Abstract
The present paper was aimed to investigate the physicochemical properties and combustion characteristics of corn straw briquette as a fuel energy. The results of physicochemical properties displayed that corn straw briquette has higher volatile matter, lower ash content, and higher heating value. Combustion characteristics and kinetic analysis were investigated using thermogravimetry analysis at various heating rates of 10, 15, and 20 °C/min. It was observed that the maximum burning rate shifted to a higher temperature as the heating rate increased. In addition, a lower heating rate would help in better heat transfer, leading to less mass residual. In contrast, the combustion characteristic index showed a nearly 2-fold increase under a higher heating rate, indicating a good combustion performance. The combustion kinetics were expressed using isoconversional methods with Kissinger–Akahira–Sunose and Ozawa–Flynn–Wall methods, which authenticated the average activation energy at 108.85 and 114.42 kJ/mol, respectively. These results can provide a theoretical basis and data support for further utilization of agriculture biomass briquette.
1. Introduction
Facing a serious challenge of the shortage problem of fossil energy coupled with environmental degradation has made the awareness of the development of alternative energy sources an urgent matter. Among various forms of renewable energy, biomass has captured attention for its huge resources, low cost, renewable nature, and low emissions.1−3 It can also become the future source of energy for its low carbon dioxides and sulfur oxides than fossil fuels.4 Biomass ranks fourth after coal, oil, and gas, providing approximately 14% of the global energy demand.5 Agriculture and forestry residues, livestock manure, municipal solid waste, sewage, wastewater, and energy plants are the main sources of biomass. China is an agricultural country, and a large amount of biomass is produced every year, which equals 460 million tons of standard coal.1 However, the low density and high costs of handling, transportation, and storage restricted its further utilization.6−9 They are combusted directly without the optimization of energy efficiency, or they are left on farming area, potentially causing loss of energy and air and water pollution.10 One effective way to solve these problems is densification of biomass into pellet or briquette. More generally, it is a physical change process of the crushed irregular shape biomass material rearranged by the mechanical and plastic deformation under specific temperature, pressure, and moisture content. Compared to raw material, the made pellet or briquette has the advantage of higher density and heating value, lower carbon dioxide and sulfur dioxide emissions, and reducing release of toxic and greenhouse gases. Meanwhile, the increased density and decreased volume reduce transport and storage costs.
Nowadays, some rural areas in China are using scattered coal for heating. However, the emissions were 10 times that of coal-fired power plants. One or more China policies point to focus on the development of biomass heating, while solving the environmental problems caused by the direct burning of scattered coal and agroforestry waste. Hence, the utilization of pellets or briquette fuel as an alternative fuel has a well future. However, accurate combustion kinetic models are essential to the full utilization. Combustion kinetics focuses on the combustion reaction mechanism, combustion reaction rate, and influencing factors, which will help to predict the combustion efficiency, combustion parameters, and pollutant emissions. It can provide an accurate chemical mechanism for the actual combustion situation and optimize the burner structure and operation parameters.
Thermogravimetry analysis (TGA) is one of the most common methods and has received great attention in the understanding of solid fuel substrate degradation to release energy.11 In addition, some studies have been carried out on the combustion characteristics of biomass solid fuel and biomass blends, such as cotton refuse,12 pine and corn straw,13 microalgal,14 pinewood sawdust,15 Azadirachta indica seeds and Phyllanthus emblica kernel,16 red pepper,17 rice husk,18 camphor,19 elephant grass,20 hazelnut husk,4 soybean straw,21 and banana peel.22
As mentioned above, researchers mostly focus on the study of biomass materials or pellets, while rarely mentioning the biomass briquette. Subsequently, the present paper purposed to enrich the studies to analyze the combustion characteristics of biomass fuel. In this paper, TGA was applied to evaluate the combustion characteristics of corn straw briquette at a heating rate of 10, 15, 20 °C/min. The isoconversional methods, Kissinger–Akahira–Sunose (KAS) and Ozawa–Flynn–Wall (OFW), were used for calculating kinetic parameters. The results can be provided for further utilization and thermochemical conversion of agriculture biomass briquettes.
2. Results and Discussion
2.1. Biomass Briquette Characteristics
Various studies confirmed that the thermochemical process largely depends on biomass characteristics.23−26 High volatile content matter facilitates the formation of more pyrolytic liquid and gaseous products. McKendry reported that the lower the ratios of O/C and H/C are, the greater the energy content of the biomass is.24 In addition, studies showed that less amount of N and S made it more environmentally friendly. Table 1 presents the proximate and ultimate analysis of the CSB. As can be seen from Table 1, the moisture content was 10.20%, indicating their suitability for combustion because the moisture content was around 10%.27 The amount of volatile matter in the CSB has 69.65%. The volatile matter can affect the ignition temperature during combustion.28 Thus, the CSB can ignite earlier and burn faster. It can also be observed that CSB has lower ash content, 8.84%. An unfavorable relationship between higher ash content and combustion tends to make poor combustion, lower heating value, as well as slagging, which is harmful to the furnace.
Table 1. Ultimate and Proximate Analysis.
| ultimate
analysis (%) |
proximate
analysis (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| item | C | H | O | N | S | moisture | volatile matter | fixed carbon | ash |
| CSB | 42.95 | 6.68 | 40.82 | 0.71 | 0 | 10.20 | 69.65 | 11.31 | 8.84 |
In addition, the ultimate analysis results showed that the main components were C and O elements, which were shown to be 42.95 and 40.82%. Higher O content was mainly because of the highly oxygenated content, such as cellulose, hemicellulose, lignin, and other substances.23 The H/C and O/C ratio is 0.15 and 0.95, respectively, resulting in an HHV of 16.79 MJ/kg, which is higher than the heating value of the pellet obtained in our previous literature.29 Thus, the made briquette can be considered to be a good fuel energy resource. However, the H, N, and S elements were found to be lower, total less than 10%. The lower N and trace S content reflected the low NOx and SOx emissions during combustion. It also indicated that the CSB is a valuable fuel for thermal utilization.
2.2. Thermogravimetric Analysis
TGA has been a mature technique for studying combustion behavior.30Figure 1 presents the TG and derivative thermogravimetric (DTG) curves of CSB under different heating rates. The TG curves revealed that changes in weight loss with increasing temperature. In addition, DTG was derived from TG, which shows the derivative weight loss curves. During combustion, the decomposition of hemicellulose and cellulose firstly generates volatile matter and separates. Then, as the ignition temperature is reached, the volatile matter will burn, followed by the combustion of coke produced by the lignin.31,32
Figure 1.
TG (a) and DTG (b) curves of CSB at different heating rates.
Figure 1a,b shows that the combustion process of CSB consists of three stages of thermal degradation. The weak peak in the DTG curve indicates that the mass loss in the first stage corresponds to the removal of moisture content, namely, the dehydration stage. Sometimes, it is also accompanied by a loss of the very small amounts of volatile matters. For the heating rate, 10 and 15 °C/min, they were started at 30 °C and ended at 150 and 155 °C, leading to a mass loss of 5.7 and 4.5%, respectively. Different from the above, the heating rate of 20 °C/min started at 30 °C and ended at 175 °C, leading to a mass loss of 6.7%. It can be seen from Figure 1b that the maximum mass loss in the first stage for the heating rate of 10 and 15 °C/min was 1.03 and 1.10%/min, which occurred at approximately 85 °C, respectively. In contrast, the maximum weight loss is 2.04%/min for a heating rate of 20 °C/min, which occurred at 100 °C. Next are considered as the main reaction stage during combustion. The second stage corresponds to the combustion of volatile matter generated by the decomposition of hemicellulose, cellulose. The thermal degradation occurred at various temperature zones ranging from 150 to 345 °C, 155 to 355 °C, and 175 to 375 °C for the heating rates of 10, 15, and 20 °C/min, contributing to 48.79, 49.37, and 47.00% of the mass loss, respectively. The DTG curve reveals that the maximum mass loss in stage 2 was 8.96, 11.31, and 13.95%/min for the heating rate of 10, 15, and 20 °C/min, which occurred at a temperature of 278, 290, and 298 °C, respectively. Those conclusions are also similar to the study of Xu et al., Braga et al., and Ahmad et al.26,33,34 The third stage ranging from 345 to 510 °C, 355 to 545 °C, and 375 to 570 °C under heating rate 10, 15, and 20 °C/min, resulting in the mass loss of 33.88, 33.07, and 29.68%/min, respectively. It was due to the combustion of the char remaining after devolatilization of the samples.
The combustion characteristic parameters, including ignition temperature, burnout temperature, and so forth, obtained from the graph are summarized in Table 2. As presented in Table 2, the data showed clearly that nearly all the parameters increase with the rise of heating rate. While compared to 10 °C/min, the Sn value of CSB increased by 1.5 and 1.9 times at a heating rate of 15 and 20 °C/min, respectively, indicating that the heating rate contributes to the improvement of the combustion performance of CSB.
Table 2. Combustion Characteristic Parameters.
| heating rate (°C/min) | 10 | 15 | 20 |
| ignition temperature (°C) | 241 | 248 | 248 |
| burnout temperature (°C) | 343 | 368 | 393 |
| maximum burning rate (%/min) | 8.96 | 11.31 | 13.95 |
| average burning rate (%/min) | 0.042 | 0.056 | 0.062 |
| combustion characteristic index (10–8) | 1.9 | 2.8 | 3.6 |
2.3. Effect of Heating Rates
It is reported that the heating rate has a significant effect on the combustion process. It can affect the maximum decomposition rate, DTG peak temperature, and final residual mass after the combustion.35−37 Depending on the heating rate, the burnouts are achieved quicker at a lower temperature or later at a higher temperature.11
Figure 1a,b vividly describes the effect of heating rate on the biomass briquette combustion at various heating rates of 10, 15, and 20 °C/min. Table 3 reveals that as the heating rate rises from 10 to 20 °C/min, the maximum decomposition rate increases from 8.95 to 13.95%/min, and the DTG peak temperature rises from 278 to 298 °C, which is similar to the study of Jayaraman et al.,38 in which the peak temperature of DTG curves was shifted to a higher value with an increase in heating rate. This is mainly due to the increase in heating rate; there is a certain difference between the internal and external temperatures of the experimental sample. It takes a long time to transfer the external heat to the internal. Thus, the hysteresis effect occurred.
Table 3. Maximum Decomposition Rate, DTG Peak Temperature, and Final Residual Mass at Different Heating Rates.
| heating rate (°C/min) | maximum decomposition rate (%/min) | peak temperature (°C) | residual mass (%) |
|---|---|---|---|
| 10 | 8.95 | 278 | 14.91 |
| 15 | 11.31 | 290 | 16.30 |
| 20 | 13.95 | 298 | 17.32 |
It can be seen from Table 3 that the residual mass increases from 14.91 to 17.32% with the rise of heating rate from 10 to 20 °C/min. It was due to the fact that the lower heating rate helps transfer heat into the interior of CSB, resulting in better combustion. This is also the reason why the termination temperature of every stage observed in Part 2.2 increases with the rise of the heating rate.
2.4. Kinetic Analysis
The combustion process can be displayed through the activation energy ranges with the conversion degree. Here, the activation energy is the energy required to transform molecules from normality to an active state in which the reaction can easily occur. Briefly, it is the minimum energy required for a reaction, whose reaction process takes place easily with lower activation energy than that of higher activation energy.
As mentioned above,
the kinetic parameters were determined using isoconversional methods
KAS and OFW based on the thermogravimetric analysis data obtained
under different heating rates. Figure 2 shows the linear fitting plots of CSB by KAS and OFW
methods. Figure 2a
shows the linear fitting plot of 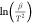 versus
versus  at the various conversion rates.
Similarly, Figure 2b gives the linear
fitting plot of ln(β) versus
at the various conversion rates.
Similarly, Figure 2b gives the linear
fitting plot of ln(β) versus 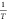 at various conversion degrees.
The conversion
rate was chosen ranging from 0.1 to 0.8 with a step size of 0.1. For
the low coefficient of determination (R2), a conversion rate beyond 0.8 was not considered. The calculated
activation energies from the slopes and intercepts of the plots are
listed in Table 4.
at various conversion degrees.
The conversion
rate was chosen ranging from 0.1 to 0.8 with a step size of 0.1. For
the low coefficient of determination (R2), a conversion rate beyond 0.8 was not considered. The calculated
activation energies from the slopes and intercepts of the plots are
listed in Table 4.
Figure 2.
Linear fitting at various α by (a) KAS and (b) OFW methods.
Table 4. Calculated Activation Energies of CSB.
| KAS |
OFW |
||||
|---|---|---|---|---|---|
| conversion rate (α) | E (kJ/mol) | R2 | E (kJ/mol) | R2 | difference (%) |
| 0.1 | 85.67 | 0.99 | 88.54 | 0.99 | 3.35 |
| 0.2 | 90.34 | 0.99 | 92.12 | 0.99 | 1.97 |
| 0.3 | 93.14 | 0.99 | 96.93 | 0.99 | 4.07 |
| 0.4 | 102.15 | 0.99 | 109.07 | 0.99 | 6.77 |
| 0.5 | 106.58 | 0.99 | 114.84 | 0.99 | 7.75 |
| 0.6 | 110.95 | 0.97 | 116.88 | 0.98 | 5.34 |
| 0.7 | 138.95 | 0.99 | 145.86 | 0.99 | 4.97 |
| 0.8 | 143.01 | 0.97 | 151.15 | 0.99 | 5.69 |
| average | 108.85 | 114.42 | 5.12 | ||
From Table 4, it can be seen that all the coefficients of determination are larger than 0.97, which indicated the accuracy of the isoconversional methods. In other words, the results of activation energies calculated by the KAS and OFW methods were reliable. The mean activation energy was calculated as 108.85 kJ/mol of the KAS method and 114.42 kJ/mol of the OFW method, respectively. Comparing the two methods, it shows that the difference in average activation energy between KAS and OFW methods was 5.12%. This is consistent with other literature, such as hazelnut husk (7.32%).4
The relationship between the activation energy versus the conversion rate is depicted in Figure 3. It can be observed that changes in the activation energy deeply depended on the conversion rate, reflecting that the combustion process was complex.35 As shown in Figure 3, the activation energy increased with the increase of conversion rate, regardless of the KAS or OFW method. With the increase of the conversion rate from 0.1 to 0.8, the activation energy ranged from 85.67 to 143.01 kJ/mol for the KAS method and 88.54 to 151.15 kJ/mol for the OFW method. The change in activation energy with the degree of conversion is essentially the result of thermal degradation of different components of biomass with increasing temperature. The weaker bonds (lower molecular weight compounds) decayed at moderate energy and lesser temperature, while degradation of stronger bonds (higher molecular weight products) needed more energy at greater temperature.
Figure 3.
Activation energy vs conversion rate.
Generally, activation energy is regarded as the minimum energy requirement during the reaction.4 Lower activation energy means faster reaction and vice versa. The kinetic parameter variation with conversion rate is referred to biomass heterogeneity. That is to say, each component has its particular characteristics, thus causing the different phenomenon of various biomass briquette.39 The interplay of multiple reaction mechanisms leads to a change in activation energy with the degree of conversion. For CSB, the increasing higher activation energy might be caused by the decomposition of chars. A study of Oyedun et al. showed that the chars in biomass had a higher activation energy.40
3. Conclusions
In this study, the combustion and thermal characteristics of corn straw briquette under three heating rates using TGA were investigated. The higher heating value and lower sulfur and nitrogen indicated that the corn straw briquette was a potential fuel. The less residual after combustion in lower heating rate was due to the better heat transfer to the interior of the corn straw briquette. With increasing heating temperature, the maximum thermal decomposition temperature shifted to a higher temperature. In addition, it also leads to the improvement of combustion performance. The combustion kinetics reveals a similar mean activation energy, 108.85 kJ/mol, calculated from the KAS method, and 114.42 kJ/mol calculated from the OFW method. High relation between conversion degree and activation energy indicated the complex combustion process. Based on these above conclusions, it provides the theoretical foundation for future application of corn straw briquette as a fuel resource of energy.
4. Materials and Methods
4.1. Materials
Agriculture biomass (corn straw) was locally collected in Zibo city (36.81 °N, 118.05 °E), Shandong province, China. The fresh feedstocks were air-dried for 7 days and then crushed and sieved to less than 0.5 mm particle size. Subsequently, they were dried at 105 °C for 12 h to a constant weight in a drying cabinet and later stored in the ventilated surroundings for briquette preparation.
The briquettes were obtained using a ram compression molding machine described in our patent.23 Briefly, the raw material was introduced to the forming mold by the screw conveyor and then stroked by a reciprocating plunger to form a briquette. Figure 4 shows a photograph of briquette. For convenience, corn straw briquette was abbreviated as CSB.
Figure 4.

Morphology of CSB.
4.2. Characteristic Analysis
Proximate analysis for CSB was carried out according to the Chinese standard GB/T 28731-2012. Ultimate analysis of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) was determined using the elemental analyzer (Vario EL Cube). The weight percent of oxygen (O) was determined by difference according to eq 1.
| 1 |
All the proximate and ultimate analysis results are presented in Table 1.
4.3. Thermogravimetry Analysis
The combustion was conducted using a thermogravimetric analyzer (Netzsch, STA 449 F5). Approximately 10 mg sample was placed in an aluminum crucible and heated from room temperature (about 26 °C) to 1000 °C with three heating rates (10, 15, and 20 °C/min). The airflow rate was maintained at 20 mL/min.
4.3.1. Combustion Characteristic Parameters
The combustion characteristic parameters mainly refer to ignition temperature (Ti), burnout temperature (Tf), and combustion characteristic index (SN). They were defined by analyzing the thermogravimetric (TG) curve and DTG curve.
The ignition temperature (Ti) and burnout temperature (Tf) were determined by the TG–DTG joint definition method as presented in Figure 5.41,42 First, a vertical green line was plotted through the peak point of the DTG curve to meet the TG curve. Then, a tangent was made to the intersection. Finally, the temperature corresponding to the intersection of the tangent and upper horizontal blue line (it was defined as the TG curve begins to lose weight) was recorded as the ignition temperature.
Figure 5.
Determination of ignition temperature (Ti) and burnout temperature (Tf).
Similarly, the temperature corresponding to the intersection of the tangent and the lower horizontal blue line (it was defined as the end of weight loss of the TG curve) was recorded as the burnout temperature.
As an important indicator of combustion performance, the combustion characteristic index (SN) was calculated according to eq 2.43,44
| 2 |
where (dα/dt)max (%/min) is the maximum burning rate, (dα/dt)mean (%/min) is the average burning rate, and Ti and Tf are the ignition and burnout temperatures, respectively.
Meanwhile, the average burning rate was determined as eq 3.
| 3 |
where β is the heating rate, αi is the percentage of the remaining samples corresponding to ignition temperature, and αf is the percentage of the remaining samples corresponding to burnout temperature.
4.3.2. Study of Kinetic Modeling
During thermal decomposition, the reaction process can be described as follows
The precipitation rate of volatiles, also named the reaction rate, can be expressed as follows
| 4 |
where α is the rate of conversion during
thermal decomposition,  is the reaction rate, t is time, T is the absolute temperature, k(T) is the temperature-dependent rate
constant, and f(α) is the reaction model.
is the reaction rate, t is time, T is the absolute temperature, k(T) is the temperature-dependent rate
constant, and f(α) is the reaction model.
Moreover, α can be written as follows
| 5 |
where mi is the initial mass of the experimental sample before combustion, mt is the mass of experimental sample at any time t during combustion, and mf is the final mass of the experimental sample at the end of the combustion.
Here, introducing the Arrhenius laws
| 6 |
where A is the pre-exponential factor, E (J/mol) is the activation energy of the combustion, R (J/k·mol) is the gas constant, and T (K) is the absolute temperature.
Thus, combining eqs 4 and 6, it then can be rearranged as follows
| 7 |
In addition, the temperature increases
with a constant heating
value. Then, take  into eq 7. Hence, eq 7 can be rearranged as follows
into eq 7. Hence, eq 7 can be rearranged as follows
| 8 |
Following is the integration function of eq 8.
4.4. KAS Method
The KAS method is presented as follows45,46
While plotting
the  vs
vs 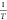 , a straight line can be drawn
and the slope
of the fitted curve is equal to
, a straight line can be drawn
and the slope
of the fitted curve is equal to  , then E can be calculated.
, then E can be calculated.
4.5. OFW Method
The OFW method is presented as follows47,48
where the plot
of lnβ against  can be drawn, and the slope of
the fitted
curve is equal to
can be drawn, and the slope of
the fitted
curve is equal to 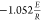 , then E can be calculated.
, then E can be calculated.
Acknowledgments
The authors thank the financial support by the Foundation of Rikaze Science and Technology Plan Project (RKZ2020KJ03).
The authors declare no competing financial interest.
References
- Liu J.; Cheng W.; Jiang X.; Khan M. U.; Zhang Q.; Cai H. Effect of extractives on the physicochemical properties of biomass pellets: Comparison of pellets from extracted and non-extracted sycamore leaves. BioResources 2020, 15, 544–556. 10.15376/biores.15.1.544-556. [DOI] [Google Scholar]
- Zhou C.; Liu G.; Wang X.; Qi C. Co-combustion of bituminous coal and biomass fuel blends: Thermochemical characterization, potential utilization and environmental advantage. Bioresour. Technol. 2016, 218, 418–427. 10.1016/j.biortech.2016.06.134. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Wang G.; Zhang J.; Lee J.-Y.; Wang H.; Wang C. Combustion behaviors and kinetics analysis of coal, biomass and plastic. Thermochim. Acta 2018, 669, 140–148. 10.1016/j.tca.2018.09.016. [DOI] [Google Scholar]
- Ceylan S.; Topçu Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. 10.1016/j.biortech.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Sudha P.; Ravindranath N. H. Land availability and biomass production potential in India. Biomass Bioenergy 1999, 16, 207–221. 10.1016/S0961-9534(98)00083-X. [DOI] [Google Scholar]
- Pradhan P.; Mahajani S. M.; Arora A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. 10.1016/j.fuproc.2018.09.021. [DOI] [Google Scholar]
- Saidur R.; Abdelaziz E. A.; Demirbas A.; Hossain M. S.; Mekhilef S. A review on biomass as a fuel for boilers. Renew. Sust. Energ. Rev. 2011, 15, 2262–2289. 10.1016/j.rser.2011.02.015. [DOI] [Google Scholar]
- Whittaker C.; Shield I. Factors affecting wood, energy grass and straw pellet durability - A review. Renew. Sust. Energ. Rev. 2017, 71, 1–11. 10.1016/j.rser.2016.12.119. [DOI] [Google Scholar]
- Kaliyan N.; Vance Morey R. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy 2009, 33, 337–359. 10.1016/j.biombioe.2008.08.005. [DOI] [Google Scholar]
- Muazu R. I.; Stegemann J. A. Effects of operating variables on durability of fuel briquettes from rice husks and corn cobs. Fuel Process. Technol. 2015, 133, 137–145. 10.1016/j.fuproc.2015.01.022. [DOI] [Google Scholar]
- Islam M. A.; Auta M.; Kabir G.; Hameed B. H. A thermogravimetric analysis of the combustion kinetics of karanja (Pongamia pinnata) fruit hulls char. Bioresour. Technol. 2016, 200, 335–341. 10.1016/j.biortech.2015.09.057. [DOI] [PubMed] [Google Scholar]
- Haykırı-Açma H. Combustion characteristics of different biomass materials. Energy Convers. Manage. 2003, 44, 155–162. 10.1016/S0196-8904(01)00200-X. [DOI] [Google Scholar]
- Mian I.; Li X.; Dacres O. D.; Wang J.; Wei B.; Jian Y.; Zhong M.; Liu J.; Ma F.; Rahman N. Combustion kinetics and mechanism of biomass pellet. Energy 2020, 205, 117909. 10.1016/j.energy.2020.117909. [DOI] [Google Scholar]
- Bach Q.-V.; Chen W.-H. A comprehensive study on pyrolysis kinetics of microalgal biomass. Energy Convers. Manage. 2017, 131, 109–116. 10.1016/j.enconman.2016.10.077. [DOI] [Google Scholar]
- Ma P.; Yang J.; Xing X.; Weihrich S.; Fan F.; Zhang X. Isoconversional kinetics and characteristics of combustion on hydrothermally treated biomass. Renewable Energy 2017, 114, 1069–1076. 10.1016/j.renene.2017.07.115. [DOI] [Google Scholar]
- Mishra R. K.; Mohanty K. Kinetic analysis and pyrolysis behaviour of waste biomass towards its bioenergy Potential. Bioresour. Technol. 2020, 311, 123480. 10.1016/j.biortech.2020.123480. [DOI] [PubMed] [Google Scholar]
- Maia A. A. D.; de Morais L. C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. 10.1016/j.biortech.2015.12.055. [DOI] [PubMed] [Google Scholar]
- Yuan R.; Yu S.; Shen Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. 10.1016/j.wasman.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Jiang L.; Yuan X.; Li H.; Xiao Z.; Liang J.; Wang H.; Wu Z.; Chen X.; Zeng G. Pyrolysis and combustion kinetics of sludge-camphor pellet thermal decomposition using thermogravimetric analysis. Energy Convers. Manage. 2015, 106, 282–289. 10.1016/j.enconman.2015.09.046. [DOI] [Google Scholar]
- Braga R. M.; Costa T. R.; Freitas J. C. O.; Barros J. M. F.; Melo D. M. A.; Melo M. A. F. Pyrolysis kinetics of elephant grass pretreated biomasses. J. Therm. Anal. Calorim. 2014, 117, 1341–1348. 10.1007/s10973-014-3884-2. [DOI] [Google Scholar]
- Huang X.; Cao J.-P.; Zhao X.-Y.; Wang J.-X.; Fan X.; Zhao Y.-P.; Wei X.-Y. Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 2016, 169, 93–98. 10.1016/j.fuel.2015.12.011. [DOI] [Google Scholar]
- Tahir M. H.; Zhao Z.; Ren J.; Rasool T.; Naqvi S. R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. 10.1016/j.biombioe.2019.01.009. [DOI] [Google Scholar]
- Cheng W. D.; Liu J. B.; Cai H. Z.; Jiang X. Y.; Yi W. M.. Flat-die plunger type biomass solidifying forming machine. CN 201910715788.7, 2020.
- Mckendry P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. 10.1016/S0960-8524(01)00118-3. [DOI] [PubMed] [Google Scholar]
- Ahmad M. S.; Mehmood M. A.; Al Ayed O. S.; Ye G.; Luo H.; Ibrahim M.; Rashid U.; Arbi Nehdi I.; Qadir G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. 10.1016/j.biortech.2016.10.090. [DOI] [PubMed] [Google Scholar]
- Ahmad M. S.; Mehmood M. A.; Ye G.; Al-Ayed O. S.; Ibrahim M.; Rashid U.; Luo H.; Qadir G.; Nehdi I. A. Thermogravimetric analyses revealed the bioenergy potential of Eulaliopsis binata. J. Therm. Anal. Calorim. 2017, 130, 1237–1247. 10.1007/s10973-017-6398-x. [DOI] [Google Scholar]
- Boubacar Laougé Z.; Merdun H. Pyrolysis and combustion kinetics of Sida cordifolia L. using thermogravimetric analysis. Bioresour. Technol. 2020, 299, 122602. 10.1016/j.biortech.2019.122602. [DOI] [PubMed] [Google Scholar]
- Toptas A.; Yildirim Y.; Duman G.; Yanik J. Combustion behavior of different kinds of torrefied biomass and their blends with lignite. Bioresour. Technol. 2015, 177, 328–336. 10.1016/j.biortech.2014.11.072. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Cheng W.; Liu J.; Xu H.; Zhang D.; Zheng Y.; Cai H. Effect of moisture content during preparation on the physicochemical properties of pellets made from different biomass materials. BioResources 2020, 15, 557–573. 10.15376/biores.15.1.557-573. [DOI] [Google Scholar]
- Zhou Z.; Hu X.; You Z.; Wang Z.; Zhou J.; Cen K. Oxy-fuel combustion characteristics and kinetic parameters of lignite coal from thermo-gravimetric data. Thermochim. Acta 2013, 553, 54–59. 10.1016/j.tca.2012.11.030. [DOI] [Google Scholar]
- Worasuwannarak N.; Sonobe T.; Tanthapanichakoon W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J. Anal. Appl. Pyrolysis 2007, 78, 265–271. 10.1016/j.jaap.2006.08.002. [DOI] [Google Scholar]
- Yan R.; Yang H.; Chin T.; Liang D. T.; Chen H.; Zheng C. Influence of temperature on the distribution of gaseous products from pyrolyzing palm oil wastes. Combust. Flame 2005, 142, 24–32. 10.1016/j.combustflame.2005.02.005. [DOI] [Google Scholar]
- Xu Y.; Chen B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. 10.1016/j.biortech.2013.07.086. [DOI] [PubMed] [Google Scholar]
- Braga R. M.; Melo D. M. A.; Aquino F. M.; Freitas J. C. O.; Melo M. A. F.; Barros J. M. F.; Fontes M. S. B. Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J. Therm. Anal. Calorim. 2014, 115, 1915–1920. 10.1007/s10973-013-3503-7. [DOI] [Google Scholar]
- Bhattacharjee N.; Biswas A. B. Physicochemical analysis and kinetic study of orange bagasse at higher heating rates. Fuel 2020, 271, 117642. 10.1016/j.fuel.2020.117642. [DOI] [Google Scholar]
- Ranjith Kumar R.; Ramesh D.; Mutanda T.; Rawat I.; Bux F. Thermal Behavior and Pyrolytic Characteristics of FreshwaterScenedesmussp. Biomass. Energy Sources, Part A 2015, 37, 1383–1391. 10.1080/15567036.2011.605428. [DOI] [Google Scholar]
- Thakur L. S.; Varma A. K.; Mondal P. Analysis of thermal behavior and pyrolytic characteristics of vetiver grass after phytoremediation through thermogravimetric analysis. J. Therm. Anal. Calorim. 2018, 131, 3053–3064. 10.1007/s10973-017-6788-0. [DOI] [Google Scholar]
- Jayaraman K.; Kök M. V.; Gökalp I. Combustion mechanism and model free kinetics of different origin coal samples: Thermal analysis approach. Energy 2020, 204, 117905. 10.1016/j.energy.2020.117905. [DOI] [Google Scholar]
- Rahib Y.; Sarh B.; Bostyn S.; Bonnamy S.; Boushaki T.; Chaoufi J. Non-isothermal kinetic analysis of the combustion of argan shell biomass. Mater. Today: Proc. 2020, 24, 11–16. 10.1016/j.matpr.2019.07.437. [DOI] [Google Scholar]
- Oyedun A. O.; Tee C. Z.; Hanson S.; Hui C. W. Thermogravimetric analysis of the pyrolysis characteristics and kinetics of plastics and biomass blends. Fuel Process. Technol. 2014, 128, 471–481. 10.1016/j.fuproc.2014.08.010. [DOI] [Google Scholar]
- Yuanyuan Z.; Yanxia G.; Fangqin C.; Kezhou Y.; Yan C. Investigation of combustion characteristics and kinetics of coal gangue with different feedstock properties by thermogravimetric analysis. Thermochim. Acta 2015, 614, 137–148. 10.1016/j.tca.2015.06.018. [DOI] [Google Scholar]
- Ma B.-G.; Li X.-G.; Xu L.; Wang K.; Wang X.-G. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Thermochim. Acta 2006, 445, 19–22. 10.1016/j.tca.2006.03.021. [DOI] [Google Scholar]
- Essenhigh R. H.; Misra M. K.; Shaw D. W. Ignition of coal particles: a review. Combust. Flame 1989, 77, 3–30. 10.1016/0010-2180(89)90101-6. [DOI] [Google Scholar]
- Zhao D.; Zhang J.; Wang G.; Conejo A. N.; Xu R.; Wang H.; Zhong J. Structure characteristics and combustibility of carbonaceous materials from blast furnace flue dust. Appl. Therm. Eng. 2016, 108, 1168–1177. 10.1016/j.applthermaleng.2016.08.020. [DOI] [Google Scholar]
- Akahira T.; Sunuse T. T.. Joint Convention of Four Electrical Institutes, Research Report; Chiba Institute of Technology, 1971; Vol. 16; pp 22–31. [Google Scholar]
- Kissinger H. E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. 10.1021/ac60131a045. [DOI] [Google Scholar]
- Ozawa T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. 10.1246/bcsj.38.1881. [DOI] [Google Scholar]
- Flynn J. H.; Wall L. A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci., Part B: Polym. Lett. 1966, 4, 323–328. 10.1002/pol.1966.110040504. [DOI] [Google Scholar]







