The authors noticed that Figure
7 of the original article is incorrect due it was reporting NICS values
instead 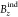 values (which is equivalent to NICSzz or
equivalent to the negative of the zz component of
the shielding tensor). In this correction, the
values (which is equivalent to NICSzz or
equivalent to the negative of the zz component of
the shielding tensor). In this correction, the 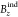 values are plotted correctly in Figure 1.
values are plotted correctly in Figure 1.
Figure 1.

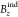 profiles are depicted and split into two
set for clearness. The orientation of the molecules has been described
in Scheme 1 of the original article: the molecular plane was placed
on the xy plane, perpendicular to the z-axis. The external magnetic field was oriented parallel to the z-axis.
profiles are depicted and split into two
set for clearness. The orientation of the molecules has been described
in Scheme 1 of the original article: the molecular plane was placed
on the xy plane, perpendicular to the z-axis. The external magnetic field was oriented parallel to the z-axis.
With this update, some changes must be considered in the main article. Thus, the first paragraph of the Induced Magnetic Field section must be reconsidered as
“The induced magnetic field was employed
in this set of
metallic cycles. As mentioned in the Computational Details section,
its z-component or  , was used in the current
work. The more
negative
, was used in the current
work. The more
negative 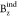 values, the more diatropic
the system is.
All the molecules analyzed in the current work presented a similar
response in shape and magnitude to the borazine’s response.
Benzene1 and borazine2 were employed as the reference of aromatic and “weak aromatic” molecules, respectively. (Borazine
is considered as “weak aromatic” or
“low aromatic” because is not equally delocalized as
benzene, due to the difference of electronegativities of boron and
nitrogen atoms.)2 All the metallabenzenoids
analyzed in this work presented a paratropic region around the center
of the ring but some of them presented a small diatropic response
around 1 Å above and below of the molecular plane. Borazine’s
value of the
values, the more diatropic
the system is.
All the molecules analyzed in the current work presented a similar
response in shape and magnitude to the borazine’s response.
Benzene1 and borazine2 were employed as the reference of aromatic and “weak aromatic” molecules, respectively. (Borazine
is considered as “weak aromatic” or
“low aromatic” because is not equally delocalized as
benzene, due to the difference of electronegativities of boron and
nitrogen atoms.)2 All the metallabenzenoids
analyzed in this work presented a paratropic region around the center
of the ring but some of them presented a small diatropic response
around 1 Å above and below of the molecular plane. Borazine’s
value of the  calculated at R = 0 (molecular
plane) was 10.4 ppm and calculated at R = 1 (1 Å
above or below of the molecular plane) was −6.1 ppm. In benzene,
the values were −16.4 and −30.7 ppm, respectively. In
borazine and benzene it is necessary to consider that σ electrons
are more localized than π electrons and their local paramagnetic
contributions generate a paratropic region around the ring’s
center, which is clearer in borazine due to the less delocalized π
electrons compared with benzene’s π electrons. Thus,
all the metallacycles could be considered as “weak aromatic” compounds in terms of the induced magnetic field.”
calculated at R = 0 (molecular
plane) was 10.4 ppm and calculated at R = 1 (1 Å
above or below of the molecular plane) was −6.1 ppm. In benzene,
the values were −16.4 and −30.7 ppm, respectively. In
borazine and benzene it is necessary to consider that σ electrons
are more localized than π electrons and their local paramagnetic
contributions generate a paratropic region around the ring’s
center, which is clearer in borazine due to the less delocalized π
electrons compared with benzene’s π electrons. Thus,
all the metallacycles could be considered as “weak aromatic” compounds in terms of the induced magnetic field.”
The second paragraph of the Conclusions section is rewritten as follows:
“According to the induced magnetic field results the molecules could be catalogued as “weak aromatic” systems. Their response was similar in shape and magnitude to borazine’s response. But phosphines groups could “interfere” in this measurement (such as in 2 and 9), due to the diatropic contribution of atomic nuclei; for this reason, the MICD analysis was performed.”
The line of the third paragraph of the Conclusions:
“The non-planar rings in 4,
and 6 are diatropic with the 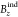 results, but the MICD values indicate a
“weak aromatic” response if the total
strength current is integrated.”
results, but the MICD values indicate a
“weak aromatic” response if the total
strength current is integrated.”
should now read
“The MICD values of 4 and 6 indicate
a “weak aromatic” response if the total
strength current is integrated. Considering this MICD values and according
with its  values and its no
planar structure, 6 could be considered as a no aromatic
compound”.
values and its no
planar structure, 6 could be considered as a no aromatic
compound”.
The authors apologize with the readers and colleagues for any confusion caused.
References
- Heine T.; Islas R.; Merino G. σ and π contributions to the induced magnetic field: Indicators for the mobility of electrons in molecules. J. Comput. Chem. 2007, 28 (1), 302–309. 10.1002/jcc.20548. [DOI] [PubMed] [Google Scholar]
- Islas R.; Chamorro E.; Robles J.; Heine T.; Santos J. C.; Merino G. Borazine: to be or not to be aromatic. Struct. Chem. 2007, 18 (6), 833–839. 10.1007/s11224-007-9229-z. [DOI] [Google Scholar]


