA(R) stereotactic robot. Stereotact Funct Neurosurg. 2014;92(4):242-250. [DOI] [PubMed] [Google Scholar]
- 13. Candela S, Vanegas MI, Darling Aet al. Frameless robot-assisted pallidal deep brain stimulation surgery in pediatric patients with movement disorders: precision and short-term clinical results. J Neurosurg Pediatr. 2018;22(4):416-425. [DOI] [PubMed] [Google Scholar]
- 14. Benabid AL, Hoffmann D, Ashraf A, Koudsie A, Esteve F, Le-Bas JF. The robotization of neurosurgery: state of the art and future outlook. Bull Acad Natl Med. 1997;181(8):1625-1635; discussion 1635-1626. [PubMed] [Google Scholar]
- 15. Lefranc M, Zouitina Y, Tir Met al. Asleep robot-assisted surgery for the implantation of subthalamic electrodes provides the same clinical improvement and therapeutic window as awake surgery. World Neurosurg. 2017;106:602-608. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez-Martinez J, Bulacio J, Thompson Set al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery. 2016;78(2):169-180. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013;54(2):323-330. [DOI] [PubMed] [Google Scholar]
- 18. Vadera S, Chan A, Lo Tet al. Frameless stereotactic robot-assisted subthalamic nucleus deep brain stimulation: case report. World Neurosurg. 2017;97:762.e711-762.e714. [DOI] [PubMed] [Google Scholar]
- 19. Richardson RM. Functional neurosurgery: deep brain stimulation and gene therapy. In: Golby A, ed. Image-Guided Neurosurgery, 1st edn. Philadelphia, PA: Elsevier; 2015. [Google Scholar]
- 20. Lee PS, Crammond DJ, Richardson RM. Deep brain stimulation of the subthalamic nucleus and globus pallidus for parkinson's disease. Prog Neurol Surg. 2018;33:207-221. [DOI] [PubMed] [Google Scholar]
- 21. Medtech . ROSA Brain: Robotized Stereotactic Assistant User Manual. Montpelier, France: Medtech SA; 2017. [Google Scholar]
- 22. Xu F, Jin H, Yang Xet al. Improved accuracy using a modified registration method of ROSA in deep brain stimulation surgery. Neurosurg Focus. 2018;45(2):E18. [DOI] [PubMed] [Google Scholar]
- 23. Crowell AL, Ryapolova-Webb ES, Ostrem JLet al. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135(pt 2):615-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondylis ED, Randazzo MJ, Alhourani Aet al. Movement-related dynamics of cortical oscillations in Parkinson's disease and essential tremor. Brain. 2016;139(pt 8):2211-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lipski WJ, Alhourani A, Pirnia Tet al. Subthalamic nucleus neurons differentially encode early and late aspects of speech production. J Neurosci. 2018;38(24):5620-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chrabaszcz A, Neumann WJ, Stretcu Oet al. Subthalamic nucleus and sensorimotor cortex activity during speech production. J Neurosci. 2019;39(14):2698-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee PS, Weiner GM, Corson Det al. Outcomes of interventional-mri versus microelectrode recording-guided subthalamic deep brain stimulation. Front Neurol. 2018;9:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson RM, Ostrem JL, Starr PA. Surgical repositioning of misplaced subthalamic electrodes in Parkinson's disease: location of effective and ineffective leads. Stereotact Funct Neurosurg. 2009;87(5):297-303. [DOI] [PubMed] [Google Scholar]
- 29. Maciunas RJ, Galloway RL Jr, Latimer JW. The application accuracy of stereotactic frames. Neurosurgery. 1994;35(4):682-695; discussion 694-685. [DOI] [PubMed] [Google Scholar]
- 30. Ho AL, Pendharkar AV, Brewster Ret al. Frameless robot-assisted deep brain stimulation surgery: an initial experience. Oper Neurosurg (Hagerstown). 2019;17(4):424-431. [DOI] [PubMed] [Google Scholar]
- 31. Lee PS, Richardson RM. Interventional MRI-guided deep brain stimulation lead implantation. Neurosurg Clin N Am. 2017;28(4):535-544. [DOI] [PubMed] [Google Scholar]