Abstract
Objectives:
Although community-acquired pneumonia (CAP) is one of the most common infections in children, no standardized risk classification exists to guide management. The objective of this study was to develop expert consensus for factors associated with various degrees of disease severity in pediatric CAP.
Methods:
Using a web-based classical Delphi process, a multidisciplinary panel of 10 childhood pneumonia experts rated the degree of severity (mild, moderate, or severe) of clinical, radiographic, and laboratory factors, as well as outcomes relevant to pediatric pneumonia. Round 1 was open-ended, with panelists freely stating all characteristics they felt determined pneumonia severity. In rounds 2 to 4, panelists used a 9-point Likert scale (1–3, mild; 4–6, moderate; 7–9, severe) to rate severity for each item. Consensus was defined as 70% or greater agreement in ranking mild, moderate, or severe.
Results:
Panelists identified 318 factors or outcomes in round 1; the panel reached consensus for 286 (90%). The majority of items without consensus straddled levels of severity (eg, mild-moderate). Notable clinical factors with consensus included age, oxygen saturation, age-based respiratory rate, and gestational age. Severity classification consensus was also reached for specific imaging and laboratory findings. Need for and duration of hospitalization, supplemental oxygen/respiratory support, and intravenous fluids/medications were considered important outcomes in classifying severity.
Conclusions:
This study presents factors deemed important for risk stratification in pediatric CAP by consensus of a multidisciplinary expert panel. This initial step toward identifying and formalizing severity criteria for CAP informs critical knowledge gaps and can be leveraged in future development of clinically meaningful risk stratification scores.
Keywords: infectious diseases, risk stratification, pneumonia
Community-acquired pneumonia (CAP) is the leading cause of postneonatal mortality in children worldwide1 anda leading cause of pediatric hospitalization in the United States.2,3 Despite this, there is significant variation in the care of childhood CAP, including hospitalization, diagnostic testing, and antibiotic use.4–8 The lack of clear definitions for mild, moderate, and severe pneumonia in children contributes to the variation in management, with resultant overuse of diagnostic testing and hospitalizations when severity is overestimated and delays in essential care when severity is underestimated. Given the importance of appropriate risk stratification to the initial treatment of children with CAP, the need for clear severity definitions was identified as a key research priority by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA).9
Most of the evidence on pneumonia severity comes from studies performed in low-middle income countries. The World Health Organization defined danger signs, including central cyanosis or oxygen saturation less than 90%, inability to drink/feed, vomiting, convulsions, lethargy/unresponsiveness or impaired consciousness, and severe respiratory distress, which have shown good discriminatory ability to predict mortality in children with pneumonia.10,11 Other scores have also outlined factors associated with pneumonia severity in low-middle income countries with fair-to-good discriminatory ability for mortality.12–14 None of these scores were derived in the developed world, and it is unclear if these factors are applicable in settings where there is ready access to pulse oximetry, radiography, and advanced technology and resources.
Although national guidelines include severity criteria to assist with clinical decision making in the developed world, the application of these criteria into clinical practice is limited.9,15,16 Most of these criteria were not derived in children but rather were modified from adult studies9,15; thus, they have limitations when applied to children. In addition, these criteria were not developed using rigorous consensus methods. A recent study found that most children classified as severe disease requiring hospitalization according to the PIDS/IDSA severity criteria were safely discharged home.17 Specific severity criteria for childhood CAP have the potential to improve risk stratification and resource allocation, thereby minimizing variation in care. One of the first steps in creating a severity score is to determine the factors to include in score development. Thus, the objective of this study was to establish expert consensus for factors that should be considered across various degrees of severity in children cared for in the developed world using the Delphi process to incorporate multidisciplinary expert perspectives.
METHODS
We applied a web-based classical Delphi process to derive and classify factors and outcomes associated with pneumonia severity into mild, moderate, or severe disease. The Delphi process is a multiround group survey technique with interround group feedback that is commonly used in health care to gain expert consensus.18
Expert Panel
An expert panel representing diverse pediatric clinical specialties was recruited through peer recommendations and literature review. We defined an expert in childhood pneumonia as an individual with at least 3 peer-reviewed publications as a primary author focused on pediatric lower respiratory tract infections and at least 5 posttraining years caring for children with pneumonia. We identified panelists by both author relationship and PubMed search using the following search criteria: (“pneumonia”) AND (“childhood” OR “pediatric”), limited to human studies published in the past 20 years. Potential panelists were reviewed by study authors (P.D. and T.F.), and all invitations were agreed upon. Of 11 potential experts approached, 10 agreed to participate. These individuals represented a range of pediatric subspecialties, including emergency medicine, hospital medicine, infectious disease, primary care, and pulmonology, to ensure diversity of clinical backgrounds and practice location (see Appendix Table I, Supplemental Digital Content 1, http://links.lww.com/PEC/A539). Panelists remained anonymous to one another to avoid undue influence of other members in the rating process and were provided a US $100 honorarium.
Study Design
We used a web-based classical Delphi process consisting of an initial open-ended round followed by 3 subsequent rounds during which panelists rated factors and outcomes developed by the panel in the first round. In round 1, panelists were asked to list the clinical, radiographic, and laboratory factors that influence pneumonia severity and relevant severity outcomes in an open-ended fashion. Consistent with prior Delphi studies where expert consensus is guided by existing evidence, panelists were provided a systematic review of the existing evidence on factors that influence pneumonia severity in children.19 Panelists were asked to be as specific as possible in an attempt to develop numerical cutoffs for quantifiable factors and outcomes.
After round 1, all study investigators analyzed panelist responses to determine common themes, which were organized into statements used during subsequent rounds. In rounds 2 to 4, panelists rated factors and outcomes generated during round 1 using a 9-point Likert scale, with values 1 to 3 representing “mild pneumonia,” 4 to 6 representing “moderate pneumonia,” and 7 to 9 representing “severe pneumonia.” Panelists were given a general frame of reference that, in the vast majority of cases, mild pneumonia should be appropriate for outpatient management, moderate pneumonia should be appropriate for hospital admission but not intensive care unit (ICU) admission, and severe pneumonia should require invasive interventions or ICU-level care. During round 2, panelists were given an additional opportunity to provide open-ended factors or outcomes that were not initially included in round 1 but should be considered in rounds 3 and 4.
We defined consensus as 70% or greater of panelists choosing values 1 to 3 (mild), 4 to 6 (moderate), or 7 to 9 (severe) for a particular factor or outcome.18 After each round, panelists were provided with their response from the prior round and the group distribution of responses (ie, how many panelists selected each numerical level of severity). They were asked to reconsider their rating based on this information. Factors and outcomes that met consensus criteria in a previous round were not presented in subsequent rounds.
Surveys were conducted electronically via Research Electronic Data Capture (REDCap). This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Statistical Analysis
After round 4, the median and interquartile ranges (IQRs) were calculated for factors and outcomes not reaching consensus. For items not reaching consensus, those with an IQR of 2 or less that straddled levels of severity were classified as associated with mild-moderate or moderate-severe disease. Analyses were performed using STATA version 15 (College Station, TX).
RESULTS
All members of the expert panel completed each round of the Delphi process. Panelist open-ended responses from round 1 were grouped into 311 factors or outcome statements that were rated in round 2 (Fig. 1). Consensus was achieved for 196 (63%) of 311 factors or outcomes in round 2. The panelists added 7 open-ended factors in round 2. The 115 items that did not reach consensus in round 2, plus these 7 additional factors, formed the 122 factors or outcomes that were presented in round 3. Consensus was reached for 68 (56%) of the 122 items in round 3 and for 22 (41%) of 54 additional items in round 4.
FIGURE 1.
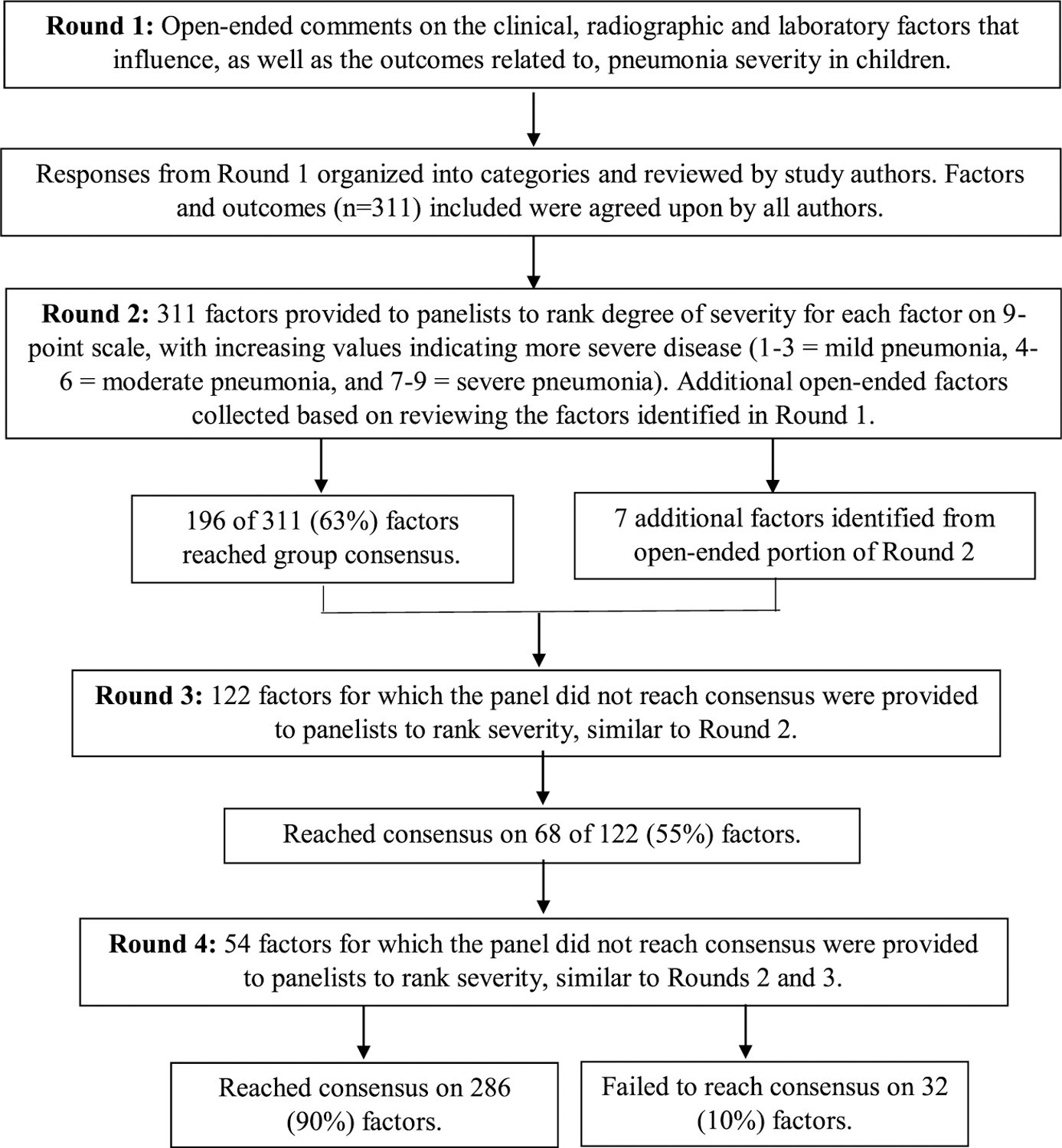
Delphi process flowchart. Consensus defined as ≥70% panelists ranking the factor in the same category (mild, moderate, or severe).
Overall, consensus was reached for 286 (90%) of 318 factors or outcomes during the Delphi process, with no consensus achieved for 32 (10%) items. All factors and outcomes for which consensus was reached, including numerical cutoffs when applicable, are listed in Tables 1–4. Gaps in values (eg, oxygen saturation [SpO2], 81–84%) indicate an inability to reach consensus for that range of values.
TABLE 1.
Clinical Factors That Reached Consensus
| Mild | Moderate | Severe | |
|---|---|---|---|
| Oxygenation/need for oxygen | |||
| Room air oxygen saturation | ≥92% | 85–91% | <80% |
| Oxygen saturation with supplemental oxygen | - | • 1–5 L oxygen by nasal cannula to maintain SpO2 > 90% | • SpO2 < 85%on ≥5 L oxygen |
| • Need for supplemental oxygen | • At least 5–10 L oxygen by nasal cannula or face mask to maintain SpO2 > 90% • PaO2/FiO2 < 250 • Oxyhemoglobin desaturation not responding to supplemental oxygen |
||
| Cyanosis | No | No | Yes |
| Respiratory rate by age | |||
| 0–2 mo | 21–60 | >60 | ≤20 |
| 2–12 mo | 21–50 | 51–80 | ≤20 and >80 |
| 1–5 y | 21–40 | 51–80 | <10 and >80 |
| 5–10 y | 10–40 | 41–50 | <10 and >60 |
| >10 y | 10–20 | — | <10 and >40 |
| Respiratory support | None | High-flow nasal cannula | BiPAP/CPAP or mechanical ventilation |
| Hydration, perfusion, and circulatory | |||
| Tolerating enteral feeding/drinking | Yes | No | — |
| Blood pressure | Normal | — | • Hypotensive • Vasoactive medications to maintain blood pressure |
| Perfusion | Normal | • Poor | • Poor despite intravenous rehydration |
| • Capillary refill >2 sec | • Vasoactive medications to maintain perfusion | ||
| Dehydration | Mild | • Moderate-severe • No urine output in 12–24 h |
— |
| Fluid requirement | — | • ≥1 intravenous fluid bolus on presentation | — |
| Dyspnea/work of breathing | |||
| Severity | • No or mildly increased work of breathing | • Moderate or severely increased work of breathing | • Concern for deterioration based on work of breathing • Exhausted/fatigued • Unable to speak |
| — | • Dyspnea that resolves with oxygen via nasal cannula, high-flow nasal cannula, or oxymask | • Dyspnea that requires noninvasive positive pressure ventilation | |
| Retraction location | — | •Subcostal • Intercostal • Supraclavicular • Posterior rib |
— |
| Other work of breathing | — | •Head bobbing • Grunting • Nasal flaring |
— |
| Mental status | |||
| Normal | Irritable but arousable | • Altered • Lethargic • Obtunded • Unconscious • Seizures |
|
| Temperature | |||
| ≥38°C | 35.1–35.9°C | — | |
| Heart rate | |||
| • 10–94th percentile for age • Tachycardia responsive to decreasing temperature |
• ≥95th percentile for age • Tachycardia responsive to fluid resuscitation |
• Persistent tachycardia despite ≥40 mL/kg intravenous fluid resuscitation | |
| Auscultation findings | |||
| • Wheezing • Focal or diffuse crackles |
— | — | |
| Appearance/other examination findings | |||
| — | • Ill appearance • Anxious with respirations |
• Septic appearance | |
| • Chest/pleuritic pain | • Rapid worsening change in clinical appearance | ||
| Comorbidities/history | |||
| Gestational age | • 28–32 wk, currently >6 mo of age |
• 28–32 wk, currently <6 mo of age | — |
| • 32–35 wk | • 24–28 wk | ||
| Medical history | • Intermittent, mild persistent, or moderate persistent asthma | • Severe persistent asthma • Structurally or physiologically significant congenital heart disease • Chronic respiratory disease (ie, cystic fibrosis, chronic lung disease) • Neuromuscular disease affecting respiration • Sickle cell disease • Immunocompromised or immunosuppressed • Antibiotic-resistant organisms (ie, MRSA) • Central airway obstruction • Rapid decompensation |
• Risk for Pneumocystis carinii pneumonia |
| Recent history | • Recent antibiotic use | • Failed antibiotics for current illness | — |
| • Duration of symptoms ≥1 d on presentation | • Worsening symptoms regardless of duration | ||
| Age | |||
| >4 mo | ≤4 mo | — | |
| Social history | |||
| • Crowding in home • <2 parent household • No transportation • No primary care physician |
• Concern for family noncompliance or Unable to obtain prescriptions | — | |
| • Tobacco smoke exposure • Current smoker • No insurance • Non–English-speaking |
• Caregiver unable to understand or follow instructions |
BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; MRSA, methicillin-resistant Staphylococcus aureus; PaO2, partial pressure of arterial oxygen.
TABLE 4.
Outcomes That Reached Consensus
| Mild | Moderate | Severe | |
|---|---|---|---|
| Hospitalization/LOS/ICU | |||
| Outpatient | Any non-ICU hospitalization regardless of LOS | Any ICU admission regardless of duration | |
| Respiratory support | |||
| Duration of supplemental O2 | <4 h | >4 h | — |
| Mode of ventilation | — | — | • Noninvasive positive pressure ventilation • Intubation/invasive positive pressure ventilation • Oscillator |
| Organ failure | |||
| — | — | • End organ replacement • Multi-organ failure |
|
| Cardiopulmonary | |||
| — | — | • Cardiopulmonary resuscitation • Extracorporeal membrane oxygenation |
|
| Fluid status | |||
| — | • Need for intravenous fluids regardless of duration | • Vasoactive medications | |
| General chemistry abnormalities | |||
| — | • Interventions to address electrolyte abnormalities | • 25% dextrose for purposes of treating hypoglycemia | |
| Antibiotic use | |||
| — | • Intravenous antibiotics for 1–5 d • Change in class of intravenous antibiotics • Persistent fever despite 48–72 h of antibiotics |
— | |
| Sepsis | |||
| — | • Sepsis (SIRS criteria + infection) without severe sepsis or septic shock | • Severe sepsis or septic shock | |
| Surgical/invasive interventions | |||
| — | •Pleural drainage or chest tube | • >1 chest tube • Video-assisted thoracoscopic surgery |
|
| • Clear (nonpurulent) chest fluid on drainage | • Need for bronchoalveolar lavage • Definitive surgery (ie, bronchopleural fistula repair) |
LOS, length of stay; SIRS, systemic inflammatory response syndrome.
Appendix Table II (Supplemental Digital Content 2, http://links.lww.com/PEC/A539) lists all items for which the panelists failed to reach consensus, broken down by category of factor/outcome and level of severity, with median and IQR of round 4 voting provided. Of the 32 items without consensus, 30 straddled two levels of severity (ie, mild-moderate or moderate-severe).
Clinical Factors
The SpO2 in room air of 92% or greater was characterized as mild disease, 85% to 91% as moderate disease, and less than 80% as severe disease. Consensus was not achieved for the range of 81% to 84%, which straddled moderate-severe classifications. Supplemental oxygen requirement and need for respiratory support were considered at least moderate severity factors, with high-flow nasal oxygen considered to be relevant to moderate disease and need for positive-pressure ventilation indicating severe disease (Table 1).
Consensus respiratory rate thresholds by age for each level of severity were also reached by the panel. For severe disease, consensus was reached for age 0 to 2 months (respiratory rate, ≤20 breaths per minute with no cutoff for tachypnea identified), 2 to 12 months (≤20 and >80 breaths per minute), 1 to 5 years (<10 and >80 breaths per minute), 5 to 10 years (<10 and >60 breaths per minute), and longer than 10 years (<10 and >40 breaths per minute). When considering age and prematurity as severity indicators, pneumonia in children younger than 4 months, infants born at 24 to 28 weeks, and infants born at 28–32 weeks if less than 6 months of age reached consensus as indicators of moderate disease.
Radiographic Factors
Effusions less than 10% of hemithorax met consensus for mild disease, with effusions 10% to 50% hemithorax or requiring drainage representing moderate disease. Unilateral effusions greater than 50% hemithorax were thought to represent moderate-severe disease and bilateral effusions greater than 50% hemithorax met consensus for severe disease. Effusions causing tracheal or mediastinal shift were also felt to reflect severe disease (Table 2).
TABLE 2.
Radiographic Factors That Reached Consensus
| Mild | Moderate | Severe |
|---|---|---|
| Pleural effusion | ||
| • Unilateral small simple effusion <10% hemithorax with or without layering out on decubitus | • Unilateral simple effusions 10–50% of hemithorax | • Effusion causing tracheal/mediastinal shift |
| • Bilateral trivial/trace effusions | • Bilateral simple effusions 10–50% of hemithorax | • Bilateral effusions encompassing >50% of hemithorax |
| • Simple effusion not requiring surgical intervention | • Simple effusion requiring surgical drainage | • Empyema >50% hemithorax diameter |
| Infiltrate pattern | ||
| • Single lobe or unilateral <50% of chest volume | • Multi-lobar with or without perihilar disease | — |
| • Any degree of perihilar, interstitial, or nodular disease | • Whole lung white out | — |
| Pneumothorax | ||
| • Small unilateral or bilateral | • Medium-large unilateral or bilateral • Tension |
|
| Secondary changes/complications | ||
| — | •Any size lung abscess | • Septic emboli • Necrotizing or cavitary pneumonia |
| • Pneumomediastinum | • Pneumopericardium • Bronchopleural fistula |
The presence of multilobar infiltrates was considered to indicate at least moderate pneumonia, whereas other infiltrate patterns were not felt to influence pneumonia severity. Complications of pneumonia meeting consensus for severe pneumonia included septic emboli, necrotizing or cavitary pneumonia, pneumopericardium, and bronchopleural fistula.
Laboratory Factors
White blood cell (WBC) count of 4000 to 25,000 cells/mm3 met consensus for mild disease, with WBC count less than 3000 or greater than 25,000 cells/mm3 for moderate disease. The panelists agreed that an absolute neutrophil count (ANC) of 300 to 1000 cells/mm3 indicated moderate disease, and less than 300 cells/mm3 indicated severe disease (Table 3).
TABLE 3.
Laboratory Factors That Reached Consensus
| Mild | Moderate | Severe | |
|---|---|---|---|
| Hematologic factors | |||
| Hematocrit (%) | ≥25 | <25 | — |
| Hemoglobin (g/dL) | ≥8 | <8 | — |
| Platelet count (/mm3) | >120,000 | 50,000–100,000 | <50,000 |
| WBC (cells/mm3) | 4000–25,000 | <3,000 or >25,000 | — |
| ANC (cells/mm3) | — | 300–1,000 | <300 |
| Inflammatory markers/lactic acid | |||
| CRP (mg/dL) | <10 | >10 | — |
| Procalcitonin (ng/mL) | <0.5 | >2 | — |
| Lactic acid | — | Elevated | — |
| General chemistries/blood gas | |||
| Sodium (mmol/L) | 130–145 | 125–130 | — |
| pH | ≥7.3 | <7.3 | — |
| pCO2 (mm Hg) | ≤50 | >50 | — |
| Creatinine | ≤2 times baseline | >2 times baseline | Renal failure requiring renal replacement therapy (using KDIGO definitions) |
| Mycoplasma positive | Acute kidney injury without need for renal replacement therapy | ||
| Microbiology | |||
| MRSA positive Influenza positive Bacteremia with any true pathogen |
KDIGO, Kidney Disease Improving Global Outcomes; pCO2, partial pressure of carbon dioxide.
Additional values reaching consensus as indicators of moderate pneumonia included C-reactive protein (CRP) greater than 10 mg/dL, procalcitonin greater than 2 ng/mL, sodium 125 to 130 mmol/L (<125 mmol/L was rated as moderate-severe pneumonia; median, 6.5; IQR, 6–7), pH less than 7.3, pCO2 greater than 50 mm Hg, and creatinine more than 2 times baseline. Methicillin-resistant Staphylococcus aureus or influenza-positive cases were also felt to suggest moderate pneumonia, as was bacteremia with any true pathogen.
Outcomes
Notable outcomes reaching consensus as moderate pneumonia were non-ICU hospitalization, supplemental oxygen longer than 4 hours, intravenous fluid use, and sepsis without shock (Table 4). Outcomes rated as severe pneumonia included ICU admission, positive pressure ventilation, vasoactive medications, and septic shock.
DISCUSSION
Pneumonia severity definitions vary by clinician and by study. As a step toward developing unified severity classifications for pediatric CAP, this study was designed to inform a gap in the literature by using formal expert consensus methods to define factors and outcomes associated with pneumonia severity in children. Our multidisciplinary panel of experts in pediatric pneumonia, chosen based on their combination of clinical and research expertise, generated 318 clinical, radiographic, and laboratory factors and outcomes important in the assessment of pneumonia severity in an open-ended fashion with the assistance of an evidence-based systematic review.14 They reached consensus in classifying 286 (90%) of these factors and outcomes as representing mild, moderate, or severe pneumonia. When considering all items for which consensus was reached and items having an IQR of 2 or less that crossed 2 levels of severity, 316 (99%) of 318 items were classified as mild, mild-moderate, moderate, moderate-severe, or severe. Given the high level of expert consensus in our study, our findings can inform the direction of future studies evaluating disease severity in pediatric CAP, including the development of a pneumonia severity score in children.
The expert panel agreed with some previously demonstrated severity criteria, as was expected, while also adding novel information that is not clearly delineated in the medical literature.9,15,16,20,21 General overarching areas in which our panel demonstrated agreement included young age, hypoxemia, tachypnea, dyspnea, altered mental status, temperature, presence of effusion, and infiltrate pattern. Challenges to the broader application of published severity criteria are that the criteria in current treatment guidelines were not derived in children and existing severity prediction models have limited generalizability, having been derived in a developing nation20 or exclusively in hospitalized children.21 The agreement of our expert panel in the criteria listed above adds support for the application of these criteria in assessing CAP severity in children in the developed world.
In addition to areas of agreement with current literature, our study also adds novel information about specific numerical cutoffs and more granular age groupings related to pneumonia severity. From a clinical standpoint, the panel’s severity classification based on SpO2 (mild, ≥92%; moderate, 85–91%; moderate-severe, 81–84%; severe, ≤80%) provides additional information to address variation of SpO2 thresholds for the definition of hypoxemia by source (PIDS/IDSA <90%; British Thoracic Society, or BTS <92%).9,15 Though we cannot make definitive conclusions about the association between these values and severity, the expert consensus in our study should inform future studies to establish more robust evidence.
With regard to respiratory rate, thresholds for tachypnea by age vary greatly throughout the literature (see Appendix Table III, Supplemental Digital Content 3, http://links.lww.com/PEC/A539),9,15,16,22,23 and the relevance to disease severity of decreasing respiratory rates progressing toward apnea is incompletely described. Our study provides more granular respiratory rate classifications than existing guidelines by separating tachypnea-by-age thresholds into moderate-severe and severe disease, considering children 5 to 10 years of age and older than 10 years as 2 separate groups, and classifying severity based on decreasing respiratory rates. Age and prematurity have an impact on disease severity, and our expert panel also provided initial consensus for age/gestational age-specific cutoffs in pediatric CAP. The PIDS/IDSA suggests admission for children younger than 3 to 6 months,9 and BTS states that chronic lung disease of prematurity should be considered in severity assessment,15 but the PIDS/IDSA guideline does not mention prematurity in relation to severity.9 Our panel provided further granularity to existing recommendations by considering children 4 months or younger (regardless of gestational age), infants born at 24 to 28 weeks (at any current age), and infants born at 28 to 32 weeks (if <6 months old) to have moderate disease. Additionally, comorbidities are incompletely described in the BTS15 and PIDS/IDSA9 severity criteria. Our panel identified the specific comorbidities they felt impacted pneumonia severity (Table 1).
From a radiographic standpoint, PIDS/IDSA considers the presence of effusion as a minor criteria for severe disease.9 The impact of effusions on severity is incompletely described in the BTS guideline.15 Our Delphi panel provided finer detail in this area, stating their opinion of severity based on effusion size (ie, <10% hemithorax: mild disease, 10–50% hemithorax: moderate disease, and >50% hemithorax: severe disease) and laterality (ie, unilateral vs bilateral). Our panel was also able to reach consensus on rare but potentially serious complications including lung abscesses and pneumomediastinum (indicators of moderate disease) and septic emboli, necrotizing pneumonia, pneumopericardium, and bronchopleural fistula (indicators of severe disease).
Our panel also reached consensus on numerical cutoffs for several commonly obtained laboratory studies used in the work-up of pneumonia. As complete blood counts are a typical component of laboratory workup in infectious processes in children, consensus for leukocytosis (WBC >25,000 cells/mm3: moderate disease), leukopenia (WBC <3,000 cells/mm3: moderate disease), and neutropenia (ANC, 300–1,000 cells/mm3: moderate disease, ANC less than 300 cells/mm3: severe disease) can assist in conceptualizing severity. Evidence is conflicting on the use of CRP and procalcitonin in the assessment of pneumonia severity in children,12,24–29 with guidelines suggesting utility in more serious cases.9,15 Our panel’s agreement that CRP greater than 10 mg/dL and procalcitonin greater than 2 ng/mL are indicators of moderate disease suggests these factors may help in risk stratification, but further objective evidence is necessary in evaluating biomarker thresholds for use in discriminating disease severity.
Our study has several limitations. Although this study achieved a high level of consensus for individual measures, it did not seek to assess how the combination of individual factors affect patients’ overall clinical presentation. Additionally, we were unable to assess how other factors may impact vital signs (ie, the impact of significant altitude on SpO2 or the impact of high fever on both heart rate and respiratory rate); thus, applications of our findings at the bedside are still limited. Future studies will need to evaluate how the combination of the factors identified by expert consensus affects patients’ overall clinical presentation. Our study does provide increased detail for different strata within individual factors (eg, the extent of an effusion to be considered mild, moderate or severe) that can be incorporated into future work or clinical criteria. Additional limitations are inherent to the design of the Delphi process. First, severity criteria were identified by panelists in an open-ended fashion without prompting, thus, fine granularity on certain factors, or additional factors that some clinicians may deem important, may not have been considered. Our choice of an electronic Delphi process did not allow for panelists to have active discussions; however, it did allow for anonymity of panelists, decreasing the potential influence of individual members on the responses of others. Generating cutoffs from continuous values is challenging, and the arbitrary nature of these cutoffs, even in expert consensus methods, can make agreement on what is an “appropriate” cutoff difficult. However, given the lack of clarity in this area, we felt it important to include this information, particularly as these cutoffs were generated by open-ended free-text answers in round 1 by the panelists themselves and were not provided a priori. Finally, although developing expert consensus on factors associated with disease severity, particularly specific numerical cutoffs for continuous variables (SpO2, respiratory rate, age, etc.), can provide clinicians a frame of reference and can inform future studies, the use of these values in clinical practice remains limited at this time. We anticipate that the utility of these consensus criteria, which are the first step in informing a gap outlined by the PIDS/IDSA CAP guideline, will initially be in the design of future research in this area. Validation through prospective prognostic studies is needed before these cutoffs can be used in a clinical context.
In conclusion, this study takes an important step in addressing variability in childhood CAP severity classification by establishing expert consensus for factors that influence, and outcomes that determine, pneumonia severity in children. We evaluated many factors, at times finding agreement with current severity criteria and other times establishing novel consensus in previously incompletely described areas. Our findings provide guidance for risk stratification of children with pneumonia and future validation work. Future research should focus on establishing a more robust evidence base to validate our findings and determine how individual factors interact to contribute to disease severity. Future research in developing practical severity scores in children with CAP, informed by this work, will guide which and how many of these factors are included in prospectively developed prognostic scores.
Supplementary Material
ACKNOWLEDGMENTS
The authors sincerely thank all members of our Delphi panel for their time and expert opinion.
Disclosure: This work was supported by the American Academy of Pediatrics Research in Residency Grant. Additional funding was provided though Cincinnati Children’s Research Foundation. Dr. Florin’s effort was supported, in part, by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (K23AI121325).
Footnotes
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pec-online.com).
REFERENCES
- 1.World Health Organization. Pneumonia Fact Sheet 2015. In:2016.
- 2.Jain S, Self WH, Wunderink RG, Cdc Epic Study Team. Community-Acquired Pneumonia Requiring Hospitalization. N Engl J Med. 2015;373:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfuntner A, Wier L, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011. In. HCUP Statistical Brief #162.: Agency for Healthcare Research and Quality. Rockville, MD.; 2013. [PubMed] [Google Scholar]
- 4.Bourgeois FT, Monuteaux MC, Stack AM, et al. Variation in emergency department admission rates in US children’s hospitals. Pediatrics. 2014; 134:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florin TA, French B, Zorc JJ, et al. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132:237–244. [DOI] [PubMed] [Google Scholar]
- 6.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handy LK, Bryan M, Gerber JS, et al. Variability in antibiotic prescribing for community-acquired pneumonia. Pediatrics. 2017;139. [DOI] [PMC free article] [PubMed]
- 8.Williams DJ, Hall M, Gerber JS, et al. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139:pii: e20163231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Pocket book of hospital care for children : guidelines for the management of common childhood illnesses. Second edition, 2013 ed. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 11.Gallagher KE, Knoll MD, Prosperi C, et al. The predictive performance of a pneumonia severity score in HIV-negative children presenting to hospital in seven low and middle-income countries. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed]
- 12.Reed C, Madhi SA, Klugman KP, et al. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. PLoS One. 2012; 7:e27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emukule GO, McMorrow M, Ulloa C, et al. Predicting mortality among hospitalized children with respiratory illness in Western Kenya, 2009–2012. PLoS One. 2014;9:e92968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooli S, Colbourn T, Lufesi N, et al. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS One. 2016; 11:e0168126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1–23. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities: Evidence Summaries. In:2014. [PubMed]
- 17.Florin TA, Brokamp C, Mantyla R, et al. Validation of the Pediatric Infectious Diseases Society-Infectious Diseases Society of America severity criteria in children with community-acquired pneumonia. Clin Infect Dis. 2018;67:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeney S, Hasson F, McKenna HP. The Delphi technique in nursing and health research. Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- 19.Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araya S, Lovera D, Zarate C, et al. Application of a prognostic scale to estimate the mortality of children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2016; 35:369–373. [DOI] [PubMed] [Google Scholar]
- 21.Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Heart Association. Pediatric Advanced Life Support Provider Manual. Dallas: American Heart Association; 2016. [Google Scholar]
- 23.Advanced Life Support Group. Advanced Paediatric Life Support: A Practical Approach to Emergencies. Sixth ed. Chichester: John Wiley and Sons; 2016. [Google Scholar]
- 24.Agnello L, Bellia C, Di Gangi M, et al. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem. 2016;49:47–50. [DOI] [PubMed] [Google Scholar]
- 25.Williams DJ, Hall M, Auger KA, et al. Association of White Blood Cell Count and C-reactive protein with outcomes in children hospitalized for community-acquired pneumonia. Pediatr Infect Dis J. 2015;34:792–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Jin YU, Li H, et al. Evaluation and significance of C-reactive protein in the clinical diagnosis of severe pneumonia. Exp Ther Med. 2015;10:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018;7:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav KK, Awasthi S, Takia L, et al. Procalcitonin and C-reactive protein in WHO defined severe and very severe community acquired pneumonia: a hospital based cross-sectional study. Clinical Epidemiology and Global Health. 2015;3:S3–S9. [Google Scholar]
- 29.Don M, Valent F, Korppi M, et al. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int. 2009;51:91–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


