Abstract
In this article, nano-CuCr2O4 (copper chromite)/ultrafine ammonium perchlorate (AP) composites were prepared by a ultrasonic dispersion method and a mechanical grinding method. A series of nano-CuCr2O4/ultrafine AP composites with different dispersions were prepared by controlling the compounding time to study the best catalytic effect of nano-CuCr2O4 on the ultrafine AP. The microstructures, surface elements, and morphologies of samples were analyzed by X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, and energy dispersion X-ray spectroscopy. The catalytic effect of nano-CuCr2O4 on the thermal decomposition of AP was investigated by differential scanning calorimetric techniques and thermogravimetric analysis. The results indicated that the mechanical ball milling method could make nano-CuCr2O4 more evenly dispersed on the ultrafine AP, and with the increase in the milling time, the uniformity of nano-CuCr2O4 on the ultrafine AP was better. When the milling time was 6–12 h, nano-CuCr2O4 was most evenly dispersed on the ultrafine AP. At this time, the decomposition temperature and Gibbs free energy of the nano-CuCr2O4/ultrafine AP composite were the lowest, which decreased by 78.1 °C and 25.16 kJ/mol compared with those of ultrafine AP, respectively. Moreover, the mechanical sensitivity of nano-CuCr2O4/ultrafine AP composites was lower than that of ultrafine AP. It showed that ball milling for 6–12 h could make nano-CuCr2O4 evenly dispersed on the ultrafine AP, and nano-CuCr2O4 could play the best catalytic effect on the ultrafine AP.
1. Introduction
Ammonium perchlorate (AP) is the most widely used oxidant in the propellant applications, and its content in propellant accounts for a large proportion, usually exceeding 60%.1,2 As a strong oxidant, the thermal decomposition of AP provides the oxygen needed for the combustion of the propellant and releases a large amount of energy during the decomposition process to promote the combustion of the propellant.3,4 Therefore, the pyrolysis of AP plays a decisive role in the combustion performance of the propellant.5 To be specific, decreasing decomposition temperatures of the AP decomposition process will lead to shorter ignition delay time and higher burning rate of the propellant.6
A lot of studies have shown that the burning rate of propellant can be increased by decreasing the particle diameter of AP7−9 as well as employing some combustion catalyst to AP.10,11 For example, Dolgoborodov et al. used a planetary ball mill method to prepare ultrafine AP of size 5–10 μm, and the temperature of the ultrafine AP thermal decomposition decreased by more than 100 °C than raw AP with a particle size of 1000 μm.12 Compared with the reduction in the particle size of AP, the addition of combustion catalysts to AP has caught most attention. Among these catalysts, copper chromite, a mixed oxide of CuO and Cr2O3, is well-known burn rate modifier for combustion of propellants, which can accelerate the thermal decomposition of AP and increase the burn rate of propellant combustion. Especially when the catalyst reaches the nanometer size, it can better exert its catalytic effect. For example, Sanoop et al. prepared copper chromite nanoparticles by the thermal decomposition of basic copper ethylamine chromate to catalyze AP decomposition and decreased AP decomposition temperature from 378.5 to 338.6 °C.13 Hosseini et al. synthesized copper chromite spinel nanoparticles (CuCr2O4 SNPs) via the sol–gel route and studied its catalytic effect on AP in CuCr2O4 SNPs/AP composite prepared by the solvent/nonsolvent route. The results showed that the addition of CuCr2O4 SNPs to AP remarkably decreased the decomposition temperature from 422.5 °C for pure AP to 338.6 °C for AP containing CuCr2O4 SNP additive with a mass ratio of 5% and improved the heat released from 879.5 J/g for pure AP to 1474.5 J/g for CuCr2O4 SNPs/AP composite.14 Further research found that uniformly compounding the nanocatalyst and AP can further enhance its catalytic effect on AP. Eslami et al. fabricated the AP/CuCr2O4 core–shell nanocomposites containing CuCr2O4 additive with a mass ratio of 6% by a feasible deposition method and found that the decomposition temperature of AP composites was at 346 °C, which decreased by 74 °C compared to AP, and the apparent decomposition heat of AP composite was about three times that of AP, changing from 450 J/g for AP to 1510 J/g for AP composite.15
Currently, little attention has been paid to the catalysis of ultrafine AP. In fact, evenly compounding the nanocatalyst with the ultrafine AP can significantly improve the thermal decomposition performance of the AP.16,17 Therefore, nano-CuCr2O4/ultrafine AP composites would be prepared by an ultrasonic dispersion method and a mechanical grinding method, and the best catalytic effect of nano-CuCr2O4 on ultrafine AP was studied in this work.
2. Experiment
2.1. Materials
All the reagents were of analytical grade except AP and directly used without further purification. AP was of industrial grade and was purchased from Dalian Perchloric Acid Ammonium Factory. CuCr2O4 was purchased from Sinopharm Chemical Reagent Co., Ltd. Ethyl acetate was purchased from Nanjing Chemical Reagent Co., Ltd. Nano-CuCr2O4 with an average diameter of 50 nm was prepared by mechanical grinding. Ultrafine AP was prepared by air flow crushing method and its particle was 1–5 μm.
2.2. Preparation of Nano-CuCr2O4/Ultrafine AP Composites
The nano-CuCr2O4/ultrafine AP composites were prepared by ultrasonic dispersion and mechanical ball milling methods. First, nano-CuCr2O4/ultrafine AP composite was prepared by the ultrasonic dispersion method. The nano-CuCr2O4 and ultrafine AP were mixed at a molar ratio of 3:97 to form a mixture of 10 g, and the mixture was added to the beaker. Ethyl acetate (5 mL) was added to the beaker, and the amount of ethyl acetate added just covered the mixture powder. The ultrasonic cleaner was turned on, and the nano-CuCr2O4 and ultrafine AP were compounded by ultrasonic action. After 30 min, the ultrasonic cleaner was turned off. After ultrasonic treatment, the sample was poured into the surface dish and then dried in a 45 °C water bath oven. Nano-CuCr2O4/ultrafine AP composite was obtained after complete drying.
Then, a series of nano-CuCr2O4/ultrafine AP composites with different dispersions were prepared by the mechanical ball milling method. The nano-CuCr2O4 and ultrafine AP were mixed at a molar ratio of 3:97 to form a mixture of 20 g. The mixture was put into 200 mL of ethyl acetate, and the mass concentration of the mixture in the slurry was kept strictly at 8–10%. The slurry was ground and crushed in nanometer pulverizer. The speed of rotation was 1000 rpm. The outlet temperature of slurry was controlled in the range of 15 °C by the chiller. To explore the best catalytic effect of nano-CuCr2O4 on ultrafine AP, the ball milling time would be controlled. Here, a series of nano-CuCr2O4/ultrafine AP composites with different dispersions would be prepared by selecting different ball milling times (30 min and 1, 3, 6, 12, 24, and 48 h). After grinding, a slurry of nano-CuCr2O4/ultrafine AP composites was obtained. The slurry supernatant was removed by siphoning after natural sedimentation of the slurry. The slurry was poured into the surface dish and then dried in a 45 °C water bath oven. Nano-CuCr2O4/ultrafine AP composites were obtained after complete drying.
Finally, nano-CuCr2O4/ultrafine AP composites with different dispersions were numbered, and their types and numbers are shown in Table 1. To facilitate the description of different samples in this paper, the samples were named as CuCr2O4/AP-1, CuCr2O4/AP-2, CuCr2O4/AP-3, CuCr2O4/AP-4, CuCr2O4/AP-5, CuCr2O4/AP-6, CuCr2O4/AP-7, and CuCr2O4/AP-8.
Table 1. Prepration of a Series of Nano-CuCr2O4/Ultrafine AP Composites with Different Dispersions.
| number of samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| nano-CuCr2O4/ultrafine AP composites | ultrasonic for 30 min | ball milling for 30 min | ball milling for 1 h | ball milling for 3 h | ball milling for 6 h | ball milling for 12 h | ball milling for 24 h | ball milling for 48 h |
2.3. Measurements and Characterizations
Powder X-ray diffraction (XRD) patterns were performed with a Bruker Advance D8 instrument equipped with Cu Kα radiation. Fourier transform infrared (FTIR) analysis of the samples was carried out (Nicolet iS10) in the range of 4000–500 cm–1. The morphology of nano-CuCr2O4/ultrafine AP composites was examined by an S-4800II cold field scanning electron microscopy (Hitachi Corporation, Japan) equipped with an energy dispersion X-ray spectrometer (EDS), and their chemical compositions were determined by EDS.
The thermogravimetric tests were carried out employing an SDT Q600 thermal analyzer with a gas flow rate of 20 mL/min in nitrogen. The selected heating rates were 5, 10, 15, and 20 °C/min. The program temperature was increased from room temperature to about 520 °C at the end. The kinetic parameters for the exothermic decomposition of AP and AP composites with different nanocatalysts were obtained by the Kissinger method.18
The sensitivity test in this paper is impact sensitivity test. The impact sensitivity of the samples is determined according to the method 601.2 in GJB 772A-1997. The dosage of the samples used is 50 mg, and the weight of a drop hammer is 5 kg. The test samples are divided into 25 times as a group, and the “lifting method” is used to measure the characteristic drop height of the samples. If there is no explosion, the height of the drop hammer will be increased, and if there is explosion, the height of the drop hammer will be reduced. The range of height is within six grades. For the measured data, use eq 1 to calculate the characteristic drop height
| 1 |
where n is the total number of experiments; d is the logarithm of step size; Y0 is the logarithm of the characteristic height when the stimulus number is “0”; i is the stimulus number, and its values are i= ±1, ±2, and so on. If the value is smaller than Y0, it is negative, and if it is larger, it is positive; and ni is the number of explosions when the stimulus number is i.
3. Results and Discussion
3.1. XRD and FTIR Analysis
The structural information of nano-CuCr2O4/ultrafine AP composites is analyzed using XRD and FTIR. Figure 1 shows the XRD pattern of ultrafine AP and nano-CuCr2O4/ultrafine AP composites, respectively. It can be seen from Figure 1 that the characteristic diffraction peaks of nano-CuCr2O4/ultrafine AP composites with different dispersions are basically the same, only the intensity of the diffraction peaks is different. According to the XRD pattern of CuCr2O4/AP-1, the characteristic diffraction peaks of its XRD pattern are completely consistent with those of the ultrafine AP. It shows that in the process of preparing nano-CuCr2O4/ultrafine AP composites, the ultrafine AP does not participate in the reaction, and the crystal form of ultrafine AP does not change, only physical and mechanical composites occur. However, the characteristic peaks of nano-CuCr2O4 are not found in the XRD spectrum of nano-CuCr2O4/ultrafine AP composites, which are mainly because the intensity of the characteristic diffraction peaks of ultrafine AP is too large, and the content of nano-CuCr2O4 is too small to reach the detection range.19 Therefore, the XRD pattern of nano-CuCr2O4 is not listed in the figure to compare with that of CuCr2O4/AP-1.
Figure 1.
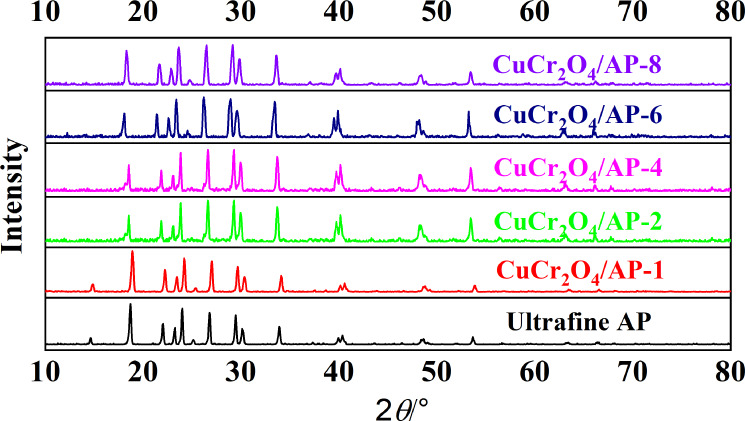
XRD patterns of ultrafine AP and nano-CuCr2O4/ultrafine AP composites.
IR spectrums of nano-CuCr2O4, ultrafine AP, and nano-CuCr2O4/ultrafine AP composites are shown in the Figure 2. As shown from the IR spectrum of nano-CuCr2O4, the observed IR band in the range of 600–540 cm–1 can be attributed to a stretching vibration of Cr–O bands of chromium atoms in the tetragonal environment of the O atom.20 The characteristic peaks of the nano-CuCr2O4 sample at 691 and 515 cm–1 could be attributed to the presence of Cr2O42– group, and the characteristic peak at 884 cm–1 refers to Cr–O chromate group.13,21 For FTIR spectrum of ultrafine AP, the broad absorption bands at 3278 and 1408 cm–1 correspond to the N–H stretching vibration and the N–H bending vibration of the AP crystal, respectively. The absorption peak at about 1050 and 622 cm–1 correspond to the Cl–O vibration and ClO4– vibration absorption, respectively. It can be seen from Figure 2 that the shape and position of the infrared absorption peaks of different nano-CuCr2O4/ultrafine AP composites are basically the same, so CuCr2O4/AP-1 is taken as the representative for the analysis. According to the FTIR spectrum of CuCr2O4/AP-1, there are four obvious infrared absorption peaks, which are consistent with the infrared absorption peaks of ultrafine AP. The stretching vibration absorption peaks of NH4+ are around 3277 and 1409 cm–1, and the stretching vibration absorption peaks of ClO4– are around 1037 and 615 cm–1.19 In addition, compared with the ultrafine AP, a new infrared absorption peak appears near 780 cm–1. Compared with the FTIR image of nano-CuCr2O4, the new infrared absorption peak is the stretching vibration absorption peak of chromate group (Cr2O42–), indicating that nano-CuCr2O4 is compounded on the surface of the ultrafine AP.15 Through FTIR spectrum, it can be confirmed that nano-CuCr2O4/ultrafine AP composites can be successfully prepared by the ultrasonic dispersion and mechanical ball milling methods, and in the preparation process, the ultrafine AP does not participate in the reaction, and the crystal form does not change, only physical and mechanical composites occur.
Figure 2.
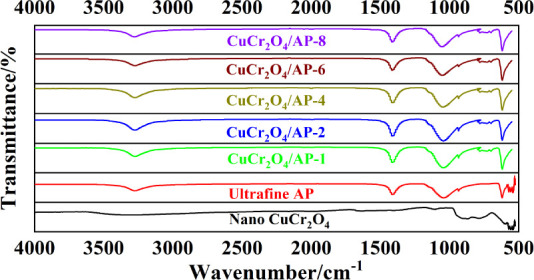
FTIR spectrums of ultrafine AP, nano-CuCr2O4, and nano-CuCr2O4/ultrafine AP composites.
3.2. SEM and EDS Analysis
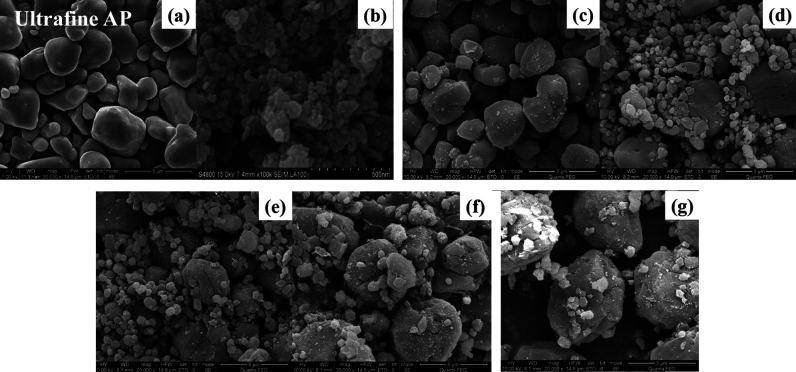
The morphologies of ultrafine AP, nano-CuCr2O4, and nano-CuCr2O4/ultrafine AP composites measured by SEM are presented in Figure 3. As shown in Figure 3a,b, the particle size of ultrafine AP and nano-CuCr2O4 is 1–5 μm, respectively. In Figure 3c, the particle size of ultrafine AP is consistent with that in Figure 3a before compounding. The particles present a spherical shape, and the surface becomes rough from smooth. It is obvious that nano-CuCr2O4 is attached to the surface of ultrafine AP. However, only a small amount of nano-CuCr2O4 is dispersed on the ultrafine AP, and the dispersion is very uneven. It can be seen from Figure 3d–g that under the force of mechanical ball milling the particle size of some ultrafine AP is further reduced, basically reaching the nanometer level. Moreover, with the increase in the milling time, more and more ultrafine AP particles are further crushed. More and more nano-CuCr2O4 is dispersed on the surface of ultrafine AP, and its distribution is more uniform. As seen from Figure 3g, the nano-AP gradually accumulates into large spherical particles with a particle size of 5–10 μm. This is mainly due to the long time grinding that some water vapor enters into the ball milling tank. The ultrafine AP is very easy to absorb water, so agglomeration occurs. The agglomeration is formed by the stacking of nano-AP, which makes the nano-CuCr2O4 on the nano-AP enter into the interior of the large spherical AP particles, so the nano-CuCr2O4 is more evenly dispersed in the ultrafine AP. With the further increase in the ball milling time, the ultrafine AP has no further change. It can be seen from Figure 3 that nano-CuCr2O4 can be considered to be very evenly dispersed in the ultrafine AP when the ball milling time is 6–12 h.
Figure 3.
SEM images of (a) ultrafine AP; (b) nano-CuCr2O4; and nano-CuCr2O4/ultrafine AP composites: (c) CuCr2O4/AP-1, (d) CuCr2O4/AP-2, (e) CuCr2O4/AP-4, (f) CuCr2O4/AP-6, and (g) CuCr2O4/AP-8.
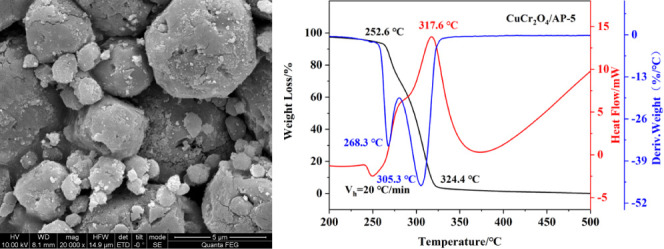
To better characterize the uniformity of nano-CuCr2O4/ultrafine AP composites, the surface element distribution characterization of CuCr2O4/AP-5 is carried out, and the image is shown in Figure 4. It is clear from Figure 4 that in nano-CuCr2O4/ultrafine AP composite the elements Cu and Cr of nano-CuCr2O4 are very evenly distributed in the elements Cl, O, and N of ultrafine AP, confirming that the nano-CuCr2O4 has good dispersion on the surface of ultrafine AP. This shows that nano-CuCr2O4 can be evenly loaded on the surface of the ultrafine AP via the mechanical grinding method.
Figure 4.
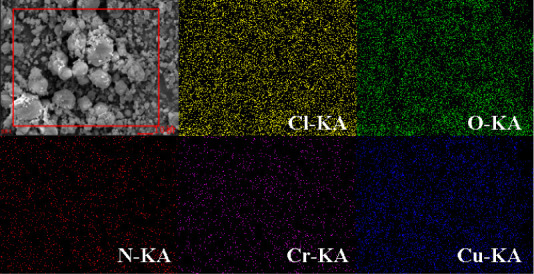
EDS mappings of CuCr2O4/AP-5.
3.3. Thermal Analysis
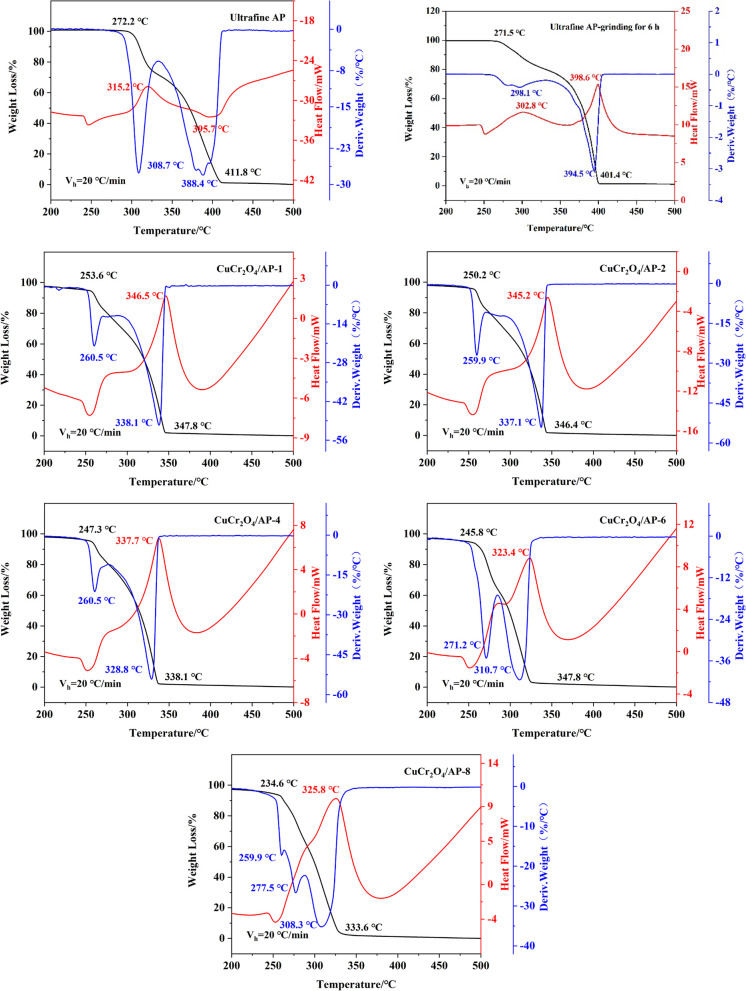
The differential scanning calorimetric (DSC)–thermogravimetric (TG)–thermogravimetric derivative (DTG) curves of ultrafine AP and different nano-CuCr2O4/ultrafine AP composites at 20 °C/min are shown in Figure 5, and the data of thermal decomposition properties are shown in Table 2. Besides, the DSC–TG–DTG curves of ultrafine AP, which was ground for 6 h, is also shown in Figure 5 for comparison. It can be seen from Figure 5 and Table 2 that after adding nano-CuCr2O4 the thermal decomposition reaction of the whole ultrafine AP is advanced. From the DSC curve, it can be seen that the low temperature decomposition peak of ultrafine AP is not obvious, and it is basically fused with high temperature decomposition peaks. From the TG curve, it is only a weight loss process. CuCr2O4/AP-1 and CuCr2O4/AP-2 prepared by the ultrasonic dispersion and mechanical ball milling methods can reduce the TH of ultrafine AP from 395.7 to 346.5 and 345.2 °C, respectively, which are decreased by 49.2 and 50.5 °C. It shows that the thermal decomposition performance of nano-CuCr2O4/ultrafine AP composite prepared by the mechanical ball milling method is better, and the catalytic effect of nano-CuCr2O4 on ultrafine AP is better. With the increase in the milling time from 0.5 to 48 h, the TH of ultrafine AP are 345.2, 340.5, 337.7, 317.6, 323.4, 339.8, and 325.8 °C,, which are decreased by 50.5, 55.2, 58.0, 78.1, 72.3, 55.9, and 69.9 °C than those without catalyst, respectively. It is indicated that the catalytic effect of nano-CuCr2O4 on ultrafine AP increases first and then decreases with the increase in the milling time. It can be seen from the DTG curve that with the increase in the ball milling time, the law of Tm of ultrafine AP is consistent with that of TH, which further confirms that the catalytic effect of nano-CuCr2O4 on ultrafine AP first increases and then decreases with the increase in the ball milling time. This is mainly because with the increase in the ball milling time, nano-CuCr2O4 disperses more evenly in ultrafine AP, showing better catalytic effect. However, when the ball milling time is too long, the catalytic effect of nano-CuCr2O4 is weakened due to the agglomeration effect. Therefore, the TH of ultrafine AP is the lowest when the milling time is 6–12 h. It is indicated that the dispersion of nano-CuCr2O4 in ultrafine AP is the best and the most uniform, so it shows the best catalytic effect.
Figure 5.
DSC–TG–DTG curves of ultrafine AP, ultrafine AP ground for 6 h, and nano-CuCr2O4/ultrafine AP composites.
Table 2. Data of Thermal Decomposition Properties of Ultrafine AP and Nano-CuCr2O4/Ultrafine AP Compositesa.
| name of samples | Te (°C) | Tc (°C) | Tm (°C) | TL (°C) | TH (°C) |
|---|---|---|---|---|---|
| ultrafine AP | 272.2 | 411.8 | 388.4 | 315.2 | 395.7 |
| CuCr2O4/AP-1 | 253.6 | 347.8 | 338.1 | 346.5 | |
| CuCr2O4/AP-2 | 250.2 | 346.4 | 337.1 | 345.2 | |
| CuCr2O4/AP-3 | 255.1 | 345.9 | 332.7 | 340.5 | |
| CuCr2O4/AP-4 | 247.3 | 338.1 | 328.8 | 337.7 | |
| CuCr2O4/AP-5 | 252.6 | 324.4 | 305.3 | 317.6 | |
| CuCr2O4/AP-6 | 245.8 | 347.8 | 310.7 | 323.4 | |
| CuCr2O4/AP-7 | 242.3 | 340.5 | 332.2 | 339.8 | |
| CuCr2O4/AP-8 | 234.6 | 333.6 | 308.3 | 325.8 |
Te is the initial decomposition temperature; Tc is the terminal decomposition temperature; Tm is the maximum weight loss temperature; TL is the low temperature decomposition temperature; and TH is the high temperature decomposition temperature.
According to Figure 5, after the ultrafine AP with an average particle size of 1–5 μm was ground for 6 h, its pyrolysis peak temperature changed from 395.7 to 398.6 °C, confirming that the decrease in the high temperature decomposition temperature of the nano-CuCr2O4/ultrafine AP composite is mainly due to the effect of nano-CuCr2O4.
The catalytic mechanism of nano-CuCr2O4 for ultrafine AP is mainly because CuCr2O4 is a p-type semiconductor with conductive holes, which shows high activity in the oxidation atmosphere and can participate in the electron transfer. The addition of CuCr2O4 to AP can accelerate the thermal decomposition of AP by a redox reaction with the high temperature decomposition products of AP. After nano-CuCr2O4 is compounded with ultrafine AP by the mechanical ball milling, the dispersion of nano-CuCr2O4 in ultrafine AP is more uniform, and the contact area between nano-CuCr2O4 and ultrafine AP is larger. This is more conducive to the reaction of holes in nano-CuCr2O4 and oxides decomposed by ultrafine AP at high temperature, thus showing better catalytic performance for ultrafine AP.
The catalytic effect of different catalysts on AP reported in the literatures is listed in Table 3. Compared with other catalysts, TH of AP decreases to the lowest when CuCr2O4 is added to AP, which indicates that CuCr2O4 shows better catalytic effect on AP than most other catalysts. Compared with the reports in other literatures, the prepared nano-CuCr2O4/ultrafine AP composite in this paper has a lower TH and better thermal decomposition performance. This is mainly due to the fact that nano-CuCr2O4 can be more evenly distributed on the ultrafine AP surface by the mechanical grinding method, so it can show better catalytic effect.
Table 3. The Comparison of Catalytic Effect of Different on AP Reported in the Literatures and This Study.
| number | type of catalysts | preparation method of AP composite | usage amount of catalyst (%) | heating rate (°C/min) | TH (°C) | references |
|---|---|---|---|---|---|---|
| 1 | CuCr2O4 | mechanical grinding | 3 | 10 | 305.4 | this study |
| 2 | CuCr2O4 | mechanical grinding | 3 | 20 | 317.6 | this study |
| 3 | CuCr2O4 | thermal decomposition | 3 | 10 | 331.1 | (13) |
| 4 | CuCr2O4 | sol–gel | 5 | 10 | 338.6 | (14) |
| 5 | CuCr2O4 | electrochemical | 2 | 10 | 349.4 | (22) |
| 6 | CuCr2O4 | chemical liquid deposition | 6 | 10 | 346.0 | (15) |
| 7 | CuO | coprecipitation | 1 | 10 | 350.4 | (23) |
| 8 | CuO | surfactant-mediated method | 1 | 10 | 317.2 | (24) |
| 9 | CuO | sol–gel | 3 | 20 | 353.1 | (25) |
| 10 | CuO | hydrothermal | 2 | 20 | 339.3 | (26) |
| 11 | CuO@Cr2O3 | mechanical grind method | 2 | 20 | 351.1 | (27) |
| 12 | CuO/Fe2O3 | solid phase method | 2 | 20 | 335.3 | (28) |
| 13 | CNTs/CuO | coprecipitation | 8 | 20 | 333.1 | (29) |
3.4. Kinetic Analysis
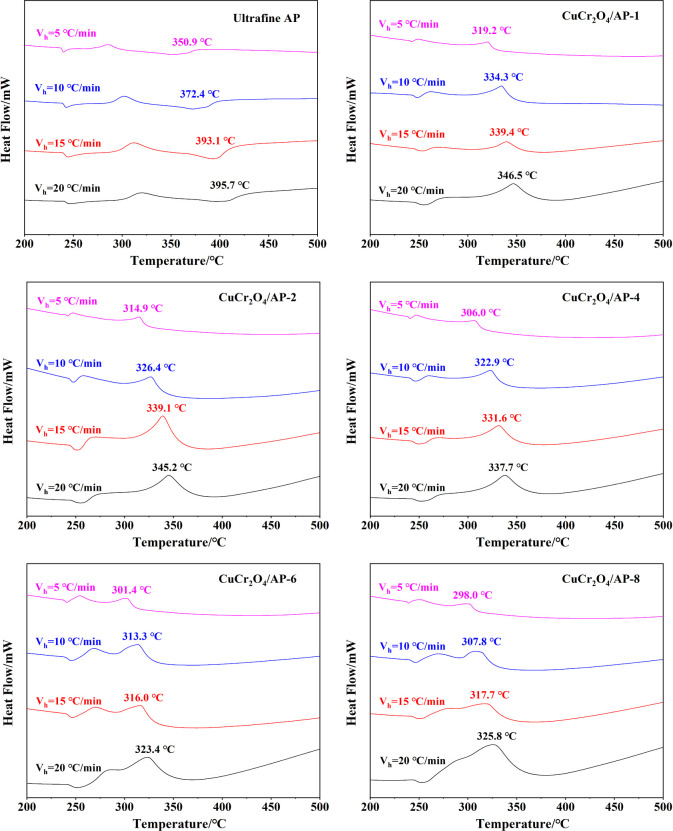
To further study the pyrolysis of nano-CuCr2O4/ultrafine AP composites, the thermal decomposition kinetic parameters are calculated. The DSC curves of ultrafine AP and different nano-CuCr2O4/ultrafine AP composites at heating rates of 5, 10, 15, and 20 °C/min are shown in Figure 6. It can be seen from Figure 6 that with the increase in the heating rate, TH of nano-CuCr2O4/ultrafine AP composites gradually increases. This may be due to the fact that with an increase in the heating rate, the heat transfer rate inside the specimen is less than the growth rate of the program temperature. Then, the decomposition rate is not able to keep up with the growth rate of the program temperature. This is so-called “temperature hysteresis phenomenon”.
Figure 6.
DSC curves of ultrafine AP and nano-CuCr2O4/ultrafine AP composites at heating rates of 5, 10, 15, and 20 °C/min.
For TH at different heating rates, the Kissinger method is used to calculate the thermal decomposition kinetic parameters of nano-CuCr2O4/ultrafine AP composites. Figure 7 shows ln(β/TP2) ∼ 1000/TP diagram of nano-CuCr2O4/ultrafine AP composites. Table 4 shows the linear fitting equations of ln(β/TP2) ∼ 1000/TP of ultrafine AP and different nano-CuCr2O4/ultrafine AP composites.
Figure 7.
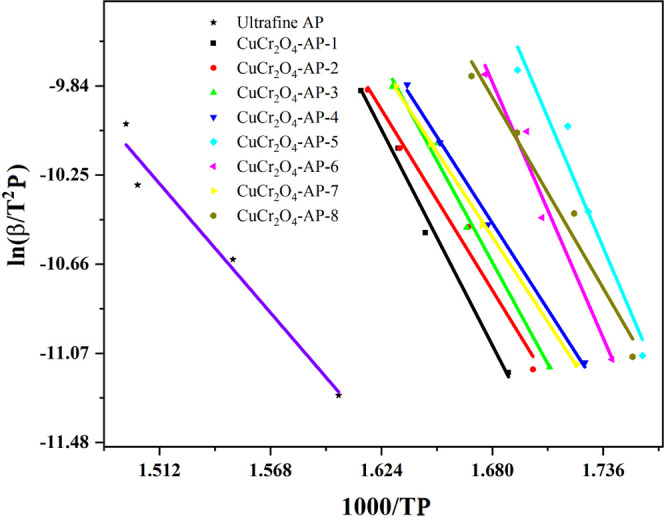
The ln(β/TP2) ∼ 1000/TP diagram of ultrafine AP and nano-CuCr2O4/ultrafine AP composites.
Table 4. Linear Fitting Equations of ln(β/TP2) ∼ 1000/TP of Ultrafine AP and Different Nano-CuCr2O4/Ultrafine AP Composites.
| name of samples | linear fitting equations | R2 |
|---|---|---|
| ultrafine AP | y = −10.59712x + 5.73371 | 0.96955 |
| CuCr2O4/AP-1 | y = −17.71734x + 18.73228 | 0.98826 |
| CuCr2O4/AP-2 | y = −14.85011x + 14.16850 | 0.98123 |
| CuCr2O4/AP-3 | y = −16.19638x + 17.40815 | 0.98723 |
| CuCr2O4/AP-4 | y = −14.19638x + 13.37943 | 0.99703 |
| CuCr2O4/AP-5 | y = −21.38111x + 26.53310 | 0.95128 |
| CuCr2O4/AP-6 | y = −21.02202x + 25.49423 | 0.96962 |
| CuCr2O4/AP-7 | y = −13.97709x + 12.94707 | 0.99923 |
| CuCr2O4/AP-8 | y = −15.6849x + 16.45807 | 0.97340 |
According to the intercept and slope in the linear fitting equation, the thermal decomposition kinetics/kinetic parameters of nano-CuCr2O4/ultrafine AP composites can be obtained by thermal decomposition calculation, as shown in Table 5.
Table 5. The Thermal Decomposition Kinetics/Kinetic Parameters of Ultrafine AP and Nano-CuCr2O4/Ultrafine AP Composites.
| name of samples | Ea (kJ/mol) | Ln A (s–1) | k(TP) (s–1) | ΔS≠ (J/mol K) | ΔH≠ (kJ/mol) | ΔG≠ (kJ/mol) |
|---|---|---|---|---|---|---|
| ultrafine AP | 88.10 | 15.00 | 4.30 × 10–1 | –126.92 | 82.54 | 167.43 |
| CuCr2O4/AP-1 | 147.30 | 28.51 | 6.22 × 10–1 | –13.85 | 142.22 | 150.68 |
| CuCr2O4/AP-2 | 123.46 | 23.77 | 5.69 × 10–1 | –53.24 | 118.39 | 150.88 |
| CuCr2O4/AP-3 | 138.86 | 27.13 | 6.54 × 10–1 | –25.25 | 133.82 | 149.12 |
| CuCr2O4/AP-4 | 118.03 | 22.94 | 5.25 × 10–1 | –60.03 | 113.03 | 149.16 |
| CuCr2O4/AP-5 | 177.76 | 36.50 | 6.30 × 10–1 | –53.04 | 172.95 | 142.27 |
| CuCr2O4/AP-6 | 174.78 | 35.45 | 5.73 × 10–1 | –44.23 | 169.93 | 144.10 |
| CuCr2O4/AP-7 | 116.21 | 22.49 | 5.49 × 10–1 | –63.82 | 111.18 | 149.81 |
| CuCr2O4/AP-8 | 130.40 | 26.12 | 4.25 × 10–1 | –33.30 | 125.57 | 144.93 |
It can be seen from Table 5 that Ea of high temperature decomposition of nano-CuCr2O4/ultrafine AP composites prepared by the mechanical ball milling method is lower than that prepared by the ultrasonic dispersion method. With the increase in the milling time, the change in Ea is not obvious. Ea is the lowest when milling for 3 h and then increases significantly at 6–12 h. But with the further milling, it continues to decrease. However, by comparing the ΔG≠ of nano-CuCr2O4/ultrafine AP composites, it can be seen that the ΔG≠ of ultrafine AP decreases significantly after adding nano-CuCr2O4 catalyst, which indicates that nano-CuCr2O4 can make the thermal decomposition reaction of ultrafine AP easier. The lowest ΔG≠ is 142.27 kJ/mol, which is 25.16 kJ/mol lower than that of ultrafine AP, when milling for 6–12 h. It is indicated that nano-CuCr2O4 has the best catalytic effect in ultrafine AP by the mechanical ball milling for 6–12 h. In the continuous pyrolysis process of AP, nano-CuCr2O4 accelerates the thermal decomposition of AP and plays the role of adsorption and catalysis. However, nano-CuCr2O4 is a kind of inorganic substance, which does not contain energy. At this time, there is no continuous heating, which is mainly reflected in the reduction of impact sensitivity. The same results have been reported in many literatures. We have also obtained the similar conclusion that nano-Cu(OH)2 could reduce the sensitivity of AP.30 In this paper, the activation energy increased, and the A has also increased at the same time, resulting in an increase in the reaction rate k, which reflected in the lower ΔG≠ value. In this case, the increase in the A (the positive effect on the reaction rate) exceeds the increase in the activation energy (the negative effect on the reaction rate), which is comprehensively reflected by the increase in the reaction rate.31
3.5. Sensitivity Analysis
The impact sensitivity data of ultrafine AP and nano-CuCr2O4/ultrafine AP composites are shown in Table 6. It can be seen from Table 6 that the impact sensitivity of nano-CuCr2O4 combined with ultrafine AP is slightly lower than that of ultrafine AP, and the impact sensitivity tends to decrease with the increase in the ball milling time. This is because nano-CuCr2O4 coated on the surface of ultrafine AP can absorb and buffer energy, thus reducing the probability of hot spot formation on the surface of ultrafine AP, the impact sensitivity is reduced. As the dispersion of nano-CuCr2O4 on the surface of ultrafine AP gets better and better, it can play a better role in reducing the sensitivity, and the effect is the best when milling for 6–12 h.
Table 6. The Impact Sensitivity Data of Ultrafine AP and Nano-CuCr2O4/Ultrafine AP Composites.
| name of samples | impact sensitivity H50 (cm) | Sdev. |
|---|---|---|
| ultrafine AP | 35.1 | 0.14 |
| CuCr2O4/AP-1 | 35.5 | 0.11 |
| CuCr2O4/AP-2 | 35.8 | 0.12 |
| CuCr2O4/AP-3 | 36.9 | 0.10 |
| CuCr2O4/AP-4 | 36.2 | 0.09 |
| CuCr2O4/AP-5 | 37.6 | 0.09 |
| CuCr2O4/AP-6 | 37.1 | 0.08 |
| CuCr2O4/AP-7 | 36.8 | 0.10 |
| CuCr2O4/AP-8 | 37.3 | 0.11 |
4. Conclusions
(1) Nano-CuCr2O4/ultrafine AP composites are successfully prepared by the ultrasonic dispersion and mechanical ball milling methods. Compared with nano-CuCr2O4/ultrafine AP composite prepared by ultrasonic method, the nano-CuCr2O4 in ultrafine AP composites prepared by the mechanical ball milling has better dispersion on the ultrafine AP. With the increase in the milling time, the dispersion of nano-CuCr2O4 on ultrafine AP becomes better and better. When milling for 6–12 h, nano-CuCr2O4 disperses most evenly in ultrafine AP.
(2) After adding nano-CuCr2O4, the thermal decomposition performance of ultrafine AP is obviously improved, which shows that the high temperature decomposition temperature is advanced and the Gibbs free energy is reduced. The high temperature decomposition temperature and Gibbs free energy of nano-CuCr2O4/ultrafine AP composite prepared by ball milling for 6–12 h are the lowest, which decrease at 78.1 °C and 25.16 kJ/mol, respectively, indicating that nano-CuCr2O4 has the best catalytic effect on ultrafine AP. Moreover, the mechanical sensitivity of nano-CuCr2O4/ultrafine AP composites is lower than that of ultrafine AP.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (Project no. 21805139), China Postdoctoral Science Foundation (no. 2020M673527), and the Fundamental Research Funds for the Central Universities (no. 30919011271).
The authors declare no competing financial interest.
References
- Zhang L. K.; Tian R. Y.; Zhang Z. W. Burning rate of AP/HTPB base-bleed composite propellant under free ambient pressure. Aerosp. Sci. Technol. 2017, 62, 31–35. 10.1016/j.ast.2016.12.004. [DOI] [Google Scholar]
- Manash A.; Kumar P. Comparison of burn rate and thermal decomposition of AP as oxidizer and PVC and HTPB as fuel binder based composite solid propellants. Def. Technol. 2019, 15, 227–232. 10.1016/j.dt.2018.08.010. [DOI] [Google Scholar]
- Wang J. H.; Zhang W. C.; Zheng Z. L.; Gao Y.; Ma K. F.; Ye J. H.; Yang Y. Enhanced thermal decomposition properties of ammonium perchlorate through addition of 3DOM core–shell Fe2O3/Co3O4 composite. J. Alloys Compd. 2017, 724, 720–727. 10.1016/j.jallcom.2017.07.033. [DOI] [Google Scholar]
- Hedman T. D.; Gross M. L. On the thermal stability of partially decomposed ammonium perchlorate. Propellants, Explos., Pyrotech. 2016, 41, 254–259. 10.1002/prep.201500067. [DOI] [Google Scholar]
- Nagendra K.; Vijay C.; Ingole M.; Ramakrishna P. A. Combustion of ammonium perchlorate monopropellant: Role of heat loss. Combust. Flame 2019, 209, 363–375. 10.1016/j.combustflame.2019.08.004. [DOI] [Google Scholar]
- Xiao X. C.; Zhang Z. Y.; Cai L. F.; Li Y. T.; Yan Z. Y.; Wang Y. D. The excellent catalytic activity for thermal decomposition of ammonium perchlorate using porous CuCo2O4 synthesized by template-free solution combustion method. J. Alloys Compd. 2019, 797, 548–557. 10.1016/j.jallcom.2019.05.074. [DOI] [Google Scholar]
- Chaturvedi S.; Dave P. N. A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2013, 17, 135–149. 10.1016/j.jscs.2011.05.009. [DOI] [Google Scholar]
- Vara J. A.; Dave P. N.; Chaturvedi S. The catalytic activity of transition metal oxide nanoparticles on thermal decomposition of ammonium perchlorate. Def. Technol. 2019, 15, 629–635. 10.1016/j.dt.2019.04.002. [DOI] [Google Scholar]
- Hu Y. H.; Tao B. W.; Shang F.; Zhou M. X.; Hao D. Y.; Fan R. Q.; Xia D. B.; Yang Y. L.; Pan A. M.; Lin K. F. Thermal decomposition of ammonium perchlorate over perovskite catalysts: Catalytic decomposition behavior, mechanism and application. Appl. Surf. Sci. 2020, 513, 145849 10.1016/j.apsusc.2020.145849. [DOI] [Google Scholar]
- Ye P.; Lu Y. W.; Xu P. F.; Hu X.; He J. X.; Wang Q.; Guo C. P. Preparation of CoFe2O4@C nano-composites and their catalytic performance for the thermal decomposition of ammonium perchlorate. Chin. J. Explos. Propell. 2019, 42, 358–362. [Google Scholar]
- Ma Z. Y.; Li C.; Wu R. J.; Chen R. Z.; Gu Z. G. Preparation and characterization of superfine ammonium perchlorate (AP) crystals through ceramic membrane anti-solvent crystallization. J. Cryst. Growth 2009, 311, 4575–4580. 10.1016/j.jcrysgro.2009.06.008. [DOI] [Google Scholar]
- Dolgoborodov A. Y.; Streletskii A. N.; Shevchenko A. A.; Vorobieva G. A.; Val’yano G. E. Thermal decomposition of mechanoactivated ammonium perchlorate. Thermochim. Acta 2018, 669, 60–65. 10.1016/j.tca.2018.09.007. [DOI] [Google Scholar]
- Sanoop A. P.; Rajeev R.; George B. K. Synthesis and characterization of a novel copper chromite catalyst for the thermal decomposition of ammonium perchlorate. Thermochim. Acta 2015, 606, 34–40. 10.1016/j.tca.2015.03.006. [DOI] [Google Scholar]
- Hosseini S. G.; Abazari R.; Gavi A. Pure CuCr2O4 nanoparticles: Synthesis, characterization and their morphological and size effects on the catalytic thermal decomposition of ammonium perchlorate. Solid State Sci. 2014, 37, 72–79. 10.1016/j.solidstatesciences.2014.08.014. [DOI] [Google Scholar]
- Eslami A.; Juibari N. M.; Hosseini S. G. Fabrication of ammonium perchlorate/copper-chromium oxides core-shell nanocomposites for catalytic thermal decomposition of ammonium perchlorate. Mater. Chem. Phys. 2016, 181, 12–20. 10.1016/j.matchemphys.2016.05.064. [DOI] [Google Scholar]
- Lu Y. W.; Zhu Y. F.; Xu P. F.; Ye P.; Gao B.; Sun Y.; Guo C. P. In situ synthesis of cobalt alginate/ammonium perchlorate composite and its low temperature decomposition performance. J. Solid State Chem. 2018, 258, 718–721. 10.1016/j.jssc.2017.12.003. [DOI] [Google Scholar]
- Zhou Z. X.; Tian S. Q.; Zeng D. W.; Tang G.; Xie C. S. MOX (M = Zn, Co, Fe)/AP shell–core nanocomposites for self-catalytical decomposition of ammonium perchlorate. J. Alloys Compd. 2012, 513, 213–219. 10.1016/j.jallcom.2011.10.021. [DOI] [Google Scholar]
- Blaine R. L.; Kissinger H. E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. 10.1016/j.tca.2012.04.008. [DOI] [Google Scholar]
- Ge Z.; Li X.; Zhang W.; Sun Q.; Chai C.; Luo Y. Preparation and characterization of ultrafine Fe-O compound/ammonium perchlorate nanocomposites via in-suit growth method. J. Solid State Chem. 2018, 258, 138–145. 10.1016/j.jssc.2017.10.012. [DOI] [Google Scholar]
- Durrani S. K.; Hussain S. Z.; Saeed K.; Khan Y.; Ahmed N. Hydrothermal synthesis and characterization of nanosized transition metal chromite spinels. Truk. J. Chem. 2012, 36, 111–120. 10.3906/kim-1104-61. [DOI] [Google Scholar]
- Appalakutti S.; Sonawane S.; Bhanvase B. A.; Mittal V.; Ashokkumar M. Process intensification of copper chromite (CuCr2O4) nanoparticle production using continuous flow microreactor. Chem. Eng. Process 2015, 89, 28–34. 10.1016/j.cep.2014.12.012. [DOI] [Google Scholar]
- Patil P. R.; Krishnamurthy V. N.; Joshi S. S. Effect of nano-copper oxide and copper chromite on the thermal decomposition of ammonium perchlorate. Propellants, Explos., Pyrotech. 2008, 33, 266–270. 10.1002/prep.200700242. [DOI] [Google Scholar]
- Singh G.; Kapoor I. P. S.; Dubey S.; Siril P. F. Preparation, characterization and catalytic activity of transition metal oxide nanocrystals. J. Sci. Conf. Proc. 2009, 1, 11–17. 10.1166/jcp.2009.002. [DOI] [Google Scholar]
- Fujimura K.; Miyake A. The effect of specific surface area of TiO2 on the thermal decomposition of ammonium perchlorate. J. Therm. Anal. Calorim. 2010, 99, 27–31. 10.1007/s10973-009-0462-0. [DOI] [Google Scholar]
- Alizadeh G. E.; Shaabani B.; Khodayari A.; Azizian-Kalandaragh Y.; Rahimi R. Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012, 217, 330–339. 10.1016/j.powtec.2011.10.045. [DOI] [Google Scholar]
- Chen L. J.; Li L. P.; Li G. S. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2008, 464, 532–536. 10.1016/j.jallcom.2007.10.058. [DOI] [Google Scholar]
- Hao G. Z.; Li L.; Gou B. W. Preparation of nano-Cu-Cr composite metal oxides via mechanical grinding method and its catalytic performance for the thermal decomposition of ammonium perchlorate. Chin. J. Explos. Propell. 2019, 42, 557–565. [Google Scholar]
- Wang Y. P.; Xia X. Y.; Zhu J. W.; Li Y.; Wang X.; Hu X. D. Catalytic activity of nanometer-sized CuO/Fe2O3 on thermal decomposition of AP and combustion of AP-based propellant. Combust. Sci. Technol. 2010, 183, 154–162. 10.1080/00102202.2010.507561. [DOI] [Google Scholar]
- Cui P.; Wang A. J. Synthesis of CNTs/CuO and its catalytic performance on the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2016, 20, 343–348. 10.1016/j.jscs.2014.09.010. [DOI] [Google Scholar]
- Hao G. Z.; Liu J.; Liu Q. E.; Xiao L.; Ke X.; Gao H.; Du P.; Jiang W.; Zhao F.; Gao H. Facile preparation of AP/Cu(OH)2 core-shell nanocomposites and its thermal decomposition behavior. Propellants, Explos., Pyrotech. 2017, 42, 947–952. 10.1002/prep.201600209. [DOI] [Google Scholar]
- Dhupe A. P.; Gokarn A. N.; Doraiswamy L. K. Investigations into the compensation effect at catalytic gasification of active charcoal by carbon dioxide. Fuel 1991, 70, 839–844. 10.1016/0016-2361(91)90192-D. [DOI] [Google Scholar]