Abstract
In Gaucher disease type 1 (GD1), genetic deficiency of lysosomal glucocerebrosidase results in the accumulation of glucosylceramide and glucosylsphingosine (GlcSph), that underlie chronic lipid-mediated metabolic inflammation. An important age-related phenotype is high risk of monoclonal gammopathy (MG), including multiple myeloma. We identified GlcSph, a pathological lyso-sphingolipid exclusively elevated in GD, as a mediator of B cell activation and as an antigenic target for GD1-associated MG. Saposin C (SapC), is a lipid-binding protein and activator of lysosomal glucocerebrosidase, which when mutated, cause a rare variant of GD. Sera of GD1 patients with MG of diverse immunoglobulin types were compared to GD patients without gammopathy for reactivity against GlcSph and SapC. We show reactivity of clonal immunoglobulin in GD1 to GlcSph but not to SapC. In two patients with GD1 and gammopathy, GlcSph-reduction therapy with eliglustat resulted in reduction in clonal Ig. Together, our data show that GlcSph but not SapC is the antigenic target in GD1-associated MG and that therapy aimed at reducing the levels of immunogenic lipid resulted in reduction of clonal immunoglobulin in vivo.
Keywords: Gaucher disease, Glucosylsphingosine, Multiple Myeloma, Saposin C
1. Introduction
In Gaucher disease (GD), biallelic mutations in GBA result in defective lysosomal glucocerebrosidase and the accumulation of glucocerebroside (GlcCer). The accumulation of GlcCer triggers its diacylation via an alternative metabolic pathway involving acid ceramidase to form glucosylsphingosine (GlcSph). Both, GlcCer and GlcSph are highly inflammatory lipids [1–3]. There is widespread involvement of myeloid cells in disease pathogenesis associated with chronic lipid-mediated inflammation. The most conspicuous manifestation of accumulation of these inflammatory lipids is seen as alternatively activated macrophages engorged with lipid-laden lysosomes classically described as Gaucher cells [2, 3]. In addition to the classical visceral, hematological and skeletal manifestations, patients with non-neuronopathic GD type 1 exhibit increased risk of hematologic malignancies, most strikingly for multiple myeloma (MM) by up to ~30-fold [4]. Furthermore, GD1 is known for highly prevalent polyclonal and monoclonal gammopathy (MG) of unknown significance (MGUS), suggesting broad B cell activation and a natural history culminating in MM [5]. Although, the factors that confer an elevated risk of MG in GD1 is still enigmatic, in our previous studies we identified CD1d-restricted GlcSph - specific type 2 NKTFH -mediated activation and proliferation of germinal center B cells. Further, we showed that one of the antigenic targets of clonal immunoglobulin (Ig) in GD-associated MG is GlcSph [2, 6, 7]. In some GD patients with gammopathy saposin C (SapC), a lipid binding protein and an activator of lysosomal glucocerebrosidase, was reported to be the target antigen [8]. Herein, we examined sera from 17 GD patients with monoclonal gammopathy of diverse Ig types and 8 GD patients without gammopathy for reactivity to GlcSph and to SapC. We show that GlcSph but not SapC is the target for clonal Ig in GD. Further, in two GD patients with MGUS, GlcSph-reduction therapy with eliglustat resulted in reduction in clonal immunoglobulin in vivo.
2. Methods:
2.1. Patients and materials
Peripheral blood samples from GD1 patients with MG were collected following informed consents approved by the institutional review board in accordance with the Declaration of Helsinki. Patients had diverse immunoglobulin types of MG: (11 IgG, 5 IgA and 1 IgM) and one kappa light chain only smoldering myeloma, with median age of 63 years; range between 37–90 years and gender distribution Male (n=17) and female (n=1). The GBA genotypes of patients were as follows: N370S/N370S (n=7), N370S/84GG (n=4), N370S/L444P (n=3), N370S/RecNcil (n=3) and N370S/? (n=1). Two patients were deemed to have smoldering myeloma. Healthy control sera were purchased from Valley Biomedical Inc, VA, and USA. Recombinant human His-tag Saposin C (SapC) protein was purchased from Enzo life Sciences. Anti- His mAb was obtained from Biolegend. Glucosylsphingosine was purchased from Matreya (catalogue no-2086, C24H47NO7).
2.2. ELISA
SapC-specific ELISA was performed as described previously by Preuss et al 2018 [8]. GlcSph specific ELISA was performed as described previously [7].
2.3. Absorption Studies
SapC-specific immunoaffinity column was prepared using anti-His antibody and recombinant His-tagged SapC protein according to the protocol described previously (2018) [8].
2.4. Statistical analyses
Statistical analyses were performed using Prism 8.0c (GraphPad) software packages. For comparison of means between groups, either unpaired t test or non-parametric Wilcoxon Mann Whitney test were used, and significance was set at P < .05.
3. Results:
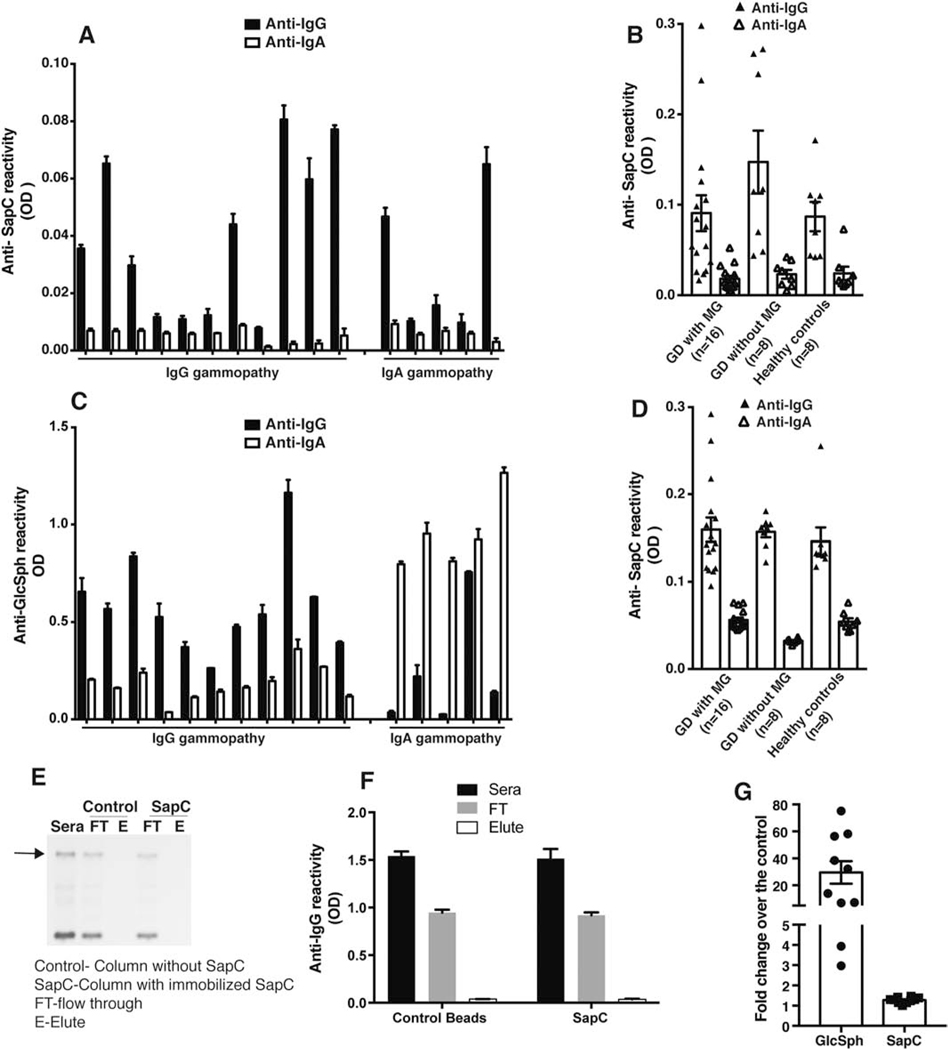
Sera of 16 GD patients with MG (11 IgG and 5 IgA), 8 GD patients without MG and 8 Healthy controls were evaluated for SapC reactivity by capture ELISA using purified recombinant His tagged SapC proteins and anti-His mAb. [8]. Immunoreactivity against SapC protein was not observed at high dilution (1:106) , which would be expected for a putative protein antigen (Figure 1 A) [8]. Reactivity against SapC was noted only at low dilutions(1:103) (data not shown). Moreover, the immunoreactivity to SapC was not found to be clonal immunoglobulin (Ig) class specific in the sera of GD1 patients with MG (Figure 1A). Furthermore, the low-level immunoreactivity to SapC was not exclusively observed in GD1 associated MG sera but equivalent SapC immunoreactivity was observed in sera of GD1 patients without gammopathy as well as sera of healthy controls at comparable dilutions (Figure 1 B). Importantly, in the same set of sera, anti-GlcSph immunoreactivity was found to be specific for clonal Ig class and only seen in GD1 patients with MG and not present in GD1 patients without gammopathy or in healthy controls (Figure 1C and Figure S1).
Figure 1. Immunoreactivity comparison between Glucosylsphingosine and Saposin C in the GD1 patient sera.
A) Capture ELISA was performed as described by Preuss et al 2018 [8] using anti-His mAb to capture recombinant purified human his tagged-saposin C (SapC) protein and Gaucher disease (GD) sera diluted 1:106. GD sera with monoclonal gammopathy (MG) with IgG (n=10) and IgA gammopathy (n=6) were used. B) Anti-SapC reactivity was compared in sera of GD sera with monoclonal gammopathy (MG) (n=16), GD sera without MG (n=8) and healthy donors (n=8). C) Glucosylsphingosine (GlcSph) reactivity for the same set of sera used for A) was analyzed by ELISA performed as earlier published paper[7] D) Direct ELISA was performed by coating purified human his tagged- SapC protein to wells of Nunc maxisorp plates followed by GD sera with monoclonal gammopathy (MG) with IgG (n=10) and IgA gammopathy (n=6) sera dilution of 1:106. E) Depletion of SapC specific clonal Igs from the sera of GD patient with MG. Representative blot shows serum protein electrophoresis (SPEP) analysis performed on sera from GD patient with MG after passing the sera through either control (without protein) or SapC immunoaffinity column prepared in accordance to Preuss et al[8]. FT-flow through; E-Elute. The experiment was repeated three times independently using at least 6 different sera from GD patients with MG. F) Flow through and elute fraction obtained from passing the sera through either the control or SapC immunoaffinity column was analyzed by clonal Ig class specific ELISA. G) Comparison of fold change of GlcSph and SapC levels in GD type 1 patient sera over the healthy controls (n=10).
Multiple complementary approaches were employed to critically examine the lack of specific high titer anti-SapC immunoreactivity in ELISA. First, the purity of the recombinant SapC protein as well as binding of SapC protein to anti-His mAb was confirmed by western blot (Figure S2). Second, to exclude the possibility that absence of specific high titer immunoreactivity towards SapC protein in sera of GD1 associated MG was due to anti-His interference in the capture assay, we performed direct ELISA to check SapC specific immunoreactivity in GD associated sera. For direct ELISA, SapC protein was directly coated to the wells of Nunc maxisorp plates followed by addition of the patient sera. Akin to the capture ELISA, there was no detectable class specific high titer immunoreactivity against SapC in GD1 associated MG sera (Figure 1D). Next, we employed SapC specific immunoaffinity columns, prepared in accordance to protocol described earlier [8], to determine whether Sap C bound clonal Igs from GD1 patient sera and thereby depleted clonal Igs from sera of GD1-MG patients. Passing sera of GD1 patients with MG (n=6) through SapC specific immunoaffinity column did not lead to depletion of clonal Igs from sera of GD1-MG patients as evident by presence of clonal Igs in flow through (FT) together with absence of corresponding clonal Ig in eluted fraction (E) by serum protein electrophoresis (SPEP) (Figure 1E). Presence of Igs in FT and elute fraction was further confirmed by clonal immunoglobulin (Ig) class specific ELISA. Similar to SPEP data in Figure 1E, clonal Igs were detected in the sera and FT fraction but not in the elute fraction (Figure 1F). Immobilization of SapC protein on the column was confirmed by taking beads after elution of clonal Igs using 0.1 M glycine pH 3.0, which were then washed and boiled (in SDS loading buffer) and run on 18% Tris/Tricine SDS PAGE followed by western blot using anti-His mAb. SapC protein was detected in the beads fraction at the same molecular weight to that of recombinant SapC protein run as positive control confirming immobilization of SapC protein on the SapC immunoaffinity column (Figure S3).Thus, despite confirmed presence of SapC on the column, lack of depletion of clonal Ig by SapC immunoaffinity column from sera of GD1 patient with MG indicates that SapC protein is not an antigenic target for clonal Igs in GD1 patients. In contrast, we have previously shown that clonal Igs from the same GD1 patients are GlcSph-specific by immunoblotting and direct binding to GlcSph containing liposomes (as physiologic antigen) and sphingosine beads [7]. Additionally, elevated levels of GlcSph (~30 fold) in GD1 patient sera as compared to healthy controls in comparison to SapC levels which remain similar to that of controls supports the notion that GlcSph is a predominant antigen for GD1 associated gammopathy (Figure 1G).Together, findings from SapC specific ELISA and SapC immunoaffinity column exclude the candidacy of SapC as the target of clonal Ig in GD.
We next investigated the in vivo role of GlcSph as the target antigen for GD-associated gammopathy by assessing the effect of GlcSph reduction therapy on clonal Igs in GD patients. Two GD patients (patient characteristics described in Table 1), one with IgM kappa MG (patient 1) and the other with kappa light chain smoldering myeloma (patient 2) with clonal Igs showing in vitro reactivity to GlcSph but not SapC were studied (Figure 2A). Immunohistochemical staining of bone marrow biopsy showed presence of CD138+ plasma cells with corresponding light chain restriction (Figure S4). In prior study, we have shown that injection with GlcSph, but not PBS, resulted in an increase of clonal Igs and plasma cells in a humanized xenograft model [7]. In prior studies, we and others have also shown that substrate reduction therapy can lead to reduction in GD-associated gammopathy in murine GD models [6, 9]. In order to test whether substrate reduction could similarly lead to reduction in clonal Igs in human GD1 patients with MG, we analyzed two patients treated with Eliglustat. Patient 1 with IgM MGUS had not been previously treated for his GD and his baseline evaluation revealed significant disease activity and markedly elevated GlcSph levels (Table 1). In contrast, patient 2 had been on enzyme replacement therapy for 23 years with residual disease activity indicated by elevated chitotriosidase and GlcSph; annual evaluations had not previously revealed evidence of MGUS (Table 1). Both the patients were treated with eliglustat 84 mg bid (they were CYP2D6 extensive metabolizers), an approved oral substrate therapy for adults with GD type 1 which effectively lowers GlcSph and reverses GD manifestations [10]. Eliglustat treatment led to a significant reduction in GlcSph levels in patient 1 (Figure 2B i) and expected improvement in the chitotriosidase, a known disease biomarker (Figure 2B ii). Of note, reduction of GlcSph levels was accompanied by decline in clonal heavy IgM as well as free kappa light chain levels in patient 1 (Figure 2B iii and iv). Patient 2 with a kappa light chain smoldering myeloma, after switching from enzyme therapy to eliglustat showed a significant reduction in GlcSph levels (Figure 2C i), chitotriosidase (Figure 2C ii) and concomitant decrease in free kappa chain levels (Figure 2C iii). Reduction in GlcSph levels post eliglustat administration was not accompanied by reduction in the Sap C levels in both patients following treatment (Figure 2D).
Table 1.
Clinical and biological features of Gaucher disease patients with MGUS and smoldering myeloma. In patient 2, the second allele was not one of common alleles. Patient was diagnosed by bone marrow examination revealing massive infiltration by Gaucher cells initially and confirmed by demonstration of low acid β -glucosidase activity.
| Patient (baseline) | Genotype | Age at GD diagnosis | Chitotriosidase (nmol/hr/ml) | GlcSph in serum (ng/ml) | Hb (gm/dl) | Platelets (x1000 u/L) | Liver (x normal) | Spleen (x normal) | Age diagnosis of MG | Treatment Status | Start (Age) for Eliglustat 84 mg BID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (NR: <78.5) | (NR: 2–5) | ||||||||||
| Pt1 | N370S/L444P | 12 | 10055.8 | 498.9 | 15.1 | 114 | 1.19 | 5.65 | 33 | Naïve | 34 |
| Pt2 | N370S/? | 25 | 2607.1 | 139 | 13.8 | 85 | 0.95 | 7.46 | 56 | On ERT | 57 |
Figure 2. In vivo effect of Glucosylsphingosine reduction therapy on clonal Igs in GD patients.
A) Immunoreactivity of clonal Ig from two GD patient; patient31 (Pt 1) with IgM MGUS and patient 2 (Pt 2) with kappa light chain smoldering myeloma was assessed against Glucosylsphingosine (GlcSph) and Saposin C (SapC) by ELISA. B) Graph represents the levels of GlcSph (ng/ml) (i) levels of Chitotriosidase (nmol/hr/min) (ii), IgM (mg/dl) (iii) and free light chains (Kappa and lambda) pre and post administration of eliglustat to patient 1 (Pt 1). C) Graph represents the levels of GlcSph (ng/ml) (i) levels of Chitotriosidase (nmol/hr/min) (ii) and free light chains (Kappa and lambda) on enzyme replacement therpay (ERT) and after switch to eliglustat to patient 2 (Pt 2) . D) Graph shows Sap C levels (ug/ml) in the sera of Pt 1 and Pt 2 pre and post eliglustat administration.
4. Discussion:
There is a significant unmet need to understand triggers for B cell lymphoproliferation and mechanisms underlying evolution of gammopathy to MM [11]. Metabolic lipid disorders associated with, obesity and GD, have been associated with an increased risk of plasma cell dyscrasias and MM [12]. The risk is most striking for GD where life-time relative risk of developing MM is as high as 33-fold [13]. Studies from several centers around the world as well as the International Gaucher registry have confirmed elevated risk [4, 14, 15]. Concomitantly, there is very high prevalence of polyclonal gammopathy and MGUS reflecting board B cell activation in GD1[15–19]. While there is general acceptance of increased risk of gammopathy in GD, variable risk estimates have been reported between different cohorts, in large part due to failure of ascertainment or adjustment for age regarding this age-related phenotype. For example, the International Gaucher Registry ICGG, study is skewed to younger patients and regular screening for gammopathy in GD had not yet entered into clinical practice at the time of the study contributing to lower ascertainment [15]. Therefore, GD is an attractive model to understand the molecular underpinnings of gammopathy and the role of lipids., but also addresses an important unmet need in an aging population of GD1 patients successfully treated with enzyme replacement therapy or substrate reduction therapy.
In our previous studies we have identified glucosylsphingosine as a mediator of CD1d-restricted activation of type 2 NKT cells (NKTFH) and B cell proliferation. Further, we showed GlcSph to be one of the antigenic targets of clonal Ig in GD1 associated MG. Recently, in some patients with GD-associated MG, SapC was reported to be a putative target antigen [8]. Identification of target antigen for MG is important as it can be therapeutically targeted to modify the natural history. Several prerequisites should be met to reliably invoke the role of a putative target antigen in gammopathy. For an antigen to be considered as a target for paraprotein/clonal Igs, the binding of the antigen to the clonal Ig should be evaluated using different complementary approaches with a focus on how the antigen is presented physiologically and importantly it’s in vivo relevance. Such rigorous examination is essential when directly comparing binding characteristics of candidate lipid vs protein antigens, owing to the distinctive physicochemical properties of lipids and proteins that will alter the binding kinetics in standard assays. Taking this into consideration, it is therefore important to delineate the physiological relevance of the putative antigen determined by in vitro binding assays, by assessing in vivo effect of antigen challenge as well as therapeutic reduction of putative antigen in patients.
Together with data reported herein and in our previous study [7], there is now compelling evidence that GlcSph is an important target of GD-associated MG based on the following observations: i) binding of clonal Igs to lysolipids presented in physiological context in the form of liposomes and beads and validation of clonal nature of lysolipids reactive Igs by protein sequencing [7], ii) assessing binding of GlcSph to recombinant monoclonal Igs cloned from single sorted plasma cells derived from a GD patient [7], iii) demonstrating in vivo reactivity of clonal plasma cells to the antigen and iv) demonstrating that therapeutic reduction of GlcSph in GD associated MG results in reduction of clonal Igs. Consistent with our findings, GlcSph reduction therapy with an oral inhibitor of glucosylceramide synthase (eliglustat, substrate reduction therapy) started early in life, prevented the development of spontaneous B-cell lymphoma and myeloma in GD mice [9]. Herein, concurrently we have examined the candidacy of SapC as antigen target in GD associated MG. We find no evidence of SapC as a target for clonal Ig in GD. Importantly, Saposin C used in our study is purified recombinant protein, which we extensively verified for its purity and molecular weight as well as binding to the anti-Histidine antibody using western blot as shown in the supplementary Figure S2. In contrast, Preuss et al [8] used total cell extracts prepared from HEK293 cell lines recombinantly expressing FLAG- tagged proteins and not purified recombinant protein to perform ELISA. Moreover, experimental evidence to show presence of Saposin C protein in the extract or the actual concentration of the extract used to coat the ELISA plates have not been explicitly described in the original report [8]. Therefore, apparent reactivity noted in that study cannot be attributed specifically to Saposin C. Collectively, the evidence provided here, and additional data from our group and others provide convincing evidence of lysolipids being the antigenic target for GD associated paraprotein.
Although current data suggest sequential progression of B cell lymphoproliferation, polyclonal gammopathy, MGUS and MM, it is not known whether progression of MGUS to MM is accelerated in GD and whether therapy to reduce immunogenic lipid alters the natural history. In two patients reported herein, GlcSph reduction therapy reduces lipid-reactive MGUS. Notably, lipid-reactive MG in GD is of diverse Ig type, even including light chain MG, similar to that seen in sporadic MGUS [20]. Further studies in larger GD patient cohorts are needed to clarify the phenotypic diversity and natural history of MG and whether therapy to reduce immunogenic lipid, prevents development of MG as reported in GD mice [9]. Moreover, larger studies should clarify whether treatment slows transition of MGUS to MM in GD.
Supplementary Material
Figure S2: Coomassie stained 18% Tris/Tricine SDS PAGE showing the purity and expected molecular weight of recombinant purified human his tagged- Saposin C (SapC) protein (upper panel) and detection of SapC protein by anti-His mAb by western blot (lower panel).
Figure S3: Immobilization of Saposin C (SapC) protein on the SapC immunoaffinity column was confirmed by taking beads after elution of paraproteins using 0.1 M glycine pH3.0 and boiling them in SDS loading buffer followed by western blot using anti-His mAb. As a control purified SapC protein was also included (lane 1).
Figure S1: Glucosylsphingosine (GlcSph) was compared in sera of GD patient with monoclonal IgG (n=10 and IgA gammopathy (n=6), GD sera without MG (n=8) and healthy controls (n=8). Same set of sera to that was used to assess Anti- SapC immunoreactivity as in Figure 1B was used.
Figure S4: Upper panel Pt1 and Lower panel Pt2 A) Hematoxylin and eosin staining showing infiltration of bone marrow by plasma cells in bone marrow biopsy. B) Immunohistochemical staining of bone marrow biopsy showing positive staining for CD138. C and D) Immunohistochemical stains of bone marrow biopsy showing staining for kappa and lambda respectively. Original magnification is indicated under each figure.
Acknowledgements:
The authors thank the patients for their support and generous participation in our study.
Funding source:
Dr. Pramod Mistry PKM is supported by NIH NINDS NS 110354 and a Center of Excellence in Clinical Translational Research in Gaucher disease by Sanofi Genzyme. MVD is supported in part by funds from NIH/NCI CA197603 and LLS.
Conflicts of Interest:
Dr. Pramod Mistry has received lecture fees and travel support and served as consultant for Sanofi Genzyme.
Abbreviations:
- GD1
Gaucher disease type 1
- GlcSph
glucosylsphingosine
- SapC
Saposin C
- MG
monoclonal gammopathy
- GlcCer
Glucocerebroside
- Igs
Immunoglobulins
- MGUS
Monoclonal Gammopathy of Unknown Significance
- MM
Multiple Myeloma
- SPEP
Serum Protein Electrophoresis
- His
Histidine
- mAb
monoclonal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, Omahdi Z, Yamaji T, Miyamoto T, Bamba T, Yamasaki S, Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity, Proc Natl Acad Sci U S A, 114 (2017) E3285–E3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nair S, Boddupalli CS, Verma R, Liu J, Yang R, Pastores GM, Mistry PK, Dhodapkar MV, Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation, Blood, 125 (2015) 1256–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pandey MK, Burrow TA, Rani R, Martin LJ, Witte D, Setchell KD, McKay MA, Magnusen AF, Zhang W, Liou B, Kohl J, Grabowski GA, Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease, Nature, 543 (2017) 108–112. [DOI] [PubMed] [Google Scholar]
- [4].Cox TM, Rosenbloom BE, Barker RA, Gaucher disease and comorbidities: B-cell malignancy and parkinsonism, Am J Hematol, 90 Suppl 1 (2015) S25–28. [DOI] [PubMed] [Google Scholar]
- [5].Marti GE, Ryan ET, Papadopoulos NM, Filling-Katz M, Barton N, Fleischer TA, Rick M, Gralnick HR, Polyclonal B-cell lymphocytosis and hypergammaglobulinemia in patients with Gaucher disease, Am J Hematol, 29 (1988) 189–194. [DOI] [PubMed] [Google Scholar]
- [6].Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV, Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma, N Engl J Med, 374 (2016) 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nair S, Sng J, Boddupalli CS, Seckinger A, Chesi M, Fulciniti M, Zhang L, Rauniyar N, Lopez M, Neparidze N, Parker T, Munshi NC, Sexton R, Barlogie B, Orlowski R, Bergsagel L, Hose D, Flavell RA, Mistry PK, Meffre E, Dhodapkar MV, Antigen-mediated regulation in monoclonal gammopathies and myeloma, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Preuss KD, Hollak CEM, Fadle N, van Oers M, Regitz E, Pfreundschuh M, Saposin C is a frequent target of paraproteins in Gaucher disease-associated MGUS/multiple myeloma, Br J Haematol, 184 (2019) 384–391. [DOI] [PubMed] [Google Scholar]
- [9].Pavlova EV, Archer J, Wang S, Dekker N, Aerts JM, Karlsson S, Cox TM, Inhibition of UDP-glucosylceramide synthase in mice prevents Gaucher disease-associated B-cell malignancy, J Pathol, 235 (2015) 113–124. [DOI] [PubMed] [Google Scholar]
- [10].Mistry PK, Lukina E, Ben Turkia H, Shankar SP, Baris H, Ghosn M, Mehta A, Packman S, Pastores G, Petakov M, Assouline S, Balwani M, Danda S, Hadjiev E, Ortega A, Gaemers SJM, Tayag R, Peterschmitt MJ, Outcomes after 18 months of eliglustat therapy in treatment-naive adults with Gaucher disease type 1: The phase 3 ENGAGE trial, Am J Hematol, 92 (2017) 1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dhodapkar MV, MGUS to myeloma: a mysterious gammopathy of underexplored significance, Blood, 128 (2016) 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Korde N, Mailankody S, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Landgren O, Kristinsson SY, Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: a population-based study, Blood Adv, 1 (2017) 2186–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taddei TH, Kacena KA, Yang M, Yang R, Malhotra A, Boxer M, Aleck KA, Rennert G, Pastores GM, Mistry PK, The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients, Am J Hematol, 84 (2009) 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mistry PK, Taddei T, vom Dahl S, Rosenbloom BE, Gaucher disease and malignancy: a model for cancer pathogenesis in an inborn error of metabolism, Crit Rev Oncog, 18 (2013) 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E, Gaucher disease and cancer incidence: a study from the Gaucher Registry, Blood, 105 (2005) 4569–4572. [DOI] [PubMed] [Google Scholar]
- [16].Arends M, van Dussen L, Biegstraaten M, Hollak CE, Malignancies and monoclonal gammopathy in Gaucher disease; a systematic review of the literature, Br J Haematol, 161 (2013) 832–842. [DOI] [PubMed] [Google Scholar]
- [17].de Fost M, Out TA, de Wilde FA, Tjin EP, Pals ST, van Oers MH, Boot RG, Aerts JF, Maas M, Vom Dahl S, Hollak CE, Immunoglobulin and free light chain abnormalities in Gaucher disease type I: data from an adult cohort of 63 patients and review of the literature, Ann Hematol, 87 (2008) 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weinreb NJ, Lee RE, Causes of death due to hematological and non-hematological cancers in 57 US patients with type 1 Gaucher Disease who were never treated with enzyme replacement therapy, Crit Rev Oncog, 18 (2013) 177–195. [DOI] [PubMed] [Google Scholar]
- [19].Zimran A, Liphshitz I, Barchana M, Abrahamov A, Elstein D, Incidence of malignancies among patients with type I Gaucher disease from a single referral clinic, Blood Cells Mol Dis, 34 (2005) 197–200. [DOI] [PubMed] [Google Scholar]
- [20].Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV, Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance, N Engl J Med, 378 (2018) 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2: Coomassie stained 18% Tris/Tricine SDS PAGE showing the purity and expected molecular weight of recombinant purified human his tagged- Saposin C (SapC) protein (upper panel) and detection of SapC protein by anti-His mAb by western blot (lower panel).
Figure S3: Immobilization of Saposin C (SapC) protein on the SapC immunoaffinity column was confirmed by taking beads after elution of paraproteins using 0.1 M glycine pH3.0 and boiling them in SDS loading buffer followed by western blot using anti-His mAb. As a control purified SapC protein was also included (lane 1).
Figure S1: Glucosylsphingosine (GlcSph) was compared in sera of GD patient with monoclonal IgG (n=10 and IgA gammopathy (n=6), GD sera without MG (n=8) and healthy controls (n=8). Same set of sera to that was used to assess Anti- SapC immunoreactivity as in Figure 1B was used.
Figure S4: Upper panel Pt1 and Lower panel Pt2 A) Hematoxylin and eosin staining showing infiltration of bone marrow by plasma cells in bone marrow biopsy. B) Immunohistochemical staining of bone marrow biopsy showing positive staining for CD138. C and D) Immunohistochemical stains of bone marrow biopsy showing staining for kappa and lambda respectively. Original magnification is indicated under each figure.